Development of Graphene Oxide-Based Anticancer Drug Combination Functionalized with Folic Acid as Nanocarrier for Targeted Delivery of Methotrexate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
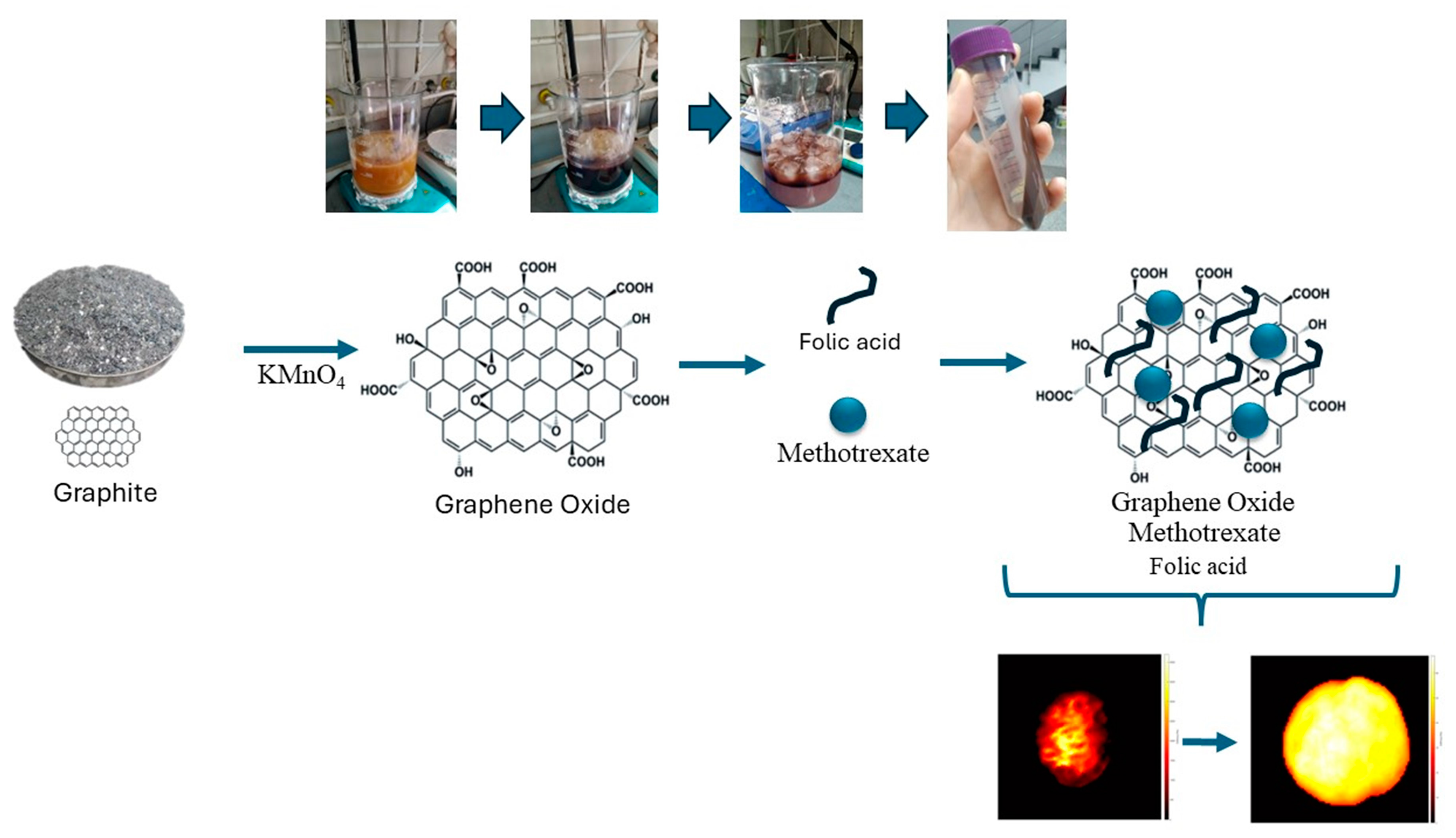
2.2. Synthesis of Graphene Oxide (GO)
2.3. Drug Loading (MTX) on GO and Conjugation of Folic Acid (FA)
2.4. Characterization of GO and Drug Delivery System
2.4.1. Particle Size and Zeta Potential Analysis
2.4.2. Morphology of the GO and Drug Delivery System
Scanning Electron Microscopy (SEM) Imaging
Transmission Electron Microscopy (TEM) Imaging
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.4. Differential Scanning Calorimetric Analysis (DSC)
2.5. In Vitro Study: Franz Diffusion Drug Delivery Kinetics
2.6. Stiffness
2.7. Cell Culture
2.8. Cytotoxicity Experiment
3. Results and Discussion
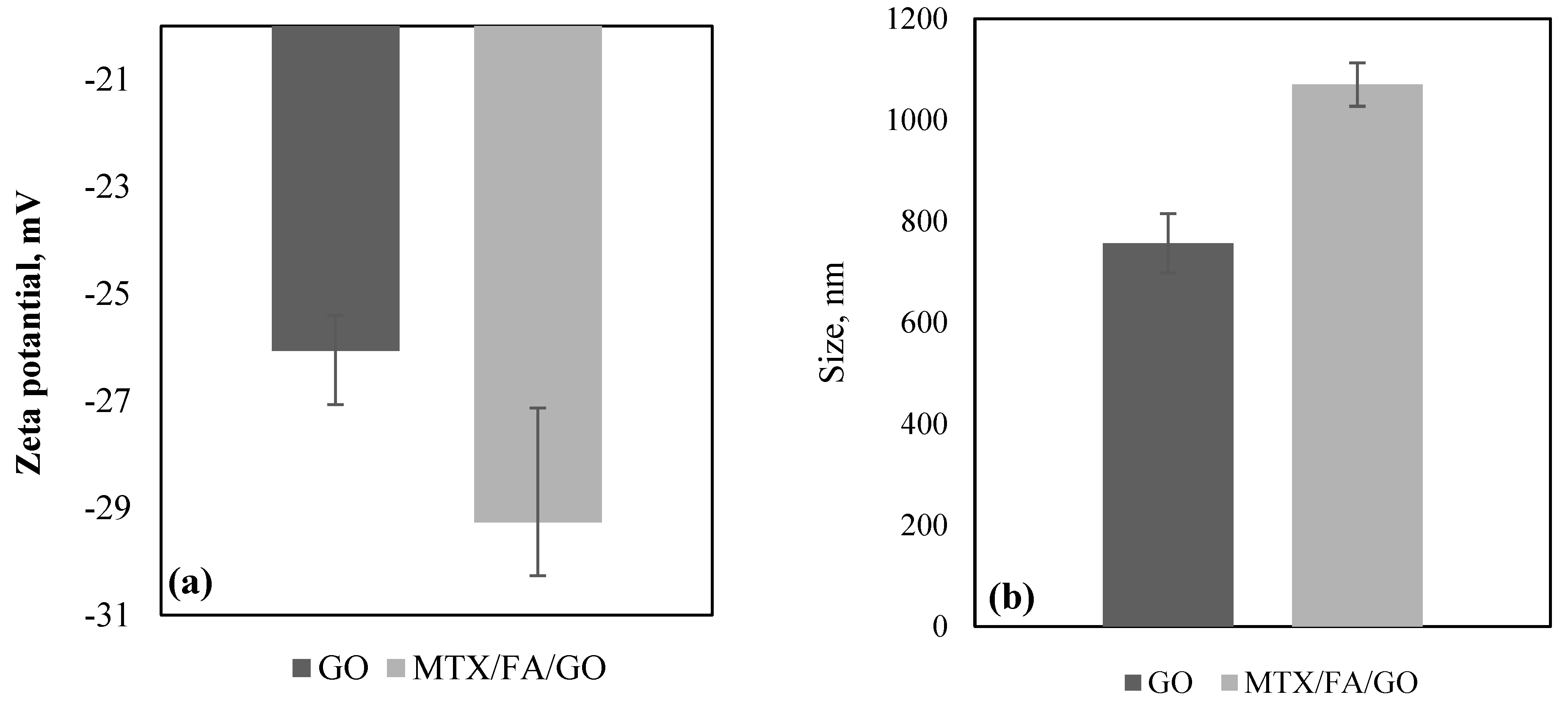
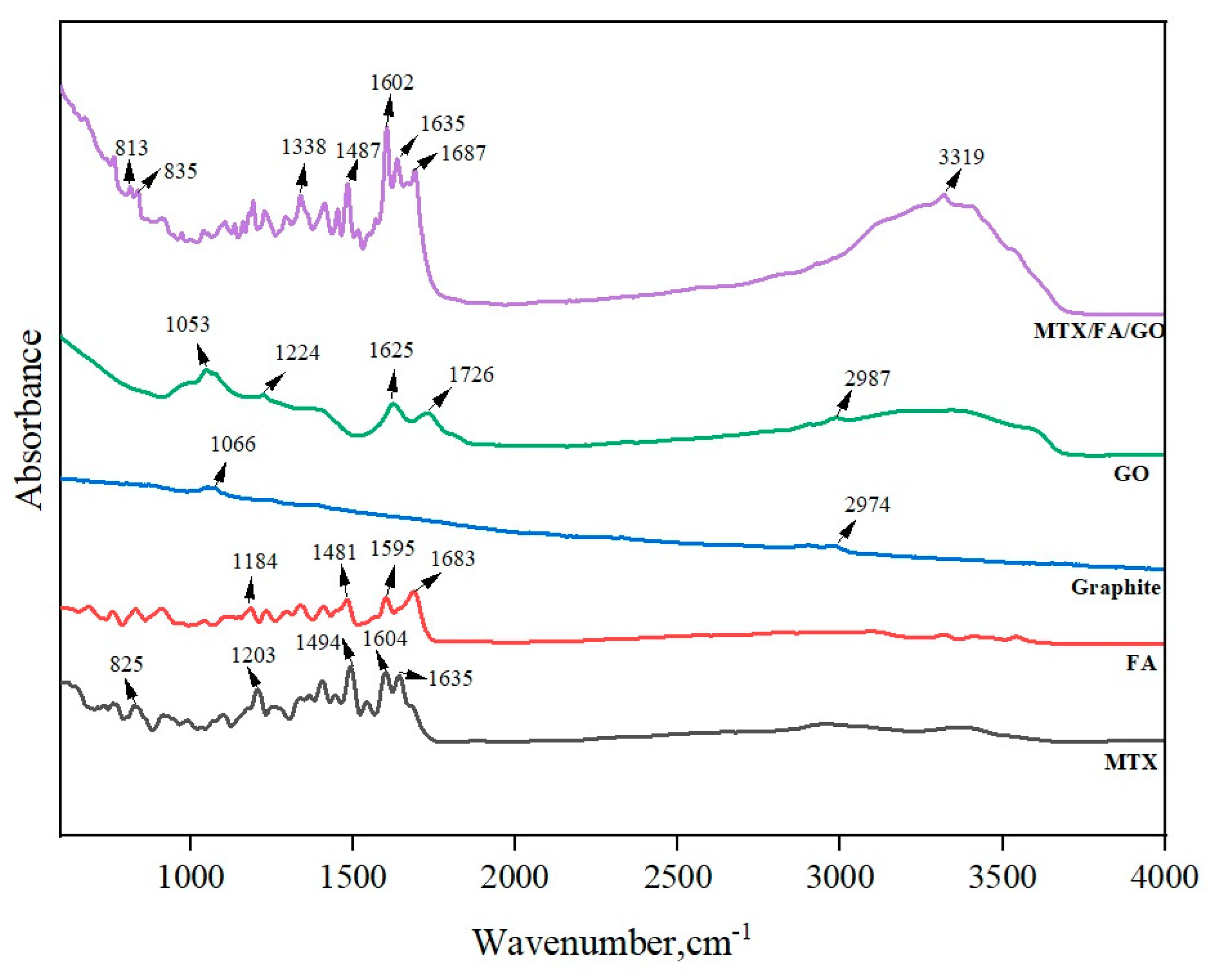
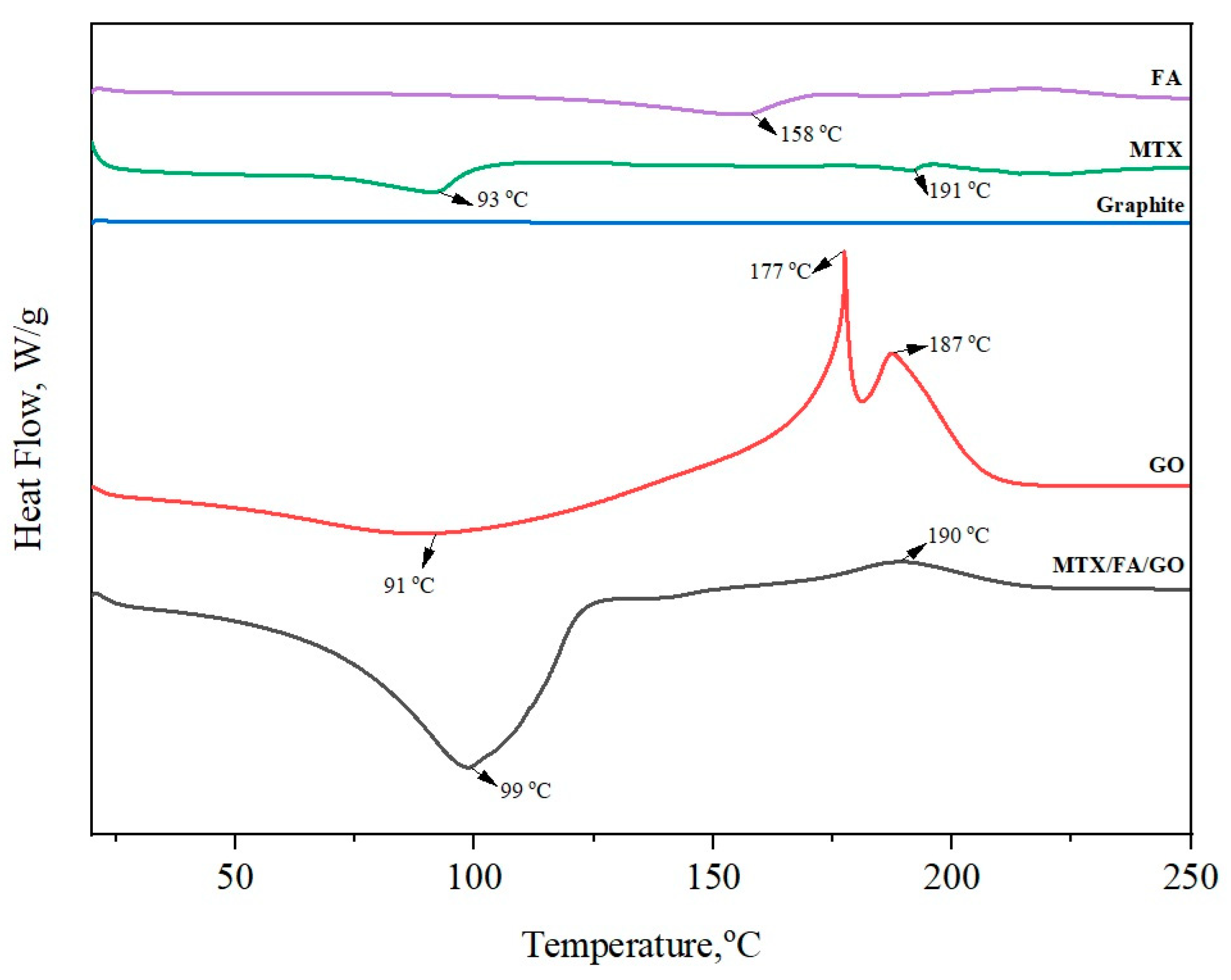
3.1. Zeta Potential, Size, and Morphology of the GO and Drug Delivery System
3.2. In Vitro Study: Franz Diffusion Drug Delivery Kinetics
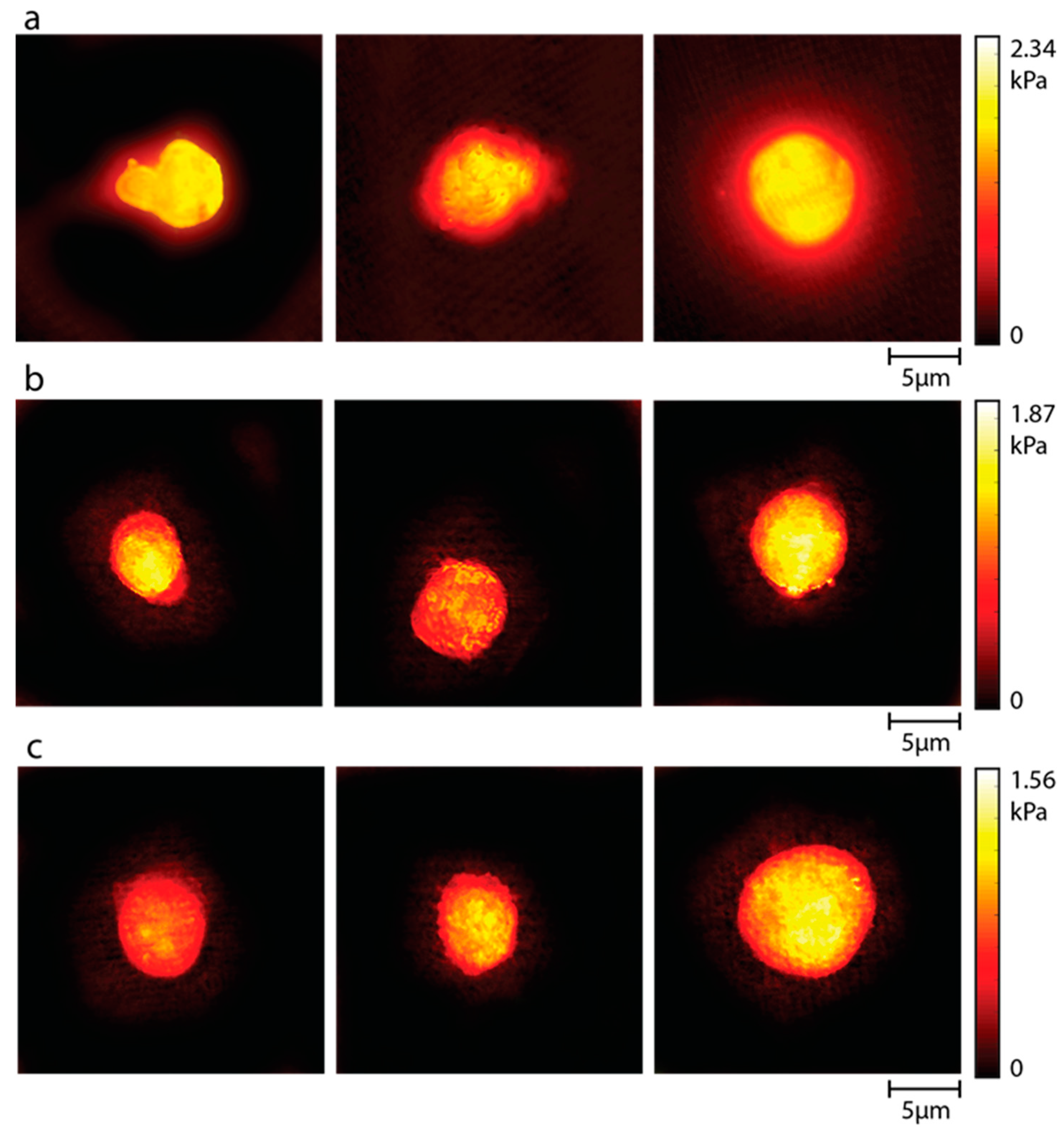
3.3. Stiffness
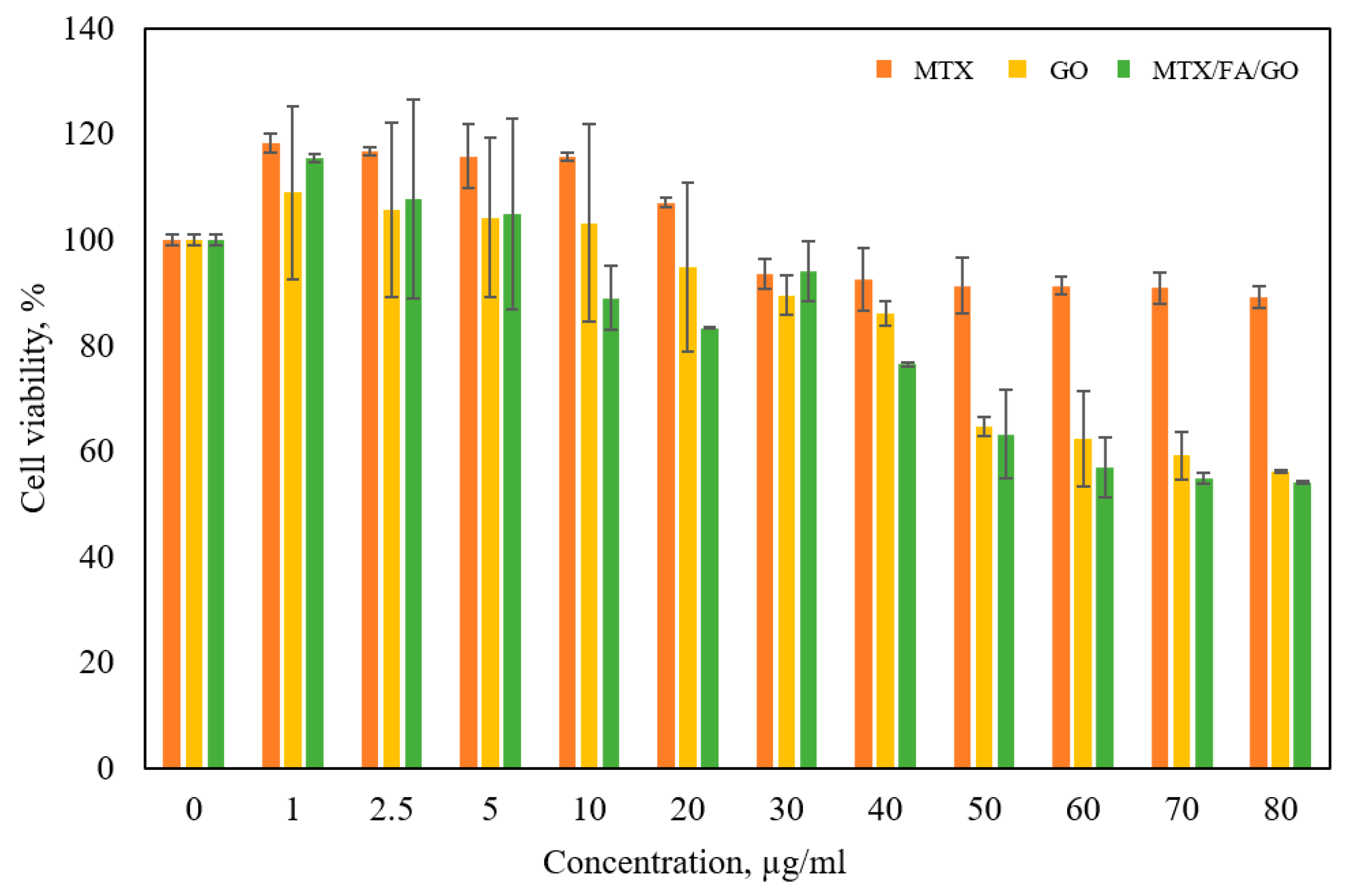
3.4. Cell Culture and Cytotoxicity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deb, A.; Vimala, R. Camptothecin Loaded Graphene Oxide Nanoparticle Functionalized with Polyethylene Glycol and Folic Acid for Anticancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 333–342. [Google Scholar] [CrossRef]
- Mishra, D.K.; Yadav, K.S.; Prabhakar, B.; Gaud, R.S. Nanocomposite for Cancer Targeted Drug Delivery. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–337. [Google Scholar]
- Lin, Q.; Huang, X.; Tang, J.; Han, Y.; Chen, H. Environmentally Friendly, One-Pot Synthesis of Folic Acid-Decorated Graphene Oxide-Based Drug Delivery System. J. Nanopart. Res. 2013, 15, 12. [Google Scholar] [CrossRef]
- Cao, X.; Feng, F.; Wang, Y.; Yang, X.; Duan, H.; Chen, Y. Folic Acid-Conjugated Graphene Oxide as a Transporter of Chemotherapeutic Drug and SiRNA for Reversal of Cancer Drug Resistance. J. Nanopart. Res. 2013, 15, 10. [Google Scholar] [CrossRef]
- Ciftci, F.; Ayan, S.; Duygulu, N.; Yilmazer, Y.; Karavelioglu, Z.; Vehapi, M.; Cakır Koc, R.; Sengor, M.; Yılmazer, H.; Ozcimen, D.; et al. Selenium and Clarithromycin Loaded PLA-GO Composite Wound Dressings by Electrospinning Method. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 898–909. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef] [PubMed]
