TB/FLU-06E Influenza Vector-Based Vaccine in the Complex Therapy of Drug-Susceptible and Drug-Resistant Experimental Tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. TB/FLU-06E Production
2.2. Laboratory Animals
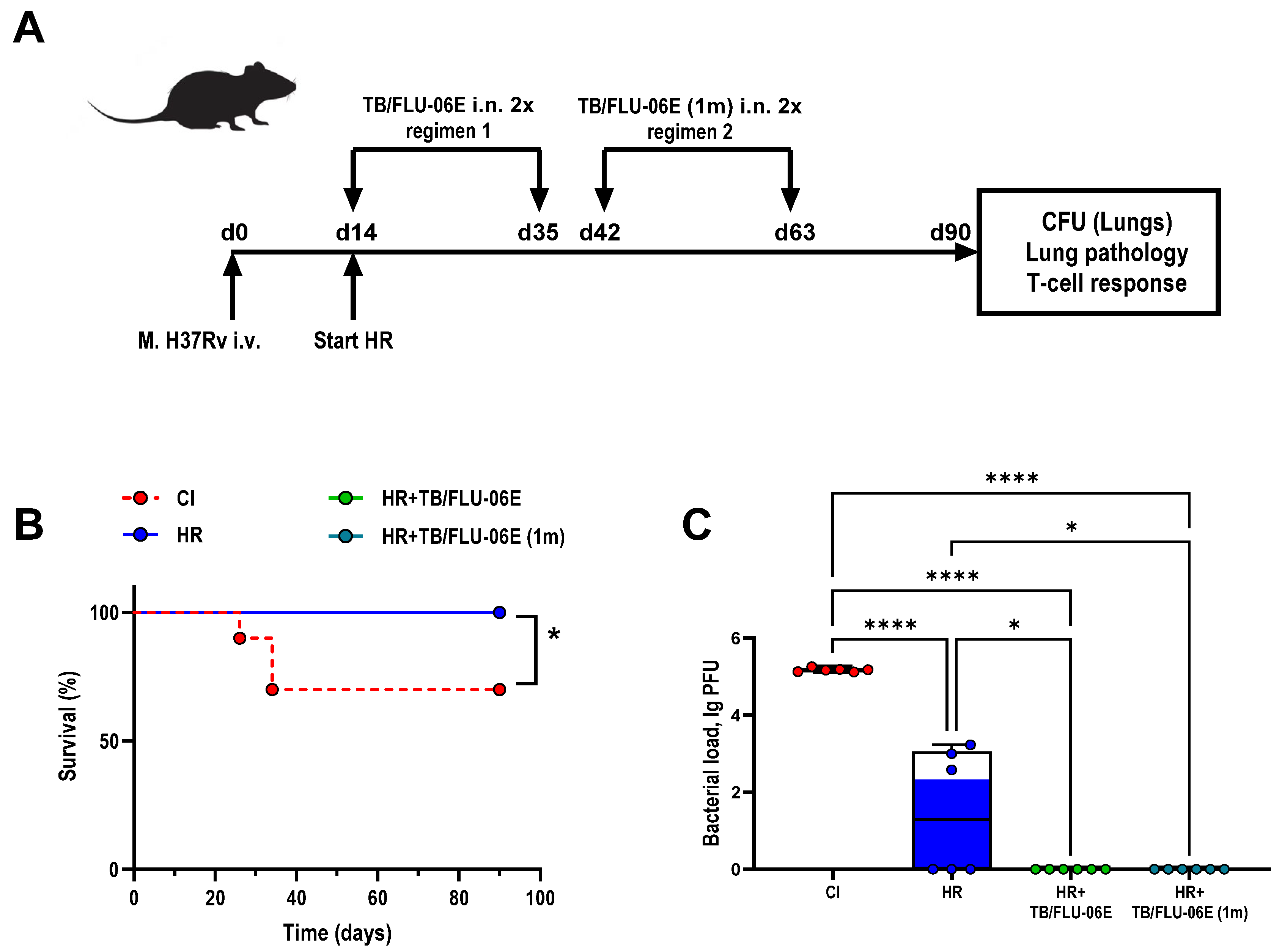
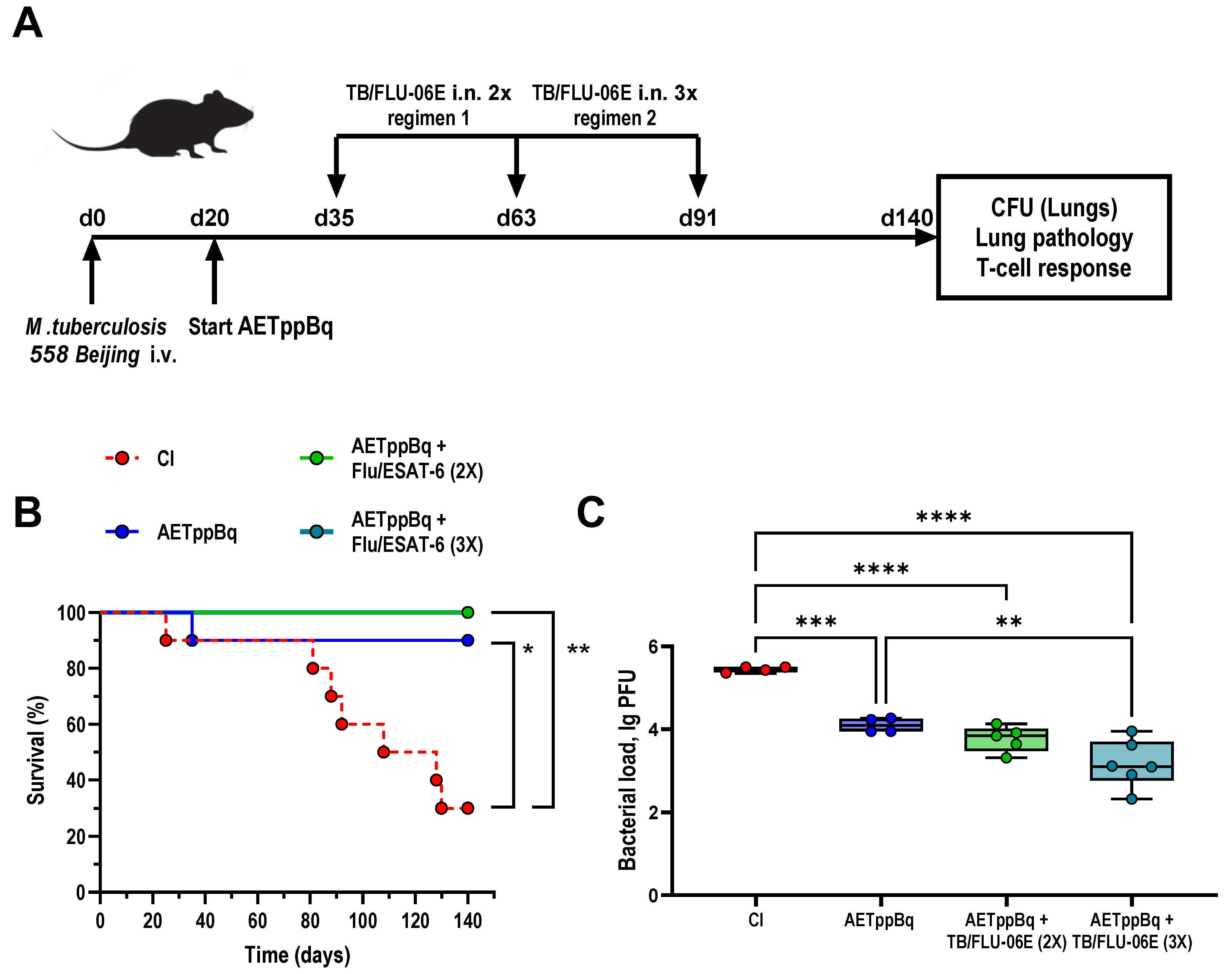
2.3. Experimental Design
2.4. Mycobacterial Load
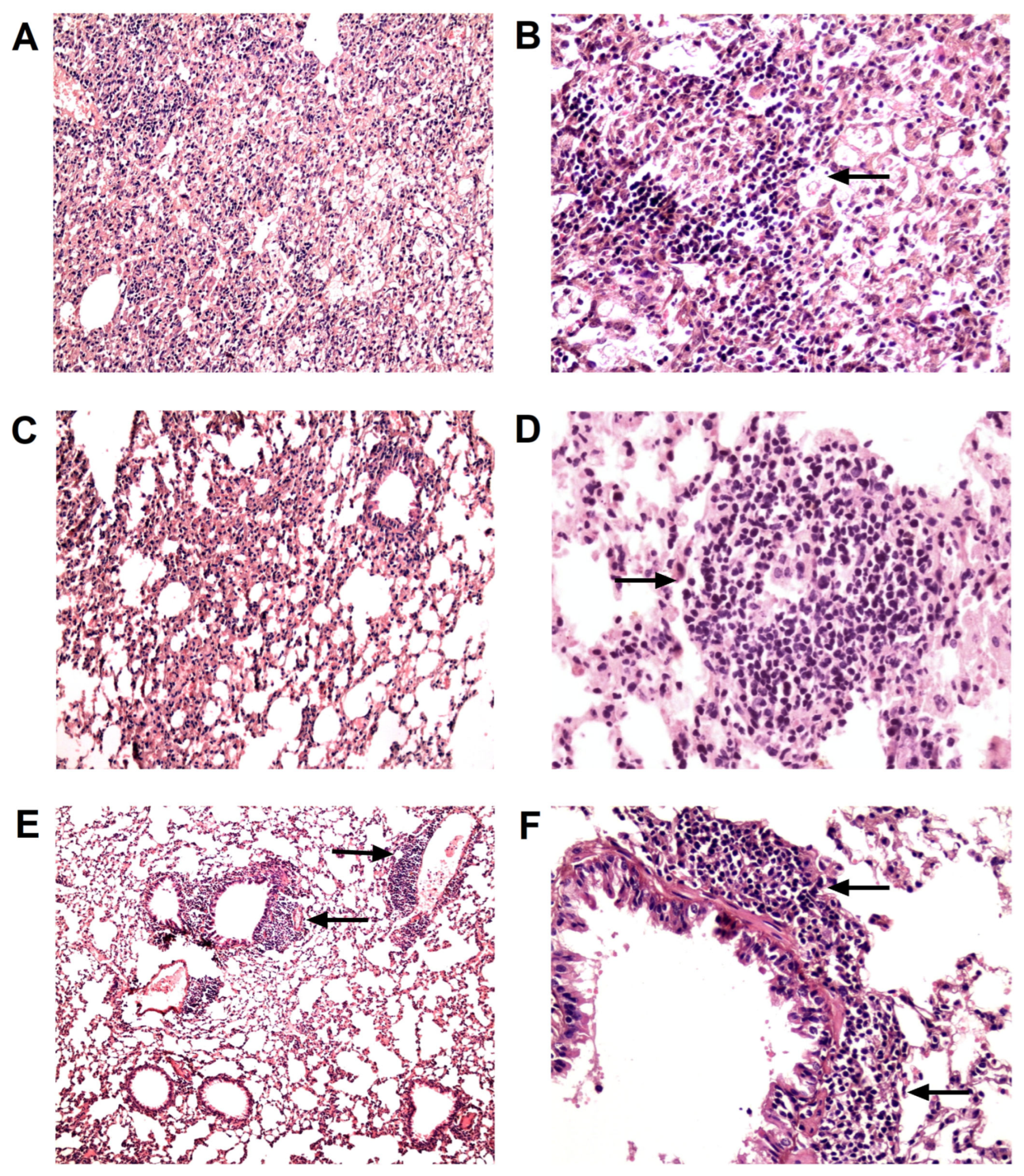
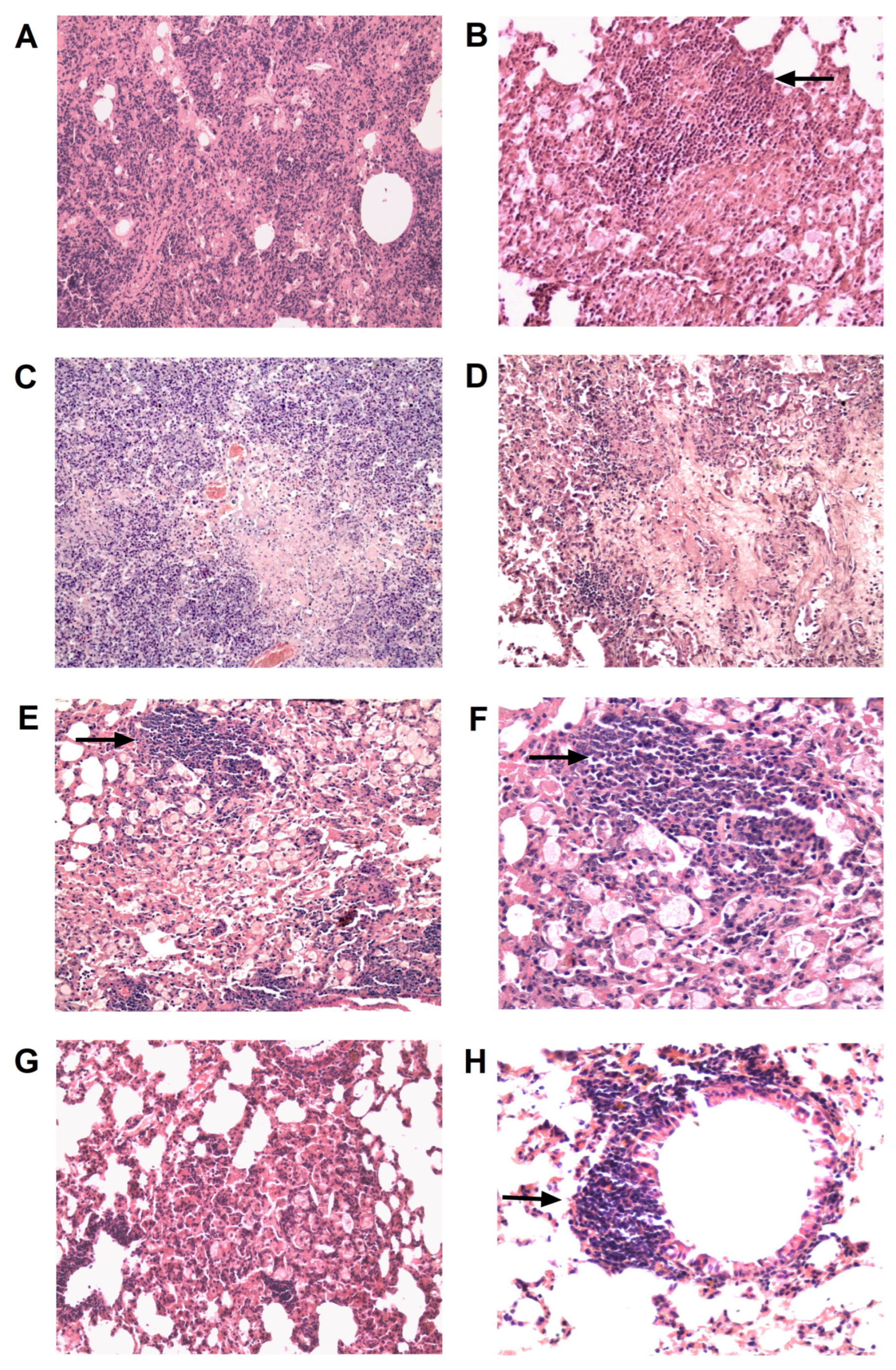
2.5. Histopathology
2.6. Intracellular Cytokine Staining
2.7. Statistical Analyses
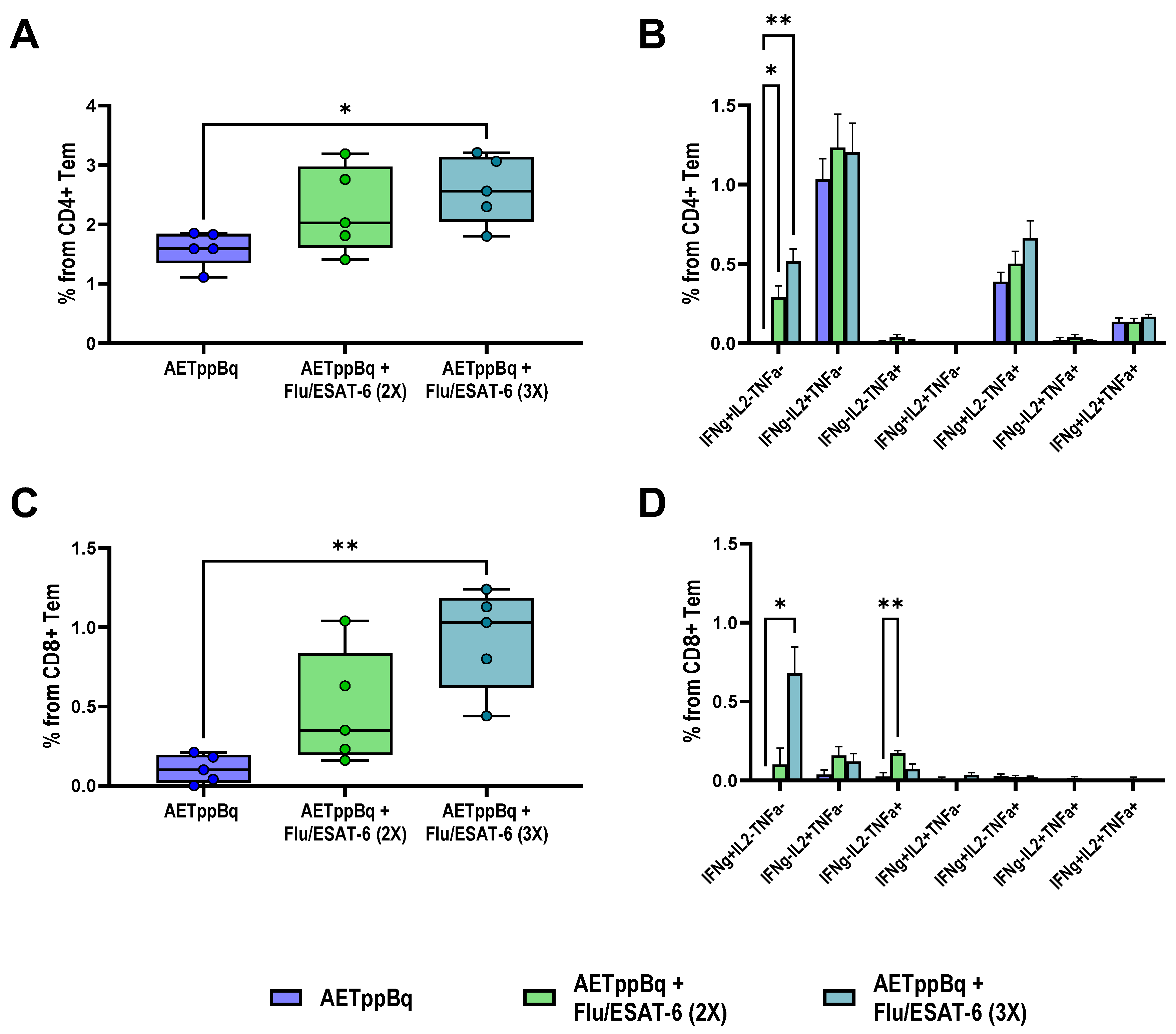
3. Results
3.1. TB/FLU-06E Vaccine Efficacy in the Complex Therapy of Drug-Susceptible Tuberculosis
3.2. TB/FLU-06E Vaccine Efficacy in the Complex Therapy of Drug-Resistant Tuberculosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dean, A.S.; Tosas Auguet, O.; Glaziou, P.; Zignol, M.; Ismail, N.; Kasaeva, T.; Floyd, K. 25 Years of Surveillance of Drug-Resistant Tuberculosis: Achievements, Challenges, and Way Forward. Lancet Infect. Dis. 2022, 22, e191–e196. [Google Scholar] [CrossRef]
- Mohammed, K.A.S.; Khudhair, G.S.; Al-Rabeai, D.B. Prevalence and Drug Resistance Pattern of Mycobacterium tuberculosis Isolated from Tuberculosis Patients in Basra, Iraq. Pol. J. Microbiol. 2022, 71, 205–215. [Google Scholar] [PubMed]
- Matteelli, A.; Rendon, A.; Tiberi, S.; Al-Abri, S.; Voniatis, C.; Carvalho, A.C.C.; Centis, R.; D’Ambrosio, L.; Visca, D.; Spanevello, A.; et al. Tuberculosis Elimination: Where Are We Now? Eur. Respir. Rev. 2018, 27, 180035. [Google Scholar] [CrossRef] [PubMed]
- Bouzeyen, R.; Javid, B. Therapeutic Vaccines for Tuberculosis: An Overview. Front. Immunol. 2022, 13, 878471. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, M.; Chen, Z.; Chen, C.; Wu, G.; Zuo, Y.; Ren, X.; Chen, Z.; Wang, W.; Pang, Y. Survival of Patients with Multidrug-Resistant Tuberculosis in Central China: A Retrospective Cohort Study. Epidemiol. Infect. 2020, 148, e50. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, F.; Niu, H.; Ma, L.; Chen, J.; Zhang, Y.; Peng, L.; Gan, C.; Ma, X.; Zhu, B. IL-2 Restores T-Cell Dysfunction Induced by Persistent Mycobacterium tuberculosis Antigen Stimulation. Front. Immunol. 2019, 10, 2350. [Google Scholar] [CrossRef]
