Advancing Tumor Therapy: Development and Utilization of Protein-Based Nanoparticles
Abstract
:1. Introduction
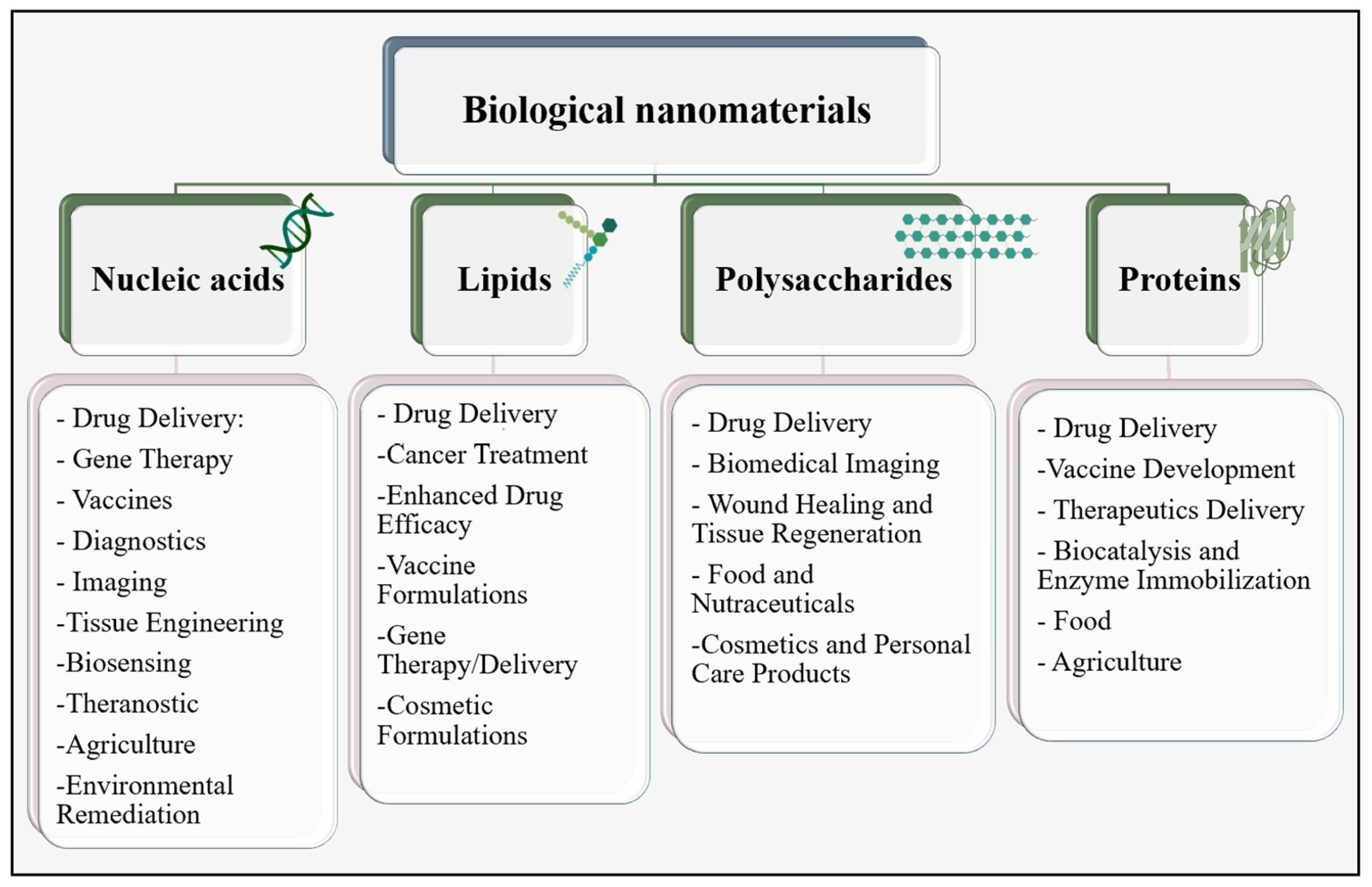
2. Nanomaterials in Biomedical Fields
PNPs in Tumor Therapy
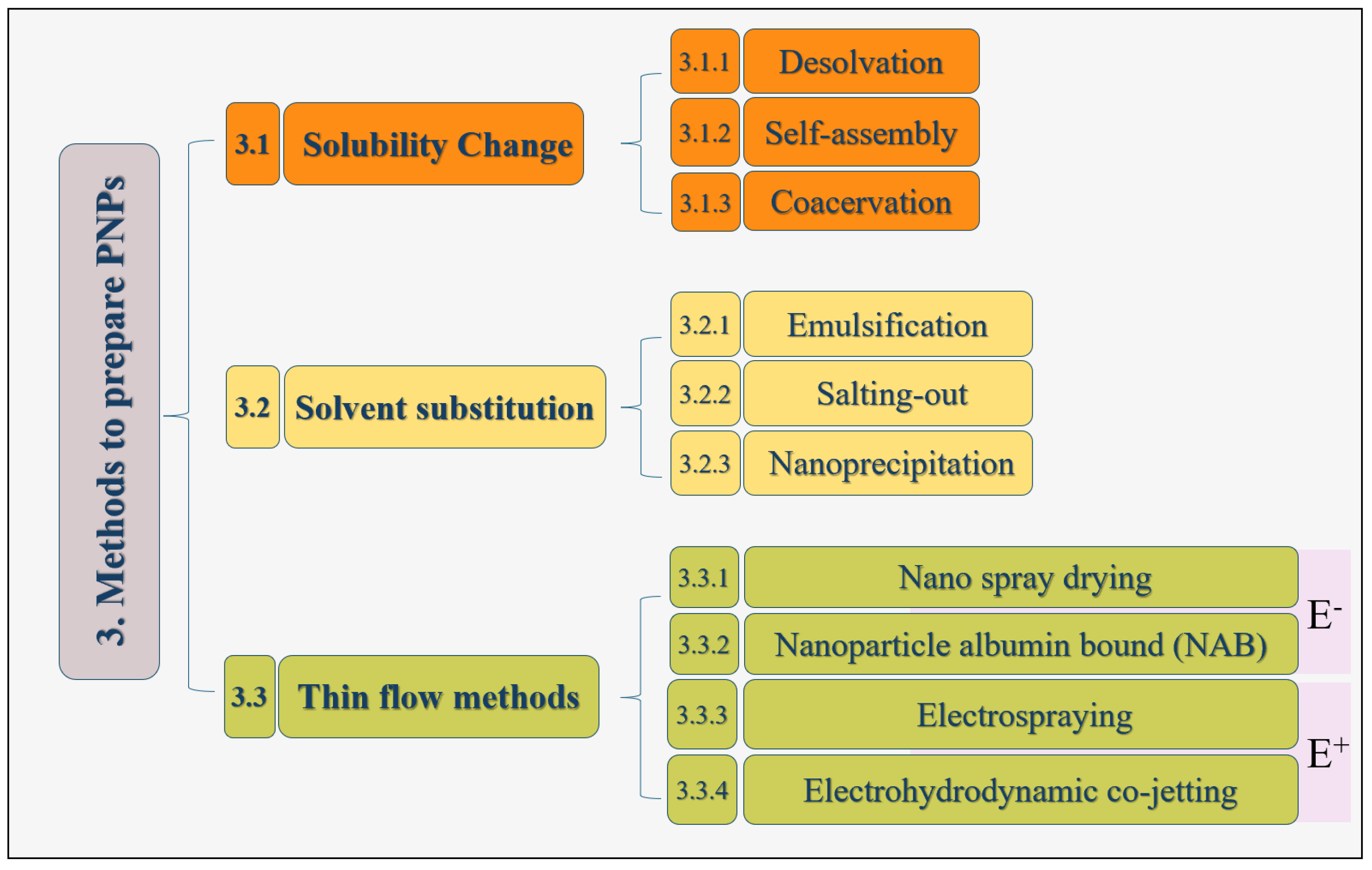
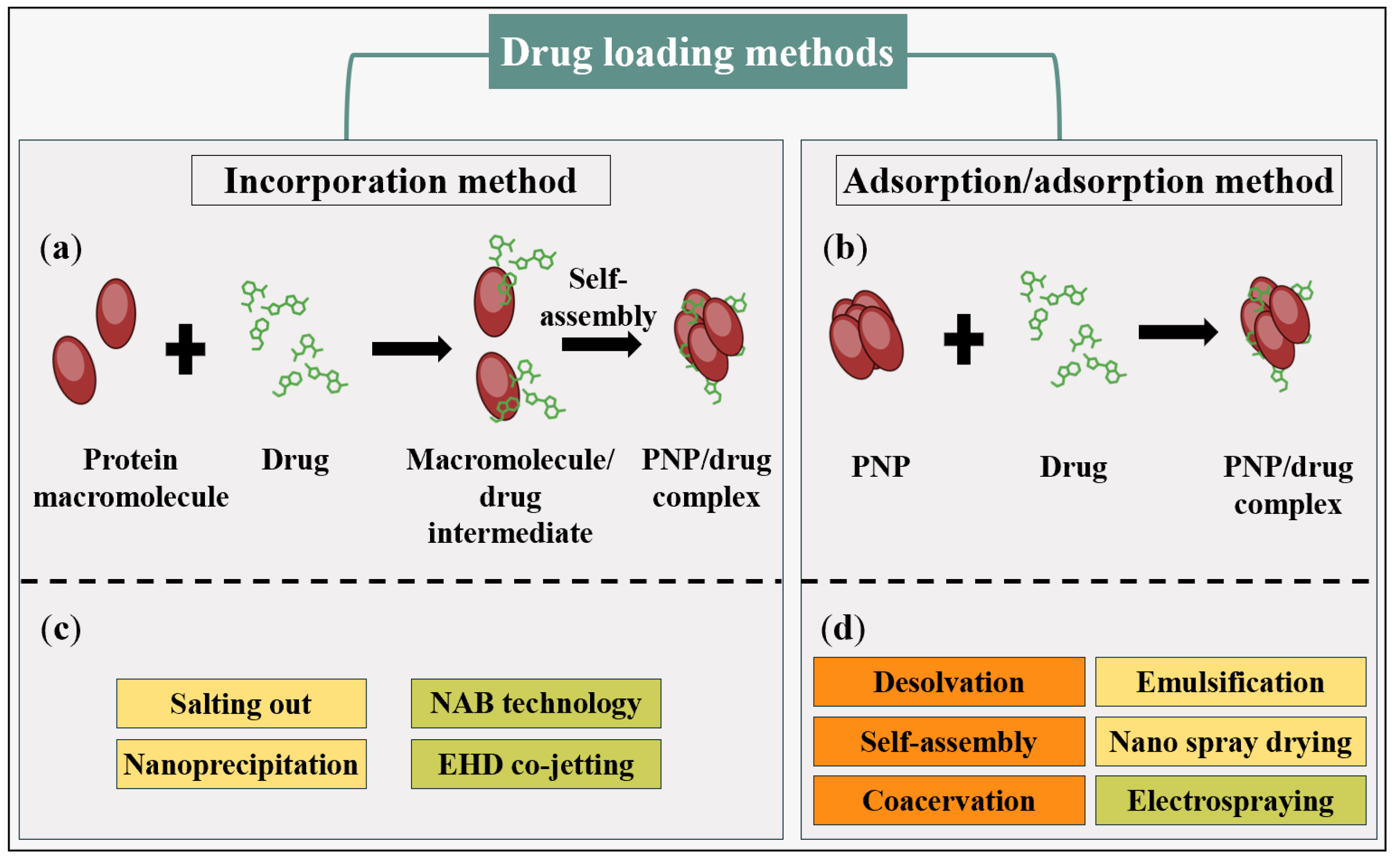
3. Methods for Generating PNPs
3.1. Solubility Change
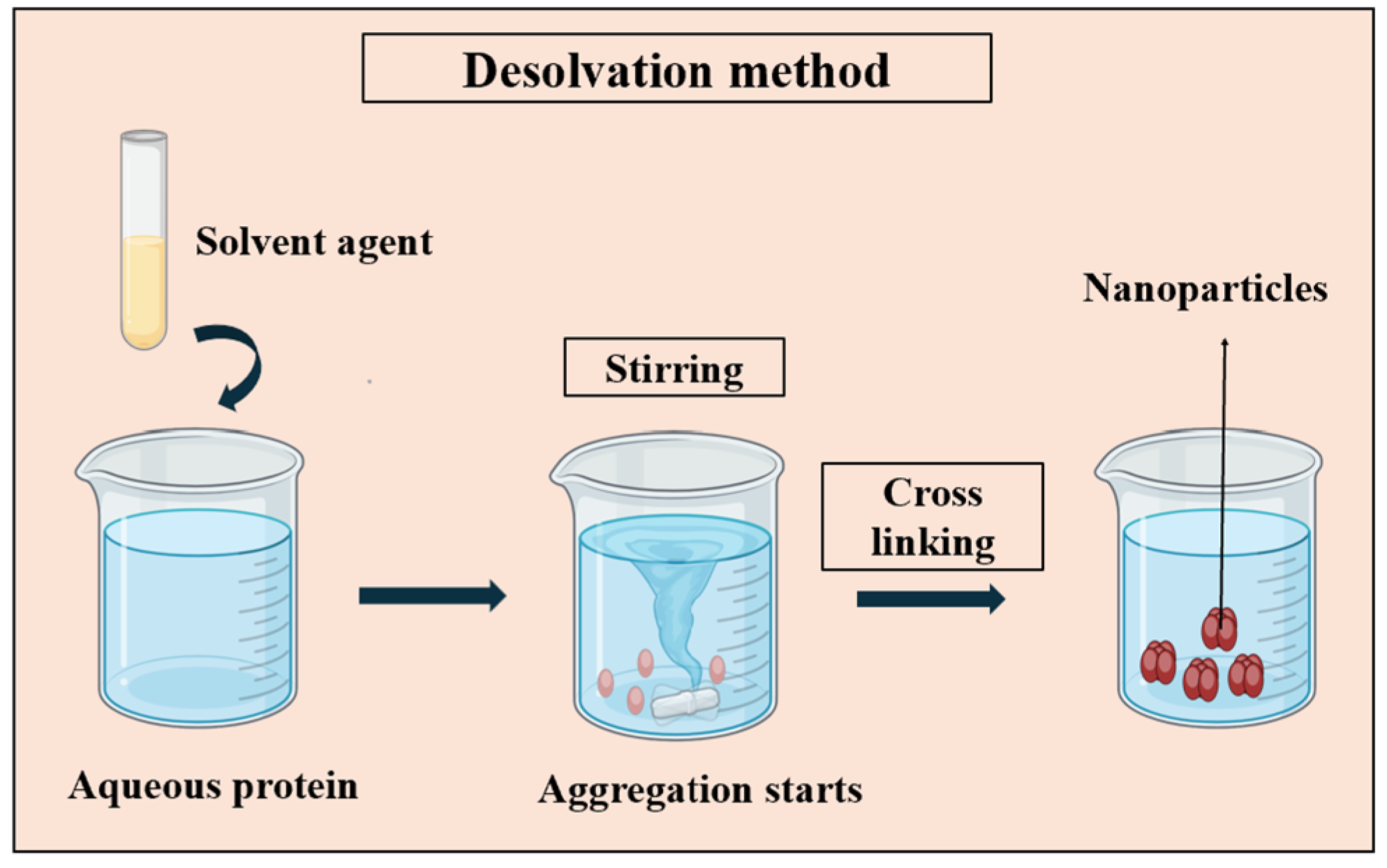
3.1.1. Desolvation
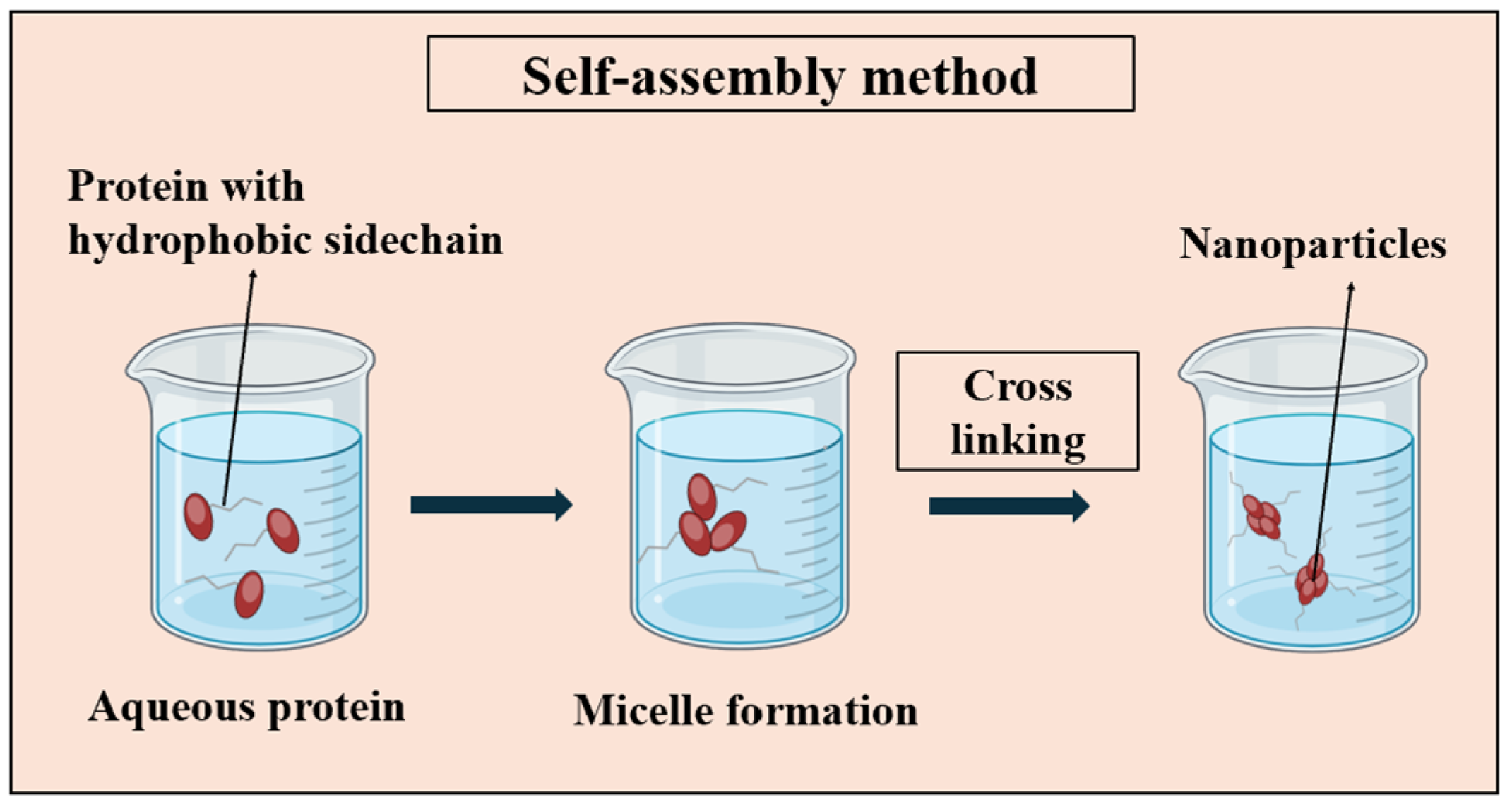
3.1.2. Self-Assembly
3.1.3. Coacervation
3.2. Solvent Substitution
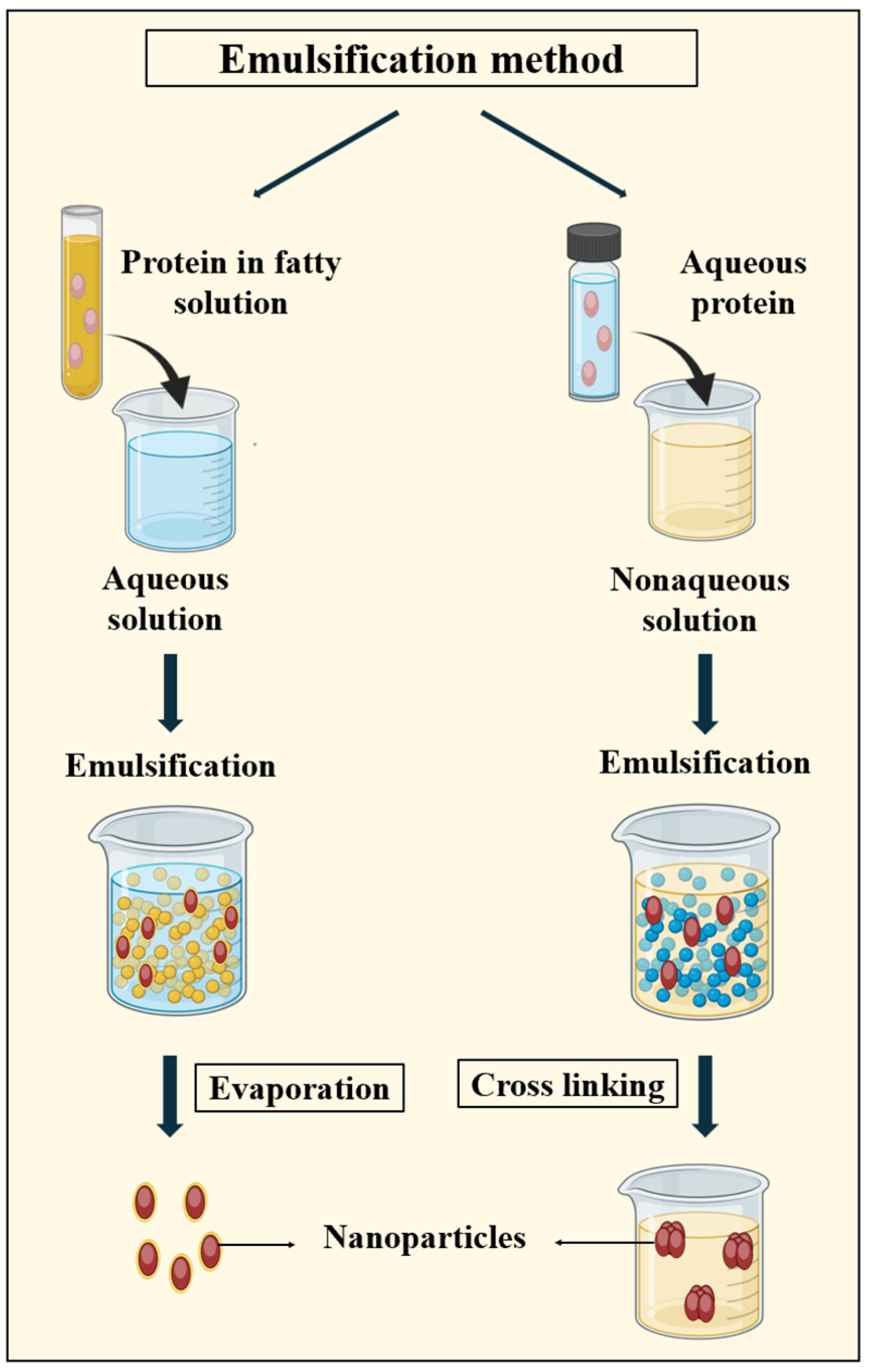
3.2.1. Emulsification Method
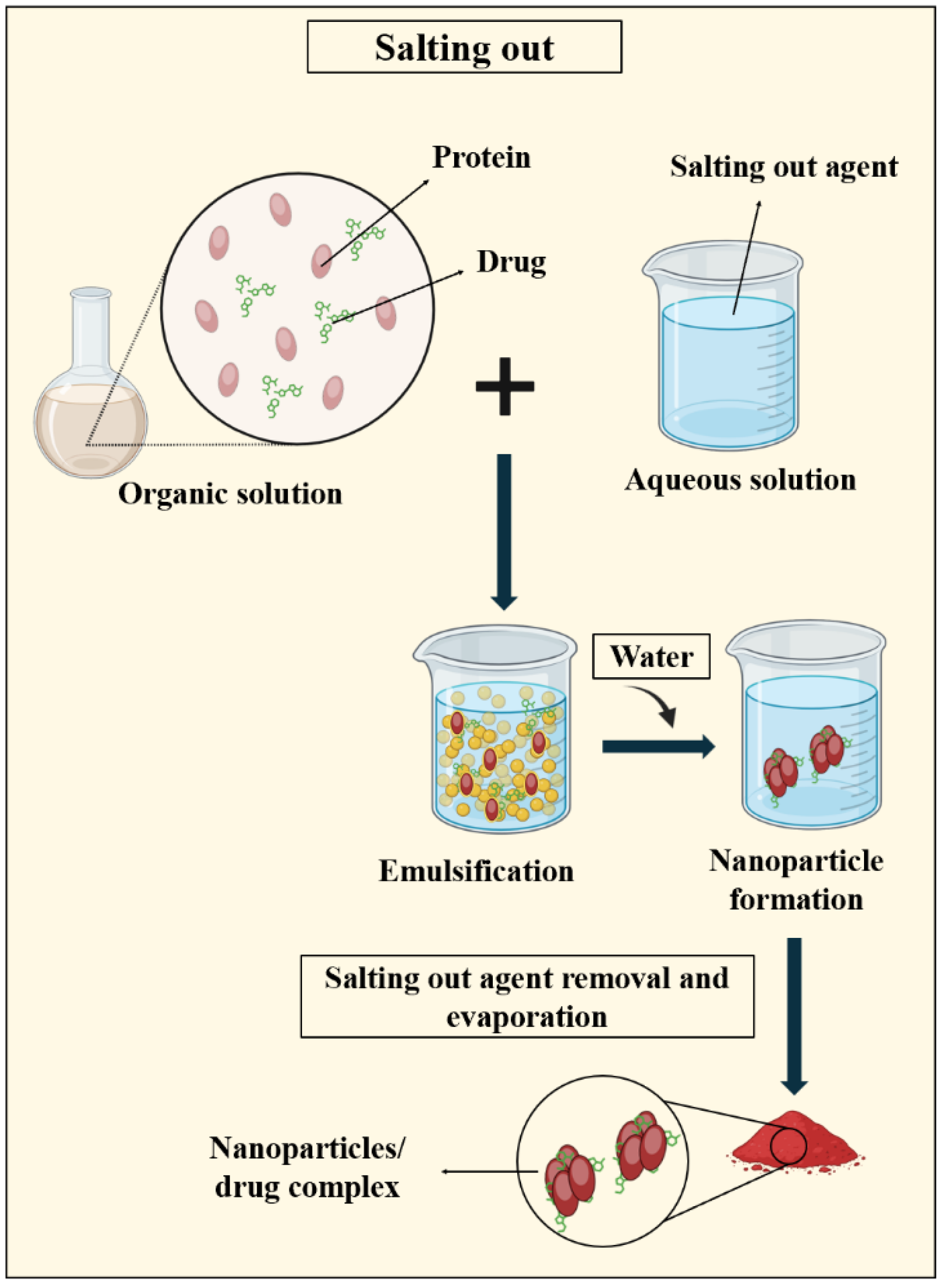
3.2.2. Salting out
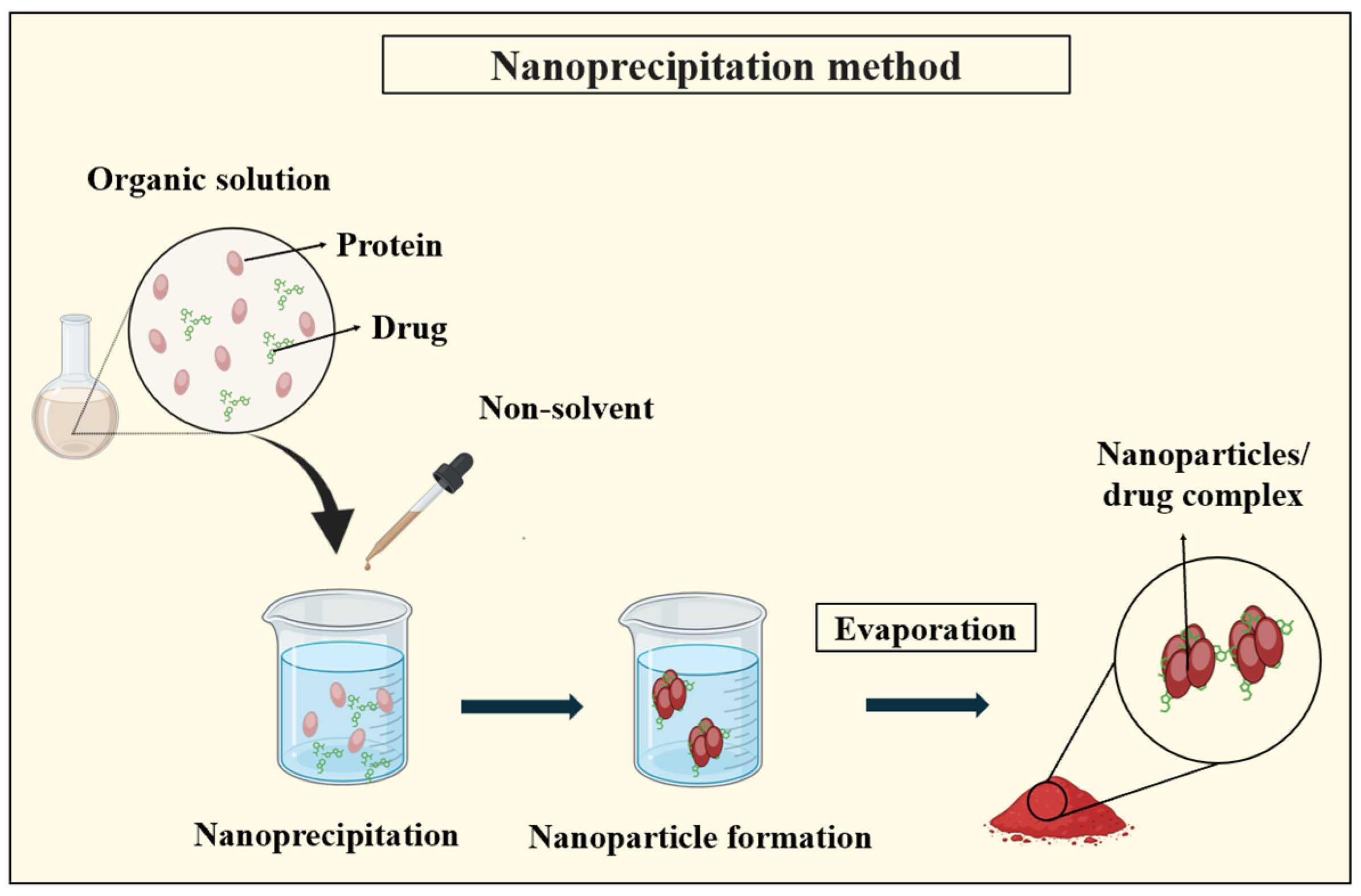
3.2.3. Nanoprecipitation
3.3. Thin Flow Methods
3.3.1. Nano Spray Drying
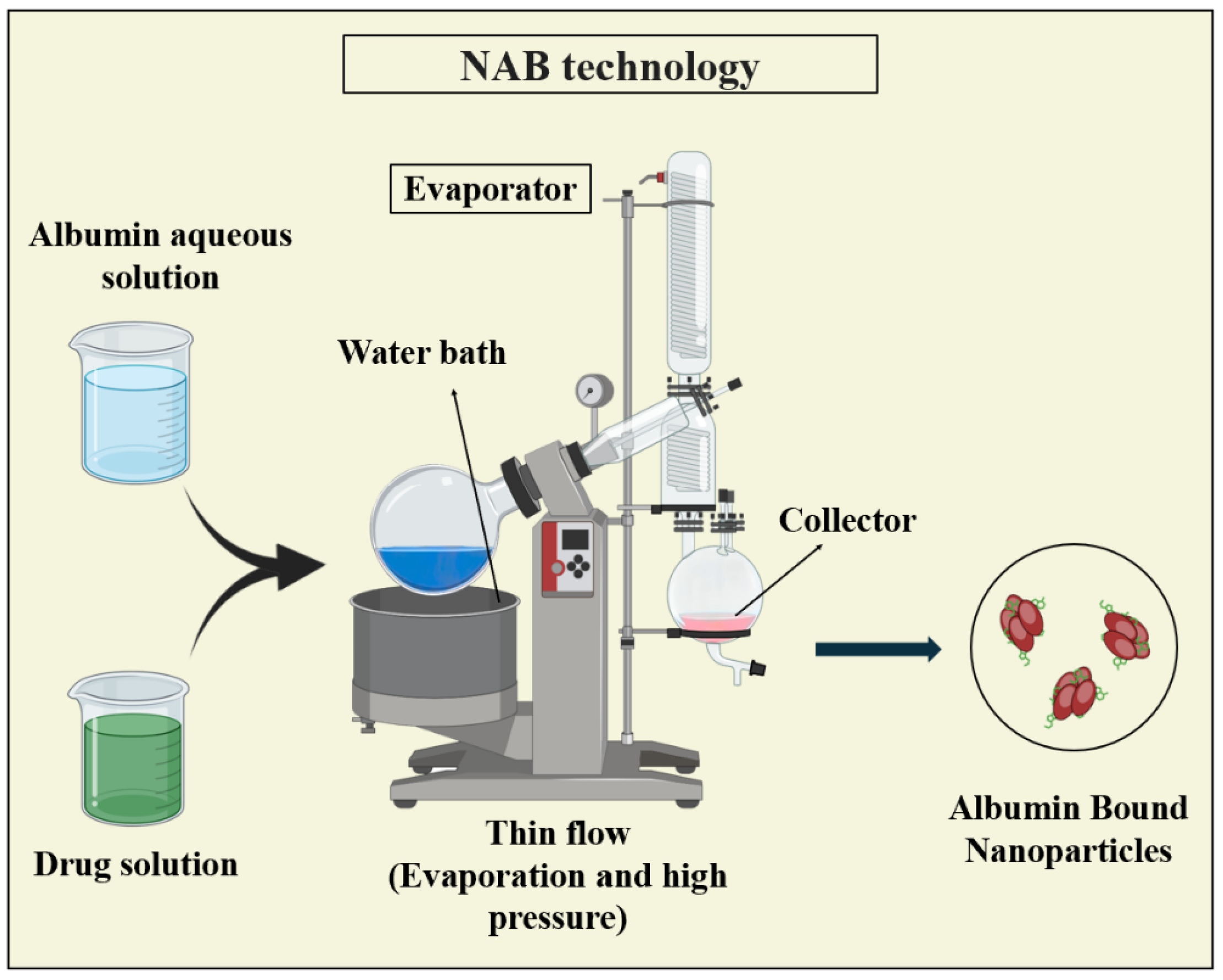
3.3.2. Nanoparticle Albumin-Bound (NAB) Technology
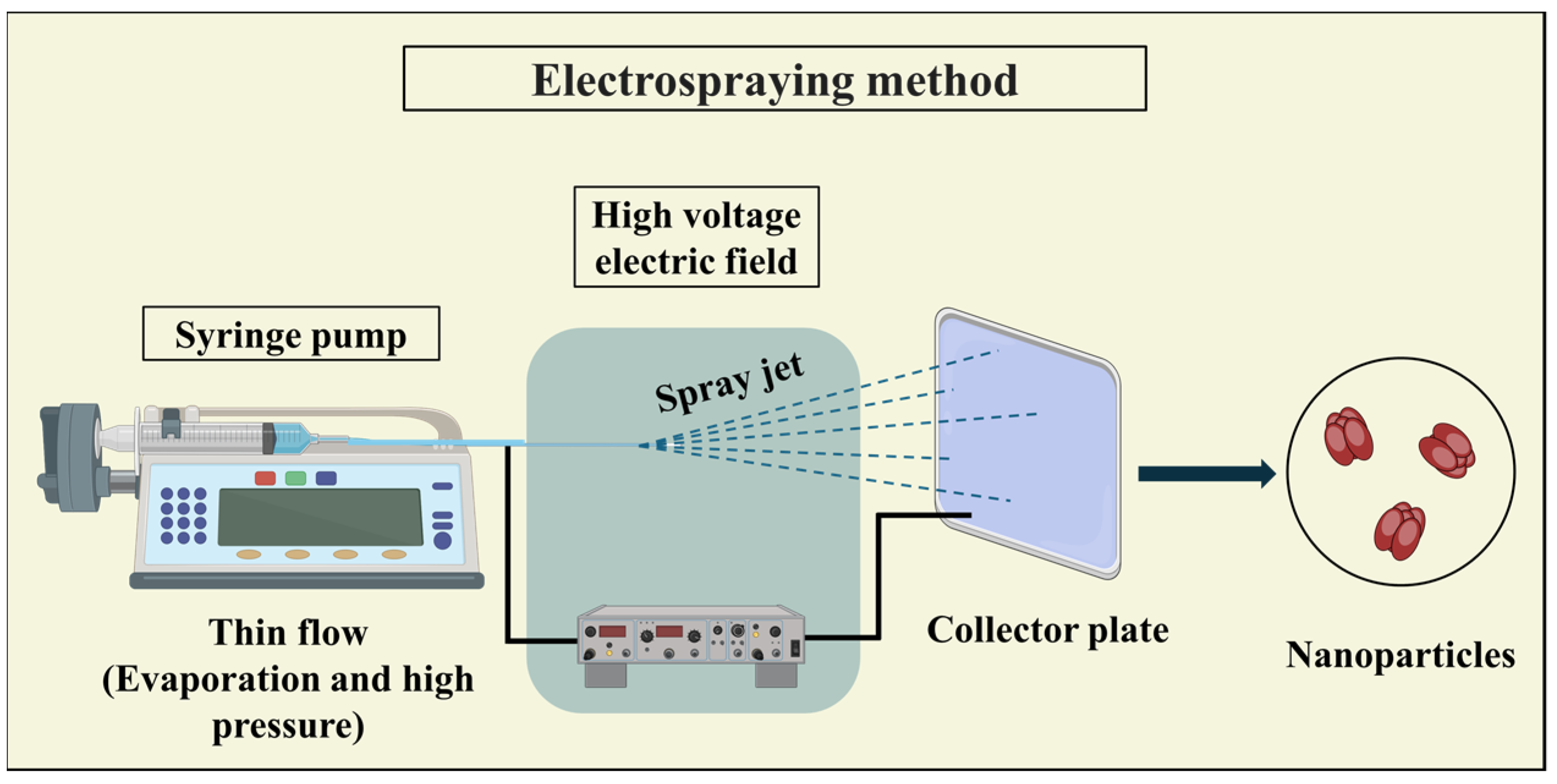
