Enhanced Oral Efficacy of Semaglutide via an Ionic Nanocomplex with Organometallic Phyllosilicate in Type 2 Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AMP-Based Nanoparticles
2.3. In Vitro Characterization
2.4. In Vitro Drug Release Studies
2.5. Gastrointestinal Stability
2.6. Transport Studies
2.7. In Vivo Efficacy Studies
2.8. HPLC Analysis
2.9. Statistical Analysis
3. Results and Discussion
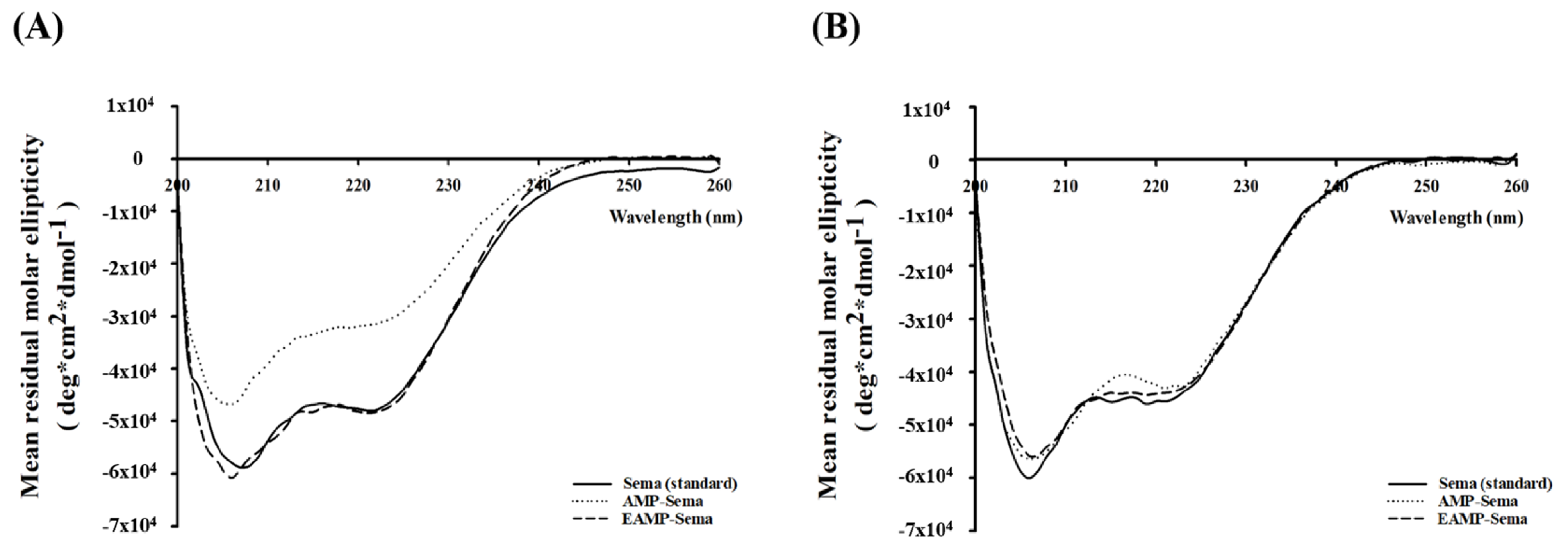
3.1. Preparation of AMP-Based Nanoparticles
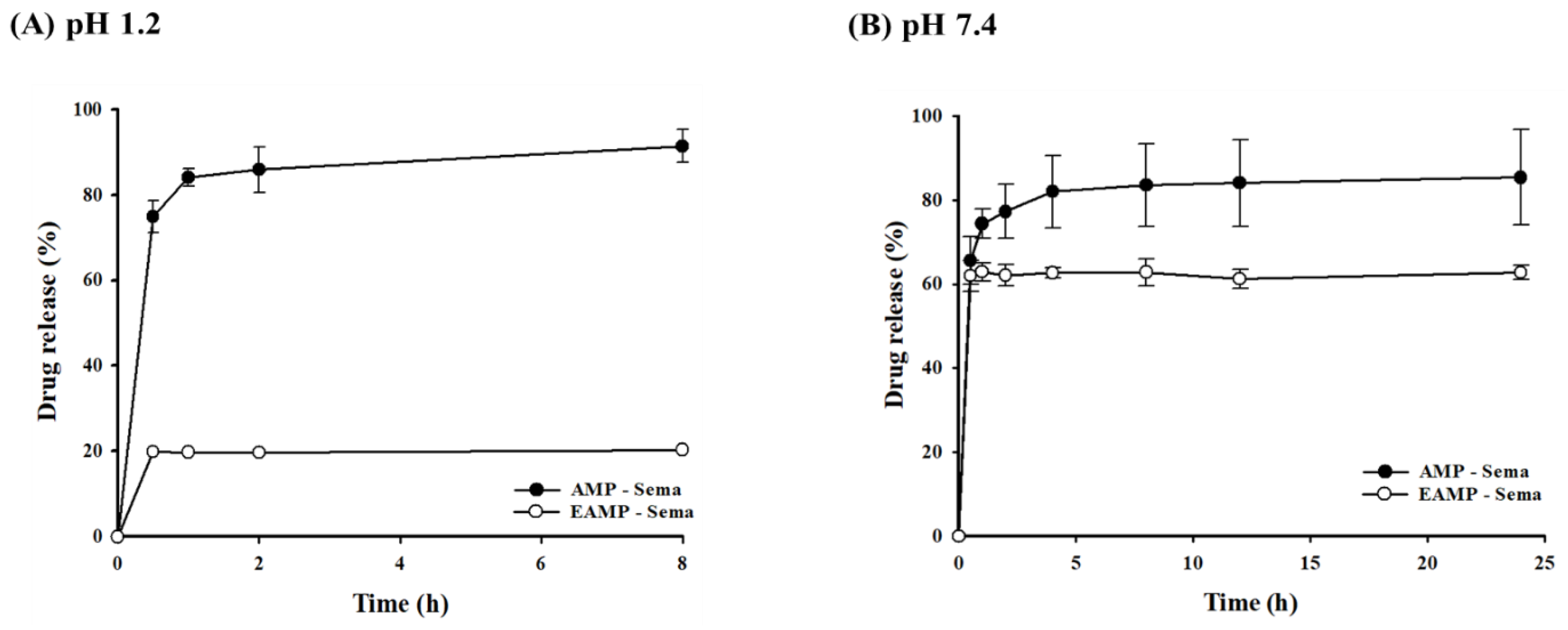
3.2. In Vitro Drug Release from AMP-Based Nanoparticles
3.3. Stability in Simulated Gastric and Intestinal Fluids
3.4. Transport Study
3.5. In Vivo Efficacy Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Sauna, Z.E.; Lagassé, H.A.D.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Kimchi-Sarfaty, C. Recent Advances in (Therapeutic Protein) Drug Development. F1000Research 2017, 6, 113. [Google Scholar] [CrossRef]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of Peptide and Protein Drug Delivery by Oral Route: Current Strategies to Improve the Bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, J.P.; Ryan, S.M.; Brayden, D.J. Oral Delivery Strategies for Nutraceuticals: Delivery Vehicles and Absorption Enhancers. Trends Food Sci. Technol. 2016, 53, 90–101. [Google Scholar] [CrossRef]
- Sadekar, S.; Thiagarajan, G.; Bartlett, K.; Hubbard, D.; Ray, A.; McGill, L.D.; Ghandehari, H. Poly(Amido Amine) Dendrimers as Absorption Enhancers for Oral Delivery of Camptothecin. Int. J. Pharm. 2013, 456, 175–185. [Google Scholar] [CrossRef]
- Bocsik, A.; Walter, F.R.; Gyebrovszki, A.; Fülöp, L.; Blasig, I.; Dabrowski, S.; Ötvös, F.; Tóth, A.; Rákhely, G.; Veszelka, S.; et al. Reversible Opening of Intercellular Junctions of Intestinal Epithelial and Brain Endothelial Cells with Tight Junction Modulator Peptides. J. Pharm. Sci. 2016, 105, 754–765. [Google Scholar] [CrossRef]
- Kim, D.; Jin, L.; Park, E.J.; Na, D.H. Peptide Permeation Enhancers for Improving Oral Bioavailability of Macromolecules. J. Pharm. Investig. 2023, 53, 59–72. [Google Scholar] [CrossRef]
