Iron-Reduced Graphene Oxide Core–Shell Micromotors Designed for Magnetic Guidance and Photothermal Therapy under Second Near-Infrared Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Solutions
2.3. Preparation of Micromotors
2.4. Photothermal Conversion Efficiency of Micromotors
2.5. Loading Doxorubicin on Micromotors
2.6. Micromotor Photothermal-Driven Release of Doxorubicin
2.7. Cell Lines and Cell Culture
2.8. Cell Viability MTT Assay
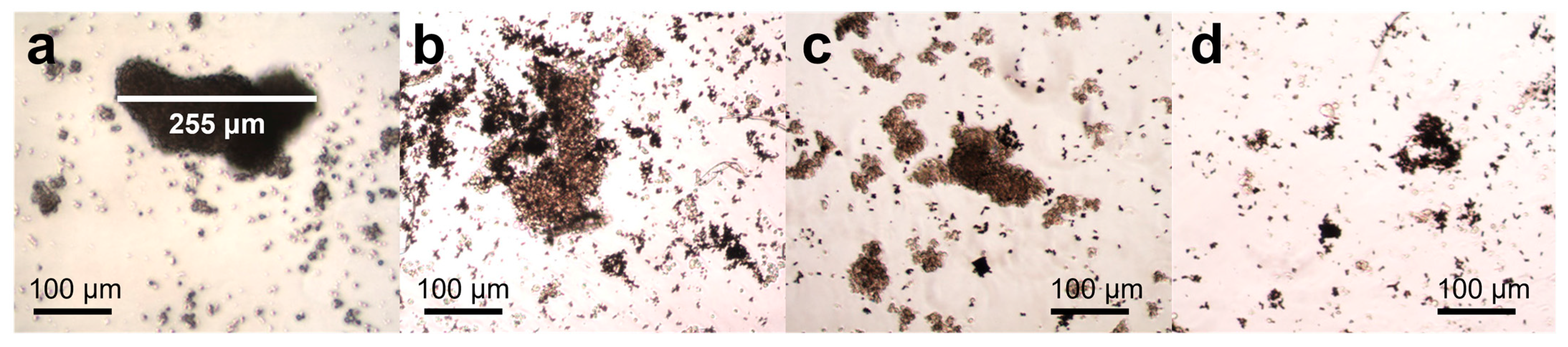
2.9. Tumoroid or 3D Model Formation Assay
2.10. Magnetic Motion
2.11. Instruments and Characterization
3. Results
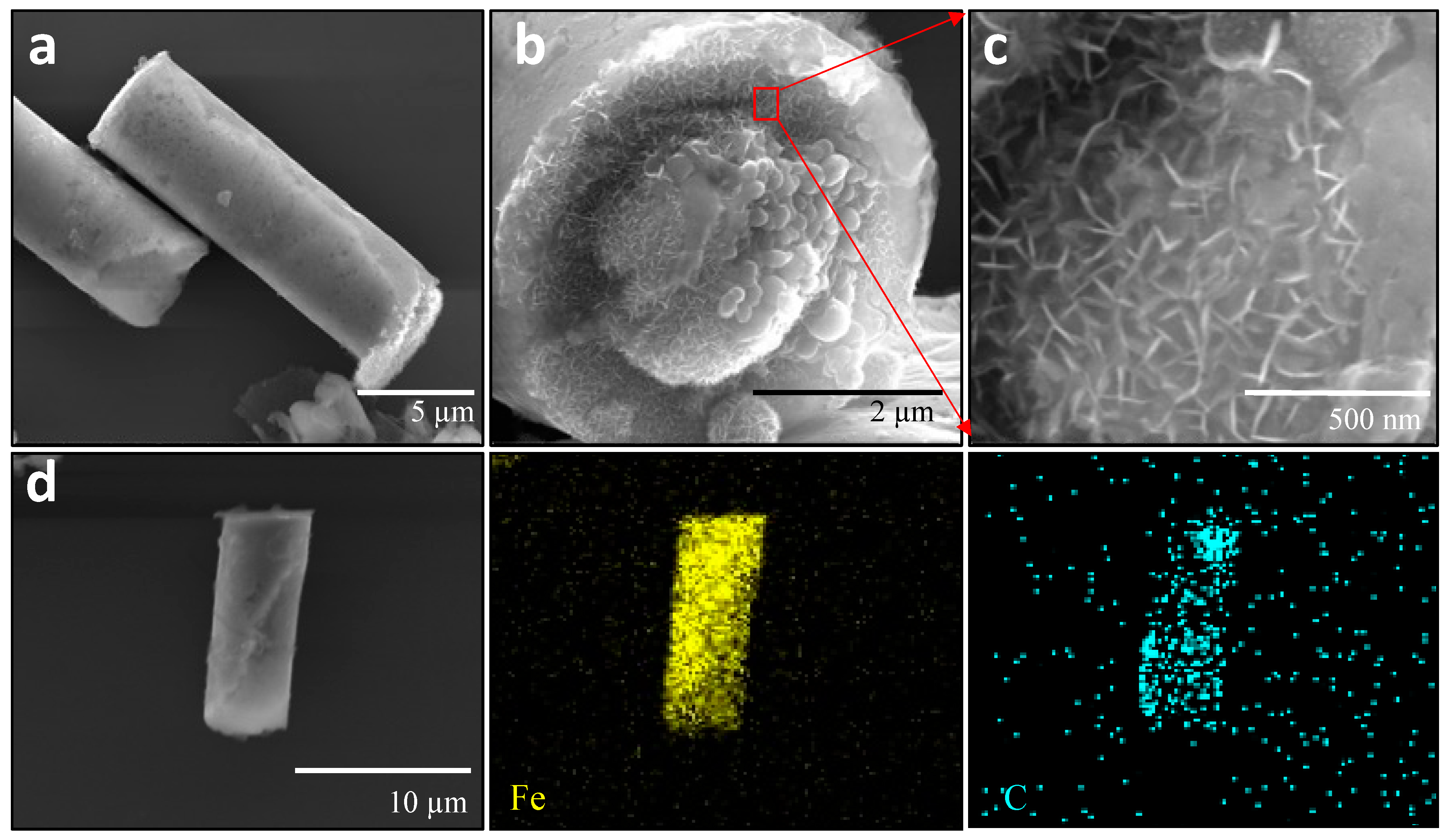
3.1. Characterization of Fe-rGO Core-Shell Micromotors
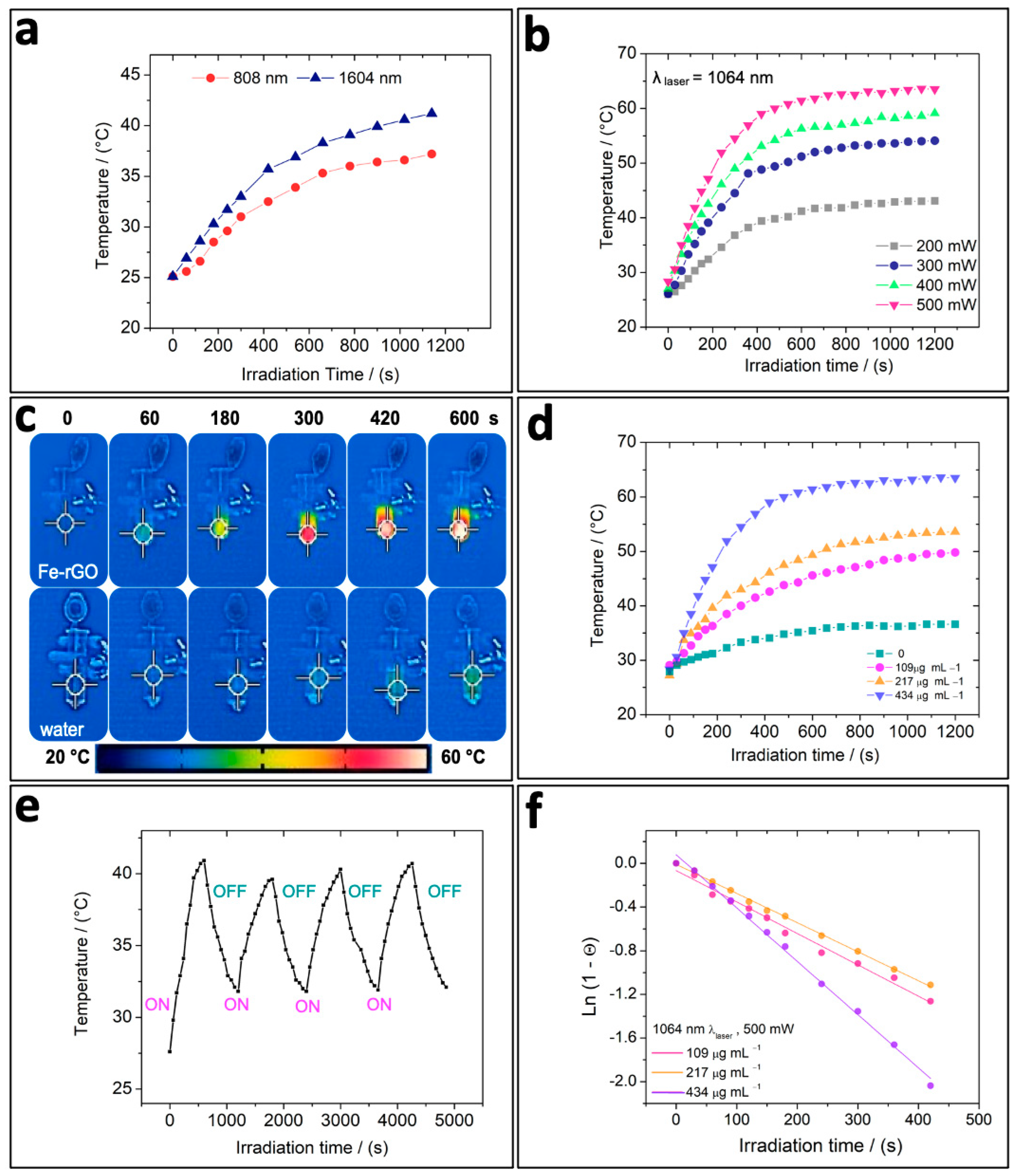
3.2. In Vitro Photothermal Capacity of Fe-rGO Micromotors
3.3. Drug Loading and Release Triggered by NIR Irradiation
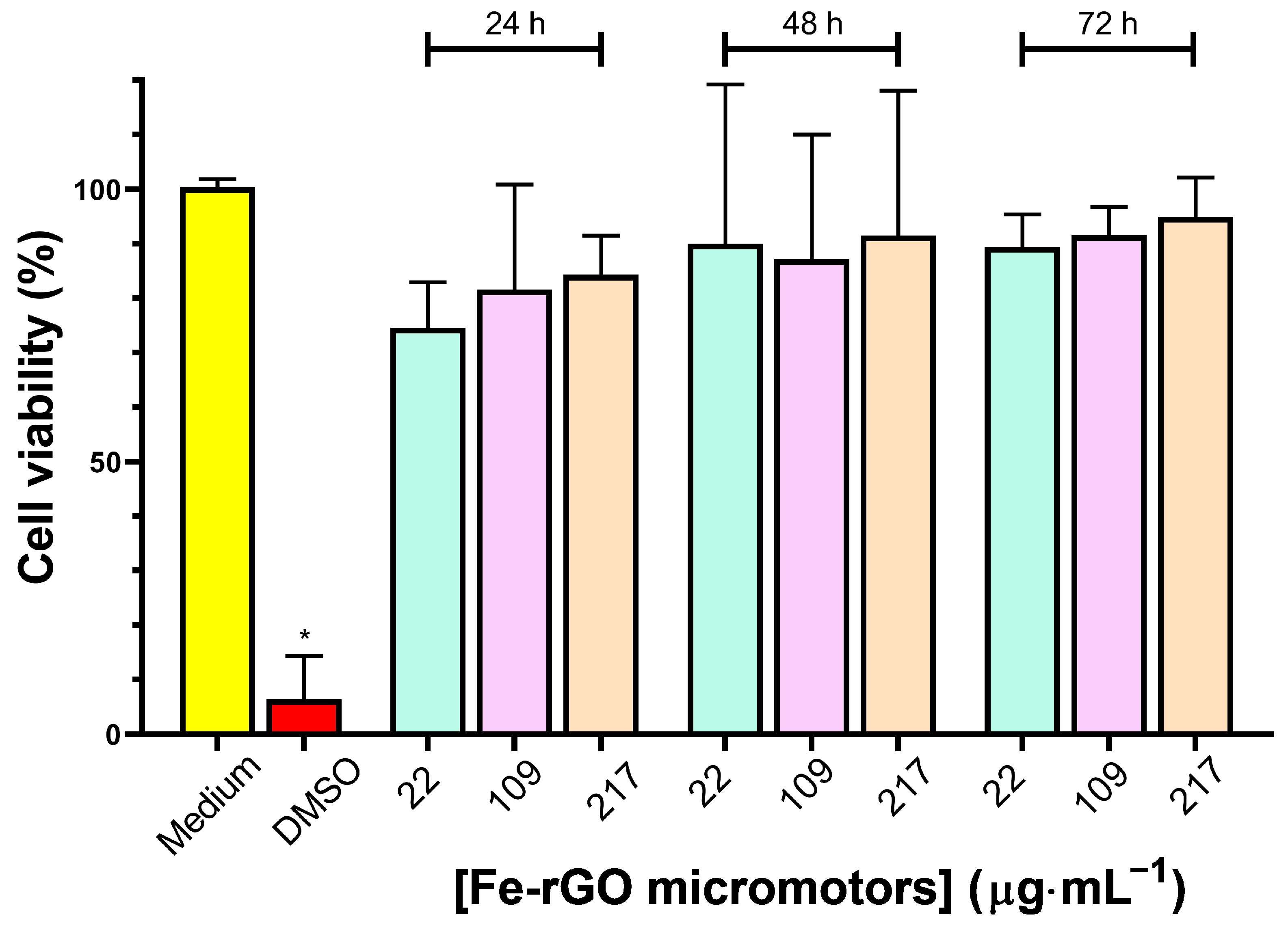
3.4. Cytotoxicity of Fe-rGO Micromotors
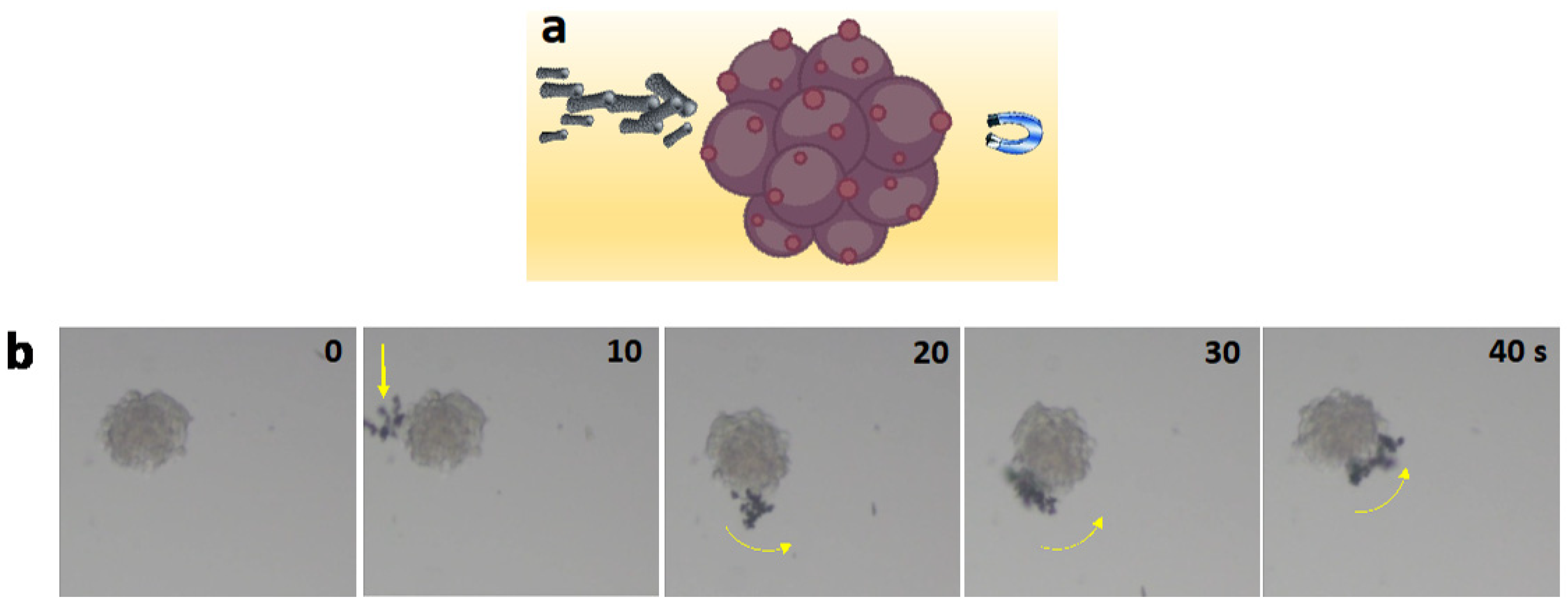
3.5. Motion Analysis of Fe-rGO Micromotors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.; Mayorga-Martinez, C.C.; Pané, S.; Zhang, L.; Pumera, M. Magnetically Driven Micro and Nanorobots. Chem. Rev. 2021, 121, 4999–5041. [Google Scholar] [CrossRef]
- Wang, B.; Chan, K.F.; Yuan, K.; Wang, Q.; Xia, X.; Yang, L.; Ko, H.; Wang, Y.X.J.; Sung, J.J.Y.; Chiu, P.W.Y.; et al. Endoscopy-Assisted Magnetic Navigation of Biohybrid Soft Microrobots with Rapid Endoluminal Delivery and Imaging. Sci. Robot. 2021, 6, eabd2813. [Google Scholar] [CrossRef]
- Venugopalan, P.L.; Ghosh, A. Investigating the Dynamics of the Magnetic Micromotors in Human Blood. Langmuir 2021, 37, 289–296. [Google Scholar] [CrossRef]
- Latiyan, S.; Suneet, K.; Jain, S. Magneto-conducting Multifunctional Janus Microbots for Intracellular Delivery of Biomolecules. J. Tissue Eng. Regen. Med. 2021, 15, 625–633. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Zhang, L. Untethered Micro/Nanorobots for Remote Sensing: Toward Intelligent Platform. Nano-Micro Lett. 2024, 16, 40. [Google Scholar] [CrossRef]
- Li, H.; Peng, F.; Yan, X.; Mao, C.; Ma, X.; Wilson, D.A.; He, Q.; Tu, Y. Medical Micro- and Nanomotors in the Body. Acta Pharm. Sin. B 2023, 13, 517–541. [Google Scholar] [CrossRef]
- Zhang, F.; Mundaca-Uribe, R.; Askarinam, N.; Li, Z.; Gao, W.; Zhang, L.; Wang, J. Biomembrane-Functionalized Micromotors: Biocompatible Active Devices for Diverse Biomedical Applications. Adv. Mater. 2022, 34, 2107177. [Google Scholar] [CrossRef]