- Lagos, K.J.; Buzzá, H.H.; Bagnato, V.S.; Romero, M.P. Carbon-Based Materials in Photodynamic and Photothermal Therapies Applied to Tumor Destruction. Int. J. Mol. Sci. 2022, 23, 22. [Google Scholar] [CrossRef]
- Doughty, A.C.V.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef]
- Chanphai, P.; Thomas, T.J.; Tajmir-Riahi, H.A. Application and Biomolecular Study of Functionalized Folic Acid-Dendrimer Nanoparticles in Drug Delivery. J. Biomol. Struct. Dyn. 2021, 39, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, M.; Jelonek, K.; Musiał-kulik, M.; Beberok, A.; Wrześniok, D.; Kasperczyk, J. Single- versus Dual-targeted Nanoparticles with Folic Acid and Biotin for Anticancer Drug Delivery. Pharmaceutics 2021, 13, 326. [Google Scholar] [CrossRef]
- Parashar, S.; Gupta, V.; Bhatnagar, R.; Kausar, A. A Clickable Folic Acid-Rhamnose Conjugate for Selective Binding to Cancer Cells. Results Chem. 2022, 4, 100409. [Google Scholar] [CrossRef]
- Felenji, H.; Johari, B.; Moradi, M.; Gharbavi, M.; Danafar, H. Folic Acid-Conjugated Iron Oxide Magnetic Nanoparticles Based on Bovine Serum Albumin (BSA) for Targeted Delivery of Curcumin to Suppress Liver Cancer Cells. Chem. Africa 2022, 5, 1627–1639. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Z.; Ma, J.; Zhou, T.; Wu, Z.; Ding, P.; Sun, N.; Liu, L.; Pei, R.; Zhu, W. Folic Acid-Modified Fluorescent-Magnetic Nanoparticles for Efficient Isolation and Identification of Circulating Tumor Cells in Ovarian Cancer. Biosensors 2022, 12, 184. [Google Scholar] [CrossRef]
- Faghfuri, E.; Sagha, M.; Faghfouri, A.H. The Cytotoxicity Effect of Curcumin Loaded Folic Acid Conjugated-Nanoparticles on Breast Cancer Cells and Its Association with Inhibition of STAT3 Phosphorylation. J. Clust. Sci. 2022, 33, 2037–2044. [Google Scholar] [CrossRef]
- Trukawka, M.; Cendrowski, K.; Konicki, W.; Mijowska, E. Folic Acid/Methotrexate Functionalized Mesoporous Silica Nanoflakes from Different Supports: Comparative Study. Appl. Sci. 2020, 10, 6465. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, J.; Li, Y.; Yang, W.; Cui, X.; Xu, H. Folic Acid Modified Glucosamine/Methotrexate Polymer Targeted Therapy for Rheumatoid Arthritis. J. Nanomater. 2022, 2022, 2443302. [Google Scholar] [CrossRef]