- Tousif, S.; Singh, D.K.; Ahmad, S.; Moodley, P.; Bhattacharyya, M.; Van Kaer, L.; Das, G. Isoniazid Induces Apoptosis of Activated CD4+ T Cells: Implications for Post-Therapy Tuberculosis Reactivation and Reinfection. J. Biol. Chem. 2014, 289, 30190–30195. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, F.; Zeng, G.; Shen, L.; Cheng, H.; Huang, D.; Wang, R.; Rong, L.; Chen, Z.W. Bis-Biguanide Dihydrochloride Inhibits Intracellular Replication of M. tuberculosis and Controls Infection in Mice. Sci. Rep. 2016, 6, 32725. [Google Scholar] [CrossRef] [PubMed]
- Surjit, M.; Liu, B.; Chow, V.T.K.; Lal, S.K. The Nucleocapsid Protein of Severe Acute Respiratory Syndrome-Coronavirus Inhibits the Activity of Cyclin-Cyclin-Dependent Kinase Complex and Blocks S Phase Progression in Mammalian Cells. J. Biol. Chem. 2006, 281, 10669–10681. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, C.; Kaushik, S.R.; Kulshreshtha, A.; Chaturvedi, S.; Nanda, R.K.; Bhaskar, A.; Chattopadhyay, D.; Das, G.; Dwivedi, V.P. The Phytochemical Bergenin as an Adjunct Immunotherapy for Tuberculosis in Mice. J. Biol. Chem. 2019, 294, 8555–8563. [Google Scholar] [CrossRef]
- Larsen, S.E.; Baldwin, S.L.; Orr, M.T.; Reese, V.A.; Pecor, T.; Granger, B.; Dubois Cauwelaert, N.; Podell, B.K.; Coler, R.N. Enhanced Anti-Mycobacterium tuberculosis Immunity over Time with Combined Drug and Immunotherapy Treatment. Vaccines 2018, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhu, S.; Zhu, L.; Kong, C.; Huang, Q.; Zhang, Z.; Wang, H.; Xu, Y. Mycobacterium tuberculosis Latent Antigen Rv2029c from the Multistage DNA Vaccine A39 Drives TH1 Responses via TLR-Mediated Macrophage Activation. Front. Microbiol. 2017, 8, 2266. [Google Scholar] [CrossRef] [PubMed]