3.3.3. Electrospraying
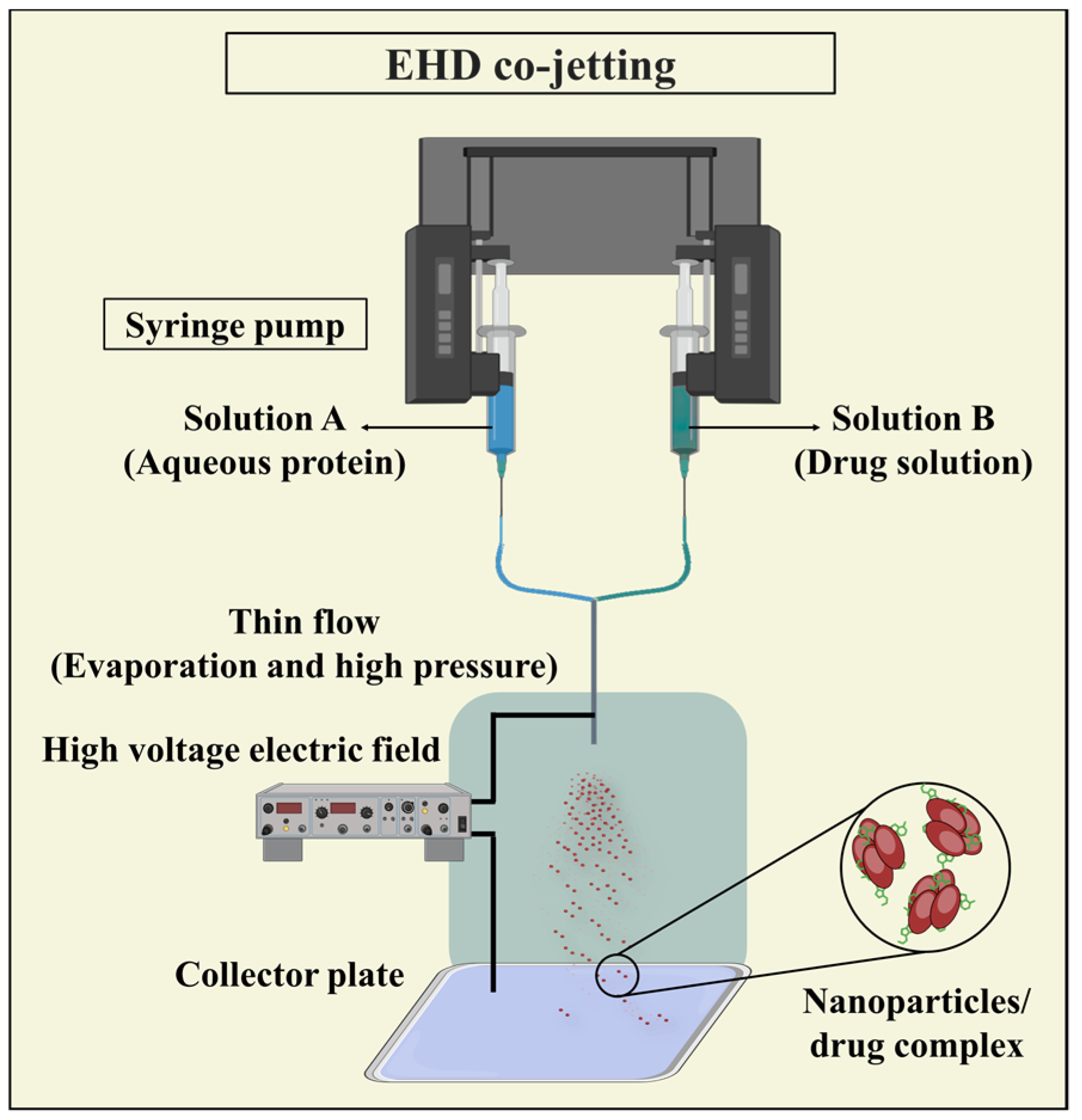
3.3.4. Electrohydrodynamic (EHD) Co-Jetting
4. Comparing PNP and PNP/Drug Complex Synthesis Methods
5. Advanced Technologies for PNP Synthesis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhodes, M. Introduction to Particle Technology; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Feynman, R.P. There’s plenty of room at the bottom [data storage]. J. Microelectromech. Syst. 1992, 1, 60–66. [Google Scholar] [CrossRef]
- Taniguchi, N. On the Basic Concept of “Nano-Technology”. In Proceedings of the International Conference on Production Engineering, Part II, Tokyo, Japan, 26–29 August 1974. [Google Scholar]
- Jose Varghese, R.; Sakho, E.; Hadji, M.; Parani, S.; Thomas, S.; Oluwafemi, O.S.; Wu, J. Introduction to nanomaterials: Synthesis and applications. In Nanomaterials for Solar Cell Applications; Thomas, S., Mamour