- Folic Acid/Methotrexate/Pyridoxine. React. Wkly. 2022, 1922, 278. [CrossRef]
- Kovalev, I.S.; Zyryanov, G.V.; Santra, S.; Majee, A.; Varaksin, M.V.; Charushin, V.N. Folic Acid Antimetabolites (Antifolates): A Brief Review on Synthetic Strategies and Application Opportunities. Molecules 2022, 27, 6229. [Google Scholar] [CrossRef]
- Prey, S.; Paul, C. Effect of Folic or Folinic Acid Supplementation on Methotrexate-Associated Safety and Efficacy in Inflammatory Disease: A Systematic Review. Br. J. Dermatol. 2009, 160, 622–628. [Google Scholar] [CrossRef]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Abdi Goushbolagh, N. Folic Acid Functionalized Nanoparticles as Pharmaceutical Carriers in Drug Delivery Systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef]
- Anarjan, F.S. Active Targeting Drug Delivery Nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370. [Google Scholar]
- He, C.; Heidari Majd, M.; Shiri, F.; Shahraki, S. Palladium and Platinum Complexes of Folic Acid as New Drug Delivery Systems for Treatment of Breast Cancer Cells. J. Mol. Struct. 2021, 1229, 129806. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Hussein, K.H. Graphene Oxide as a Carrier for Drug Delivery of Methotrexate. Biointerface Res. Appl. Chem. 2021, 11, 14726–14735. [Google Scholar]
- Khodashenas, B.; Ardjmand, M.; Rad, A.S.; Esfahani, M.R. Gelatin-Coated Gold Nanoparticles as an Effective PH-Sensitive Methotrexate Drug Delivery System for Breast Cancer Treatment. Mater. Today Chem. 2021, 20, 100474. [Google Scholar] [CrossRef]
- Bessar, H.; Venditti, I.; Benassi, L.; Vaschieri, C.; Azzoni, P.; Pellacani, G.; Magnoni, C.; Botti, E.; Casagrande, V.; Federici, M.; et al. Functionalized Gold Nanoparticles for Topical Delivery of Methotrexate for the Possible Treatment of Psoriasis. Colloids Surf. B Biointerfaces 2016, 141, 141–147. [Google Scholar] [CrossRef]
- Attari, E.; Nosrati, H.; Danafar, H.; Kheiri Manjili, H. Methotrexate Anticancer Drug Delivery to Breast Cancer Cell Lines by Iron Oxide Magnetic Based Nanocarrier. J. Biomed. Mater. Res. Part A 2019, 107, 2492–2500. [Google Scholar] [CrossRef]
- Dhanka, M.; Shetty, C.; Srivastava, R. Methotrexate Loaded Gellan Gum Microparticles for Drug Delivery. Int. J. Biol. Macromol. 2018, 110, 346–356. [Google Scholar] [CrossRef]