- Billeskov, R.; Lindenstrøm, T.; Woodworth, J.; Vilaplana, C.; Cardona, P.-J.; Cassidy, J.P.; Mortensen, R.; Agger, E.M.; Andersen, P. High Antigen Dose Is Detrimental to Post-Exposure Vaccine Protection against Tuberculosis. Front. Immunol. 2017, 8, 1973. [Google Scholar] [CrossRef] [PubMed]
- Stukova, M.A.; Sereinig, S.; Zabolotnyh, N.V.; Ferko, B.; Kittel, C.; Romanova, J.; Vinogradova, T.I.; Katinger, H.; Kiselev, O.I.; Egorov, A. Vaccine Potential of Influenza Vectors Expressing Mycobacterium tuberculosis ESAT-6 Protein. Tuberculosis 2006, 86, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Sereinig, S.; Stukova, M.; Zabolotnyh, N.; Ferko, B.; Kittel, C.; Romanova, J.; Vinogradova, T.; Katinger, H.; Kiselev, O.; Egorov, A. Influenza Virus NS Vectors Expressing the Mycobacterium tuberculosis ESAT-6 Protein Induce CD4+ Th1 Immune Response and Protect Animals against Tuberculosis Challenge. Clin. Vaccine Immunol. 2006, 13, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Passos, B.B.S.; Araújo-Pereira, M.; Vinhaes, C.L.; Amaral, E.P.; Andrade, B.B. The Role of ESAT-6 in Tuberculosis Immunopathology. Front. Immunol. 2024, 15, 1383098. [Google Scholar] [CrossRef] [PubMed]
- Anes, E.; Pires, D.; Mandal, M.; Azevedo-Pereira, J.M. ESAT-6 a Major Virulence Factor of Mycobacterium tuberculosis. Biomolecules 2023, 13, 968. [Google Scholar] [CrossRef]
- Mir, S.A.; Verma, I.; Sharma, S. Immunotherapeutic Potential of Recombinant ESAT-6 Protein in Mouse Model of Experimental Tuberculosis. Immunol. Lett. 2014, 158, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Sharma, S. Immunotherapeutic Potential of N-Terminally Formylated ESAT-6 Protein in Murine Tuberculosis. Int. J. Mycobacteriol. 2022, 11, 108–112. [Google Scholar] [CrossRef]
- Clemmensen, H.S.; Knudsen, N.P.H.; Billeskov, R.; Rosenkrands, I.; Jungersen, G.; Aagaard, C.; Andersen, P.; Mortensen, R. Rescuing ESAT-6 Specific CD4 T Cells from Terminal Differentiation Is Critical for Long-Term Control of Murine Mtb Infection. Front. Immunol. 2020, 11, 585359. [Google Scholar] [CrossRef]