- Walsh, E.G.; Adamczyk, B.E.; Chalasani, K.B.; Maher, S.; O’Toole, E.B.; Fox, J.S.; Leonard, T.W.; Brayden, D.J. Oral Delivery of Macromolecules: Rationale Underpinning Gastrointestinal Permeation Enhancement Technology (GIPET®). Ther. Deliv. 2011, 2, 1595–1610. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral Delivery of Therapeutic Peptides and Proteins: Technology Landscape of Lipid-Based Nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef]
- Noh, G.; Keum, T.; Bashyal, S.; Seo, J.E.; Shrawani, L.; Kim, J.H.; Lee, S. Recent Progress in Hydrophobic Ion-Pairing and Lipid-Based Drug Delivery Systems for Enhanced Oral Delivery of Biopharmaceuticals. J. Pharm. Investig. 2022, 52, 75–93. [Google Scholar] [CrossRef]
- Tran, P.; Park, J.S. Alginate-Coated Chitosan Nanoparticles Protect Protein Drugs from Acid Degradation in Gastric Media. J. Pharm. Investig. 2022, 52, 465–476. [Google Scholar] [CrossRef]
- Røder, M.E. Clinical Potential of Treatment with Semaglutide in Type 2 Diabetes Patients. Drugs Context. 2019, 8, 212585. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.T.; Bækdal, T.A.; Vegge, A.; Maarbjerg, S.J.; Pyke, C.; Ahnfelt-Rønne, J.; Madsen, K.G.; Schéele, S.G.; Alanentalo, T.; Kirk, R.K.; et al. Transcellular Stomach Absorption of a Derivatized Glucagon-like Peptide-1 Receptor Agonist. Sci. Transl. Med. 2018, 10, eaar7047. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.P.; Triplitt, C.L.; Solis-Herrera, C.D. Management of Type 2 Diabetes with Oral Semaglutide: Practical Guidance for Pharmacists. Am. J. Heal. Pharm. 2021, 78, 556–567. [Google Scholar] [CrossRef]
- van Hout, M.; Forte, P.; Jensen, T.B.; Boschini, C.; Bækdal, T.A. Effect of Various Dosing Schedules on the Pharmacokinetics of Oral Semaglutide: A Randomised Trial in Healthy Subjects. Clin. Pharmacokinet. 2023, 62, 635–644. [Google Scholar] [CrossRef]
- Gallwitz, B.; Giorgino, F. Clinical Perspectives on the Use of Subcutaneous and Oral Formulations of Semaglutide. Front. Endocrinol. 2021, 12, 645507. [Google Scholar] [CrossRef]
- Solis-herrera, C.; Kane, M.P.; Triplitt, C. Current Understanding of Sodium N-(8-[2-Hydroxylbenzoyl] Amino) Caprylate (SNAC) as an Absorption Enhancer: The Oral Semaglutide Experience. Clin. Diabetes 2024, 42, 74–86. [Google Scholar] [CrossRef]