- How, K.N.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 549139. [Google Scholar] [CrossRef]
- Shariare, M.H.; Masum, A.A.; Alshehri, S.; Alanazi, F.K.; Uddin, J.; Kazi, M. Preparation and Optimization of Pegylated Nano Graphene Oxide-Based Delivery System for Drugs with Different Molecular Structures Using Design of Experiment (DoE). Molecules 2021, 26, 1457. [Google Scholar] [CrossRef]
- Yu, W.J.; Huang, D.X.; Liu, S.; Sha, Y.L.; Gao, F.H.; Liu, H. Polymeric Nanoscale Drug Carriers Mediate the Delivery of Methotrexate for Developing Therapeutic Interventions Against Cancer and Rheumatoid Arthritis. Front. Oncol. 2020, 10, 1734. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Candido, K.A.; Cao, Z.; Nigavekar, S.S.; Majoros, I.J.; Thomas, T.P.; Balogh, L.P.; Khan, M.K.; Baker, J.R. Nanoparticle Targeting of Anticancer Drug Improves Therapeutic Response in Animal Model of Human Epithelial Cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar] [CrossRef]
- Wong, P.T.; Choi, S.K. Mechanisms and Implications of Dual-Acting Methotrexate in Folate-Targeted Nanotherapeutic Delivery. Int. J. Mol. Sci. 2015, 16, 1772–1790. [Google Scholar] [CrossRef]
- Samiei Foroushani, M.; Zahmatkeshan, A.; Arkaban, H.; Karimi Shervedani, R.; Kefayat, A. A Drug Delivery System Based on Nanocomposites Constructed from Metal-Organic Frameworks and Mn3O4 Nanoparticles: Preparation and Physicochemical Characterization for BT-474 and MCF-7 Cancer Cells. Colloids Surf. B Biointerfaces 2021, 202, 111712. [Google Scholar] [CrossRef]
- Ozder, M.N.; Ciftci, F.; Rencuzogullari, O.; Arisan, E.D.; Ustündag, C.B. In Situ Synthesis and Cell Line Studies of Nano-Hydroxyapatite/Graphene Oxide Composite Materials for Bone Support Applications. Ceram. Int. 2023, 49, 14791–14803. [Google Scholar] [CrossRef]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.; Zhang, C.; Zhou, X.; Guo, S.; Cui, D. Folic Acid-Conjugated Graphene Oxide Loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics 2012, 1, 240–250. [Google Scholar] [CrossRef]
- Sapino, S.; Oliaro-Bosso, S.; Zonari, D.; Zattoni, A.; Ugazio, E. Mesoporous Silica Nanoparticles as a Promising Skin Delivery System for Methotrexate. Int. J. Pharm. 2017, 530, 239–248. [Google Scholar] [CrossRef]