- Peng, X.; Urso, M.; Kolackova, M.; Huska, D.; Pumera, M. Biohybrid Magnetically Driven Microrobots for Sustainable Removal of Micro/Nanoplastics from the Aquatic Environment. Adv. Funct. Mater. 2023, 34, 2307477. [Google Scholar] [CrossRef]
- Chen, B.; Sun, H.; Zhang, J.; Xu, J.; Song, Z.; Zhan, G.; Bai, X.; Feng, L. Cell-Based Micro/Nano-Robots for Biomedical Applications: A Review. Small 2024, 20, e2304607. [Google Scholar] [CrossRef]
- Wang, J.; Dong, R.; Wu, H.; Cai, Y.; Ren, B. A Review on Artificial Micro/Nanomotors for Cancer-Targeted Delivery, Diagnosis, and Therapy. Nano-Micro Lett. 2020, 12, 11. [Google Scholar] [CrossRef]
- Yuan, K.; Jiang, Z.; Jurado-Sánchez, B.; Escarpa, A. Nano/Micromotors for Diagnosis and Therapy of Cancer and Infectious Diseases. Chem.-Eur. J. 2020, 26, 2309–2326. [Google Scholar] [CrossRef]
- Rastmanesh, A.; Tavakkoli Yaraki, M.; Wu, J.; Wang, Z.; Ghoderao, P.; Gao, Y.; Tan, Y.N. Bioinspired Micro/Nanomotors towards a Self-Propelled Noninvasive Diagnosis and Treatment of Cancer. Mol. Syst. Des. Eng. 2021, 6, 566–593. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C. A Journey of Nanomotors for Targeted Cancer Therapy: Principles, Challenges, and a Critical Review of the State-of-the-Art. Adv. Healthc. Mater. 2021, 10, e2001236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Guo, W.; Fang, D.; Li, T.; Chen, L.; Mao, C.; Wan, M.; Shen, J. Mg-Based Micromotors for Efficient Electrochemical Detection of Circulating Tumor Cells. Sens. Actuators B Chem. 2023, 390, 133933. [Google Scholar] [CrossRef]
- Gao, W.; de Ávila, B.E.-F.; Zhang, L.; Wang, J. Targeting and Isolation of Cancer Cells Using Micro/Nanomotors. Adv. Drug Deliv. Rev. 2018, 125, 94–101. [Google Scholar] [CrossRef]
- Hou, K.; Zhang, Y.; Bao, M.; Xin, C.; Wei, Z.; Lin, G.; Wang, Z. A Multifunctional Magnetic Red Blood Cell-Mimetic Micromotor for Drug Delivery and Image-Guided Therapy. ACS Appl. Mater. Interfaces 2022, 14, 3825–3837. [Google Scholar] [CrossRef]
- Li, Z.; Fu, S.; Li, H.; Chen, B.; Xie, D.; Fu, D.; Feng, Y.; Gao, C.; Liu, S.; Wilson, D.A.; et al. Light-Driven Micromotor Swarm Induced in-Situ Polymerization and Synergistic Photothermal Therapy. Chem. Eng. J. 2023, 468, 143393. [Google Scholar] [CrossRef]
- Gao, C.; Feng, Y.; Wilson, D.A.; Tu, Y.; Peng, F. Micro-Nano Motors with Taxis Behavior: Principles, Designs, and Biomedical Applications. Small 2022, 18, e2106263. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Cai, L.; Fan, L.; Zhao, Y. Dip-Printed Microneedle Motors for Oral Macromolecule Delivery. Research 2022, 2022, 9797482. [Google Scholar] [CrossRef]