Sakho, E.H., Kalarikkal, N., Oluwatobi Oluwafemi, S., Wu, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 75–95. [Google Scholar]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Trotta, F.; Mele, A. Nanomaterials: Classification and Properties. In Nanosponges; Trotta, F., Mele, A., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 1–26. [Google Scholar]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Select. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M. Nanomaterials: Types, properties, recent advances, and toxicity concerns. Curr. Opin. Environ. Sci. Health 2022, 25, 100319. [Google Scholar] [CrossRef]
- Rao, N.; Singh, R.; Bashambu, L. Carbon-based nanomaterials: Synthesis and prospective applications. Mater. Today Proc. 2021, 44, 608–614. [Google Scholar] [CrossRef]
- Bhunchu, S.; Rojsitthisak, P. Biopolymeric alginate-chitosan nanoparticles as drug delivery carriers for cancer therapy. Pharmazie 2014, 69, 563–570. [Google Scholar]
- Zhang, Q.; Yang, X.; Guan, J. Applications of Magnetic Nanomaterials in Heterogeneous Catalysis. ACS Appl. Nano Mater. 2019, 2, 4681–4697. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, L.; Yang, M.; Bai, L.; Dai, Y.; Yu, Z.; Pan, Y. Functional magnetic hybrid nanomaterials for biomedical diagnosis and treatment. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1476. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, S.; Sahu, J.; Srivastava, S.; Singh, M.R. Ceramic nanoparticles: Recompense, cellular uptake and toxicity concerns. Artif. Cells Nanomed. Biotechnol. 2016, 44, 401–409. [Google Scholar] [CrossRef]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Fu, R.; Fu, G.-D. Polymeric nanomaterials from combined click chemistry and controlled radical polymerization. Polym. Chem. 2011, 2, 465–475. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, N.; Lyu, Z.; Zhu, W.; Chang, Y.-C.; Hu, X.; Du, D.; Lin, Y. Protein-based nanomaterials and nanosystems for biomedical applications: A review. Mater. Today 2021, 43, 166–184. [Google Scholar] [CrossRef]
- Hunter, P. Nucleic acid-based nanotechnology. EMBO Rep. 2018, 19, 13–17. [Google Scholar] [CrossRef]
- Katyal, P.; Meleties, M.; Montclare, J.K. Self-Assembled Protein- and Peptide-Based Nanomaterials. ACS Biomater. Sci. Eng. 2019, 5, 4132–4147. [Google Scholar] [CrossRef]
- Wang, H.; Yang, R.; Yang, L.; Tan, W. Nucleic Acid Conjugated Nanomaterials for Enhanced Molecular Recognition. ACS Nano 2009, 3, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- ISO 18115-2:2021(en); Surface Chemical Analysis—Vocabulary—Part 2: Terms Used in Scanning-Probe Microscopy. International Organization for Standardization: Geneva, Switzerland, 2021.