- Yang, F.; Kamiya, N.; Goto, M. Transdermal Delivery of the Anti-Rheumatic Agent Methotrexate Using a Solid-in-Oil Nanocarrier. Eur. J. Pharm. Biopharm. 2012, 82, 158–163. [Google Scholar] [CrossRef]
- Kapoor, K.; Pandit, V.; Nagaich, U. Development and Characterization of Sustained Release Methotrexate Loaded Cubosomes for Topical Delivery in Rheumatoid Arthritis. Int. J. Appl. Pharm. 2020, 12, 33–39. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Delivery of Methotrexate and Characterization of Skin Treated by Fabricated PLGA Microneedles and Fractional Ablative Laser. Pharm. Res. 2018, 35, 1–20. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Prajapati, P.; Patel, M.; Kansara, Y.; Shah, P.; Pulusu, V.S.; Shah, S. Green LC-MS/MS method for in-vivo pharmacokinetics of mirabegron-encapsulated nanostructured lipid carriers in rat plasma: Integrating white analytical chemistry and analytical quality by design approach. Sustain. Chem. Pharm. 2024, 39, 101523. [Google Scholar] [CrossRef]
- Insel, M.A.; Karakuş, S.; Temelcan, G.; Kocken, H.G.; Albayrak, I. Handling uncertainty in rheological properties of green eggshell nanocomposites by a fuzzy-hybrid modeling approach: A comparative study. Phys. Scr. 2023, 98, 035001. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Bedir, T.; Baykara, D.; Yildirim, R.; Calikoglu Koyuncu, A.C.; Sahin, A.; Kaya, E.; Bosgelmez Tinaz, G.; Insel, M.A.; Topuzogulları, M.; Gunduz, O.; et al. Three-Dimensional-Printed GelMA-KerMA Composite Patches as an Innovative Platform for Potential Tissue Engineering of Tympanic Membrane Perforations. Nanomaterials 2024, 14, 563. [Google Scholar] [CrossRef]
- Varol, R.; Karavelioglu, Z.; Omeroglu, S.; Aydemir, G.; Karadag, A.; Meco, H.E.; Demircali, A.A.; Yilmaz, A.; Kocal, G.C.; Gencoglan, G.; et al. Acousto-Holographic Reconstruction of Whole-Cell Stiffness Maps. Nat. Commun. 2022, 13, 7351. [Google Scholar] [CrossRef]
- Raudenska, M.; Kratochvilova, M.; Vicar, T.; Gumulec, J.; Balvan, J.; Polanska, H.; Pribyl, J.; Masarik, M. Cisplatin Enhances Cell Stiffness and Decreases Invasiveness Rate in Prostate Cancer Cells by Actin Accumulation. Sci. Rep. 2019, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- Karavelioglu, Z.; Cakir-Koc, R. Preparation of Chitosan Nanoparticles as Ginkgo Biloba Extract Carrier: In Vitro Neuroprotective Effect on Oxidative Stress-Induced Human Neuroblastoma Cells (SH-SY5Y). Int. J. Biol. Macromol. 2021, 192, 675–683. [Google Scholar] [CrossRef]