- Maric, T.; Adamakis, V.; Zhang, Z.; Milián-Guimerá, C.; Thamdrup, L.H.E.; Stamate, E.; Ghavami, M.; Boisen, A. Microscopic Cascading Devices for Boosting Mucus Penetration in Oral Drug Delivery—Micromotors Nesting Inside Microcontainers. Small 2023, 19, e2206330. [Google Scholar] [CrossRef]
- Lopez-Ramirez, M.A.; Soto, F.; Wang, C.; Rueda, R.; Shukla, S.; Silva-Lopez, C.; Kupor, D.; McBride, D.A.; Pokorski, J.K.; Nourhani, A.; et al. Built-In Active Microneedle Patch with Enhanced Autonomous Drug Delivery. Adv. Mater. 2020, 32, e1905740. [Google Scholar] [CrossRef]
- Gordón, J.; Arruza, L.; Ibáñez, M.D.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. On the Move-Sensitive Fluorescent Aptassay on Board Catalytic Micromotors for the Determination of Interleukin-6 in Ultra-Low Serum Volumes for Neonatal Sepsis Diagnostics. ACS Sens. 2022, 7, 3144–3152. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Angell, C.; Soto, F.; Lopez-Ramirez, M.A.; Báez, D.F.; Xie, S.; Wang, J.; Chen, Y. Acoustically Propelled Nanomotors for Intracellular siRNA Delivery. ACS Nano 2016, 10, 4997–5005. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Ye, Z.; Lin, Z.; Ma, X.; He, Q. Biomedical Micro-/Nanomotors: From Overcoming Biological Barriers to In Vivo Imaging. Adv. Mater. 2021, 33, 2000512. [Google Scholar] [CrossRef]
- Van de Walle, A.; Figuerola, A.; Espinosa, A.; Abou-Hassan, A.; Estrader, M.; Wilhelm, C. Emergence of Magnetic Nanoparticles in Photothermal and Ferroptotic Therapies. Mater. Horiz. 2023, 10, 4757–4775. [Google Scholar] [CrossRef]
- Villa, K.; Krejčová, L.; Novotný, F.; Heger, Z.; Sofer, Z.; Pumera, M. Cooperative Multifunctional Self-Propelled Paramagnetic Microrobots with Chemical Handles for Cell Manipulation and Drug Delivery. Adv. Funct. Mater. 2018, 28, e1804343. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Li, Y.; Xu, D.; Pan, X.; Chen, Y.; Zhou, D.; Wang, B.; Feng, H.; Ma, X. Magnetic Nanomotor-Based Maneuverable SERS Probe. Research 2020, 2020, 7962024. [Google Scholar] [CrossRef]
- Qin, F.; Wu, J.; Fu, D.; Feng, Y.; Gao, C.; Xie, D.; Fu, S.; Liu, S.; Wilson, D.A.; Peng, F. Magnetically Driven Helical Hydrogel Micromotor for Tumor DNA Detection. Appl. Mater. Today 2022, 27, 101456. [Google Scholar] [CrossRef]
- Vyskočil, J.; Mayorga-Martinez, C.C.; Jablonská, E.; Novotný, F.; Ruml, T.; Pumera, M. Cancer Cells Microsurgery via Asymmetric Bent Surface Au/Ag/Ni Microrobotic Scalpels through a Transversal Rotating Magnetic Field. ACS Nano 2020, 14, 8247–8256. [Google Scholar] [CrossRef]
