Spirothiazolidine-Derivative on Silver Nanoparticles and Carbon Nanotubes: Evaluation of Antibacterial, Anti-Fungal, Anti-Inflammatory, Antioxidant and Gastroprotective Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.1.1. Chemicals
2.1.2. Preparation of Spiro-Thiazolidine (ST)
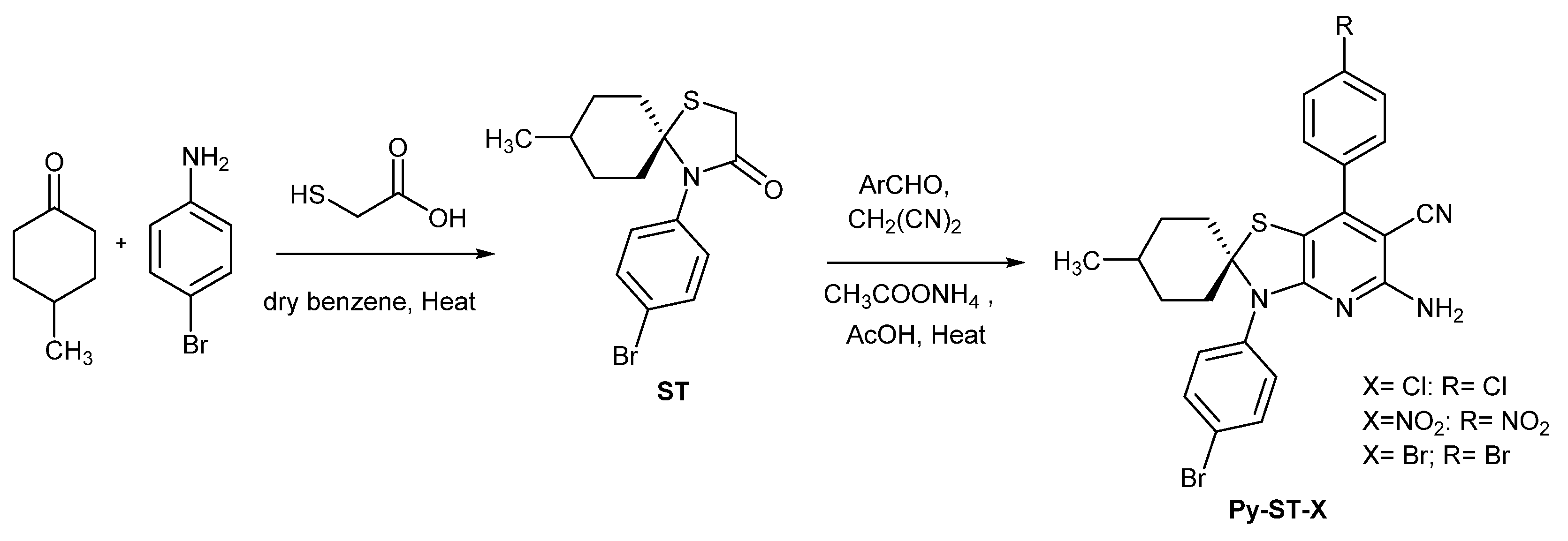
2.1.3. Preparation of Pyridinespirothiazolidines (Py-ST-X)
2.1.4. Preparation of AgNPs
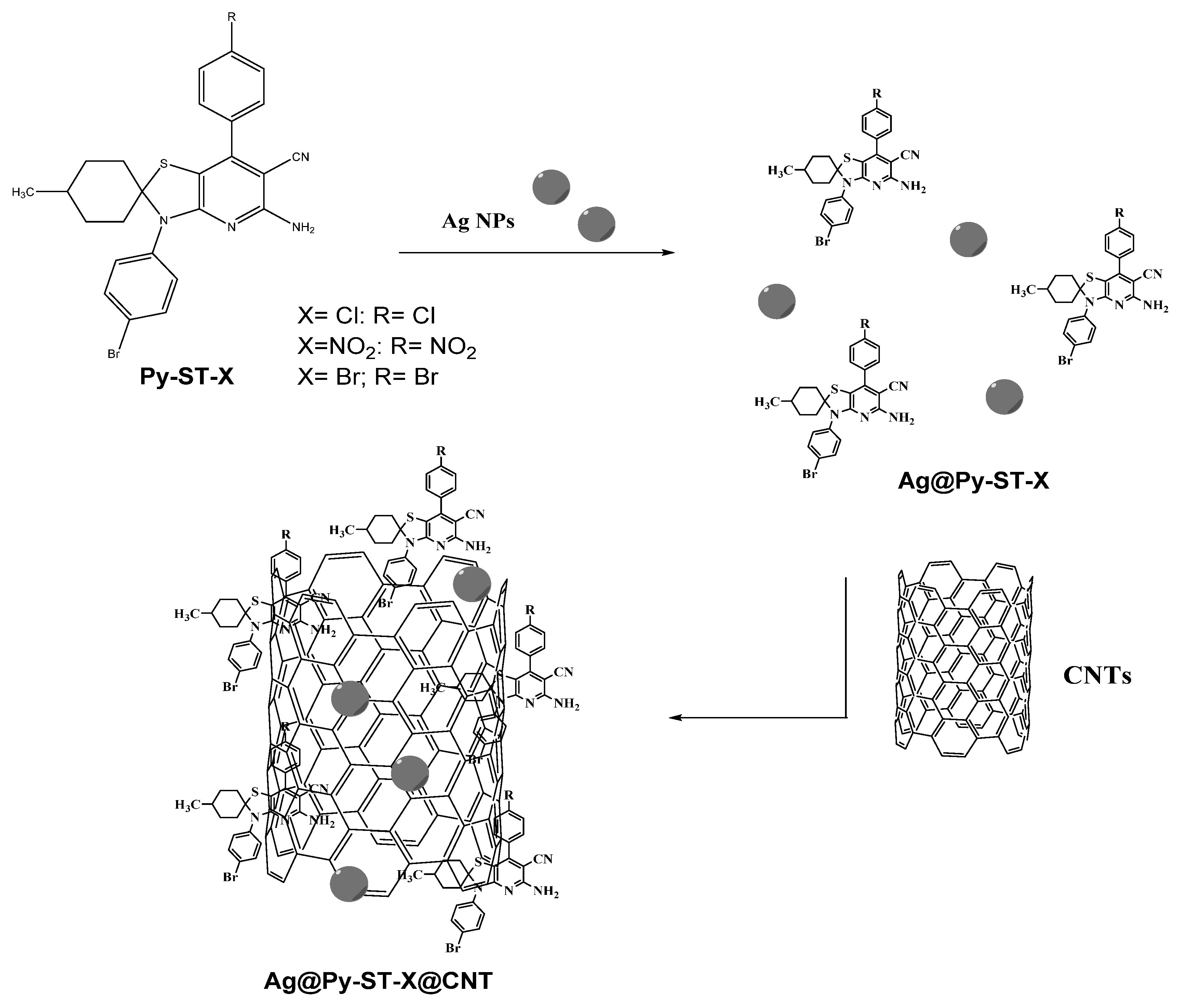
2.1.5. Preparation of Ag@Py-ST-X Composites
2.1.6. Preparation of Ag@Py-ST-X@CNT Composites
2.2. Material Characterization
2.3. Animals and Experimental Study
2.4. Evaluation of Toxicity of Compounds on Rats
2.5. Evaluation of Antibacterial Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Py-ST-X
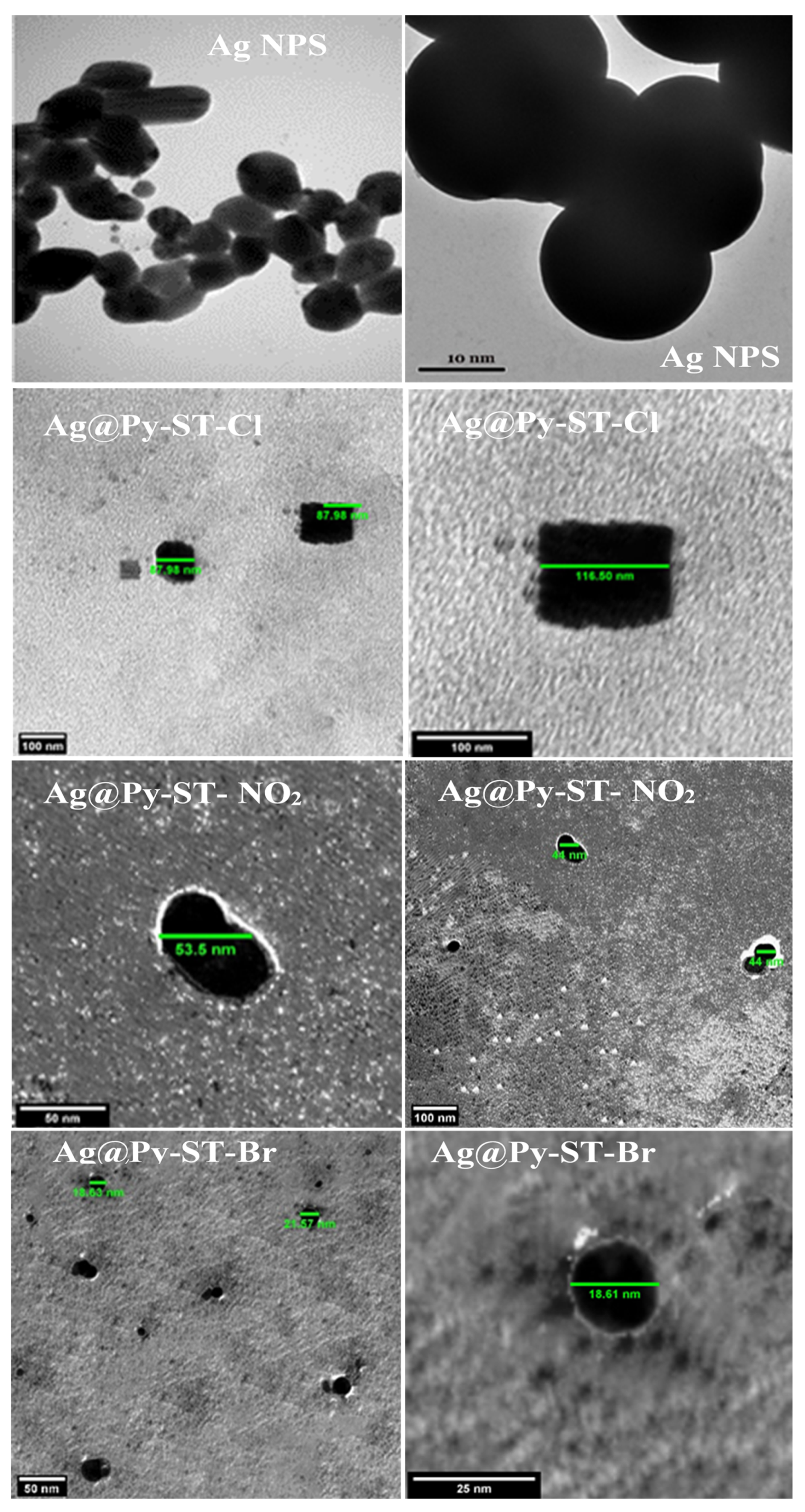
3.2. Morphology and Structure of AgNPs and CNTs