- Hoang, T.; Aagaard, C.; Dietrich, J.; Cassidy, J.P.; Dolganov, G.; Schoolnik, G.K.; Lundberg, C.V.; Agger, E.M.; Andersen, P. ESAT-6 (EsxA) and TB10.4 (EsxH) Based Vaccines for Pre- and Post-Exposure Tuberculosis Vaccination. PLoS ONE 2013, 8, e80579. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.-E.; Ahn, J.-H.; Min, S.; Kim, H.; Seo, J.; Yeo, S.-G.; Ko, H.-J. Development of New Preventive and Therapeutic Vaccines for Tuberculosis. Immune Netw. 2018, 18, e17. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, K.A.; Yukhneva, M.A.; Shurygina, A.-P.S.; Stukova, M.A.; Egorov, A.Y. Enhancement of the Immunogenicity of Influenza A Virus by the Inhibition of Immunosuppressive Function of NS1 Protein. Microbiol. Indep. Res. J. (MIR J.) 2018, 5, 36–47. [Google Scholar] [CrossRef]
- Shurygina, A.-P.; Zabolotnykh, N.; Vinogradova, T.; Khairullin, B.; Kassenov, M.; Nurpeisova, A.; Sarsenbayeva, G.; Sansyzbay, A.; Vasilyev, K.; Buzitskaya, J.; et al. Preclinical Evaluation of TB/FLU-04L—An Intranasal Influenza Vector-Based Boost Vaccine against Tuberculosis. Int. J. Mol. Sci. 2023, 24, 7439. [Google Scholar] [CrossRef] [PubMed]
- Manjelievskaia, J.; Erck, D.; Piracha, S.; Schrager, L. Drug-Resistant TB: Deadly, Costly and in Need of a Vaccine. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chibale, K. Strategies to Combat Multi-Drug Resistance in Tuberculosis. Acc. Chem. Res. 2021, 54, 2361–2376. [Google Scholar] [CrossRef]
- Rosser, A.; Marx, F.M.; Pareek, M. Recurrent Tuberculosis in the Pre-Elimination Era. Int. J. Tuberc. Lung Dis. 2018, 22, 139–150. [Google Scholar] [CrossRef]
- Zabolotnykh, N.V.; Vinogradova, T.I.; Stukova, M.A.; Vasil’eva, S.N.; Vitovskaia, M.L.; Egorov, A.I. The effectiveness of influenza vectors expressing the protective mycobacterial antigen ESAT-6 in the complex therapy of generalized tuberculosis in mice. Probl. Tuberk. Bolezn. Legk. 2008, 12, 30–34. [Google Scholar]
