In Vitro and In Vivo Antipsoriatic Efficacy of Protected and Unprotected Sugar–Zinc Phthalocyanine Conjugates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Analytical Methods
2.2. Synthesis of [1(4),8(11),15(18),22(25)-Tetrakis(1,2:5,6-tetrahydroxy-α-D-glucofuranosyl)-phthalocyaninato]zinc(II) (Glu-4-ZnPc)
2.3. Cell Culture
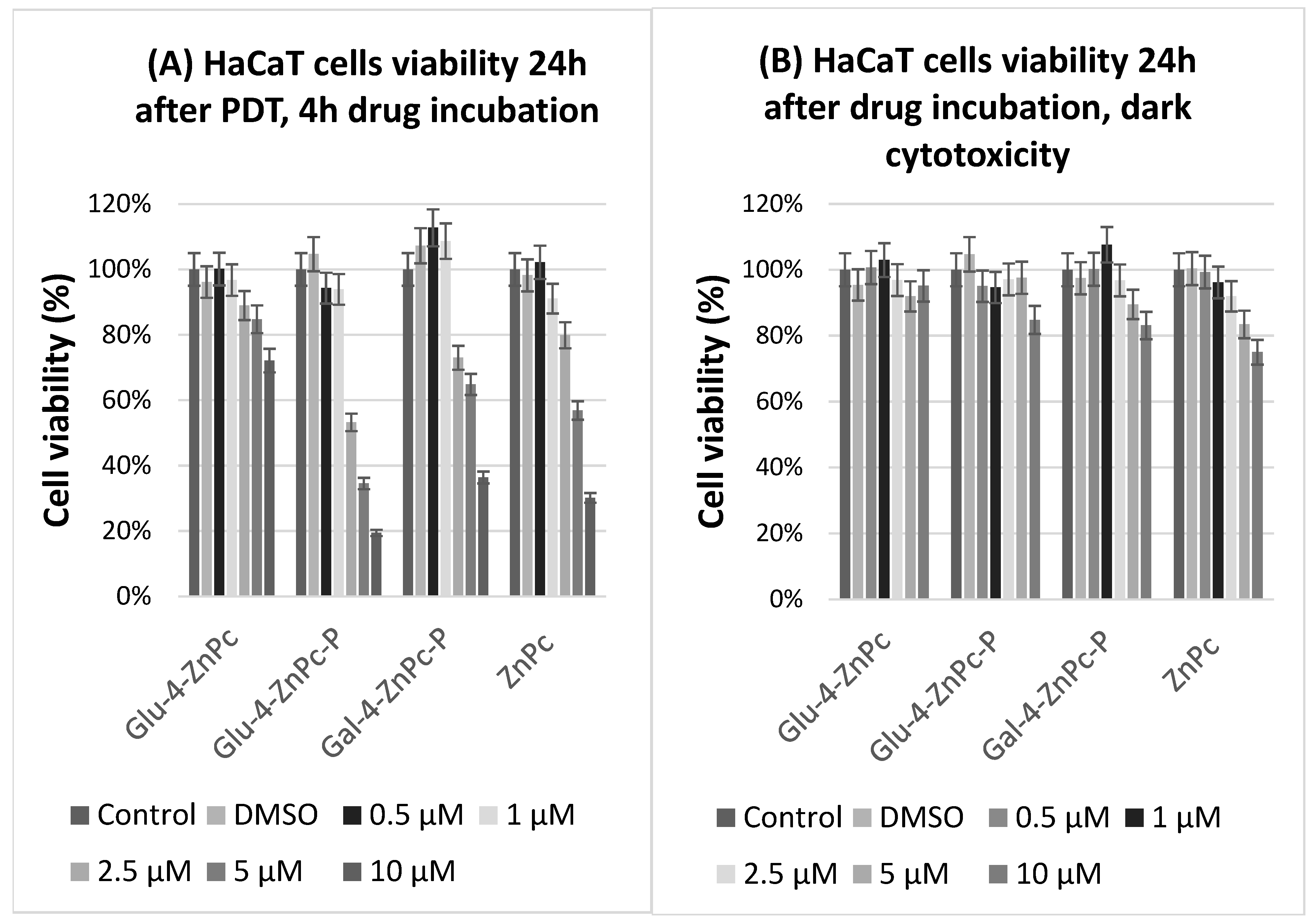
2.4. Dark Cytotoxicity and Cell Proliferation Assay
2.5. Cellular Uptake by Flow Cytometry
2.6. Fluorescent Microscopy
2.7. ROS Generation In Vitro after PDT
2.8. Animal Experiments
2.9. Histology
2.10. Determination of Serum Cytokine Levels (ELISA)
2.11. Statistical Analysis
3. Results and Discussion
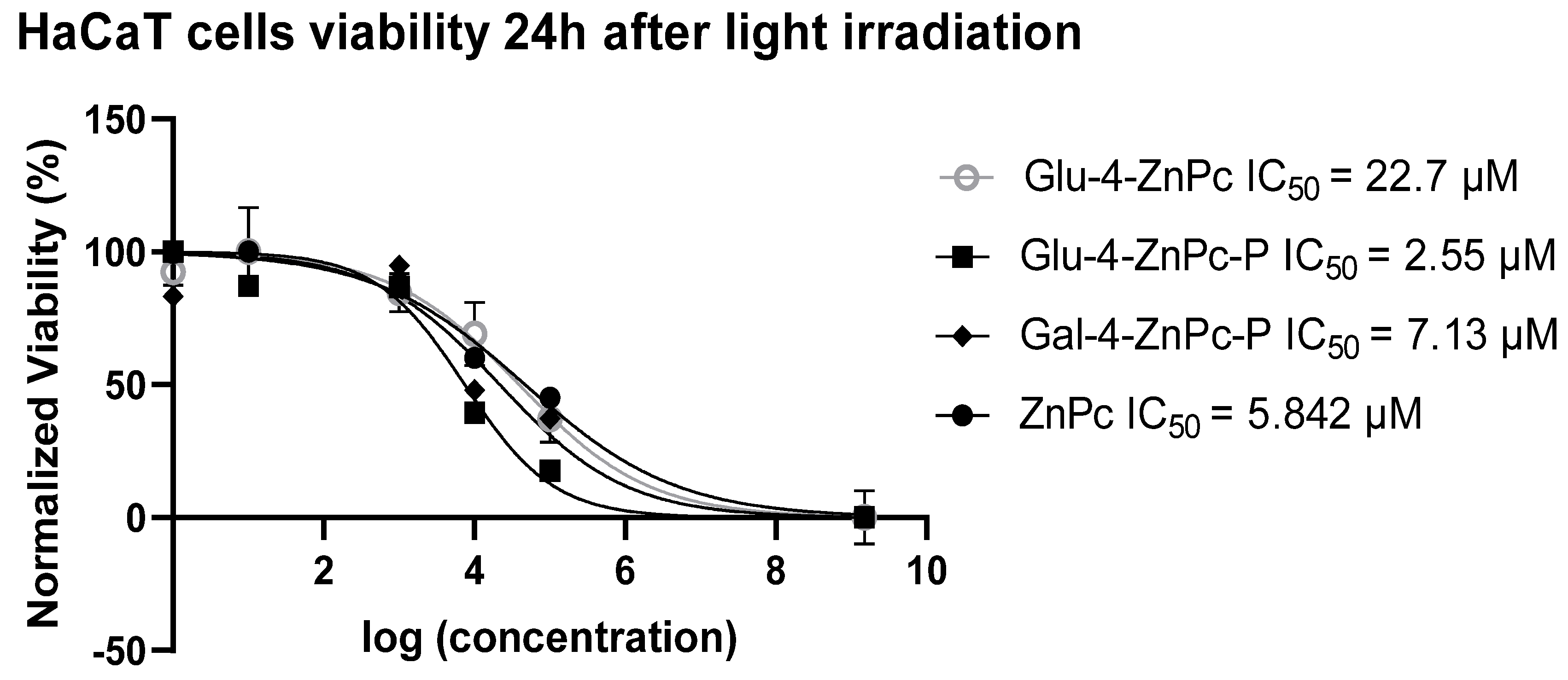
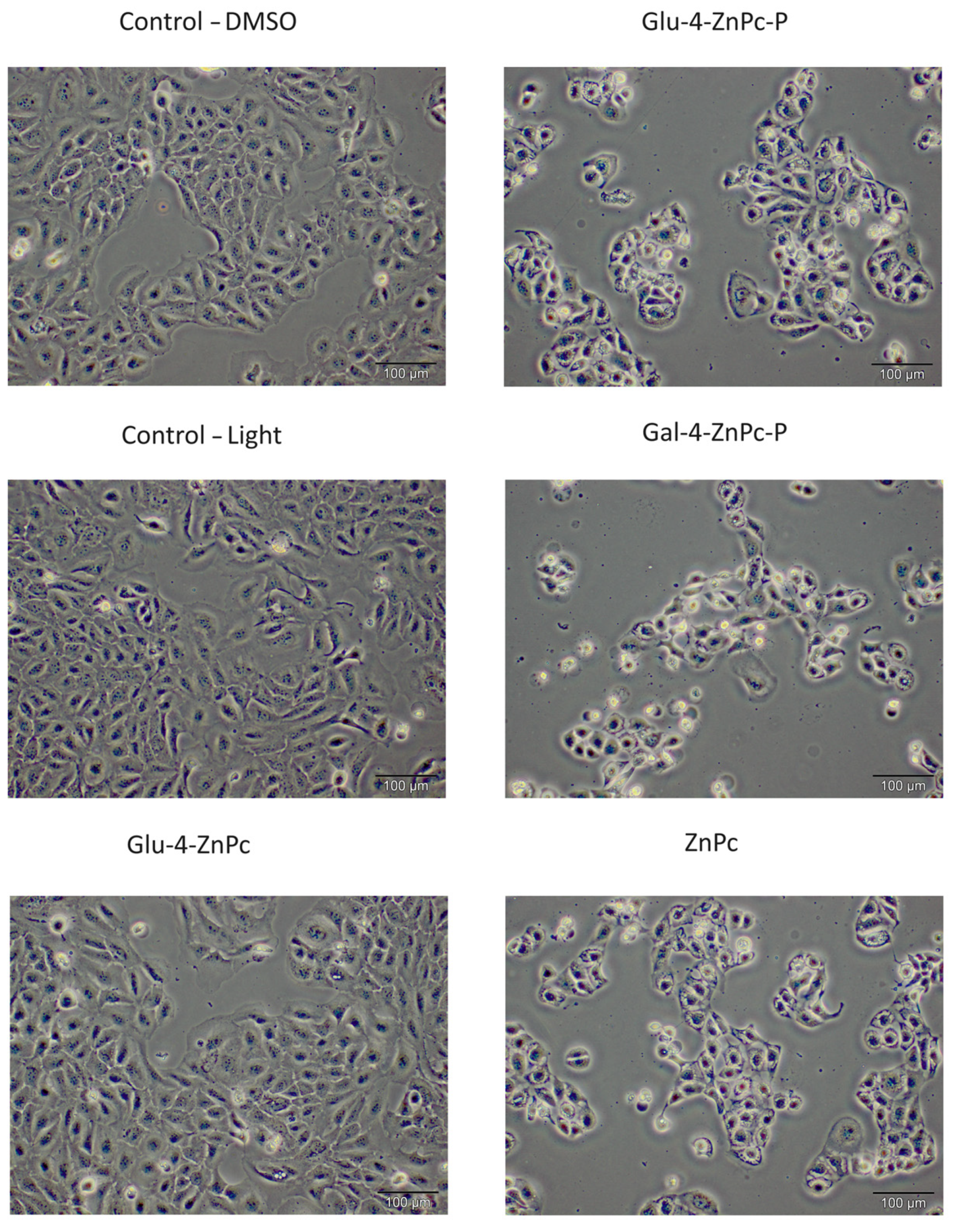
3.1. Glu-4-ZnPc-P Shows Better Photocytotoxic Activity Compared to Glu-4-ZnPc, Gal-4-ZnPc-P, and ZnPc
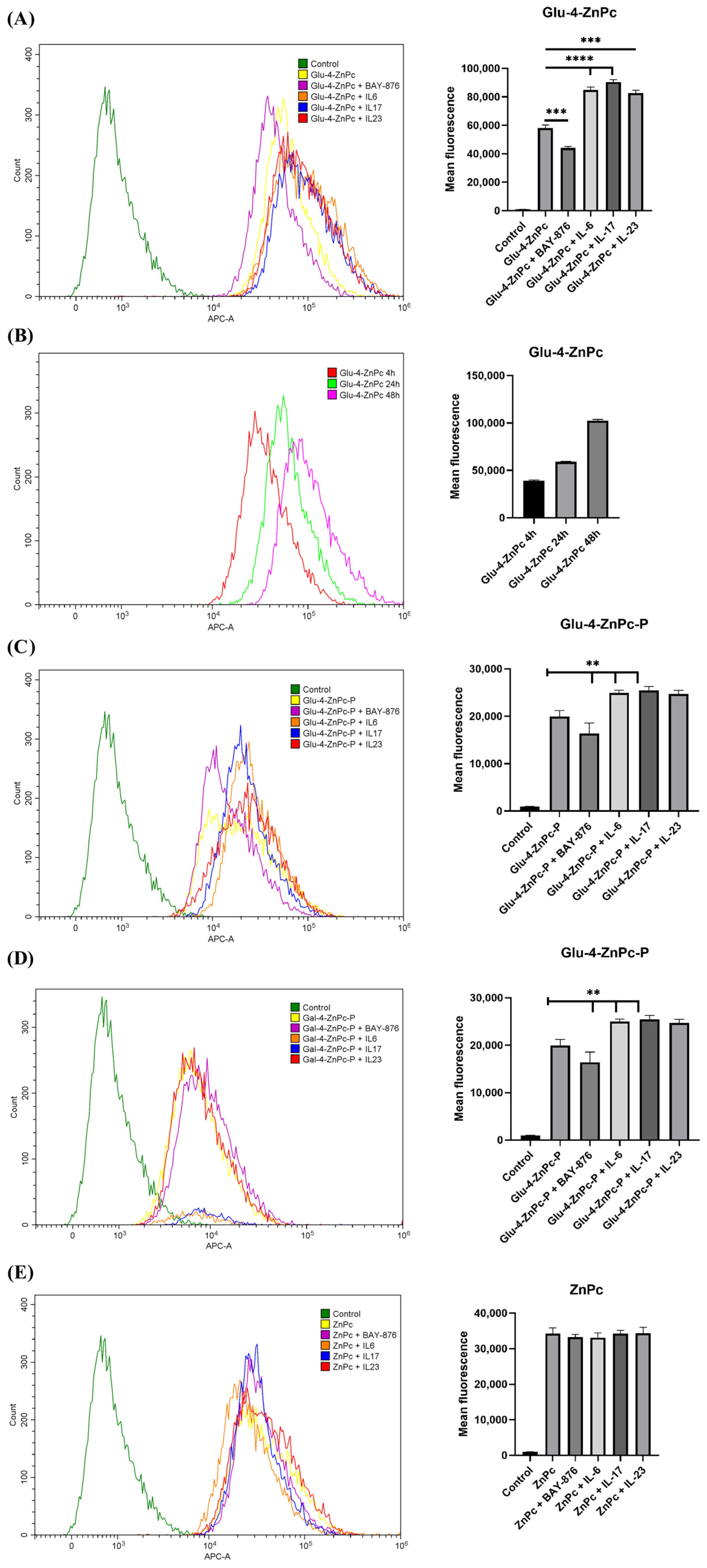
3.2. Proinflammatory Cytokines IL-6, IL-17A, and IL-23 Enhance the Cellular Uptake of Glu-4-ZnPc and Glu-4-ZnPc-P, While the Selective GLUT1 Inhibitor BAY-876 Reduces the Accumulation of the Compounds in HaCaT Cells
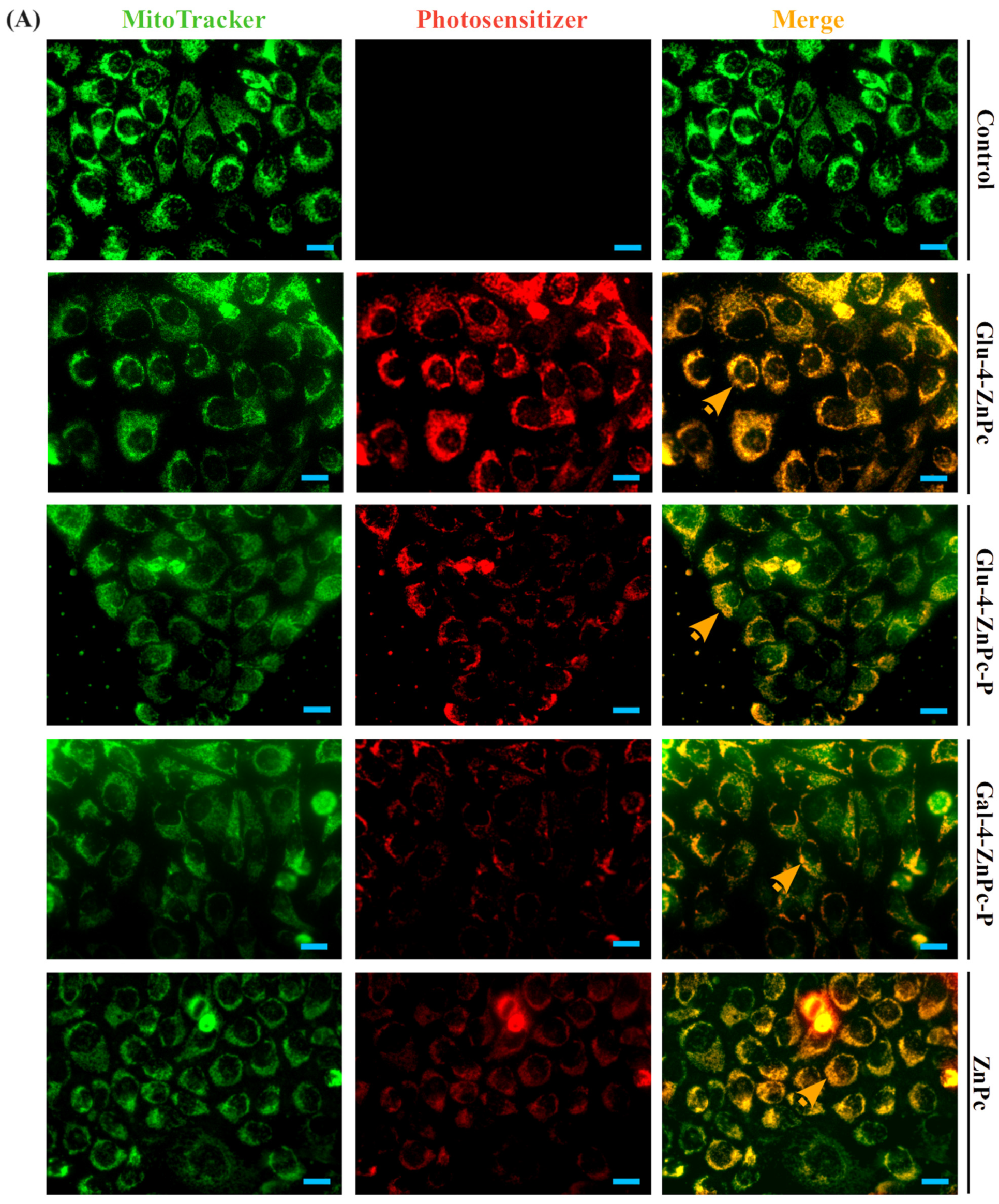
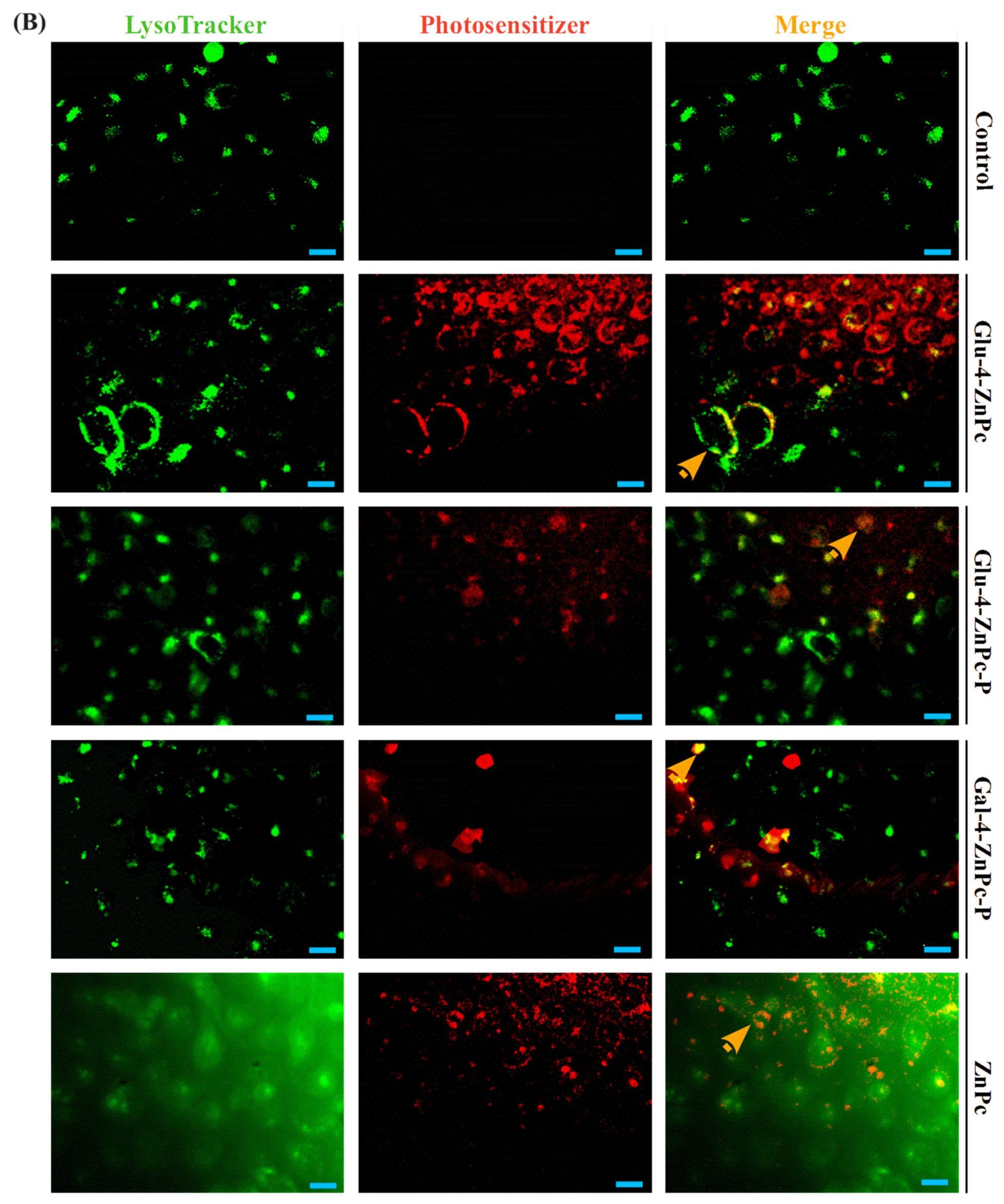
3.3. Sugar–Zinc Phthalocyanine Conjugates Are Preferentially Accumulated in Mitochondria