3.3. Biological Activities
3.3.1. Evaluation of the Toxicity of Bioactive Derivatives with Bromine (Br) or Nitro (NO2) with Carbon Nanotubes
3.3.2. Effect of New Derivatives of Spiro-Thiazolidine-Carbonitrile on Key Enzyme Related to Gastric Acidity and Ulceration as H+/K+ATPase
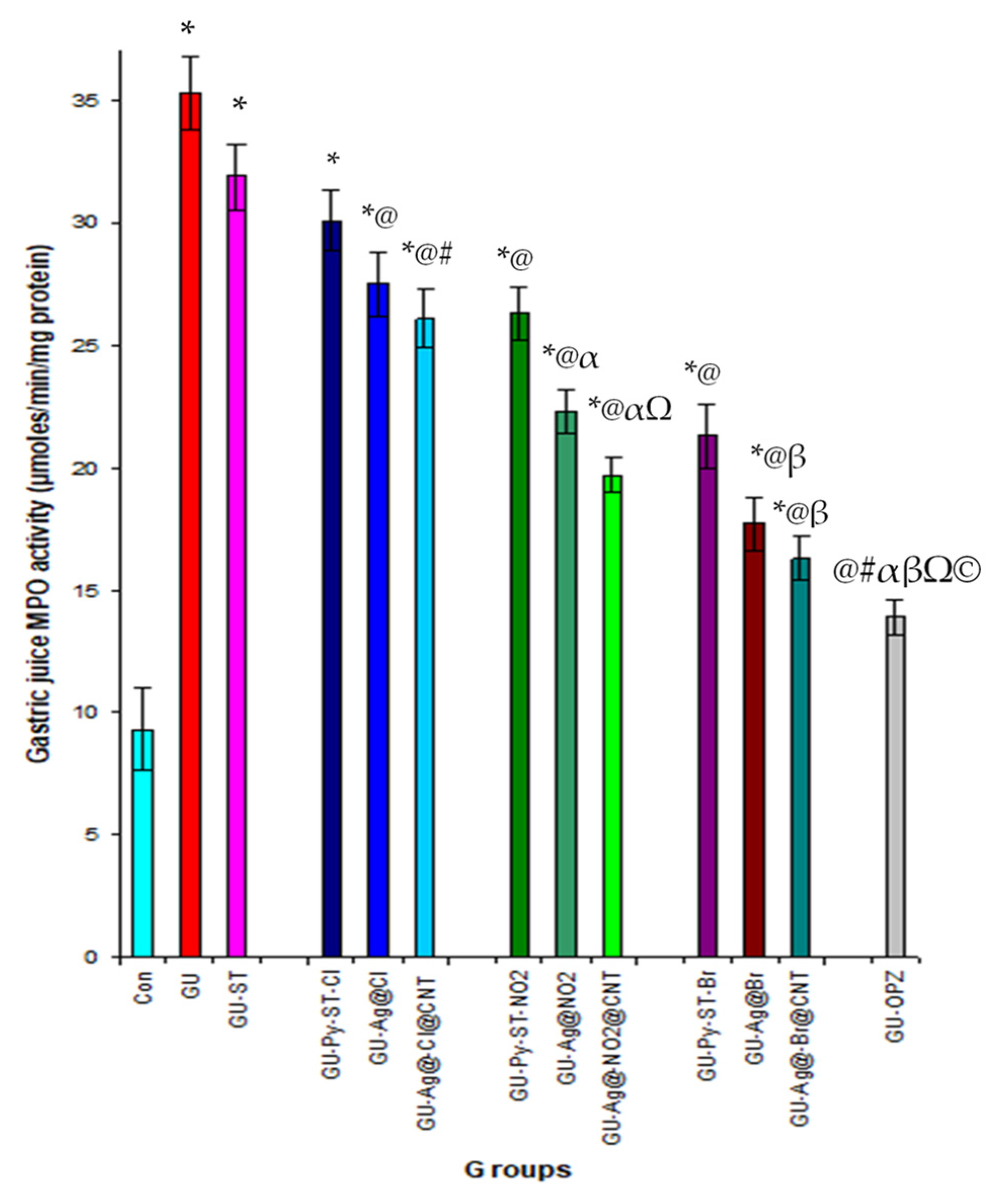
3.3.3. Effect of New Derivatives of Spiro-Thiazolidine-Carbonitrile on Gastric Inflammation
3.3.4. Effect of New Derivatives of Spiro-Thiazolidine-Carbonitrile Nanocomposites Stress Oxidant in Gastric Tissues
3.3.5. Effect of New Derivatives of Spiro-Thiazolidine-Carbonitrile Nanocomposites on Gastric Tissues Ulceration
3.3.6. Effect of New Derivatives of Spiro-Thiazolidine-Carbonitrile Nanocomposites on Histological Changes in Gastric Tissue Architecture
