Cationic Liposomes Carrying HPV16 E6-siRNA Inhibit the Proliferation, Migration, and Invasion of Cervical Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
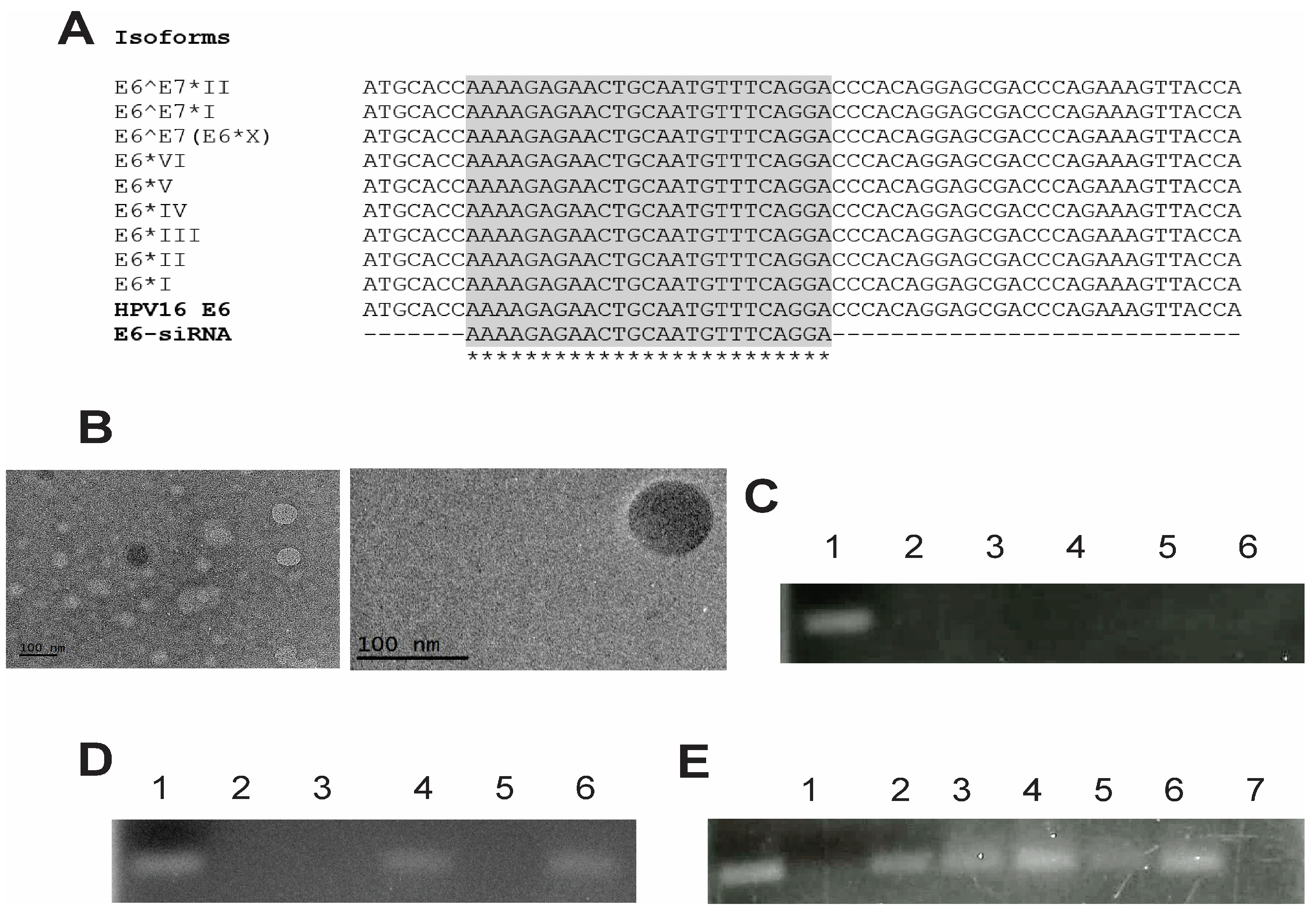
2.2. Design of the E6 siRNAs
2.3. Preparation of Liposomes
2.4. Preparation of Lipoplexes
2.5. Preparation of Pegylated Liposomes
2.6. Particle Size and Zeta Potential Measurements
2.7. Evaluation of Particle Morphology
2.8. Characterization of Lipoplexes and Encapsulation Efficiency
2.8.1. Gel Retardation Assay
2.8.2. Quant-iTTM RiboGreen® RNA Assay
2.9. Stability Study
2.9.1. siRNA Integrity
2.9.2. Loss of the siRNA
2.9.3. siRNA Protection from RNase
2.10. Cultured Cell Lines
2.11. Transfection with Lipoplexes
2.12. Western Blot
2.13. Cell Viability and Proliferation Assays
2.14. Cell Migration Assay
2.15. Matrigel Invasion Assay
2.16. Statistical Analysis
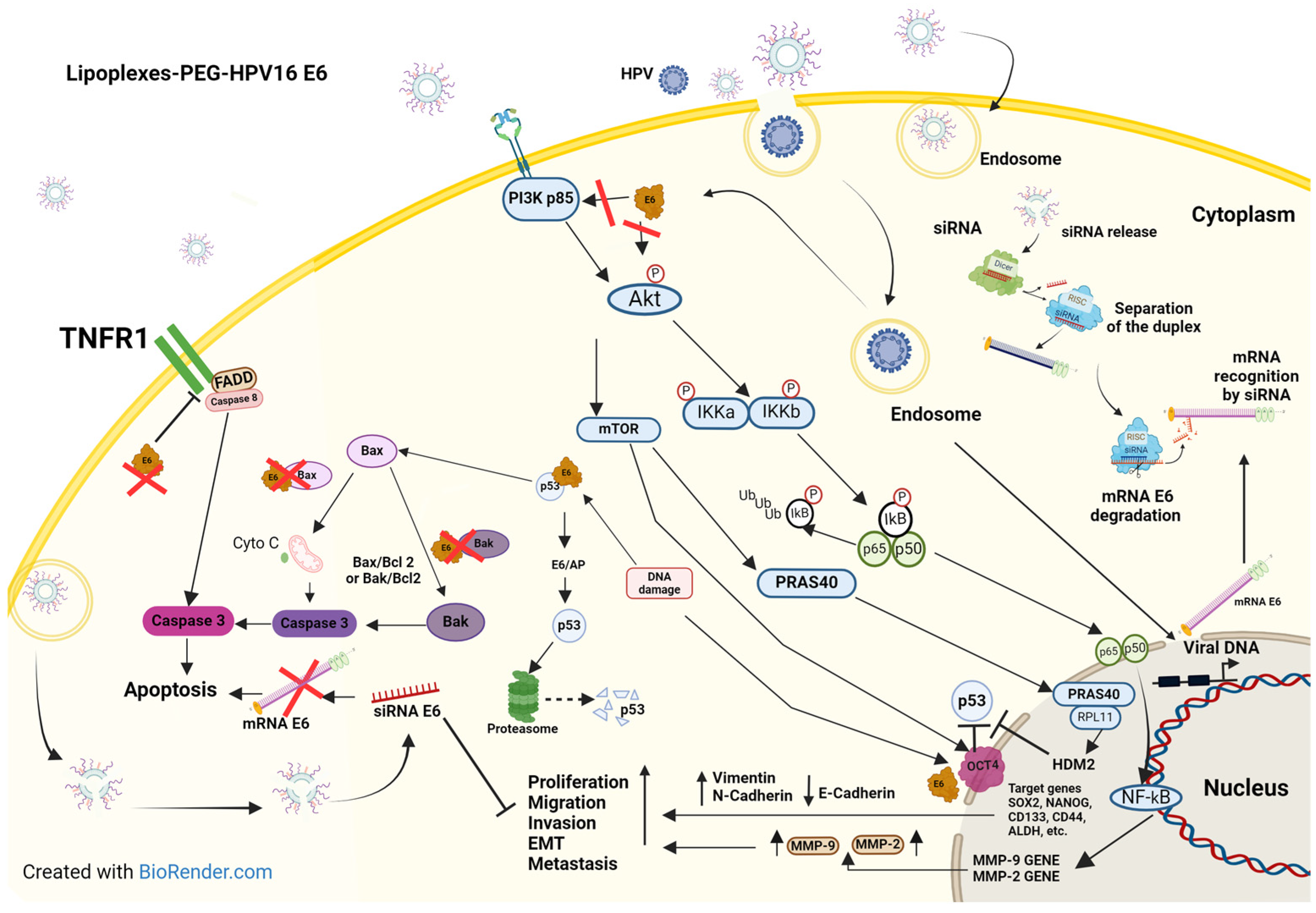
3. Results and Discussion
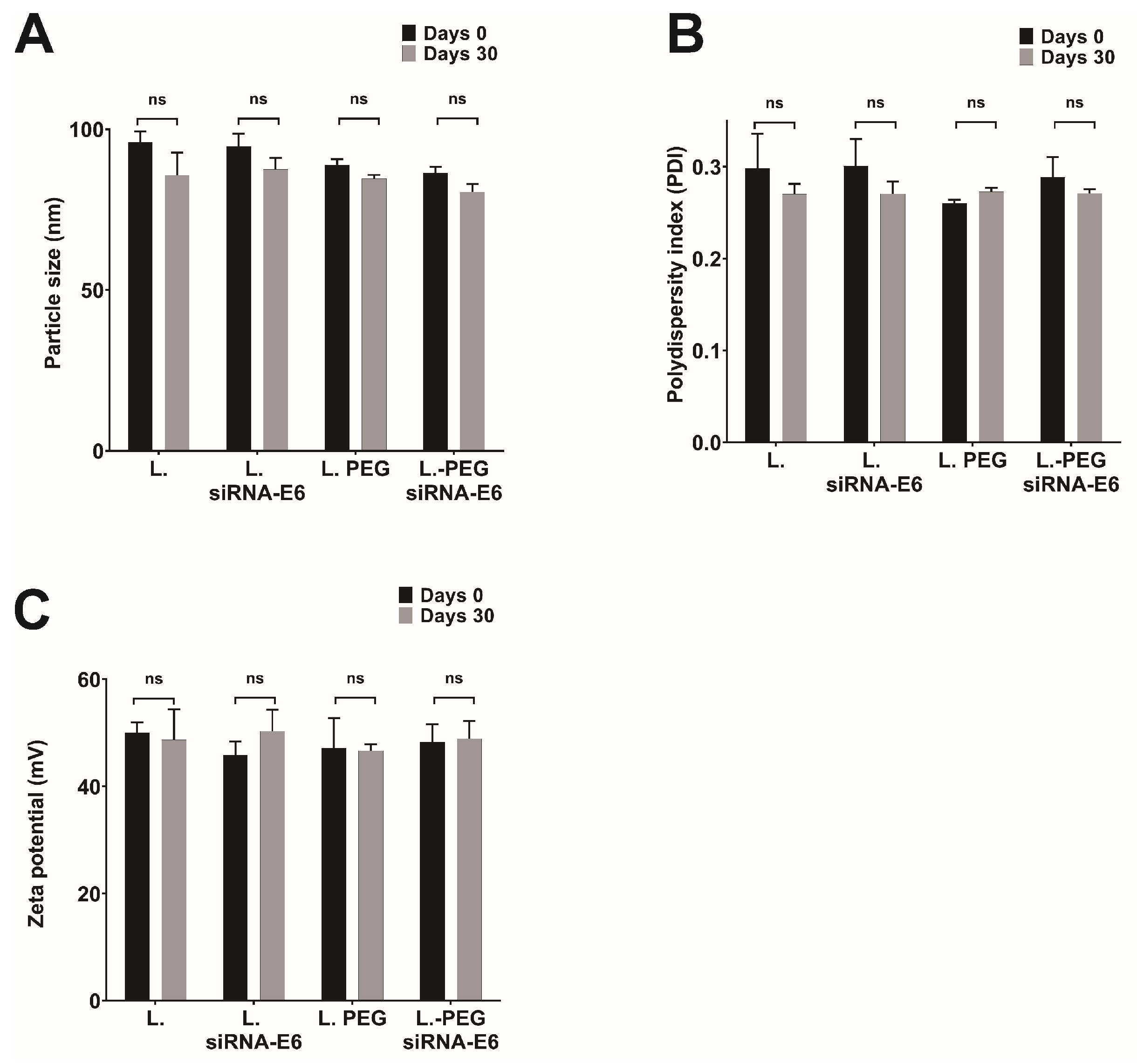
3.1. Comprehensive Characterization of Liposomes and Lipoplexes: Elucidating the Key Features for Effective Drug Delivery
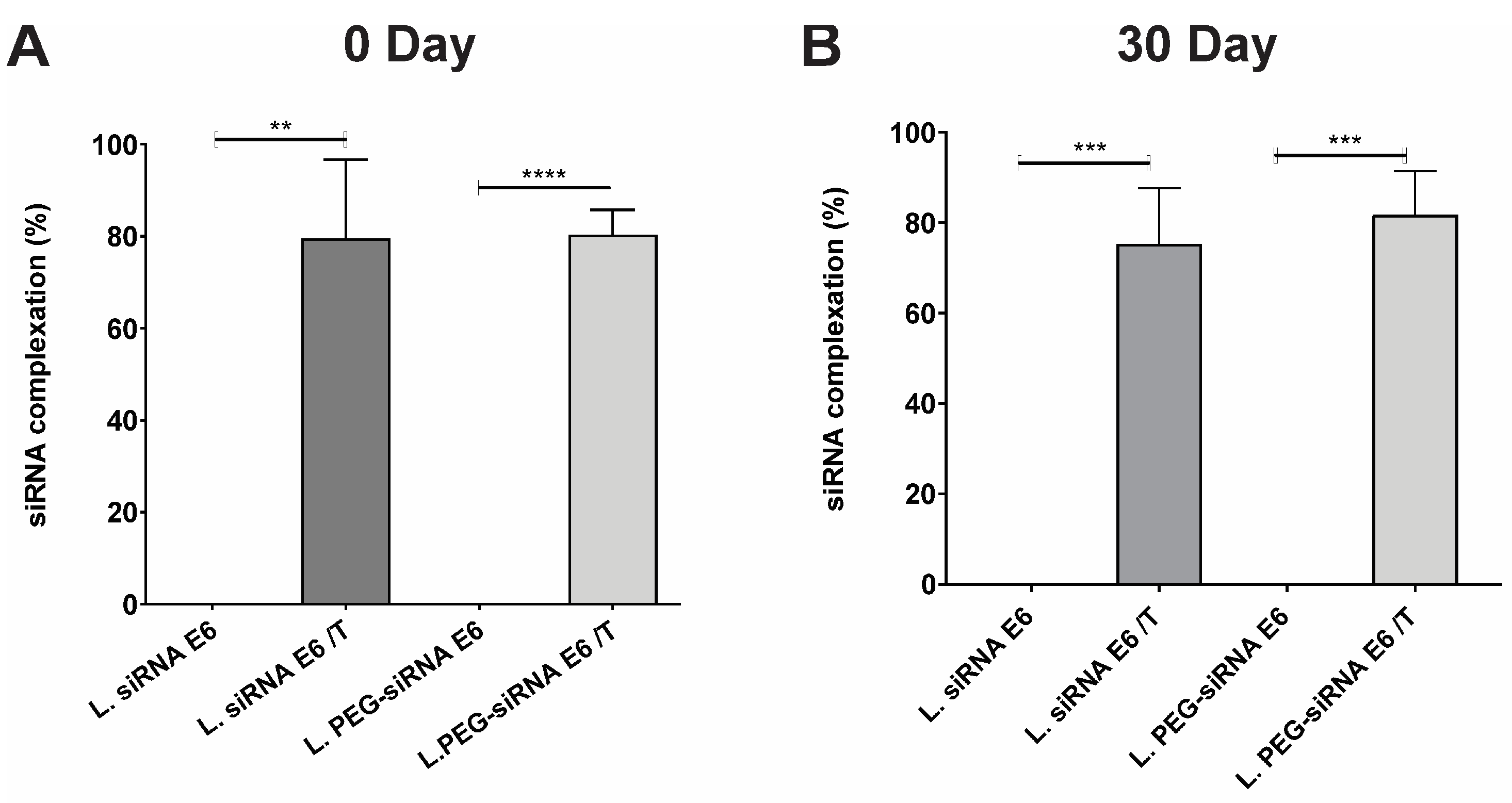
3.2. Ensuring the Stability of Lipoplexes-HPV16 E6 Is Crucial for Their Successful Application as siRNA Delivery Systems
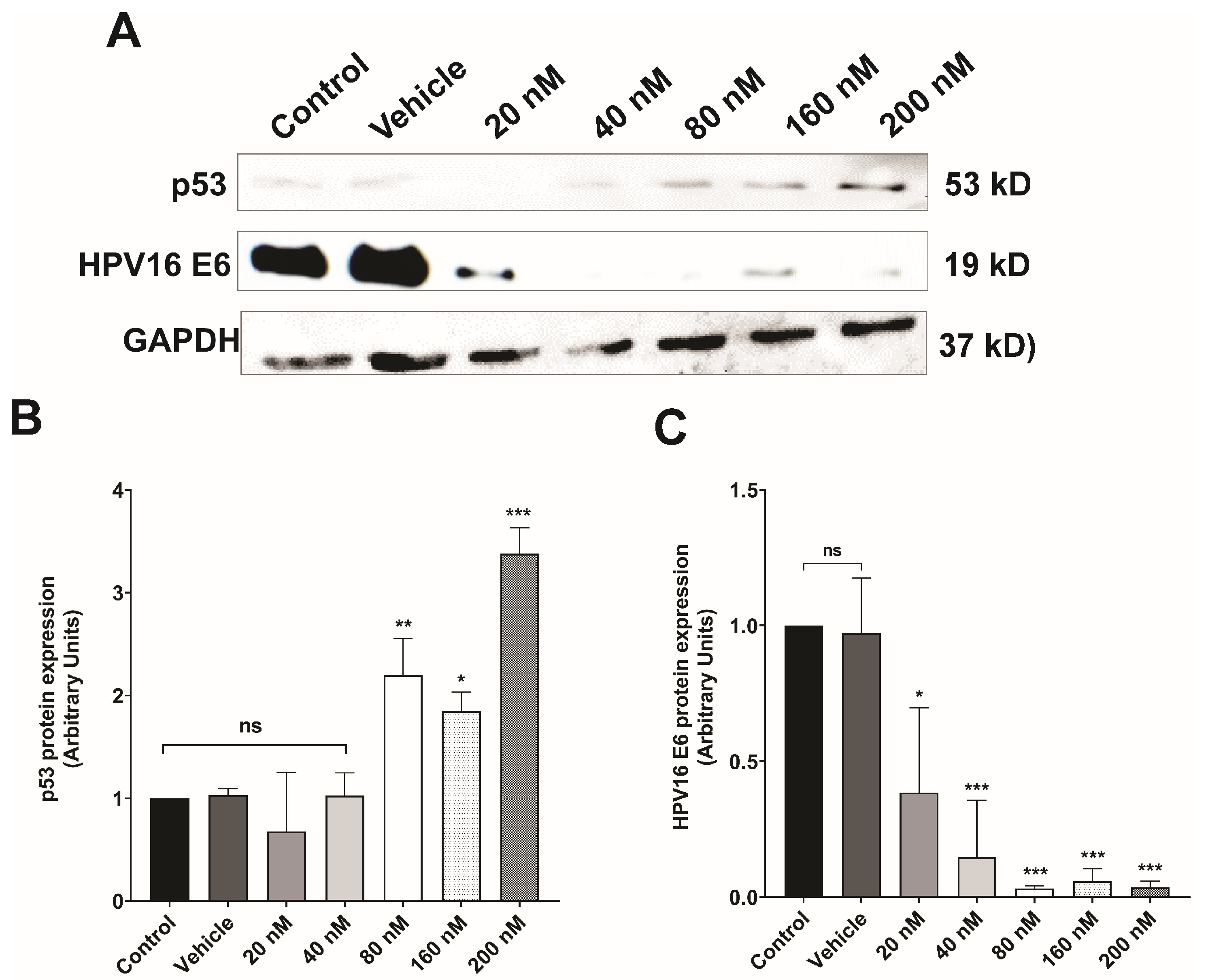
3.3. Silencing of E6 Oncoprotein Expression in CaSki Cells
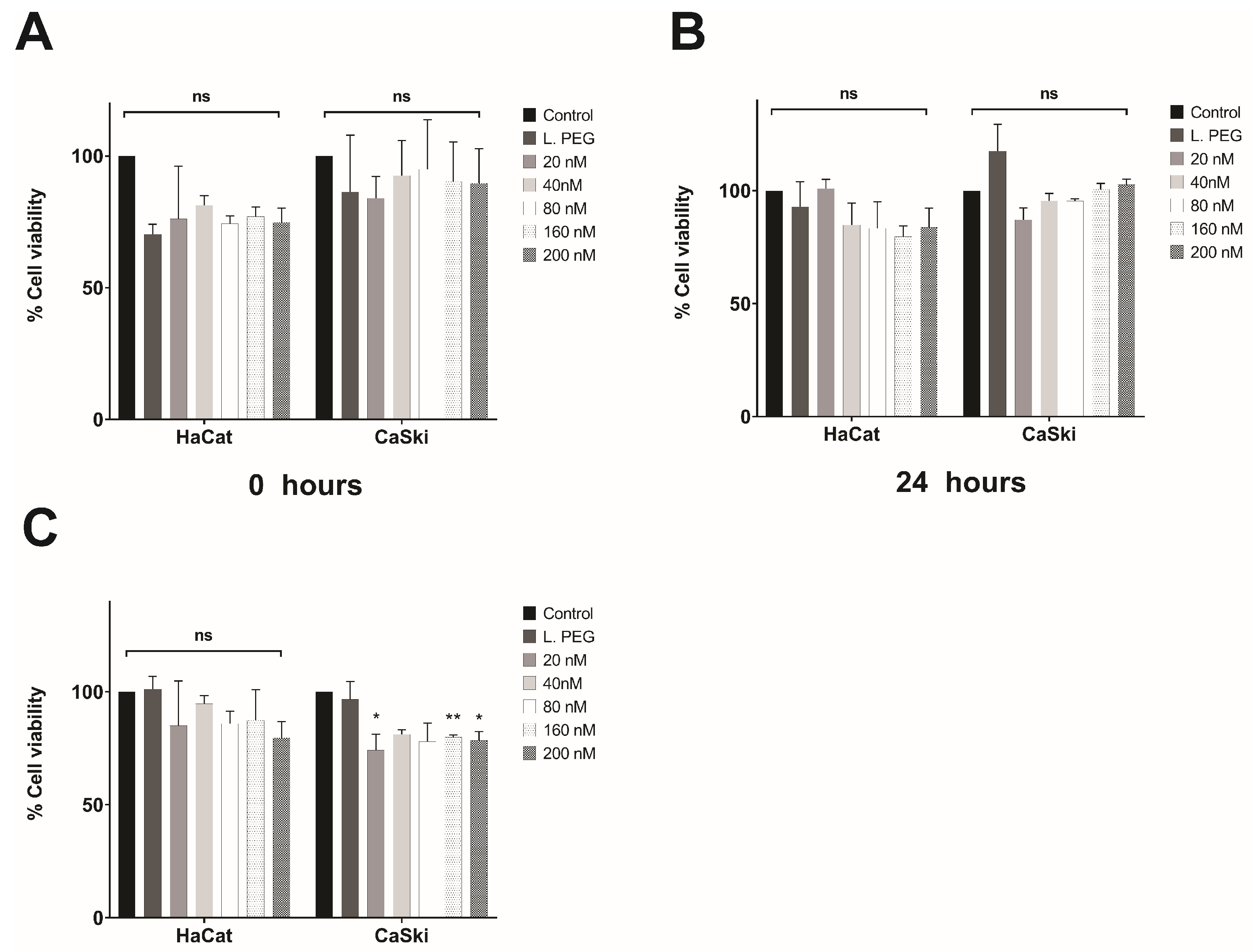
3.4. In Vitro Evaluation of the Effect of Lipoplexes-HPV16 E6 on Cell Viability
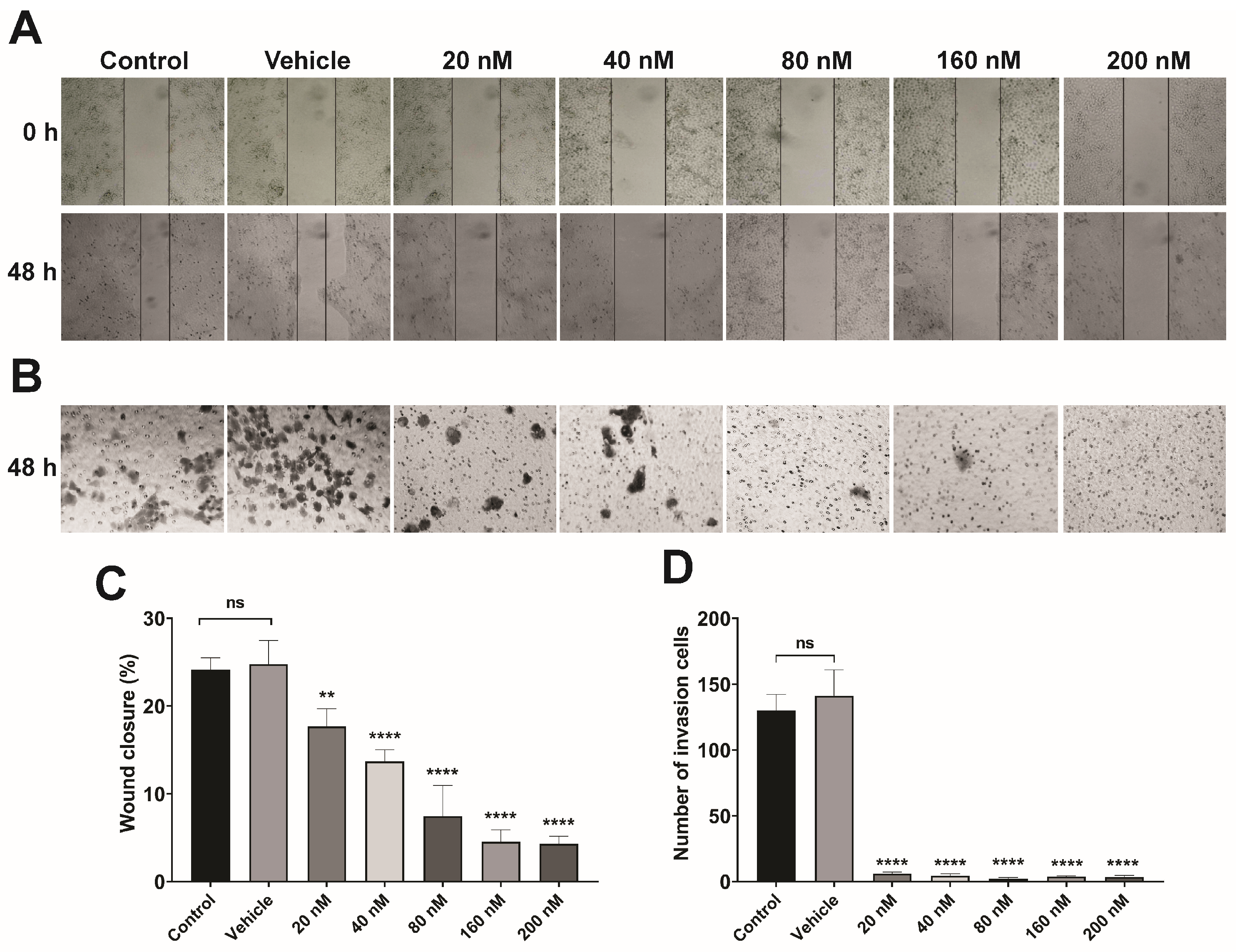
3.5. Lipoplex Treatment Suppresses the Migration and Invasion of CaSki Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.; Franceschi, S.; Diaz, M.; Muñoz, N.; Villa, L.L. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006, 24, S26–S34. [Google Scholar] [CrossRef]
- Illades-Aguiar, B.; Alarcón-Romero, L.d.C.; Antonio-Véjar, V.; Zamudio-López, N.; Sales-Linares, N.; Flores-Alfaro, E.; Fernández-Tilapa, G.; Vences-Velázquez, A.; Muñoz-Valle, J.F.; Leyva-Vázquez, M.-A. Prevalence and distribution of human papillomavirus types in cervical cancer, squamous intraepithelial lesions, and with no intraepithelial lesions in women from Southern Mexico. Gynecol. Oncol. 2010, 117, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Narisawa-Saito, M.; Kiyono, T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007, 98, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xia, S.; Wang, F.; Jin, Q.; Yu, T.; He, L.; Chen, Y.; Liu, Y.; Li, S.; Tan, X.; et al. Polymeric Nanomedicine for Combined Gene/Chemotherapy Elicits Enhanced Tumor Suppression. Mol. Pharm. 2016, 13, 663–676. [Google Scholar] [CrossRef]