- Smits, M.M.; Raalte, D.H. Van Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef]
- Isaacs, D.M.; Kruger, D.F.; Spollett, G.R. Optimizing Therapeutic Outcomes with Oral Semaglutide: A Patient-Centered Approach. Diabetes Spectr. 2021, 34, 7–19. [Google Scholar] [CrossRef]
- Aroda, V.R.; Blonde, L.; Pratley, R.E. A New Era for Oral Peptides: SNAC and the Development of Oral Semaglutide for the Treatment of Type 2 Diabetes. Rev. Endocr. Metab. Disord. 2022, 23, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.J.; Mann, S. Self-Assembly of Bio-Inorganic Nanohybrids Using Organoclay Building Blocks. J. Mater. Chem. 2008, 18, 4605–4615. [Google Scholar] [CrossRef]
- Lee, S.H.; Back, S.Y.; Song, J.G.; Han, H.K. Enhanced Oral Delivery of Insulin via the Colon-Targeted Nanocomposite System of Organoclay/Glycol Chitosan/Eudragit®S100. J. Nanobiotechnol. 2020, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Song, J.G.; Han, H.K. Development of PH-Responsive Organic-Inorganic Hybrid Nanocomposites as an Effective Oral Delivery System of Protein Drugs. J. Control Release 2019, 311–312, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Bajracharya, R.; Min, J.Y.; Han, J.; Park, B.J. Strategic Approaches for Colon Targeted Drug Delivery: An Overview of Recent Advancements. Pharmaceutics 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.K.R.; Achari, A.; Eswaramoorthy, M. Aminoclay: A Functional Layered Material with Multifaceted Applications. J. Mater. Chem. A 2013, 1, 6707–6718. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Park, D.; Lee, Y.C. Aminoclays for Biological and Environmental Applications: An Updated Review. Chem. Eng. J. 2018, 336, 757–772. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, 5–47. [Google Scholar] [CrossRef]
- Pham, D.T.; Nguyen, D.X.T.; Nguyen, N.Y.; Nguyen, T.T.L.; Nguyen, T.Q.C.; Tu, A.V.T.; Nguyen, N.H.; Thuy, B.T.P. Development of PH-Responsive Eudragit S100-Functionalized Silk Fibroin Nanoparticles as a Prospective Drug Delivery System. PLoS ONE 2024, 19, e0303177. [Google Scholar] [CrossRef]