- Huzum, B.; Puha, B.; Necoara, R.M.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.D.; Alexa, O. Biocompatibility assessment of biomaterials used in orthopedic devices: An overview (Review). Exp. Ther. Med. 2021, 22, 1315. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Moosavi-Nejad, Z.; Mohammadi, P. A new nanobiotic: Synthesis and characterization of an albumin nanoparticle with intrinsic antibiotic activity. Iran. J. Microbiol. 2023, 15, 697–704. [Google Scholar] [CrossRef]
- Gonzalez, L.; Loza, R.J.; Han, K.-Y.; Sunoqrot, S.; Cunningham, C.; Purta, P.; Drake, J.; Jain, S.; Hong, S.; Chang, J.-H. Nanotechnology in Corneal Neovascularization Therapy—A Review. J. Ocul. Pharmacol. Ther. 2013, 29, 124–134. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, Z.; Afroz, H. Prospects and applications of nanobiotechnology: A medical perspective. J. Nanobiotechnol. 2012, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Moshed, A.M.A.; Sarkar, M.K.I.; Khaleque, M.A. The Application of Nanotechnology in Medical Sciences: New Horizon of Treatment. Am. J. Biomed. Sci. 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C. Nanotechnology: Convergence with modern biology and medicine. Curr. Opin. Biotechnol. 2003, 14, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Lye Chew, C.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Chin Wei, L. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef]
- Sudha, P.N.; Sangeetha, K.; Vijayalakshmi, K.; Barhoum, A. Nanomaterials history, classification, unique properties, production and market. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–384. [Google Scholar]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and inorganic nanomaterials: Fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef]
- Saallah, S.; Lenggoro, I.W. Nanoparticles Carrying Biological Molecules: Recent Advances and Applications. KONA Powder Part. J. 2018, 35, 89–111. [Google Scholar] [CrossRef]
- Shatrohan Lal, R.K. Synthesis of Organic Nanoparticles and their Applications in Drug Delivery and Food Nanotechnology: A Review. J. Nanomater. Mol. Nanotechnol. 2014, 3, 2. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Y.; Zhu, L.; Chu, H.; Shao, X.; Asakiya, C.; Huang, K.; Xu, W. Insights into nucleic acid-based self-assembling nanocarriers for targeted drug delivery and controlled drug release. J. Control. Release 2022, 341, 869–891. [Google Scholar] [CrossRef] [PubMed]
- Kohman, R.E.; Cha, S.S.; Man, H.-Y.; Han, X. Light-Triggered Release of Bioactive Molecules from DNA Nanostructures. Nano Lett. 2016, 16, 2781–2785. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Oliva, N.; Atilano, M.; Song, H.S.; Artzi, N. Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment. Nat. Mater. 2016, 15, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Chandler, M.; Afonin, K.A. Nucleic acid nanoparticles (NANPs) as molecular tools to direct desirable and avoid undesirable immunological effects. Adv. Drug Deliv. Rev. 2021, 173, 427–438. [Google Scholar] [CrossRef] [PubMed]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, S.; Melvin, G.J.H.; Rahman, M.M. Nanotechnology: Applications in Energy, Drug and Food; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Chakravarty, A.; Singh, P.; Aggarwal, S.; Ikram, S. Nanopolysaccharides (polysaccharide-based nanoparticles): Perspectives and applications. In Innovation in Nano-Polysaccharides for Eco-Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–20. [Google Scholar]
- Wu, Y.; Liang, X.; Mao, C.; Jiang, Y. The Distinct Properties of Polysaccharide Nanoparticles Tune Immune Responses against mRNA Antigen via Stimulator of Interferon Genes-Mediated Autophagy and Inflammasome. ACS Nano 2023, 17, 21782–21798. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef]
- Kim, D.; Islam, M.S.; Tam, M.K.C. The Use of Nano-Polysaccharides in Biomedical Applications. In Advanced Functional Materials from Nanopolysaccharides; Springer: Singapore, 2019; pp. 171–219. [Google Scholar]
- Zeng, Y.; Xiang, Y.; Sheng, R.; Tomás, H.; Rodrigues, J.; Gu, Z.; Zhang, H.; Gong, Q.; Luo, K. Polysaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mater. 2021, 6, 3358–3382. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, M.; Benlyamani, I.; Hamdani, S.; Agusti, G.; Fessi, H.; Greige-Gerges, H.; Bentaher, A.; Elaissari, A. Protein-Based Nanoparticle Preparation via Nanoprecipitation Method. Materials 2018, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm. 2018, 2018, 9285854. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar] [PubMed]
- Chen, Z.; Ye, L. Controlling size and uniformity of molecularly imprinted nanoparticles using auxiliary template. J. Mol. Recognit. 2012, 25, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, M.; Moosavi-Nejad, Z.; Kermanshahi, R.K.; Hosano, H. Self-assembled uniform keratin nanoparticles as building blocks for nanofibrils and nanolayers derived from industrial feather waste. J. Clean. Prod. 2022, 335, 130331. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Prostate Cancer; National Comprehensive Cancer Network Inc.: Plymouth Meeting, PA, USA, 2005. [Google Scholar]
- Horwitz, S.B. Personal Recollections on the Early Development of Taxol. J. Nat. Prod. 2004, 67, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, L.; Chen, G.; Xu, F.; Yang, F.; Yu, H.; Li, L.; Dong, X.; Han, J.; Cao, C.; et al. A Review on Drug Delivery System for Tumor Therapy. Front. Pharmacol. 2021, 12, 735446. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Fu, R.; Yang, J.; Ma, P.; Liang, L.; Mi, Y.; Fan, D. Folic acid-modified ginsenoside Rg5-loaded bovine serum albumin nanoparticles for targeted cancer therapy in vitro and in vivo. Int. J. Nanomed. 2019, 14, 6971–6988. [Google Scholar] [CrossRef] [PubMed]
- Martín, M. Nab-Paclitaxel dose and schedule in breast cancer. Breast Cancer Res. 2015, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Moein Moghimi, S. Recent Developments in Polymeric Nanoparticle Engineering and Their Applications in Experimental and Clinical Oncology. Anticancer Agents Med. Chem. 2006, 6, 553–561. [Google Scholar] [CrossRef]
- Paál, K.; Müller, J.; Hegedûs, L. High affinity binding of paclitaxel to human serum albumin. Eur. J. Biochem. 2001, 268, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Rothweiler, F.; Anhorn, M.G.; Sauer, D.; Riemann, I.; Weiss, E.C.; Katsen-Globa, A.; Michaelis, M.; Cinatl, J.; Schwartz, D.; et al. Enhanced drug targeting by attachment of an anti αv integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials 2010, 31, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Battogtokh, G.; Kang, J.H.; Ko, Y.T. Long-circulating self-assembled cholesteryl albumin nanoparticles enhance tumor accumulation of hydrophobic anticancer drug. Eur. J. Pharm. Biopharm. 2015, 96, 96–105. [Google Scholar] [CrossRef]
- Ibrahim, N.K.; Desai, N.; Legha, S.; Soon-Shiong, P.; Theriault, R.L.; Rivera, E.; Esmaeli, B.; Ring, S.E.; Bedikian, A.; Hortobagyi, G.N.; et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin. Cancer Res. 2002, 8, 1038–1044. [Google Scholar]