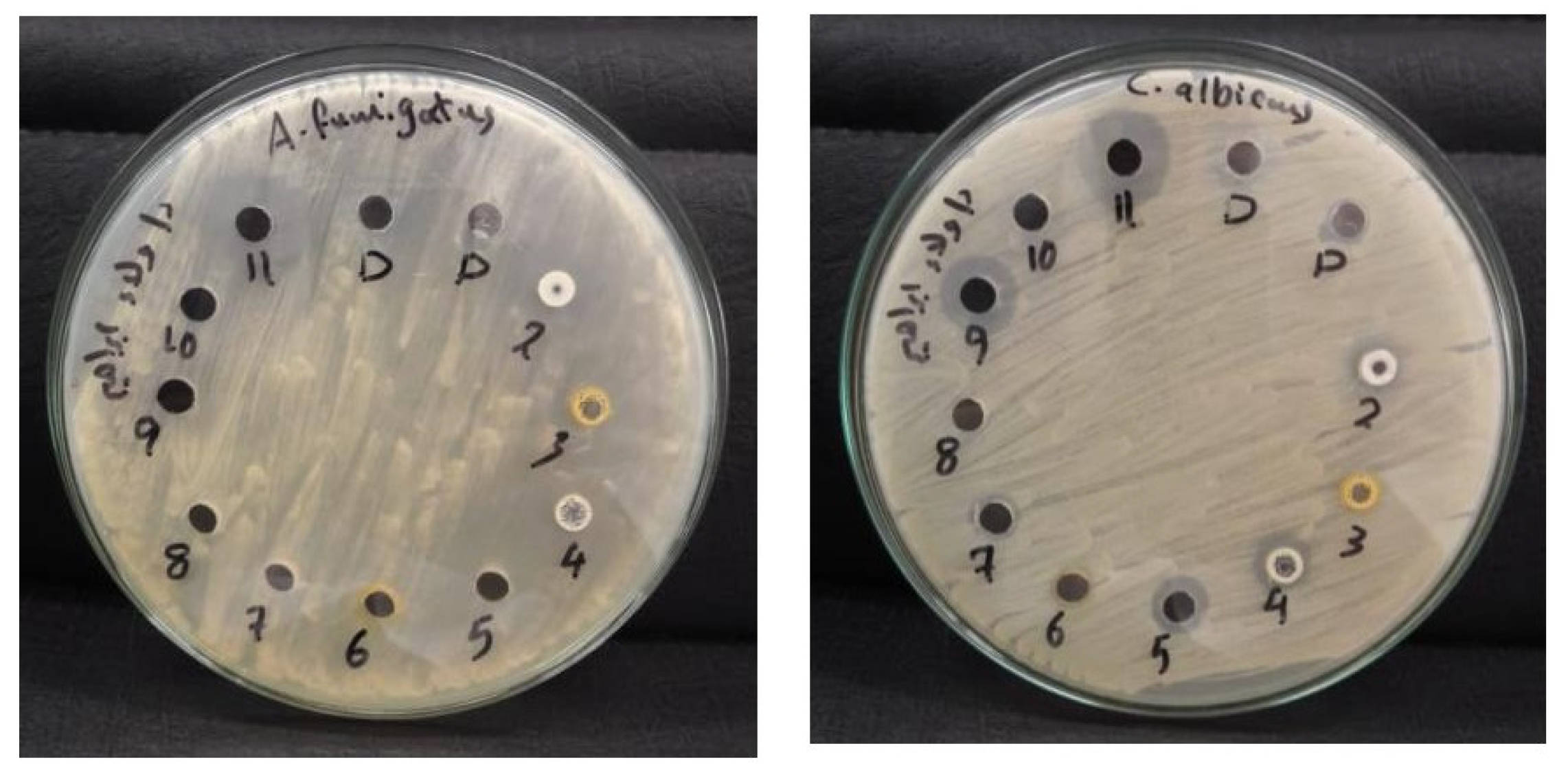
3.3.7. Assessment of the Antifungal Efficacy of Novel Spiro-Thiazolidine-Carbonitrile Derivatives
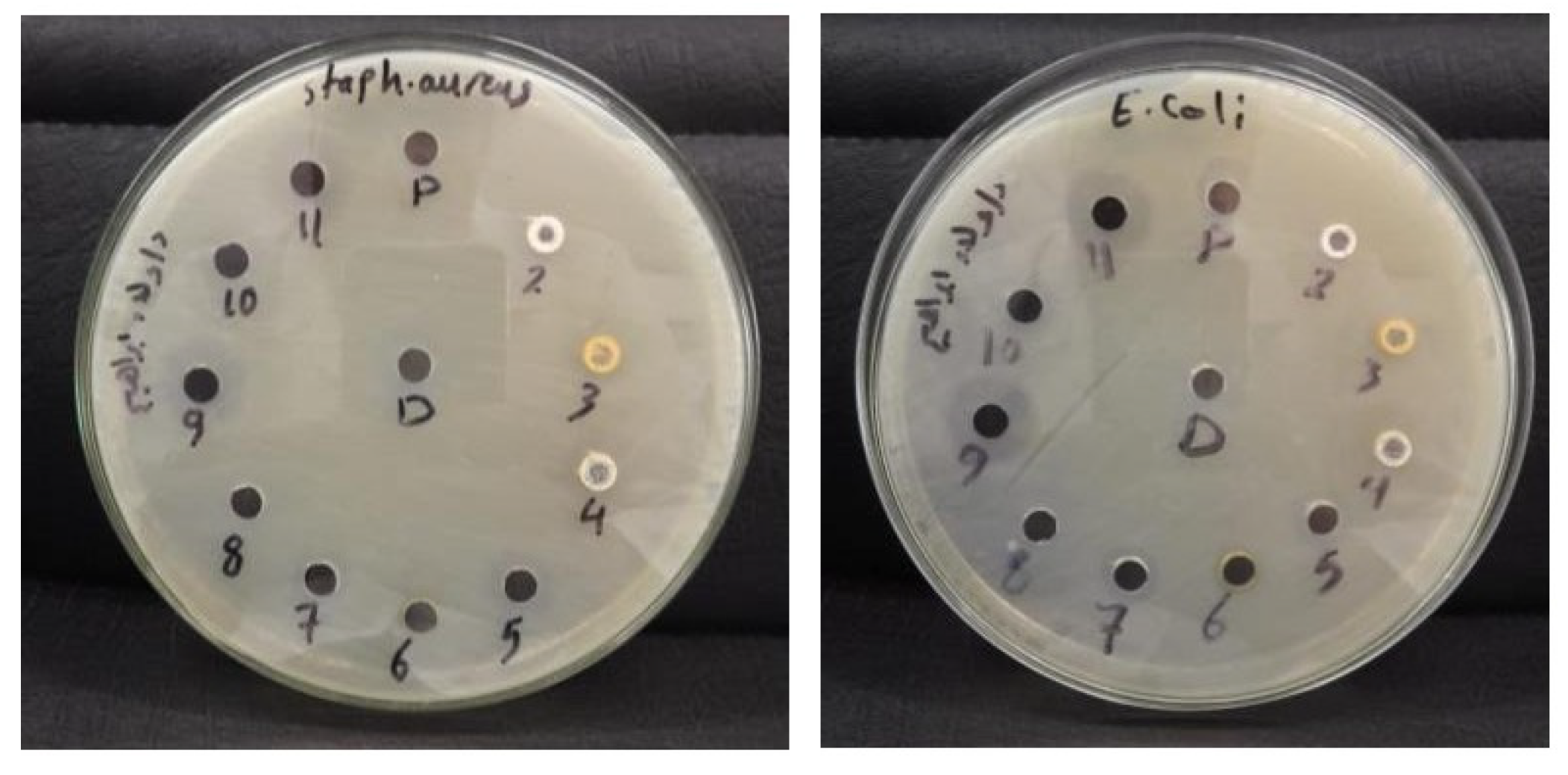
3.3.8. Assessment of the Antibacterial Efficacy of Novel Spiro-Thiazolidine-Carbonitrile Derivatives
3.3.9. Structure–Activity Relationship
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adib, M.; Peytam, F.; Rahmanian-Jazi, M.; Mohammadi-Khanaposhtani, M.; Mahernia, S.; Bijanzadeh, H.R.; Jahani, M.; Imanparast, S.; Faramarzi, M.A.; Mahdavi, M. Design, Synthesis and in Vitro α-Glucosidase Inhibition of Novel Coumarin-Pyridines as Potent Antidiabetic Agents. New J. Chem. 2018, 42, 17268–17278. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; El-Shahat, M.; Rabie, S.T.; Flefel, E.M.; Abd-Elshafy, D.N. New Pyrimidine and Fused Pyrimidine Derivatives: Synthesis and Anti Hepatitis A Virus (HAV) Evaluation. Int. J. Pharm. 2015, 5, 69–79. [Google Scholar]