- Collins, M.; Thrasher, A. Gene therapy: Progress and predictions. Proc. Biol. Sci. 2015, 282, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, L.; Rossi, J.J. RNAi therapeutics: Principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007, 59, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.; Peng, H.; Chen, Y.; Zhu, P.; Huang, Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv. Drug Deliv. Rev. 2015, 81, 142–160. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Gemeinhart, R.A. Progress in microRNA delivery. J Control. Release 2013, 172, 962–974. [Google Scholar] [CrossRef]
- Roberts, T.C.; Ezzat, K.; El Andaloussi, S.; Weinberg, M.S. Synthetic SiRNA Delivery: Progress and Prospects. Methods Mol. Biol. 2016, 1364, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y. Progress in the development of Lipoplex and Polyplex modified with Anionic Polymer for efficient Gene Delivery. J. Genet. Med. Gene Ther. 2017, 1, 003–018. [Google Scholar] [CrossRef]
- Young, L.S.; Searle, P.F.; Onion, D.; Mautner, V. Viral gene therapy strategies: From basic science to clinical application. J. Pathol. 2006, 208, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J. Seeking the Cause of Induced Leukemias in X-SCID Trial. Science 2003, 299, 495. [Google Scholar] [CrossRef]
- Glover, D.J.; Lipps, H.J.; Jans, D.A. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 2005, 6, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Jaison, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Zhou, J.; Shum, K.-T.; Burnett, C.J.; Rossi, J.J. Nanoparticle-Based Delivery of RNAi Therapeutics: Progress and Challenges. Pharmaceuticals 2013, 6, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhi, D.; Huang, L. Lipid-based vectors for siRNA delivery. J. Drug Target. 2012, 20, 724–735. [Google Scholar] [CrossRef]
- Hwang, S.J.; Davis, M.E. Cationic polymers for gene delivery: Designs for overcoming barriers to systemic administration. Curr. Opin. Mol. Ther. 2001, 3, 183–191. [Google Scholar]
- Liu, Z.; Liu, D.; Wang, L.; Zhang, J.; Zhang, N. Docetaxel-Loaded Pluronic P123 Polymeric Micelles: In vitro and in vivo Evaluation. Int. J. Mol. Sci. 2011, 12, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Butz, K.; Ristriani, T.; Hengstermann, A.; Denk, C.; Scheffner, M.; Hoppe-Seyler, F. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 2003, 22, 5938–5945. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.H.S.; Alexander, K.A. RNA Interference of Human Papillomavirus Type 18 E6 and E7 Induces Senescence in HeLa Cells. J. Virol. 2003, 77, 6066–6069. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Milner, J. Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene 2002, 21, 6041–6048. [Google Scholar] [CrossRef]