- Sun, M.; Fan, X.; Meng, X.; Song, J.; Chen, W.; Sun, L.; Xie, H. Magnetic Biohybrid Micromotors with High Maneuverability for Efficient Drug Loading and Targeted Drug Delivery. Nanoscale 2019, 11, 18382–18392. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; Jodra, A.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. Magnetic Reduced Graphene Oxide/Nickel/Platinum Nanoparticles Micromotors for Mycotoxin Analysis. Chem.-Eur. J. 2018, 24, 7172–7176. [Google Scholar] [CrossRef] [PubMed]
- Báez, D.F.; Ramos, G.; Corvalán, A.; Cordero, M.L.; Bollo, S.; Kogan, M.J. Effects of Preparation on Catalytic, Magnetic and Hybrid Micromotors on Their Functional Features and Application in Gastric Cancer Biomarker Detection. Sens. Actuators B Chem. 2020, 310, 127843. [Google Scholar] [CrossRef]
- Schwarz, L.; Medina-Sanchez, M.; Schmidt, O.G. Magnetic Micromotors for Resilient and Reversible Cargo Transport in and between Microfluidic Environments. In Proceedings of the 2019 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Helsinki, Finland, 1–5 July 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Yang, L.; Chen, X.; Wang, L.; Hu, Z.; Xin, C.; Hippler, M.; Zhu, W.; Hu, Y.; Li, J.; Wang, Y.; et al. Targeted Single-Cell Therapeutics with Magnetic Tubular Micromotor by One-Step Exposure of Structured Femtosecond Optical Vortices. Adv. Funct. Mater. 2019, 29, e1905745. [Google Scholar] [CrossRef]
- Xu, H.; Medina-Sánchez, M.; Maitz, M.F.; Werner, C.; Schmidt, O.G. Sperm Micromotors for Cargo Delivery through Flowing Blood. ACS Nano 2020, 14, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- More, S.L.; Kovochich, M.; Lyons-Darden, T.; Taylor, M.; Schulte, A.M.; Madl, A.K. Review and Evaluation of the Potential Health Effects of Oxidic Nickel Nanoparticles. Nanomaterials 2021, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, B.A.; Minigaliyeva, I.A.; Panov, V.G.; Privalova, L.I.; Varaksin, A.N.; Gurvich, V.B.; Sutunkova, M.P.; Shur, V.Y.; Shishkina, E.V.; Valamina, I.E.; et al. Some Patterns of Metallic Nanoparticles’ Combined Subchronic Toxicity as Exemplified by a Combination of Nickel and Manganese Oxide Nanoparticles. Food Chem. Toxicol. 2015, 86, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Mondal, M.H.; Bhattarai, A.; Saha, B. A Comprehensive Review on the Sources, Essentiality and Toxicological Profile of Nickel. RSC Adv. 2022, 12, 9139–9153. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv. Healthc. Mater. 2020, 9, 1901058. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.L. Synthesis, Properties, and Applications of Iron Nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef]