- Rajeev, M.R.; Manjusha, V.; Anirudhan, T.S. Transdermal delivery of doxorubicin and methotrexate from polyelectrolyte three layer nanoparticle of graphene oxide/polyethyleneimine/dextran sulphate for chemotherapy: In vitro and in vivo studies. J. Chem. Eng. 2023, 466, 143244. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Asgari, S.; Hosseini, S.H. Graphene oxide functionalized with oxygen-rich polymers as a pH-sensitive carrier for co-delivery of hydrophobic and hydrophilic drugs. J. Drug Deliv. Sci. Technol. 2020, 56, 101542. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Tajiki, A.; Abdouss, M.; Sadjadi, S.; Mazinani, S.; Ramakrishna, S. Photo-induced green synthesis of bimetallic Ag/Pd nanoparticles decorated reduced graphene oxide/nitrogen-doped graphene quantum dots nanocomposite as an amperometric sensor for nitrite detection. Anal. Bioanal. Chem. 2021, 413, 6289–6301. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef] [PubMed]
- Muniyalakshmi, M.; Sethuraman, K.; Silambarasan, D. Synthesis and characterization of graphene oxide nanosheets. Mater. Today Proc. 2020, 21, 408–410. [Google Scholar] [CrossRef]
- Hidayah, N.M.S.; Liu, W.W.; Lai, C.W.; Noriman, N.Z.; Khe, C.S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conf. Proc. 2017, 1892, 1. [Google Scholar]
- Liou, Y.J.; Huang, W.J. High temperature phase transitions of graphene oxide paper from graphite oxide solution. J. Mater. Sci. Technol. 2014, 30, 1088–1091. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Jin, X. Sulfuric acid intercalated graphite oxide for graphene preparation. Sci. Rep. 2013, 3, 3439. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zou, X.; Su, H.; Li, C. Methotrexate-loaded folic acid of solid-phase synthesis conjugated gold nanoparticles targeted treatment for rheumatoid arthritis. Eur. J. Pharm. Sci. 2022, 170, 106101. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Xiao, X.; Xi, N.; Wang, Y. Effects of Methotrexate on the Viscoelastic Properties of Single Cells Probed by Atomic Force Microscopy. J. Biol. Phys. 2016, 42, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Soleymani, H.; Hashemzadeh, H.; Mortezazadeh, S.; Sedghi, M.; Shojaeilangari, S.; Allahverdi, A.; Naderi-Manesh, H. Microfluidic Investigation of the Effect of Graphene Oxide on Mechanical Properties of Cell and Actin Cytoskeleton Networks: Experimental and Theoretical Approaches. Sci. Rep. 2021, 11, 16216. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, M.; Gao, M.; Zhang, Z.; Xu, Y.; Xia, T.; Liu, S. Graphene Oxide Induced Perturbation to Plasma Membrane and Cytoskeletal Meshwork Sensitize Cancer Cells to Chemotherapeutic Agents. ACS Nano 2017, 11, 2637–2651. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef]
- Zeng, H.; Xia, C.; Zhao, B.; Zhu, M.; Zhang, H.Y.; Zhang, D.; Rui, X.; Li, H.; Yuan, Y. Folic Acid–Functionalized Metal-Organic Framework Nanoparticles as Drug Carriers Improved Bufalin Antitumor Activity Against Breast Cancer. Front. Pharmacol. 2022, 12, 747992. [Google Scholar] [CrossRef]
- Kim, S.J.; Zhang, C.X.W.; Demsky, R.; Armel, S.; Kim, Y.I.; Narod, S.A.; Kotsopoulos, J. Folic Acid Supplement Use and Breast Cancer Risk in BRCA1 and BRCA2 Mutation Carriers: A Case–control Study. Breast Cancer Res. Treat. 2019, 174, 741–748. [Google Scholar] [CrossRef]
- Dutta, B.; Barick, K.C.; Hassan, P.A. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv. Colloid Interface Sci. 2021, 296, 102509. [Google Scholar] [CrossRef]
- Watanabe, K.; Kaneko, M.; Maitani, Y. Functional coating of liposomes using a folateconjugate to target folate receptors. Int. J. Nanomed. 2012, 7, 3679–3688. [Google Scholar]
- Kim, S.H.; Jeong, J.H.; Chun, K.W.; Park, T.G. Target-specific cellular uptake of PLGA nanoparticles coated with poly(L-lysine)-poly(ethylene glycol)-folate conjugate. Langmuir 2005, 21, 8852–8857. [Google Scholar] [CrossRef]
- Giusto, E.; Žárská, L.; Beirne, D.F.; Rossi, A.; Bassi, G.; Ruffini, A.; Montesi, M.; Montagner, D.; Ranc, V.; Panseri, S. Graphene Oxide Nanoplatforms to Enhance Cisplatin-Based Drug Delivery in Anticancer Therapy. Nanomaterials 2022, 12, 2372. [Google Scholar] [CrossRef]
- De, D.; Das, C.K.; Mandal, D.; Mandal, M.; Pawar, N.; Chandra, A.; Gupta, A.N. Curcumin Complexed with Graphene Derivative for Breast Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 6284–6296. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Wang, Y.; Zhao, L.; Li, Y.; Liu, C. Formation of Graphene Oxide-Hybridized Nanogels for Combinative Anticancer Therapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2387–2395. [Google Scholar] [CrossRef]