- Yamato, K.; Fen, J.; Kobuchi, H.; Nasu, Y.; Yamada, T.; Nishihara, T.; Ikeda, Y.; Kizaki, M.; Yoshinouchi, M. Induction of cell death in human papillomavirus 18-positive cervical cancer cells by E6 siRNA. Cancer Gene. Ther. 2006, 13, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yoshinouchi, M.; Yamada, T.; Kizaki, M.; Fen, J.; Koseki, T.; Ikeda, Y.; Nishihara, T.; Yamato, K. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by e6 siRNA. Mol. Ther. 2003, 8, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Yamashita, A.; Saito, M.; Ichino, M.; Kinjo, T.; Mizuki, N.; Klinman, D.M.; Okuda, K. The human papillomavirus E6 protein targets apoptosis-inducing factor (AIF) for degradation. Sci. Rep. 2020, 10, 2045–2322. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Howley, P.M. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 1997, 94, 4412–4417. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Banks, L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene 1998, 17, 2943–2954. [Google Scholar] [CrossRef]
- Goodwin, E.C.; DiMaio, D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc. Natl. Acad. Sci. USA 2000, 97, 12513–12518. [Google Scholar] [CrossRef]
- Goodwin, E.C.; Yang, E.; Lee, C.-J.; Lee, H.-W.; DiMaio, D.; Hwang, E.-S. Rapid induction of senescence in human cervical carcinoma cells. Proc. Natl. Acad. Sci. USA 2000, 97, 10978–10983. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, S.F.; Abrams, L.; Glick, A.; Lambert, P.F. Persistence of High-Grade Cervical Dysplasia and Cervical Cancer Requires the Continuous Expression of the Human Papillomavirus Type 16 E7 Oncogene. Cancer Res. 2009, 69, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Jonson, A.L.; Rogers, L.M.; Ramakrishnan, S.; Downs, L.S. Gene silencing with siRNA targeting E6/E7 as a therapeutic intervention in a mouse model of cervical cancer. Gynecol. Oncol. 2008, 111, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Filippova, M.; Evans, W.; Aragon, R.; Filippov, V.; Williams, V.M.; Hong, L.; Reeves, M.E.; Duerksen-Hughes, P. The small splice variant of HPV16 E6, E6, reduces tumor formation in cervical carcinoma xenografts. Virology 2014, 450–451, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Meissner, J.D. Nucleotide sequences and further characterization of human papillomavirus DNA present in the CaSki, SiHa and HeLa cervical carcinoma cell lines. J. Gen. Virol. 