- Wu, Y.; Kong, L. Advance on Toxicity of Metal Nickel Nanoparticles. Environ. Geochem. Health 2020, 42, 2277–2286. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Bolaños, K.; Sánchez-Navarro, M.; Giralt, E.; Acosta, G.; Albericio, F.; Kogan, M.J.; Araya, E. NIR and Glutathione Trigger the Surface Release of Methotrexate Linked by Diels-Alder Adducts to Anisotropic Gold Nanoparticles. Mater. Sci. Eng. C 2021, 131, 112512. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Khaniabadi, P.M.; Jameel, M.S.; Oladzadabbasabadi, N.; Rahman, A.A.; Braim, F.S.; Mehrdel, B. Gold Nanoparticles-Based Photothermal Therapy for Breast Cancer. Photodiagnosis Photodyn. Ther. 2023, 42, 103312. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and Bioactive Engineered Nanomaterials for Targeted Tumor Photothermal Therapy: A Review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef]
- Duan, S.; Hu, Y.; Zhao, Y.; Tang, K.; Zhang, Z.; Liu, Z.; Wang, Y.; Guo, H.; Miao, Y.; Du, H.; et al. Nanomaterials for Photothermal Cancer Therapy. RSC Adv. 2023, 13, 14443–14460. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Liu, T.; Zhang, Y.; Wang, C.; Xie, B. Ultrasmall Graphene Oxide for Combination of Enhanced Chemotherapy and Photothermal Therapy of Breast Cancer. Colloids Surf. B Biointerfaces 2023, 225, 113288. [Google Scholar] [CrossRef]
- Báez, D.F. Graphene-Based Nanomaterials for Photothermal Therapy in Cancer Treatment. Pharmaceutics 2023, 15, 2286. [Google Scholar] [CrossRef]
- Hao, L.; Song, H.; Zhan, Z.; Lv, Y. Multifunctional Reduced Graphene Oxide-Based Nanoplatform for Synergistic Targeted Chemo-Photothermal Therapy. ACS Appl. Bio Mater. 2020, 3, 5213–5222. [Google Scholar] [CrossRef]
- Báez, D.; Pardo, H.; Laborda, I.; Marco, J.; Yáñez, C.; Bollo, S. Reduced Graphene Oxides: Influence of the Reduction Method on the Electrocatalytic Effect towards Nucleic Acid Oxidation. Nanomaterials 2017, 7, 168. [Google Scholar] [CrossRef]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene Oxide Mediated Delivery of Methylene Blue for Combined Photodynamic and Photothermal Therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second Near-Infrared Photothermal Materials for Combinational Nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Lopez-Ramirez, M.A.; Báez, D.F.; Jodra, A.; Singh, V.V.; Kaufmann, K.; Wang, J. Aptamer-Modified Graphene-Based Catalytic Micromotors: Off–On Fluorescent Detection of Ricin. ACS Sens. 2016, 1, 217–221. [Google Scholar] [CrossRef]