- Sparreboom, A.; Scripture, C.D.; Trieu, V.; Williams, P.J.; De, T.; Yang, A.; Beals, B.; Figg, W.D.; Hawkins, M.; Desai, N. Comparative Preclinical and Clinical Pharmacokinetics of a Cremophor-Free, Nanoparticle Albumin-Bound Paclitaxel (ABI-007) and Paclitaxel Formulated in Cremophor (Taxol). Clin. Cancer Res. 2005, 11, 4136–4143. [Google Scholar] [CrossRef]
- Ibrahim, N.K.; Samuels, B.; Page, R.; Doval, D.; Patel, K.M.; Rao, S.C.; Nair, M.K.; Bhar, P.; Desai, N.; Hortobagyi, G.N. Multicenter Phase II Trial of ABI-007, an Albumin-Bound Paclitaxel, in Women with Metastatic Breast Cancer. J. Clin. Oncol. 2005, 23, 6019–6026. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared with Polyethylated Castor Oil–Based Paclitaxel in Women with Breast Cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef] [PubMed]
- Palombarini, F.; Di Fabio, E.; Boffi, A.; Macone, A.; Bonamore, A. Ferritin Nanocages for Protein Delivery to Tumor Cells. Molecules 2020, 25, 825. [Google Scholar] [CrossRef] [PubMed]
- Calisti, L.; Trabuco, M.C.; Boffi, A.; Testi, C.; Montemiglio, L.C.; des Georges, A.; Benni, I.; Ilari, A.; Taciak, B.; Białasek, M.; et al. Engineered ferritin for lanthanide binding. PLoS ONE 2018, 13, e0201859. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, S.; Shenoy, D.B.; Amiji, M.M. Gelatin Nanoparticles and Their Biofunctionalization. In Nanotechnologies for the Life Sciences; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Tseng, C.-L.; Su, W.-Y.; Yen, K.-C.; Yang, K.-C.; Lin, F.-H. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009, 30, 3476–3485. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; Sebti, S.M.; Malafa, M.P. Abstract 1699: Delta-tocotrienol potentiates the antitumor activity of standard chemotherapy with gemcitabine and abraxane in metastatic pancreatic cancer. Cancer Res. 2014, 74, 1699. [Google Scholar] [CrossRef]
- Rong, J.; Niu, Z.; Lee, L.A.; Wang, Q. Chemistry and Materials Development of Protein-Based Nanoparticles. In Comprehensive Nanoscience and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 153–174. [Google Scholar]
- Habibi, N.; Mauser, A.; Ko, Y.; Lahann, J. Protein Nanoparticles: Uniting the Power of Proteins with Engineering Design Approaches. Adv. Sci. 2022, 9, 2104012. [Google Scholar] [CrossRef] [PubMed]
- Namakka, M.; Rahman, M.R.; Bin Said, K.A.M.; Abdul Mannan, M.; Patwary, A.M. A review of nanoparticle synthesis methods, classifications, applications, and characterization. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100900. [Google Scholar] [CrossRef]
- Srivastava, A.; Prajapati, A. Albumin and functionalized albumin nanoparticles: Production strategies, characterization, and target indications. Asian Biomed. 2020, 14, 217–242. [Google Scholar] [CrossRef]
- Kovács, A.N.; Varga, N.; Gombár, G.; Hornok, V.; Csapó, E. Novel feasibilities for preparation of serum albumin-based core-shell nanoparticles in flow conditions. J. Flow. Chem. 2020, 10, 497–505. [Google Scholar] [CrossRef]
- Pathak, Y.; Thassu, D. Drug Delivery Nanoparticles Formulation and Characterization; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Miladi, K.; Ibraheem, D.; Iqbal, M.; Sfar, S.; Fessi, H.; Elaissari, A. Particles from preformed polymers as carriers for drug delivery. EXCLI J. 2014, 13, 28–57. [Google Scholar] [PubMed]
- Marty, J.J.; Oppenheim, R.C.; Speiser, P. Nanoparticles—A new colloidal drug delivery system. Pharm. Acta Helv. 1978, 53, 17–23. [Google Scholar] [PubMed]
- Coester, C.J.; Langer, K.; Von Briesen, H.; Kreuter, J. Gelatin nanoparticles by two step desolvation a new preparation method, surface modifications and cell uptake. J. Microencapsul. 2000, 17, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Sozer, S.C.; Egesoy, T.O.; Basol, M.; Cakan-Akdogan, G.; Akdogan, Y. A simple desolvation method for production of cationic albumin nanoparticles with improved drug loading and cell uptake. J. Drug Deliv. Sci. Technol. 2020, 60, 101931. [Google Scholar] [CrossRef]
- Khramtsov, P.; Burdina, O.; Lazarev, S.; Novokshonova, A.; Bochkova, M.; Timganova, V.; Kiselkov, D.; Minin, A.; Zamorina, S.; Rayev, M. Modified Desolvation Method Enables Simple One-Step Synthesis of Gelatin Nanoparticles from Different Gelatin Types with Any Bloom Values. Pharmaceutics 2021, 13, 1537. [Google Scholar] [CrossRef] [PubMed]
- Herrera Estrada, L.P.; Champion, J.A. Protein nanoparticles for therapeutic protein delivery. Biomater. Sci. 2015, 3, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, S.A.; Ahmadi, Z.; Moosavi-Nejad, Z. The albumin-based nanoparticle formation in relation to protein aggregation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 252, 119489. [Google Scholar] [CrossRef]
- Lai, Y.-T.; Tsai, K.-L.; Sawaya, M.R.; Asturias, F.J.; Yeates, T.O. Structure and Flexibility of Nanoscale Protein Cages Designed by Symmetric Self-Assembly. J. Am. Chem. Soc. 2013, 135, 7738–7743. [Google Scholar] [CrossRef]
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocoll. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Patel, A.R.; Bouwens, E.C.M.; Velikov, K.P. Sodium Caseinate Stabilized Zein Colloidal Particles. J. Agric. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Huo, M.; Zhou, J.; Zhang, Y.; Peng, X.; Yu, D.; Zhang, H.; Li, J. Synthesis, characterization, drug-loading capacity and safety of novel octyl modified serum albumin micelles. Int. J. Pharm. 2009, 376, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Helmy, M.W.; Samy, W.M.; Elgindy, N.A. Micellar Delivery of Flutamide Via Milk Protein Nanovehicles Enhances its Anti-Tumor Efficacy in Androgen-Dependent Prostate Cancer Rat Model. Pharm. Res. 2013, 30, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhou, F.; Shen, P.; Zhang, Y.; Lin, L.; Zhao, M. Self-assembled soy protein nanoparticles by partial enzymatic hydrolysis for pH-Driven Encapsulation and Delivery of Hydrophobic Cargo Curcumin. Food Hydrocoll. 2021, 120, 106759. [Google Scholar] [CrossRef]
- Salaün, F. Microencapsulation technology for smart textile coatings. In Active Coatings for Smart Textiles; Elsevier: Amsterdam, The Netherlands, 2016; pp. 179–220. [Google Scholar]
- Oppenheim, R.C.; Stewart, N.F. The Manufacture and Tumour Cell Uptake of Nanoparticles Labelled with Fluorescein Isothiocyanate. Drug Dev. Ind. Pharm. 1979, 5, 563–571. [Google Scholar] [CrossRef]
- Leo, E.; Angela Vandelli, M.; Cameroni, R.; Forni, F. Doxorubicin-loaded gelatin nanoparticles stabilized by glutaraldehyde: Involvement of the drug in the cross-linking process. Int. J. Pharm. 1997, 155, 75–82. [Google Scholar] [CrossRef]
- Satya Prakash, S. Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: Preparation and in vitro analysis. Int. J. Nanomed. 2010, 5, 525. [Google Scholar] [CrossRef] [PubMed]
- Meiguni, M.S.M.; Salami, M.; Rezaei, K.; Aliyari, M.A.; Ghaffari, S.-B.; Emam-Djomeh, Z.; Kennedy, J.F.; Ghasemi, A. Fabrication and characterization of a succinyl mung bean protein and arabic gum complex coacervate for curcumin encapsulation. Int. J. Biol. Macromol. 2023, 224, 170–180. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef]