1999, 80, 1725–1733. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human papillomavirus oncoproteins: Pathways to transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Tamaki, K.; Ozaki, K.-I.; Kawano, K.; Onishi, H. Optimized combination of cationic lipids and neutral helper lipids in cationic liposomes for siRNA delivery into the lung by intravenous injection of siRNA lipoplexes. J. Drug Deliv. Sci. Technol. 2019, 52, 1042–1050. [Google Scholar] [CrossRef]
- Grantham, J.; Ruddock, L.W.; Roobol, A.; Carden, M.J. Eukaryotic chaperonin containing T-complex polypeptide 1 interacts with filamentous actin and reduces the initial rate of actin polymerization in vitro. Cell Stress Chaperones 2002, 7, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Barichello, J.M.; Kizuki, S.; Tagami, T.; Soares, L.A.L.; Ishida, T.; Kikuchi, H.; Kiwada, H. Agitation during lipoplex formation harmonizes the interaction of siRNA to cationic liposomes. Int. J. Pharm. 2012, 430, 359–365. [Google Scholar] [CrossRef]
- Tagami, T.; Nakamura, K.; Shimizu, T.; Ishida, T.; Kiwada, H. Effect of siRNA in PEG-coated siRNA-lipoplex on anti-PEG IgM production. J. Control. Release 2009, 137, 234–240. [Google Scholar] [CrossRef]
- Kim, H.-K.; Davaa, E.; Myung, C.-S.; Park, J.-S. Enhanced siRNA delivery using cationic liposomes with new polyarginine-conjugated PEG-lipid. Int. J. Pharm. 2010, 392, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Tamaki, K.; Sakasai, S.; Ozaki, K.; Onishi, H. Effects of PEG anchors in PEGylated siRNA lipoplexes on in vitro gene-silencing effects and siRNA biodistribution in mice. Mol. Med. Rep. 2020, 22, 4183–4196. [Google Scholar] [CrossRef]
- Khatri, N.; Baradia, D.; Vhora, I.; Rathi, M.; Misra, A. cRGD grafted liposomes containing inorganic nano-precipitate complexed siRNA for intracellular delivery in cancer cells. J. Control. Release 2014, 182, 45–57. [Google Scholar] [CrossRef]
- Lechanteur, A.; Furst, T.; Evrard, B.; Delvenne, P.; Hubert, P.; Piel, G. Development of anti-E6 pegylated lipoplexes for mucosal application in the context of cervical preneoplastic lesions. Int. J. Pharm. 2015, 483, 268–277. [Google Scholar] [CrossRef]