- Rangel-Moreno, J.; Carragher, D.M.; de la Luz Garcia-Hernandez, M.; Hwang, J.Y.; Kusser, K.; Hartson, L.; Kolls, J.K.; Khader, S.A.; Randall, T.D. The Development of Inducible Bronchus-Associated Lymphoid Tissue Depends on IL-17. Nat. Immunol. 2011, 12, 639–646. [Google Scholar] [CrossRef]
- Marin, N.D.; Dunlap, M.D.; Kaushal, D.; Khader, S.A. Friend or Foe: The Protective and Pathological Roles of Inducible Bronchus-Associated Lymphoid Tissue in Pulmonary Diseases. J. Immunol. 2019, 202, 2519–2526. [Google Scholar] [CrossRef]
- Moyron-Quiroz, J.E.; Rangel-Moreno, J.; Kusser, K.; Hartson, L.; Sprague, F.; Goodrich, S.; Woodland, D.L.; Lund, F.E.; Randall, T.D. Role of Inducible Bronchus Associated Lymphoid Tissue (IBALT) in Respiratory Immunity. Nat. Med. 2004, 10, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Carow, B.; Hauling, T.; Qian, X.; Kramnik, I.; Nilsson, M.; Rottenberg, M.E. Spatial and Temporal Localization of Immune Transcripts Defines Hallmarks and Diversity in the Tuberculosis Granuloma. Nat. Commun. 2019, 10, 1823. [Google Scholar] [CrossRef]
- Basile, J.I.; Liu, R.; Mou, W.; Gao, Y.; Carow, B.; Rottenberg, M.E. Mycobacteria-Specific T Cells Are Generated in the Lung during Mucosal BCG Immunization or Infection With Mycobacterium tuberculosis. Front. Immunol. 2020, 11, 566319. [Google Scholar] [CrossRef]
- Jones, G.W.; Hill, D.G.; Jones, S.A. Understanding Immune Cells in Tertiary Lymphoid Organ Development: It Is All Starting to Come Together. Front. Immunol. 2016, 7, 401. [Google Scholar] [CrossRef]
- Zeng, B.; Xing, R.; Dong, C.; Xing, F. Commentary: Group 3 Innate Lymphoid Cells Mediate Early Protective Immunity against Tuberculosis. Front. Immunol. 2020, 11, 1925. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, M.D.; Prince, O.A.; Rangel-Moreno, J.; Thomas, K.A.; Scordo, J.M.; Torrelles, J.B.; Cox, J.; Steyn, A.J.C.; Zúñiga, J.; Kaushal, D.; et al. Formation of Lung Inducible Bronchus Associated Lymphoid Tissue Is Regulated by Mycobacterium tuberculosis Expressed Determinants. Front. Immunol. 2020, 11, 1325. [Google Scholar] [CrossRef]