- Tsai, S.W.; Yu, D.S.; Tsao, S.W.; Hsu, F.Y. Hyaluronan-Cisplatin Conjugate Nanoparticles Embedded in Eudragit S100-Coated Pectin/Alginate Microbeads for Colon Drug Delivery. Int. J. Nanomed. 2013, 8, 2399–2407. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cheon, J.H.; Lee, S.H.; Min, J.Y.; Back, S.Y.; Song, J.G.; Kim, D.H.; Lim, S.J.; Han, H.K. Ternary Nanocomposite Carriers Based on Organic Clay-Lipid Vesicles as an Effective Colon-Targeted Drug Delivery System: Preparation and in Vitro/in Vivo Characterization. J. Nanobiotechnol. 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Pan, H.; Wang, J.; Liu, D.; Pan, W. Eudragit S100 Coated Nanodiamond-Based Nanoparticles as an Oral Chemo-Photothermal Delivery System for Local Treatment of Colon Cancer. Colloids Surf. B Biointerfaces 2024, 237, 113849. [Google Scholar] [CrossRef] [PubMed]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A Technology Evaluation. Expert. Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Kavimandan, N.J. Nanoscale Analysis of Protein and Peptide Absorption: Insulin Absorption Using Complexation and PH-Sensitive Hydrogels as Delivery Vehicles. Eur. J. Pharm. Sci. 2006, 29, 183–197. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Dabholkar, N.; Agrawal, M.; Singhvi, G.; Alexander, A.; Bapat, R.A.; Kesharwani, P. Oral Peptide Delivery: Challenges and the Way Ahead. Drug Discov. Today 2021, 26, 931–950. [Google Scholar] [CrossRef]
- Kralj, S.; Rojnik, M.; Romih, R.; Jagodič, M.; Kos, J.; Makovec, D. Effect of Surface Charge on the Cellular Uptake of Fluorescent Magnetic Nanoparticles. J. Nanopart. Res. 2012, 14, 1151. [Google Scholar] [CrossRef]
- Villalobos-labra, R.E.; Yue, L.; Chen, S.; Ren, Q.; Niu, S.; Pan, X.; Chen, X.; Li, Z.; Chen, X. Effects of Semaglutide on Vascular Structure and Proteomics in High-Fat Diet-Induced Obese Mice. Front. Endocrinol. 2022, 7, 995007. [Google Scholar] [CrossRef]
- Peterson, R.G.; Van Jackson, C.; Zimmerman, K.M.; Alsina-Fernandez, J.; Michael, M.D.; Emmerson, P.J.; Coskun, T. Glucose Dysregulation and Response to Common Anti-Diabetic Agents in the FATZO/Pco Mouse. PLoS ONE 2017, 12, e0179856. [Google Scholar] [CrossRef] [PubMed]