- Lechanteur, A.; Furst, T.; Evrard, B.; Delvenne, P.; Hubert, P.; Piel, G. PEGylation of lipoplexes: The right balance between cytotoxicity and siRNA effectiveness. Eur. J. Pharm. Sci. 2016, 93, 493–503. [Google Scholar] [CrossRef]
- Bao, Y.; Jin, Y.; Chivukula, P.; Zhang, J.; Liu, Y.; Liu, J.; Clamme, J.-P.; Mahato, R.I.; Ng, D.; Ying, W.; et al. Effect of PEGylation on Biodistribution and Gene Silencing of siRNA/Lipid Nanoparticle Complexes. Pharm. Res. 2013, 30, 342–351. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.C.P.; Simões, S.; Pires, P.; Faneca, H.; Düzgüneş, N. Cationic lipid–DNA complexes in gene delivery: From biophysics to biological applications. Adv. Drug Deliv. Rev. 2001, 47, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Dang, U.J.; Yuan, Y.; Psaras, A.M.; Osipitan, O.; Brooks, T.A.; Lu, F.; Di Pasqua, A.J. Optimization of DOTAP/chol Cationic Lipid Nanoparticles for mRNA, pDNA, and Oligonucleotide Delivery. AAPS PharmSciTech 2022, 23, 135. [Google Scholar] [CrossRef]
- Beauchamp, C.O.; Gonias, S.L.; Menapace, D.P.; Pizzo, S.V. A new procedure for the synthesis of polyethylene glycol-protein adducts; Effects on function, receptor recognition, and clearance of superoxide dismutase, lactoferrin, and α2-macroglobulin. Anal. Biochem. 1983, 131, 25–33. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M.R. Entrapment of rosemary extract by liposomes formulated by Mozafari method: Physicochemical characterization and optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef]
- Angart, P.; Vocelle, D.; Chan, C.; Walton, S.P. Design of siRNA Therapeutics from the Molecular Scale. Pharmaceuticals 2013, 6, 440–468. [Google Scholar] [CrossRef] [PubMed]
- Schramm, G.; Ramey, R. siRNA design including secondary structure target site prediction. Nat. Methods 2005, 2, 632. [Google Scholar] [CrossRef]
- Frantz, S. Eight steps to silence. Nat. Rev. Drug Discov. 2004, 3, 204. [Google Scholar] [CrossRef]
- Doorbar, J.; Parton, A.; Hartley, K.; Banks, L.; Crook, T.; Stanley, M.; Crawford, L. Detection of novel splicing patterns in a HPV16-containing keratinocyte cell line. Virology 1990, 178, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Ajiro, M.; Zheng, Z.M. E6^E7, a novel splice isoform protein of human papillomavirus 16, stabilizes viral E6 and E7 oncoproteins via HSP90 and GRP78. mBio 2015, 6, e02068-14. [Google Scholar] [CrossRef] [PubMed]