- Marrella, A.; Giannoni, P.; Pulsoni, I.; Quarto, R.; Raiteri, R.; Scaglione, S. Topographical Features of Graphene-Oxide-Functionalized Substrates Modulate Cancer and Healthy Cell Adhesion Based on the Cell Tissue of Origin. ACS Appl. Mater. Interfaces 2018, 10, 41978–41985. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.H.; Jeong, J.H.; Shin, B.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Silver nanoparticle-embedded graphene oxide-methotrexate for targeted cancer treatment. Colloids Surf. B Biointerfaces 2017, 153, 95–103. [Google Scholar] [CrossRef]
- Wojtoniszak, M.; Urbas, K.; Perużyńska, M.; Kurzawski, M.; Droździk, M.; Mijowska, E. Covalent conjugation of graphene oxide with methotrexate and its antitumor activity. Chem. Phys. Lett. 2013, 568, 151–156. [Google Scholar] [CrossRef]

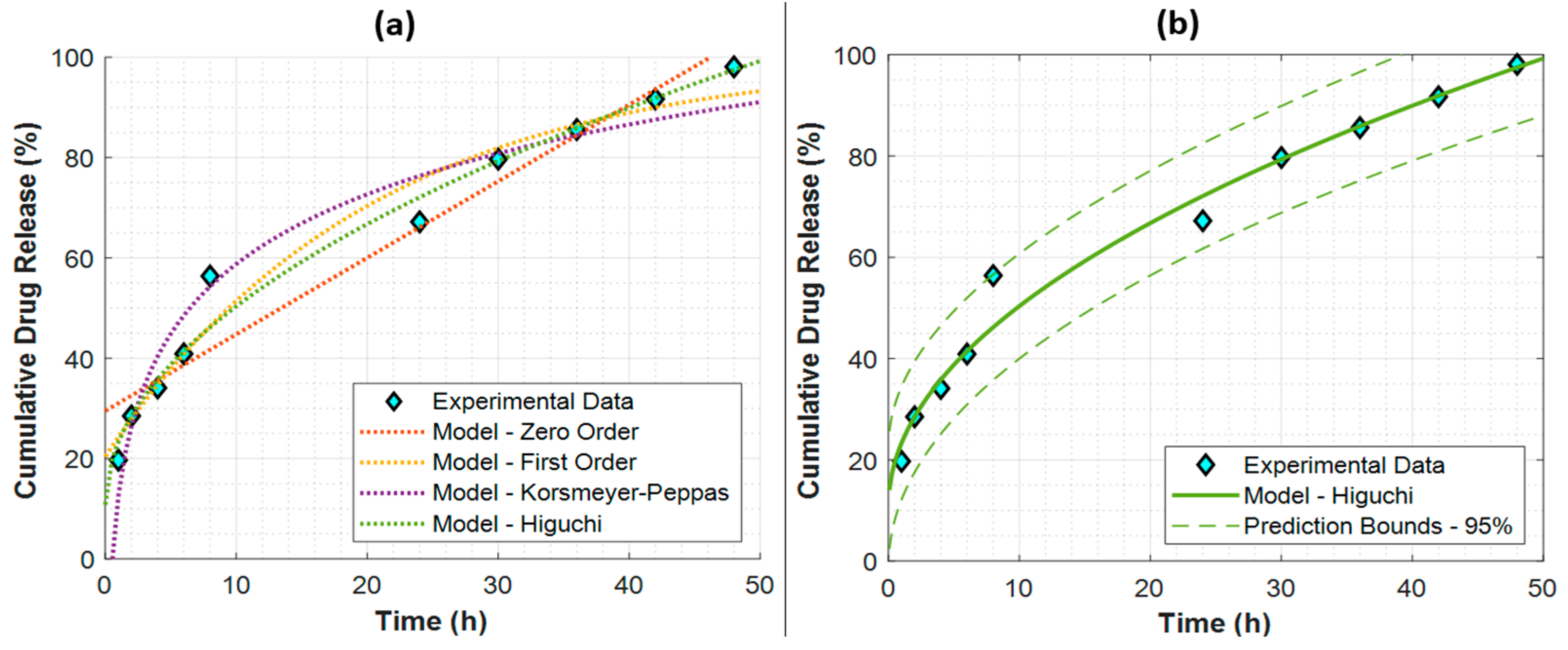
| Model | Parameters | Metrics | ||
|---|---|---|---|---|
| K (%) | Q0 (%) | R2 | RMSE (%) | |
| Zero-Order | 1.5259 1/h | 29.5187 | 0.9425 | 7.2031 |
| First-Order | 4.9400 1/h | 20.4237 | 0.9677 | 5.3938 |
| Korsmeyer–Peppas | 20.0631 | 12.5957 | 0.9554 | 6.3418 |
| Higuchi | 12.5140 1/h 0.5 | 10.7882 | 0.9799 | 4.2599 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanikoglu, R.; Karakas, C.Y.; Ciftci, F.; Insel, M.A.; Karavelioglu, Z.; Varol, R.; Yilmaz, A.; Cakir, R.; Uvet, H.; Ustundag, C.B. Development of Graphene Oxide-Based Anticancer Drug Combination Functionalized with Folic Acid as Nanocarrier for Targeted Delivery of Methotrexate. Pharmaceutics 2024, 16, 837. https://doi.org/10.3390/pharmaceutics16060837
Yanikoglu R, Karakas CY, Ciftci F, Insel MA, Karavelioglu Z, Varol R, Yilmaz A, Cakir R, Uvet H, Ustundag CB. Development of Graphene Oxide-Based Anticancer Drug Combination Functionalized with Folic Acid as Nanocarrier for Targeted Delivery of Methotrexate. Pharmaceutics. 2024; 16(6):837. https://doi.org/10.3390/pharmaceutics16060837
Chicago/Turabian StyleYanikoglu, Reyhan, Canan Yagmur Karakas, Fatih Ciftci, Mert Akın Insel, Zeynep Karavelioglu, Rahmetullah Varol, Abdurrahim Yilmaz, Rabia Cakir, Hüseyin Uvet, and Cem Bulent Ustundag. 2024. "Development of Graphene Oxide-Based Anticancer Drug Combination Functionalized with Folic Acid as Nanocarrier for Targeted Delivery of Methotrexate" Pharmaceutics 16, no. 6: 837. https://doi.org/10.3390/pharmaceutics16060837