- Kloock, S.; Haerting, N.; Herzog, G.; Oertel, M.; Geiger, N.; Geier, A.; Sequeira, V.; Nickel, A.; Kohlhaas, M.; Fassnacht, M.; et al. Effects of NPY-2 Receptor Antagonists, Semaglutide, PYY3-36, and Empagliflozin on Early MASLD in Diet-Induced Obese Rats. Nutrients 2024, 16, 904. [Google Scholar] [CrossRef]
- Bhargah, V. High Level of Individual Lipid pro Fi Le and Lipid Ratio as a Predictive Marker of Poor Glycemic Control in Type-2 Diabetes Mellitus. Vasc. Health Risk Manag. 2019, 15, 149–157. [Google Scholar] [CrossRef]
- Le, A.R.T.I.C.; Zheng, D.; Dou, J.; Liu, G.; Pan, Y.; Yan, Y.; Liu, F.; Gaisano, H.Y.; Lu, J.; He, Y. Association Between Triglyceride Level and Glycemic Control Among Insulin-Treated Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 1211–1220. [Google Scholar] [CrossRef]
- Dhillon, S. Semaglutide: First Global Approval. Drugs 2018, 78, 275–284. [Google Scholar] [CrossRef] [PubMed]




| Formulation | Size (nm) | PDI | Zeta Potential (mV) | EE (%) |
|---|---|---|---|---|
| AMP—Sema | 162 ± 3.63 | 0.272 ± 0.032 | −14.7 ± 0.35 | 99.7 ± 0.03 |
| EAMP—Sema | 318 ± 1.23 * | 0.235 ± 0.051 | −23.7 ± 0.53 * | 92.5 ± 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.L.; Song, J.G.; Han, H.-K. Enhanced Oral Efficacy of Semaglutide via an Ionic Nanocomplex with Organometallic Phyllosilicate in Type 2 Diabetic Rats. Pharmaceutics 2024, 16, 886. https://doi.org/10.3390/pharmaceutics16070886
Kim GL, Song JG, Han H-K. Enhanced Oral Efficacy of Semaglutide via an Ionic Nanocomplex with Organometallic Phyllosilicate in Type 2 Diabetic Rats. Pharmaceutics. 2024; 16(7):886. https://doi.org/10.3390/pharmaceutics16070886
Chicago/Turabian StyleKim, Gyu Lin, Jae Geun Song, and Hyo-Kyung Han. 2024. "Enhanced Oral Efficacy of Semaglutide via an Ionic Nanocomplex with Organometallic Phyllosilicate in Type 2 Diabetic Rats" Pharmaceutics 16, no. 7: 886. https://doi.org/10.3390/pharmaceutics16070886
APA StyleKim, G. L., Song, J. G., & Han, H.-K. (2024). Enhanced Oral Efficacy of Semaglutide via an Ionic Nanocomplex with Organometallic Phyllosilicate in Type 2 Diabetic Rats. Pharmaceutics, 16(7), 886. https://doi.org/10.3390/pharmaceutics16070886