- Ajiro, M.; Jia, R.; Zhang, L.; Liu, X.; Zheng, Z.-M. Intron definition and a branch site adenosine at nt 385 control RNA splicing of HPV16 E6*I and E7 expression. PLoS ONE 2012, 7, e46412. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Dasgupta, H.; Roychowdhury, A.; Bhattacharya, R.; Mukherjee, N.; Roy, A.; Mandal, G.K.; Alam, N.; Biswas, J.; Mandal, S.; et al. Study of association and molecular analysis of human papillomavirus in breast cancer of Indian patients: Clinical and prognostic implication. PLoS ONE 2017, 12, e0172760. [Google Scholar] [CrossRef]
- McFarlane, M.; MacDonald, A.I.; Stevenson, A.; Graham, S.V. Human Papillomavirus 16 Oncoprotein Expression Is Controlled by the Cellular Splicing Factor SRSF2 (SC35). J Virol. 2015, 89, 5276–5287. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Dalstein, V.; Waterboer, T.; Clavel, C.; Gissmann, L.; Pawlita, M. Diagnosing cervical cancer and high-grade precursors by HPV16 transcription patterns. Cancer Res. 2010, 70, 249–256. [Google Scholar] [CrossRef]
- Fisher, R.K.; Mattern-Schain, S.I.; Best, M.D.; Kirkpatrick, S.S.; Freeman, M.B.; Grandas, O.H.; Mountain, D.J. Improving the efficacy of liposome-mediated vascular gene therapy via lipid surface modifications. J. Surg. Res. 2017, 219, 136–144. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Forouzanfar, T.; Helder, M.N.; Zandieh-Doulabi, B. Preparation of PEGylated cationic nanoliposome-siRNA complexes for cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 684–692. [Google Scholar] [CrossRef]
- Naito, Y.; Ui-Tei, K. siRNA Design Software for a Target Gene-Specific RNA Interference. Front. Genet. 2012, 3, 102. [Google Scholar] [CrossRef]
- Tungteakkhun, S.S.; Duerksen-Hughes, P.J. Cellular binding partners of the human papillomavirus E6 protein. Arch. Virol. 2008, 153, 397–408. [Google Scholar] [CrossRef]
- Yeo-Teh, N.S.L.; Ito, Y.; Jha, S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int. J. Mol. Sci. 2018, 19, 1706. [Google Scholar] [CrossRef]
- Mantovani, F.; Banks, L. Inhibition of E6 induced degradation of p53 is not sufficient for stabilization of p53 protein in cervical tumour derived cell lines. Oncogene 1999, 18, 3309–3315. [Google Scholar] [CrossRef]
- Chang, J.T.-C.; Kuo, T.-F.; Chen, Y.-J.; Chiu, C.-C.; Lu, Y.-C.; Li, H.-F.; Shen, C.-R.; Cheng, A.-J. Highly potent and specific siRNAs against E6 or E7 genes of HPV16- or HPV18-infected cervical cancers. Cancer Gene Ther. 2010, 17, 827–836. [Google Scholar] [CrossRef]
- Aghbash, P.S.; Hemmat, N.; Baradaran, B.; Baghi, H.B. siRNA-E6 sensitizes HPV-16-related cervical cancer through Oxaliplatin: An in vitro study on anti-cancer combination therapy. Eur. J. Med. Res. 2023, 28, 42. [Google Scholar] [CrossRef]