- Griffiths, K.L.; Ahmed, M.; Das, S.; Gopal, R.; Horne, W.; Connell, T.D.; Moynihan, K.D.; Kolls, J.K.; Irvine, D.J.; Artyomov, M.N.; et al. Targeting Dendritic Cells to Accelerate T-Cell Activation Overcomes a Bottleneck in Tuberculosis Vaccine Efficacy. Nat. Commun. 2016, 7, 13894. [Google Scholar] [CrossRef]
- Genoula, M.; Marín Franco, J.L.; Dupont, M.; Kviatcovsky, D.; Milillo, A.; Schierloh, P.; Moraña, E.J.; Poggi, S.; Palmero, D.; Mata-Espinosa, D.; et al. Formation of Foamy Macrophages by Tuberculous Pleural Effusions Is Triggered by the Interleukin-10/Signal Transducer and Activator of Transcription 3 Axis through ACAT Upregulation. Front. Immunol. 2018, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, D.; Foreman, T.W.; Gautam, U.S.; Alvarez, X.; Adekambi, T.; Rangel-Moreno, J.; Golden, N.A.; Johnson, A.-M.F.; Phillips, B.L.; Ahsan, M.H.; et al. Mucosal Vaccination with Attenuated Mycobacterium tuberculosis Induces Strong Central Memory Responses and Protects against Tuberculosis. Nat. Commun. 2015, 6, 8533. [Google Scholar] [CrossRef]
- Petruccioli, E.; Petrone, L.; Vanini, V.; Sampaolesi, A.; Gualano, G.; Girardi, E.; Palmieri, F.; Goletti, D. IFNγ/TNFα Specific-Cells and Effector Memory Phenotype Associate with Active Tuberculosis. J. Infect. 2013, 66, 475–486. [Google Scholar] [CrossRef]
- Pathakumari, B.; Devasundaram, S.; Raja, A. Altered Expression of Antigen-Specific Memory and Regulatory T-Cell Subsets Differentiate Latent and Active Tuberculosis. Immunology 2018, 153, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Tonaco, M.M.; Moreira, J.D.; Nunes, F.F.C.; Loures, C.M.G.; Souza, L.R.; Martins, J.M.; Silva, H.R.; Porto, A.H.R.; Toledo, V.P.C.P.; Miranda, S.S.; et al. Evaluation of Profile and Functionality of Memory T Cells in Pulmonary Tuberculosis. Immunol. Lett. 2017, 192, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Flynn, J.L. CD8 T Cells and Mycobacterium Tuberculosis Infection. Semin. Immunopathol. 