- Akhtar, A.; Ijaz, M.; Batool, F.; Pervaiz, J. Nanomedicine in the Treatment of Viral Diseases. In Nanomedicine in Treatment of Diseases; Springer: Berlin, Germany, 2024; pp. 123–149. [Google Scholar]
- Yazdani, S.; Mozaffarian, M.; Pazuki, G.; Hadidi, N.; Villate-Beitia, I.; Zárate, J.; Puras, G.; Pedraz, J.L. Carbon-Based Nanostructures as Emerging Materials for Gene Delivery Applications. Pharmaceutics 2024, 16, 288. [Google Scholar] [CrossRef] [PubMed]
- Nouh, R.A.; Kamal, A.; Oyewole, O.; Abbas, W.A.; Abib, B.; Omar, A.; Mansour, S.T.; Abdelnaser, A. Unveiling the Potential of Cannabinoids in Multiple Sclerosis and the Dawn of Nano-Cannabinoid Medicine. Pharmaceutics 2024, 16, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, J.; Xue, P.; Zhang, J. Collagenase-Responsive Hydrogel Loaded with GSK2606414 Nanoparticles for Periodontitis Treatment through Inhibiting Inflammation-Induced Expression of PERK of Periodontal Ligament Stem Cells. Pharmaceutics 2023, 15, 2503. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Sha, X. Nanoparticle-Mediated Strategies for Enhanced Drug Penetration and Retention in the Airway Mucosa. Pharmaceutics 2023, 15, 2457. [Google Scholar] [CrossRef] [PubMed]
- Dhadda, S.; Sharma, S.; Jakhar, P.; Sharma, H. Contemporary Progress in the Green Synthesis of Spiro-Thiazolidines and Their Medicinal Significance: A Review. RSC Adv. 2023, 13, 3723–3742. [Google Scholar] [CrossRef] [PubMed]