- Yang, T.-J.; Wang, L.; Zhang, Y.; Zheng, J.-D.; Liu, L. LncRNA UCA1 regulates cervical cancer survival and EMT occurrence by targeting miR-155. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9869–9879. [Google Scholar] [CrossRef]
- Jayamohan, S.; Kannan, M.; Moorthy, R.K.; Rajasekaran, N.; Jung, H.S.; Shin, Y.K.; Arockiam, A.J.V. Dysregulation of miR-375/AEG-1 Axis by Human Papillomavirus 16/18-E6/E7 Promotes Cellular Proliferation, Migration, and Invasion in Cervical Cancer. Front. Oncol. 2019, 9, 847–861. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Lai, S.-Y.; Huang, L.-J.; Zhang, Z.-F.; Liu, J.; Zheng, S.-R.; Ding, K.; Bai, X.; Zhou, J.-Y. Myosin 1b promotes cell proliferation, migration, and invasion in cervical cancer. Gynecol. Oncol. 2018, 149, 188–197. [Google Scholar] [CrossRef]
- Jayshree, R.; Adurthi, S.; Maliekal, T.; Krishna, S. Cell intrinsic & extrinsic factors in cervical carcinogenesis. Indian J. Med. Res. 2009, 130, 286–295. [Google Scholar] [PubMed]
- Spangle, J.M.; Münger, K. The Human Papillomavirus Type 16 E6 Oncoprotein Activates mTORC1 Signaling and Increases Protein Synthesis. J. Virol. 2010, 84, 9398–9407. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Li, Z.; Liu, L.; Ying, X.; Zhang, Y.; Wang, T.; Zhou, X.; Jiang, P.; Lv, W. HPV16 E6-Activated OCT4 Promotes Cervical Cancer Progression by Suppressing p53 Expression via Co-Repressor NCOR1. Front. Oncol. 2022, 12, 900856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, Z.; Zhu, Y.; Chen, J.; Li, W. The Role and Specific Mechanism of OCT4 in Cancer Stem Cells: A Review. Int. J. Stem. Cells 2020, 13, 312–325. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Wang, X.; Yang, Y. Secretory leukocyte protease inhibitor suppresses HPV E6-expressing HNSCC progression by mediating NF-κB and Akt pathways. Cancer Cell Int. 2019, 19, 220–239. [Google Scholar] [CrossRef] [PubMed]
- Filippova, M.; Parkhurst, L.; Duerksen-Hughes, P.J. The Human Papillomavirus 16 E6 Protein Binds to Fas-associated Death Domain and Protects Cells from Fas-triggered Apoptosis. J. Biol. Chem. 2004, 279, 25729–25744. [Google Scholar] [CrossRef]
- Galati, L.; Chiocca, S.; Duca, D.; Tagliabue, M.; Simoens, C.; Gheit, T.; Arbyn, M.; Tommasino, M. HPV and head and neck cancers: Towards early diagnosis and prevention. Tumour. Virus Res. 2022, 14, 200245. [Google Scholar] [CrossRef]