2015, 37, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, N.; Guggino, G.; Joosten, S.A.; Gelsomino, G.; Di Carlo, P.; Titone, L.; Galati, D.; Bocchino, M.; Matarese, A.; Salerno, A.; et al. Multifunctional CD4+ T Cells Correlate with Active Mycobacterium tuberculosis Infection. Eur. J. Immunol. 2010, 40, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.M.; Yang, J.D.; Lee, J.; Barreira-Silva, P.; Behar, S.M. Vaccine-Elicited Memory CD4+ T Cell Expansion Is Impaired in the Lungs during Tuberculosis. PLoS Pathog. 2017, 13, e1006704. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.M.; Nunes-Alves, C.; Booty, M.G.; Way, S.S.; Behar, S.M. A Higher Activation Threshold of Memory CD8+ T Cells Has a Fitness Cost That Is Modified by TCR Affinity during Tuberculosis. PLoS Pathog. 2016, 12, e1005380. [Google Scholar] [CrossRef] [PubMed]
- Winchell, C.G.; Nyquist, S.K.; Chao, M.C.; Maiello, P.; Myers, A.J.; Hopkins, F.; Chase, M.; Gideon, H.P.; Patel, K.V.; Bromley, J.D.; et al. CD8+ Lymphocytes Are Critical for Early Control of Tuberculosis in Macaques. J. Exp. Med. 2023, 220, e20230707. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, U.; Bucsan, A.N.; Ganatra, S.R.; Ibegbu, C.; Quezada, M.; Blair, R.V.; Alvarez, X.; Velu, V.; Kaushal, D.; Rengarajan, J. Pulmonary Mycobacterium tuberculosis Control Associates with CXCR3- and CCR6-Expressing Antigen-Specific Th1 and Th17 Cell Recruitment. JCI Insight 2020, 5, e137858. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, N.; Pietra, G.; Sullivan, L.C.; Brooks, A.G.; Prezzemolo, T.; La Manna, M.P.; Di Liberto, D.; Joosten, S.A.; van Meijgaarden, K.E.; Di Carlo, P.; et al. Human CD8 T Lymphocytes Recognize Mycobacterium tuberculosis Antigens Presented by HLA-E during Active Tuberculosis and Express Type 2 Cytokines. Eur. J. Immunol. 2015, 45, 1069–1081. [Google Scholar] [CrossRef]