3.4. Glu-4-ZnPc-P Photogenerates Intracellular ROS the Most Efficiently in HaCaT Cells
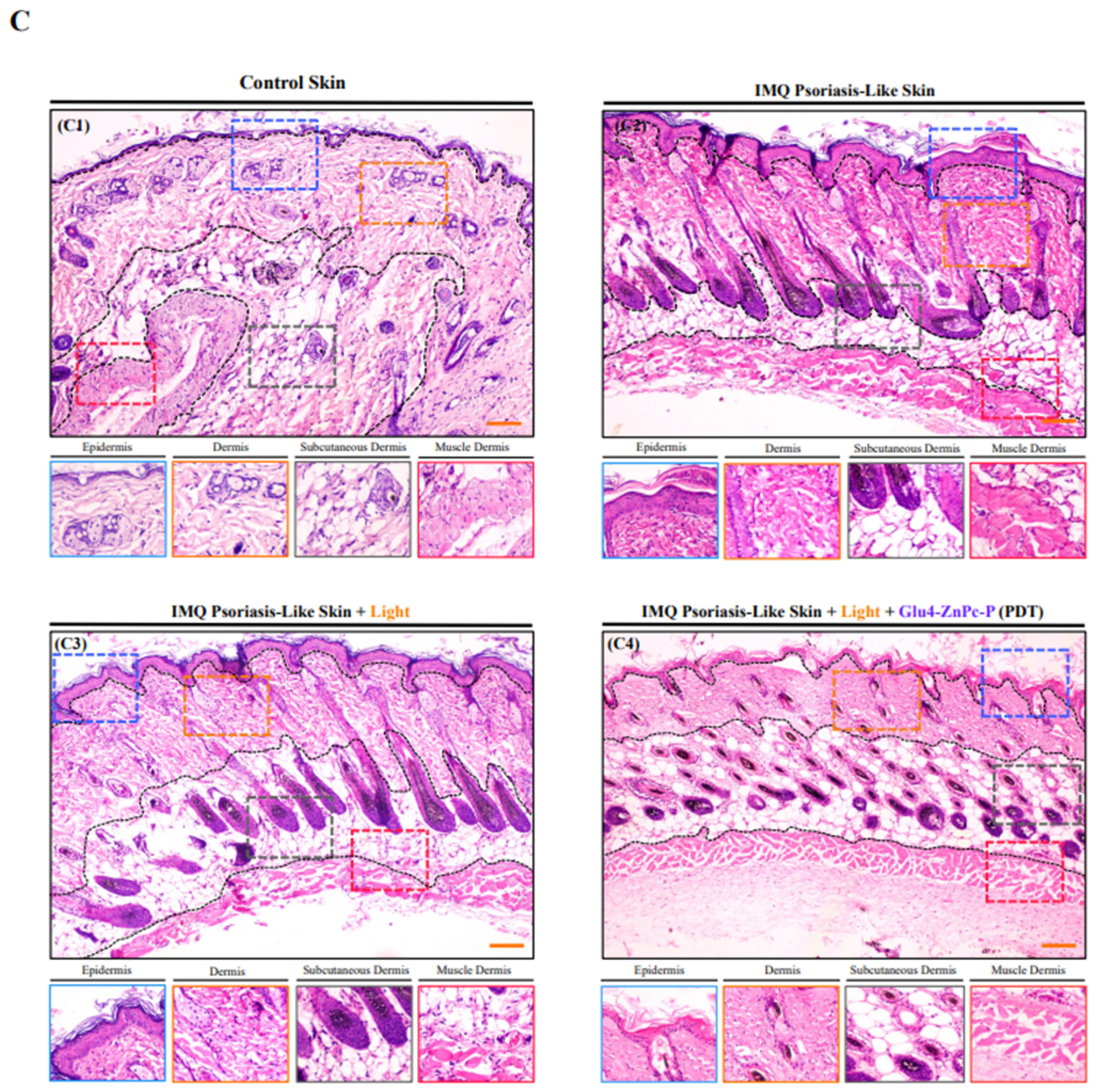
3.5. In Vivo Antipsoriatic Activity of Glu-4-ZnPc-P
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, Regional, and Worldwide Epidemiology of Psoriasis: Systematic Analysis and Modelling Study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA—J. Am. Med. Assoc. 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and Therapy of Psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Yamanaka, K.; Yamamoto, O.; Honda, T. Pathophysiology of Psoriasis: A Review. J. Dermatol. 2021, 48, 722–731. [Google Scholar] [CrossRef]
- Menter, A.; Gelfand, J.M.; Connor, C.; Armstrong, A.W.; Cordoro, K.M.; Davis, D.M.R.; Elewski, B.E.; Gordon, K.B.; Gottlieb, A.B.; Kaplan, D.H.; et al. Joint American Academy of Dermatology–National Psoriasis Foundation Guidelines of Care for the Management of Psoriasis with Systemic Nonbiologic Therapies. J. Am. Acad. Dermatol. 2020, 82, 1445–1486. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer—A Review of the Current Clinical Status. Front. Chem. 2021, 9, 608. [Google Scholar] [CrossRef]
- Choi, Y.M.; Adelzadeh, L.; Wu, J.J. Photodynamic Therapy for Psoriasis. J. Dermatol. Treat. 2015, 26, 202–207. [Google Scholar] [CrossRef]