- Havel, J.J.; Li, Z.; Cheng, D.; Peng, J.; Fu, H. Nuclear PRAS40 couples the Akt/mTORC1 signaling axis to the RPL11-HDM2-p53 nucleolar stress response pathway. Oncogene 2015, 34, 1487–1498. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Lee, C.-H.; Chiou, H.-L.; Lin, C.-L.; Chen, P.-N.; Lin, M.-T.; Hsieh, Y.-H.; Chou, M.-C. Praeruptorin-B Inhibits 12-O-Tetradecanoylphorbol-13-Acetate-Induced Cell Invasion by Targeting AKT/NF-κB via Matrix Metalloproteinase-2/-9 Expression in Human Cervical Cancer Cells. Cell Physiol. Biochem. 2019, 52, 1255–1266. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Cai, N.; Wu, X.-L.; Cao, H.-Z.; Xie, L.-L.; Zheng, P.-S. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis. 2013, 4, e760. [Google Scholar] [CrossRef]
- Mohan, A.; Raj R, R.; Mohan, G.; P., P.K.; Maliekal, T.T. Reporters of Cancer Stem Cells as a Tool for Drug Discovery. Front. Oncol. 2021, 11, 669250. [Google Scholar] [CrossRef] [PubMed]
| DOTAP:DOPE:Chol (2:1:1) | %PEG | Particle Size (nm) | PDI | Z-Potential (mV) |
|---|---|---|---|---|
| Liposome | 0 | 95.93 ± 5.89 | 0.298 ± 0.065 | 50.03 ± 3.33 |
| Liposome + siRNA E6 (lipoplexes) | 0 | 94.66 ± 6.87 | 0.300 ± 0.051 | 45.9 ± 4.33 |
| Liposome-PEG | 5 | 88.96 ± 2.98 | 0.264 ± 0 | 47.16 ± 9.73 |
| Liposome-PEG + siRNA E6 (lipoplexes) | 5 | 86.42 ± 3.19 | 0.288 ± 0.038 | 48.3 ± 5.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Meza, L.V.; Bello-Rios, C.; Eloy, J.O.; Gómez-Gómez, Y.; Leyva-Vázquez, M.A.; Petrilli, R.; Bernad-Bernad, M.J.; Lagunas-Martínez, A.; Medina, L.A.; Serrano-Bello, J.; et al. Cationic Liposomes Carrying HPV16 E6-siRNA Inhibit the Proliferation, Migration, and Invasion of Cervical Cancer Cells. Pharmaceutics 2024, 16, 880. https://doi.org/10.3390/pharmaceutics16070880
Sánchez-Meza LV, Bello-Rios C, Eloy JO, Gómez-Gómez Y, Leyva-Vázquez MA, Petrilli R, Bernad-Bernad MJ, Lagunas-Martínez A, Medina LA, Serrano-Bello J, et al. Cationic Liposomes Carrying HPV16 E6-siRNA Inhibit the Proliferation, Migration, and Invasion of Cervical Cancer Cells. Pharmaceutics. 2024; 16(7):880. https://doi.org/10.3390/pharmaceutics16070880
Chicago/Turabian StyleSánchez-Meza, Luz Victoria, Ciresthel Bello-Rios, Josimar O. Eloy, Yazmín Gómez-Gómez, Marco Antonio Leyva-Vázquez, Raquel Petrilli, María Josefa Bernad-Bernad, Alfredo Lagunas-Martínez, Luis Alberto Medina, Janeth Serrano-Bello, and et al. 2024. "Cationic Liposomes Carrying HPV16 E6-siRNA Inhibit the Proliferation, Migration, and Invasion of Cervical Cancer Cells" Pharmaceutics 16, no. 7: 880. https://doi.org/10.3390/pharmaceutics16070880