- Behar, S.M. Antigen-Specific CD8+ T Cells and Protective Immunity to Tuberculosis. Adv. Exp. Med. Biol. 2013, 783, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Prezzemolo, T.; Guggino, G.; La Manna, M.P.; Di Liberto, D.; Dieli, F.; Caccamo, N. Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis. Front. Immunol. 2014, 5, 180. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurygina, A.-P.S.; Zabolotnykh, N.V.; Vinogradova, T.I.; Vitovskaya, M.L.; Dogonadze, M.Z.; Vasilyev, K.A.; Buzitskaya, Z.V.; Yablonskiy, P.K.; Lioznov, D.A.; Stukova, M.A. TB/FLU-06E Influenza Vector-Based Vaccine in the Complex Therapy of Drug-Susceptible and Drug-Resistant Experimental Tuberculosis. Pharmaceutics 2024, 16, 857. https://doi.org/10.3390/pharmaceutics16070857
Shurygina A-PS, Zabolotnykh NV, Vinogradova TI, Vitovskaya ML, Dogonadze MZ, Vasilyev KA, Buzitskaya ZV, Yablonskiy PK, Lioznov DA, Stukova MA. TB/FLU-06E Influenza Vector-Based Vaccine in the Complex Therapy of Drug-Susceptible and Drug-Resistant Experimental Tuberculosis. Pharmaceutics. 2024; 16(7):857. https://doi.org/10.3390/pharmaceutics16070857
Chicago/Turabian StyleShurygina, Anna-Polina S., Natalia V. Zabolotnykh, Tatiana I. Vinogradova, Maria L. Vitovskaya, Marine Z. Dogonadze, Kirill A. Vasilyev, Zhanna V. Buzitskaya, Petr K. Yablonskiy, Dmitriy A. Lioznov, and Marina A. Stukova. 2024. "TB/FLU-06E Influenza Vector-Based Vaccine in the Complex Therapy of Drug-Susceptible and Drug-Resistant Experimental Tuberculosis" Pharmaceutics 16, no. 7: 857. https://doi.org/10.3390/pharmaceutics16070857
APA StyleShurygina, A.-P. S., Zabolotnykh, N. V., Vinogradova, T. I., Vitovskaya, M. L., Dogonadze, M. Z., Vasilyev, K. A., Buzitskaya, Z. V., Yablonskiy, P. K., Lioznov, D. A., & Stukova, M. A. (2024). TB/FLU-06E Influenza Vector-Based Vaccine in the Complex Therapy of Drug-Susceptible and Drug-Resistant Experimental Tuberculosis. Pharmaceutics, 16(7), 857. https://doi.org/10.3390/pharmaceutics16070857