- Vilela, D.; Parmar, J.; Zeng, Y.; Zhao, Y.; Sanchez, S. Graphene Based Microbots for Toxic Heavy Metal Removal and Recovery from Water. Nano Lett. 2016, 16, 2860–2866. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, H.; Wen, H.; Zhao, H.; Liu, X.; Cai, Y.; Wang, H.; Dong, R. Graphene Oxide Induced Enhancement of Light-Driven Micromotor with Biocompatible Fuels. Appl. Mater. Today 2021, 22, 100943. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Tan, L.; Zhang, Y.; Jiao, Y. Near-Infrared Light-Steered Graphene Aerogel Micromotor with High Speed and Precise Navigation for Active Transport and Microassembly. ACS Appl. Mater. Interfaces 2020, 12, 23134–23144. [Google Scholar] [CrossRef]
- Palomar-Pardavé, M.; Mostany, J.; Muñoz-Rizo, R.; Botello, L.E.; Aldana-González, J.; Arce-Estrada, E.M.; de Oca-Yemha, M.G.M.; Ramírez-Silva, M.T.; Romo, M.R. Electrochemical Study and Physicochemical Characterization of Iron Nanoparticles Electrodeposited onto HOPG from Fe(III) Ions Dissolved in the Choline Chloride-Urea Deep Eutectic Solvent. J. Electroanal. Chem. 2019, 851, 113453. [Google Scholar] [CrossRef]
- Martín, A.; Jurado-Sánchez, B.; Escarpa, A.; Wang, J. Template Electrosynthesis of High-Performance Graphene Microengines. Small 2015, 11, 3568–3574. [Google Scholar] [CrossRef]
- Yoshida, Y.; Langouche, G. Mössbauer Spectroscopy; Springuer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-32219-8. [Google Scholar]
- Ordoukhanian, J.; Karami, H.; Nezhadali, A. One Step Paired Electrochemical Synthesis of Iron and Iron Oxide Nanoparticles. Mater. Sci.-Pol. 2016, 34, 655–658. [Google Scholar] [CrossRef]
- Krajewski, M.; Lin, W.S.; Lin, H.M.; Brzozka, K.; Lewinska, S.; Nedelko, N.; Slawska-Waniewska, A.; Borysiuk, J.; Wasik, D. Structural and Magnetic Properties of Iron Nanowires and Iron Nanoparticles Fabricated through a Reduction Reaction. Beilstein J. Nanotechnol. 2015, 6, 1652–1660. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, X.-Q.; Wang, Y. Ultradeep Photothermal Therapy Strategies. J. Phys. Chem. Lett. 2022, 13, 9564–9572. [Google Scholar] [CrossRef]
- Liu, P.; Ye, M.; Wu, Y.; Wu, L.; Lan, K.; Wu, Z. Hyperthermia Combined with Immune Checkpoint Inhibitor Therapy: Synergistic Sensitization and Clinical Outcomes. Cancer Med. 2023, 12, 3201–3221. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, G.H.; Kim, K.S.; Hahn, S.K. Light-Guided Nanomotor Systems for Autonomous Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 2338–2346. [Google Scholar] [CrossRef]