- Wöhrle, D.; Schnurpfeil, G.; Makarov, S.G.; Kazarin, A.; Suvorova, O.N. Practical Applications of Phthalocyanines—From Dyes and Pigments to Materials for Optical, Electronic and Photo-Electronic Devices. Macroheterocycles 2012, 5, 191–202. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. (Eds.) The Porphyrin Handbook; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Durmuş, M.; Nyokong, T. Synthesis, Photophysical and Photochemical Studies of New Water-Soluble Indium(III) Phthalocyanines. Photochem. Photobiol. Sci. 2007, 6, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Roguin, L.P.; Chiarante, N.; García Vior, M.C.; Marino, J. Zinc(II) Phthalocyanines as Photosensitizers for Antitumor Photodynamic Therapy. Int. J. Biochem. Cell Biol. 2019, 114, 105575. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, X.; Zhang, B.; Kang, H.; Du, L.; Li, M. Nanostructures of an Amphiphilic Zinc Phthalocyanine Polymer Conjugate for Photodynamic Therapy of Psoriasis. Colloids Surf. B Biointerfaces 2015, 128, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Bächle, F.; Siemens, N.; Ziegler, T. Glycoconjugated Phthalocyanines as Photosensitizers for PDT—Overcoming Aggregation in Solution. Eur. J. Org. Chem. 2019, 2019, 7089–7116. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Prandini, J.A.; Biazzotto, J.C.; Tomé, J.P.C.; da Silva, R.S.; Lourenço, L.M.O. The Surprisingly Positive Effect of Zinc-Phthalocyanines With High Photodynamic Therapy Efficacy of Melanoma Cancer. Front. Chem. 2022, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Kuzyniak, W.; Schmidt, J.; Glac, W.; Berkholz, J.; Steinemann, G.; Hoffmann, B.; Ermilov, E.A.; Görek, A.G.; Ahsen, V.; Nitzsche, B.; et al. Novel Zinc Phthalocyanine as a Promising Photosensitizer for Photodynamic Treatment of Esophageal Cancer. Int. J. Oncol. 2017, 50, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Makuch, S.; Dróżdż, M.; Makarec, A.; Ziółkowski, P.; Woźniak, M. An Update on Photodynamic Therapy of Psoriasis—Current Strategies and Nanotechnology as a Future Perspective. Int. J. Mol. Sci. 2022, 23, 9845. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Wang, Y.M.; Li, W.F.; Li, C.; Jiang, Z.H.; Bao, J.; Wei, J.F.; Jin, H.T.; Wang, A.P. Anti-Psoriasis Effects and Mechanisms of A-(8-Quinolinoxy) Zinc Phthalocyanine-Mediated Photodynamic Therapy. Cell. Physiol. Biochem. 2017, 44, 200–214. [Google Scholar] [CrossRef]