- Wilde, P.; Mackie, A.; Husband, F.; Gunning, P.; Morris, V. Proteins and emulsifiers at liquid interfaces. Adv. Colloid Interface Sci. 2004, 108–109, 63–71. [Google Scholar] [CrossRef]
- Leroux, J.-C.; Allemann, E.; Doelker, E.; Gurny, R. New approach for the preparation of nanoparticles by an emulsification-diffusion method. Eur. J. Pharm. Biopharm. 1995, 41, 14–18. [Google Scholar]
- Cascone, M.G.; Lazzeri, L.; Carmignani, C.; Zhu, Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J. Mater. Sci. Mater. Med. 2002, 13, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Helmy, M.W.; Samy, W.M.; Elgindy, N.A. Novel ionically crosslinked casein nanoparticles for flutamide delivery: Formulation, characterization, and in vivo pharmacokinetics. Int. J. Nanomed. 2013, 8, 1721. [Google Scholar] [CrossRef]
- Girgis, S.; Elkordy, A.A.; Faheem, A.; Elkordy, A. Preparation of poly ɛ: Caprolactone nanoparticles containing bovine serum albumin by emulsification technique. Pharma Innov. J. 2019, 8, 238–243. [Google Scholar]
- Elzoghby, A.O.; Elgohary, M.M.; Kamel, N.M. Implications of Protein- and Peptide-Based Nanoparticles as Potential Vehicles for Anticancer Drugs. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2015; pp. 169–221. [Google Scholar]
- Nagavarma, B.; Yadav, H.; Ayaz, A.; Vasudha, L.; Shivakumar, H. Different techniques for preparation of polymeric nanoparticles—A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Allémann, E.; Gurny, R.; Doelker, E. Preparation of aqueous polymeric nanodispersions by a reversible salting-out process: Influence of process parameters on particle size. Int. J. Pharm. 1992, 87, 247–253. [Google Scholar] [CrossRef]
- Lammel, A.S.; Hu, X.; Park, S.-H.; Kaplan, D.L.; Scheibel, T.R. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010, 31, 4583–4591. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Patel, A.; Hu, Y.; Tiwari, J.K.; Velikov, K.P. Synthesis and characterisation of zein–curcumin colloidal particles. Soft Matter 2010, 6, 6192. [Google Scholar] [CrossRef]
- Lee, E.J.; Khan, S.A.; Lim, K.-H. Gelatin Nanoparticle Preparation by Nanoprecipitation. J. Biomater. Sci. Polym. Ed. 2011, 22, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Schneider, M. Nanoprecipitation versus two step desolvation technique for the preparation of gelatin nanoparticles. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 2–7 February 2013; p. 85950H. [Google Scholar]
- Zhao, H.; Wang, M.; Wang, X.; Liu, J.; Xing, M.; Huang, H.; Cohen Stuart, M.A.; Wang, J. Controlled fabrication of drug-loaded protein nanoparticles via flash nanoprecipitation. AIChE J. 2023, 69, e17941. [Google Scholar] [CrossRef]
- Aljohani, M.A.; Jimack, P.K.; Walkley, M.A. A faster optimal solver for thin film flows. Appl. Numer. Math. 2023, 184, 357–370. [Google Scholar] [CrossRef]
- Peltonen, L.; Valo, H.; Kolakovic, R.; Laaksonen, T.; Hirvonen, J. Electrospraying, spray drying and related techniques for production and formulation of drug nanoparticles. Expert Opin. Drug Deliv. 2010, 7, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Heng, D.; Ng, W.K.; Chan, H.-K.; Tan, R.B.H. Nano spray drying: A novel method for preparing protein nanoparticles for protein therapy. Int. J. Pharm. 2011, 403, 192–200. [Google Scholar] [CrossRef]
- Desai, N. Nanoparticle albumin bound (nab) technology: Targeting tumors through the endothelial gp60 receptor and SPARC. Nanomedicine 2007, 3, 339. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, J.; Zhang, W.; Sui, X.; Yan, Z.; He, Z. Nanoparticle Albumin–Bound (NAB) Technology is a Promising Method for Anti-Cancer Drug Delivery. Recent. Pat. Anticancer Drug Discov. 2009, 4, 262–272. [Google Scholar] [CrossRef]
- Joshi, M.; Nagarsenkar, M.; Prabhakar, B. Albumin nanocarriers for pulmonary drug delivery: An attractive approach. J. Drug Deliv. Sci. Technol. 2020, 56, 101529. [Google Scholar] [CrossRef]
- Tanhaei, A.; Mohammadi, M.; Hamishehkar, H.; Hamblin, M.R. Electrospraying as a novel method of particle engineering for drug delivery vehicles. J. Control. Release 2021, 330, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Lahann, J. Recent Progress in Nano-biotechnology: Compartmentalized Micro- and Nanoparticles via Electrohydrodynamic Co-jetting. Small 2011, 7, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Ashraf, S.; Hartmann, R.; Dishman, A.F.; Zyuzin, M.V.; Yu, C.K.J.; Parak, W.J.; Lahann, J. Engineering of nanoparticle size via electrohydrodynamic jetting. Bioeng. Transl. Med. 2016, 1, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C. Development of High-Drug-Loading Nanoparticles. ChemPlusChem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Ganash, E.A. Synthesis of silver nanoparticles using pulsed laser ablation in liquid: A review. Laser Phys. Lett. 2023, 20, 013001. [Google Scholar] [CrossRef]
- González-Rubio, G.; Guerrero-Martínez, A.; Liz-Marzán, L.M. Reshaping, Fragmentation, and Assembly of Gold Nanoparticles Assisted by Pulse Lasers. Acc. Chem. Res. 2016, 49, 678–686. [Google Scholar] [CrossRef] [PubMed]
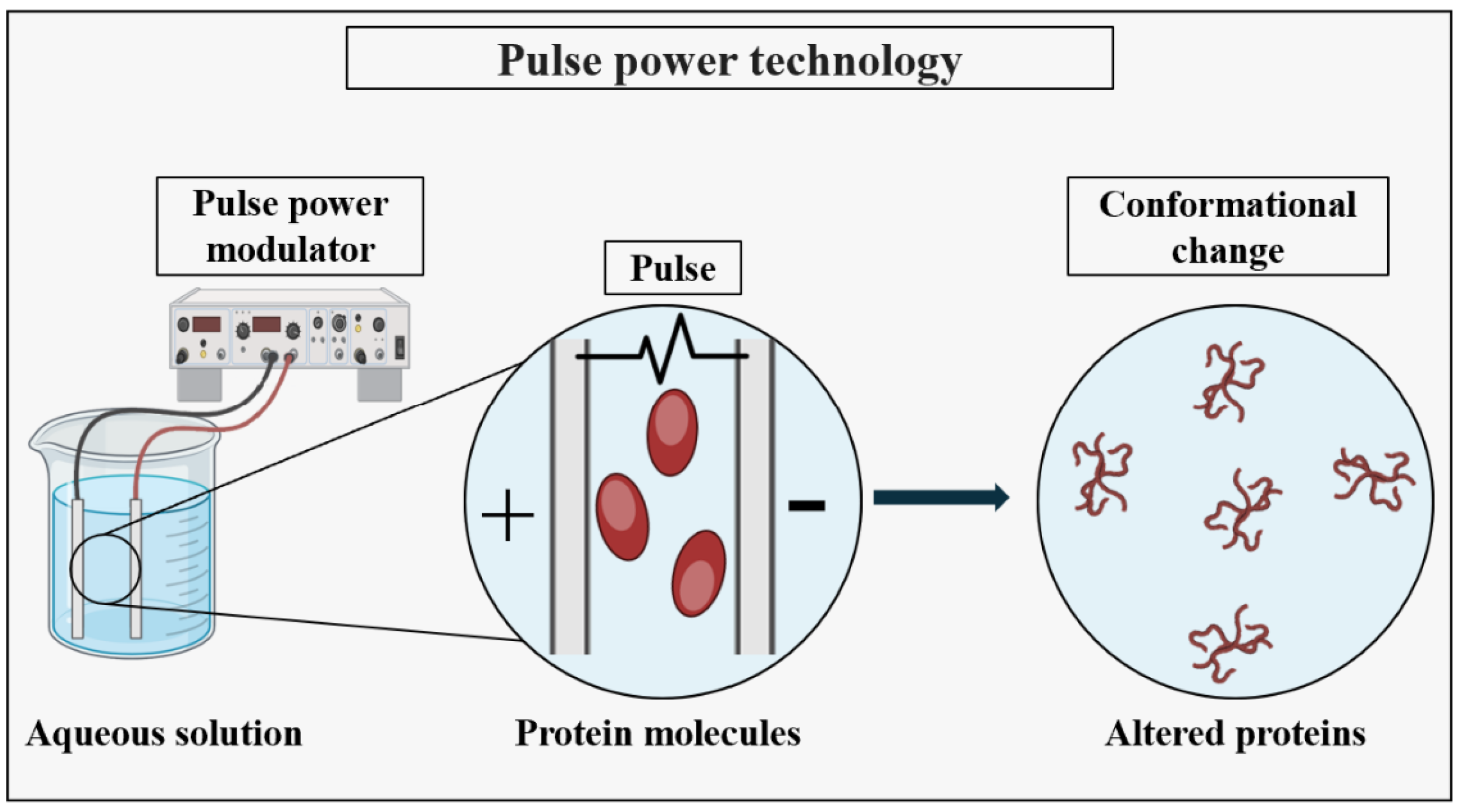
- Takaki, K.; Takahashi, K.; Guionet, A.; Ohshima, T. Pulsed Power Applications for Protein Conformational Change and the Permeabilization of Agricultural Products. Molecules 2021, 26, 6288. [Google Scholar] [CrossRef] [PubMed]
- Urabe, G.; Sato, T.; Nakamura, G.; Kobashigawa, Y.; Morioka, H.; Katsuki, S. 1.2 MV/cm pulsed electric fields promote transthyretin aggregate degradation. Sci. Rep. 2020, 10, 12003. [Google Scholar] [CrossRef]
- Urabe, G.; Shimada, M.; Ogata, T.; Katsuki, S. Pulsed Electric Fields Promote Liposome Buddings. Bioelectricity 2021, 3, 68–76. [Google Scholar] [CrossRef]


| PNPs Name | Drug Name | Targeted Cancer | Clinical Trials | References |
|---|---|---|---|---|
| Albumin (HSA and BSA) | Doxorubicin Paclitaxel | Breast cancer Melanoma cells Pancreatic cancer | Phase I Phase II Phase III | [67,68,69,70,71,72,77] |
| Ferritin | Doxorubicin | Most cancer cells with high expression of the transferrin receptor TfR1 | N/A | [73,74] |
| Gelatin | Cisplatin | Lung cancer | N/A | [75,76] |
| Method | PNPs Size (nm) | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Solubility change | 24–1000< |
|
| [26,78,85,86,87,88,89,90,92,93,96,98,99,100,101,102] |
| Solvent substitution | 49–900< |
|
| [26,105,106,107,108,109,110,111,112,114,115,116,117] |
| Thin flow methods | 87–1000< |
|
| [26,38,78,109,120,122,124,126,127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khakpour, S.; Hosano, N.; Moosavi-Nejad, Z.; Farajian, A.A.; Hosano, H. Advancing Tumor Therapy: Development and Utilization of Protein-Based Nanoparticles. Pharmaceutics 2024, 16, 887. https://doi.org/10.3390/pharmaceutics16070887
Khakpour S, Hosano N, Moosavi-Nejad Z, Farajian AA, Hosano H. Advancing Tumor Therapy: Development and Utilization of Protein-Based Nanoparticles. Pharmaceutics. 2024; 16(7):887. https://doi.org/10.3390/pharmaceutics16070887
Chicago/Turabian StyleKhakpour, Shirin, Nushin Hosano, Zahra Moosavi-Nejad, Amir A. Farajian, and Hamid Hosano. 2024. "Advancing Tumor Therapy: Development and Utilization of Protein-Based Nanoparticles" Pharmaceutics 16, no. 7: 887. https://doi.org/10.3390/pharmaceutics16070887