- Cheon, Y.A.; Bae, J.H.; Chung, B.G. Reduced Graphene Oxide Nanosheet for Chemo-Photothermal Therapy. Langmuir 2016, 32, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The Influence of Surface Chemistry and Size of Nanoscale Graphene Oxide on Photothermal Therapy of Cancer Using Ultra-Low Laser Power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Vinh, D.; Dai, H. Ultrasmall Reduced Graphene Oxide with High Near-Infrared Absorbance for Photothermal Therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wu, X.; Xing, Y.; Lilak, S.; Wu, M.; Zhao, J.X. Enhanced Synergetic Antibacterial Activity by a Reduce Graphene Oxide/Ag Nanocomposite through the Photothermal Effect. Colloids Surf. B Biointerfaces 2020, 185, 110616. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Wang, J.; Wang, S.; Wang, Y.; Jin, D.; Chen, P.; Du, W.; Zhang, L.; Liu, B.F. Graphene-Based Helical Micromotors Constructed by “Microscale Liquid Rope-Coil Effect” with Microfluidics. ACS Nano 2020, 14, 16600–16613. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Zhou, L.; Shang, L.; Su, Z. Reduced Graphene Oxide (rGO) Hybridized Hydrogel as a near-Infrared (NIR)/pH Dual-Responsive Platform for Combined Chemo-Photothermal Therapy. J. Colloid Interface Sci. 2019, 536, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Beniwal, N.; Verma, A.; Putta, C.L.; Rengan, A.K. Recent Trends in Bio-Nanomaterials and Non-Invasive Combinatorial Approaches of Photothermal Therapy against Cancer. Nanotheranostics 2024, 8, 219–238. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Yang, Y.; Yu, Y.; Zhang, Y.; Zhu, D.; Yu, X.; Ouyang, X.; Xie, Z.; Zhao, Y.; et al. Recent Advances in Nanomaterials-Based Chemo-Photothermal Combination Therapy for Improving Cancer Treatment. Front. Bioeng. Biotechnol. 2019, 7, 293. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Z.; Dou, Q.; Hui, S.; Zhang, P.; Liu, R.; Wang, D.; Jiang, S. NIR-Light-Responsive Chemo-Photothermal Hydrogel System with Controlled DOX Release and Photothermal Effect for Cancer Therapy. Colloids Surf. Physicochem. Eng. Asp. 2023, 667, 131407. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. In Neuronal Cell Culture; Amini, S., White, M.K., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1078, pp. 9–21. ISBN 978-1-62703-639-9. [Google Scholar]