- Zhang, Z.; Zi, Z.; Lee, E.E.; Zhao, J.; Contreras, D.C.; South, A.P.; Abel, E.D.; Chong, B.F.; Vandergriff, T.; Hosler, G.A.; et al. Differential Glucose Requirement in Skin Homeostasis and Injury Identifies a Therapeutic Target for Psoriasis Article. Nat. Med. 2018, 24, 617–627. [Google Scholar] [CrossRef]
- Hodeib, A.A.H.; Neinaa, Y.M.E.H.; Zakaria, S.S.; Alshenawy, H.A.S. Glucose Transporter-1 (GLUT-1) Expression in Psoriasis: Correlation with Disease Severity. Int. J. Dermatol. 2018, 57, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-F.; Huang, J.-D.; Lo, P.-C.; Fong, W.-P.; Ng, D.K.P. Glycosylated Zinc(Ii) Phthalocyanines as Efficient Photosensitisers for Photodynamic Therapy. Synthesis, Photophysical Properties and in Vitro Photodynamic Activity. Org. Biomol. Chem. 2008, 6, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.O.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Iamamoto, Y.; Torres, T. [1,2,3,4-Tetrakis(α/β-d-Galactopyranos-6-Yl)Phthalocyaninato]Zinc(II): A Water-Soluble Phthalocyanine. Tetrahedron Lett. 2006, 47, 9177–9180. [Google Scholar] [CrossRef]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A Practical Guide to Evaluating Colocalization in Biological Microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ishii, K. Photochemical Properties of Phthalocyanines with Transition Metal Ions. Coord. Chem. Rev. 2022, 468, 214626. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, C.; Zhu, C.H.; Zhang, Z.H.; Xue, J.P. Preparation and In Vitro Photodynamic Activity of Glucosylated Zinc(II) Phthalocyanines as Underlying Targeting Photosensitizers. Molecules 2017, 22, 845. [Google Scholar] [CrossRef] [PubMed]
- Kimani, S.G.; Shmigol, T.A.; Hammond, S.; Phillips, J.B.; Bruce, J.I.; MacRobert, A.J.; Malakhov, M.V.; Golding, J.P. Fully Protected Glycosylated Zinc (II) Phthalocyanine Shows High Uptake and Photodynamic Cytotoxicity in MCF-7 Cancer Cells. Photochem. Photobiol. 2013, 89, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Makuch, S.; Kupczyk, P.; Makarec, A.; Chodaczek, G.; Ziółkowski, P.; Woźniak, M. The Impact of Proinflammatory Cytokines and Imiquimod on GLUT1 in HaCaT Keratinocytes—A Potential Anti-Psoriatic Therapeutic Target? Cell. Physiol. Biochem. 2023, 57, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Makuch, S.; Wozniak, M.; Krawczyk, M.; Pastuch-Gawołek, G.; Szeja, W.; Agrawal, S. Glycoconjugation as a Promising Treatment Strategy for Psoriasis. J. Pharmacol. Exp. Ther. 2020, 373, 204–212. [Google Scholar] [CrossRef]