- Lopez-Suarez, L.; Awabdh, S.A.; Coumoul, X.; Chauvet, C. The SH-SY5Y Human Neuroblastoma Cell Line, a Relevant in Vitro Cell Model for Investigating Neurotoxicology in Human: Focus on Organic Pollutants. NeuroToxicology 2022, 92, 131–155. [Google Scholar] [CrossRef]
- Eguchi, T.; Sheta, M.; Fujii, M.; Calderwood, S.K. Cancer Extracellular Vesicles, Tumoroid Models, and Tumor Microenvironment. Semin. Cancer Biol. 2022, 86, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Pape, J.; Emberton, M.; Cheema, U. 3D Cancer Models: The Need for a Complex Stroma, Compartmentalization and Stiffness. Front. Bioeng. Biotechnol. 2021, 9, 660502. [Google Scholar] [CrossRef] [PubMed]
- Durán-Jara, E.; Del Campo, M.; Gutiérrez, V.; Wichmann, I.; Trigo, C.; Ezquer, M.; Lobos-González, L. Lactadherin Immunoblockade in Small Extracellular Vesicles Inhibits sEV-Mediated Increase of pro-Metastatic Capacities. Biol. Res. 2024, 57, 1. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donoso-González, O.; Riveros, A.L.; Marco, J.F.; Venegas-Yazigi, D.; Paredes-García, V.; Olguín, C.F.; Mayorga-Lobos, C.; Lobos-González, L.; Franco-Campos, F.; Wang, J.; et al. Iron-Reduced Graphene Oxide Core–Shell Micromotors Designed for Magnetic Guidance and Photothermal Therapy under Second Near-Infrared Light. Pharmaceutics 2024, 16, 856. https://doi.org/10.3390/pharmaceutics16070856
Donoso-González O, Riveros AL, Marco JF, Venegas-Yazigi D, Paredes-García V, Olguín CF, Mayorga-Lobos C, Lobos-González L, Franco-Campos F, Wang J, et al. Iron-Reduced Graphene Oxide Core–Shell Micromotors Designed for Magnetic Guidance and Photothermal Therapy under Second Near-Infrared Light. Pharmaceutics. 2024; 16(7):856. https://doi.org/10.3390/pharmaceutics16070856
Chicago/Turabian StyleDonoso-González, Orlando, Ana L. Riveros, José F. Marco, Diego Venegas-Yazigi, Verónica Paredes-García, Camila F. Olguín, Cristina Mayorga-Lobos, Lorena Lobos-González, Felipe Franco-Campos, Joseph Wang, and et al. 2024. "Iron-Reduced Graphene Oxide Core–Shell Micromotors Designed for Magnetic Guidance and Photothermal Therapy under Second Near-Infrared Light" Pharmaceutics 16, no. 7: 856. https://doi.org/10.3390/pharmaceutics16070856
APA StyleDonoso-González, O., Riveros, A. L., Marco, J. F., Venegas-Yazigi, D., Paredes-García, V., Olguín, C. F., Mayorga-Lobos, C., Lobos-González, L., Franco-Campos, F., Wang, J., Kogan, M. J., Bollo, S., Yañez, C., & Báez, D. F. (2024). Iron-Reduced Graphene Oxide Core–Shell Micromotors Designed for Magnetic Guidance and Photothermal Therapy under Second Near-Infrared Light. Pharmaceutics, 16(7), 856. https://doi.org/10.3390/pharmaceutics16070856