- Ge, Y.; Weng, X.; Tian, T.; Ding, F.; Huang, R.; Yuan, L.; Wu, J.; Wang, T.; Guo, P.; Zhou, X. A Mitochondria-Targeted Zinc(II) Phthalocyanine for Photodynamic Therapy. RSC Adv. 2013, 3, 12839–12846. [Google Scholar] [CrossRef]
- Muli, D.K.; Rajaputra, P.; You, Y.; McGrath, D.V. Asymmetric ZnPc-Rhodamine B Conjugates for Mitochondrial Targeted Photodynamic Therapy. Bioorg. Med. Chem. Lett. 2014, 24, 4496–4500. [Google Scholar] [CrossRef] [PubMed]
- Nash, G.T.; Luo, T.; Lan, G.; Ni, K.; Kaufmann, M.; Lin, W. Nanoscale Metal-Organic Layer Isolates Phthalocyanines for Efficient Mitochondria-Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143, 2194–2199. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zheng, K.; Liu, X. Mitochondria-Targeting Phthalocyanines and Porphyrins for Enhanced Photodynamic Tumor Therapy. ChemistrySelect 2023, 8, e202205022. [Google Scholar] [CrossRef]
- Mizuguchi, S.; Gotoh, K.; Nakashima, Y.; Setoyama, D.; Takata, Y.; Ohga, S.; Kang, D. Mitochondrial Reactive Oxygen Species Are Essential for the Development of Psoriatic Inflammation. Front. Immunol. 2021, 12, 714897. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makuch, S.; Kupczyk, P.; Woźniak, M.; Makarec, A.; Lipińska, M.; Klyta, M.; Sulecka-Zadka, J.; Szeja, W.; Gani, M.; Rapozzi, V.; et al. In Vitro and In Vivo Antipsoriatic Efficacy of Protected and Unprotected Sugar–Zinc Phthalocyanine Conjugates. Pharmaceutics 2024, 16, 838. https://doi.org/10.3390/pharmaceutics16060838
Makuch S, Kupczyk P, Woźniak M, Makarec A, Lipińska M, Klyta M, Sulecka-Zadka J, Szeja W, Gani M, Rapozzi V, et al. In Vitro and In Vivo Antipsoriatic Efficacy of Protected and Unprotected Sugar–Zinc Phthalocyanine Conjugates. Pharmaceutics. 2024; 16(6):838. https://doi.org/10.3390/pharmaceutics16060838
Chicago/Turabian StyleMakuch, Sebastian, Piotr Kupczyk, Marta Woźniak, Alicja Makarec, Maja Lipińska, Magdalena Klyta, Joanna Sulecka-Zadka, Wiesław Szeja, Mariachiara Gani, Valentina Rapozzi, and et al. 2024. "In Vitro and In Vivo Antipsoriatic Efficacy of Protected and Unprotected Sugar–Zinc Phthalocyanine Conjugates" Pharmaceutics 16, no. 6: 838. https://doi.org/10.3390/pharmaceutics16060838
APA StyleMakuch, S., Kupczyk, P., Woźniak, M., Makarec, A., Lipińska, M., Klyta, M., Sulecka-Zadka, J., Szeja, W., Gani, M., Rapozzi, V., Ziółkowski, P., & Smoleński, P. (2024). In Vitro and In Vivo Antipsoriatic Efficacy of Protected and Unprotected Sugar–Zinc Phthalocyanine Conjugates. Pharmaceutics, 16(6), 838. https://doi.org/10.3390/pharmaceutics16060838