- Demir Atlı, D.; Aksu, D. Air Stable N-Heterocyclic Carbene Silver Complexes: Synthesis, Characterization, and Antibacterial Activity. J. Coord. Chem. 2023, 76, 1497–1506. [Google Scholar] [CrossRef]
- Xiong, J.; Ding, R.; Liu, Z.; Zheng, H.; Li, P.; Chen, Z.; Yan, Q.; Zhao, X.; Xue, F.; Peng, Q. High-Strength, Super-Tough, and Durable Nacre-Inspired MXene/Heterocyclic Aramid Nanocomposite Films for Electromagnetic Interference Shielding and Thermal Management. Chem. Eng. J. 2023, 474, 145972. [Google Scholar] [CrossRef]
- Aghababaei Tafreshi, O.; Saadatnia, Z.; Ghaffari-Mosanenzadeh, S.; Kumar, A.; Salari, M.; Mohseni Taromsari, S.; Rastegardoost, M.M.; Park, C.B.; Naguib, H.E. Flexible, Thermally Stable, and Ultralightweight Polyimide-CNT Aerogel Composite Films for Energy Storage Applications. ACS Appl. Mater. Interfaces 2023, 15, 50360–50377. [Google Scholar] [CrossRef]
- Li, D.; Ke, Z.; Xu, K.; Dai, F.; Wang, M.; Chen, C.; Qian, G.; Yu, Y. Mechanically Strong Polyimide Aerogels Containing Benzimidazole Groups with Excellent Flame-Retardant, Thermal Insulation and High Service Temperature. Chem. Eng. J. 2023, 461, 141722. [Google Scholar] [CrossRef]
- Rogers-Nieman, G.M.; Dinu, C.Z. Therapeutic Applications of Carbon Nanotubes: Opportunities and Challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 327–337. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Darwesh, O.M.; El-Shahat, M. Titanium-Based Metal-Organic Framework Capsulated with Magnetic Nanoparticles: Antimicrobial and Photocatalytic Degradation of Pesticides. Microporous Mesoporous Mater. 2023, 354, 112543. [Google Scholar] [CrossRef]
- El-Sofany, W.I.; El-sayed, W.A.; Abd-Rabou, A.A.; El-Shahat, M. Synthesis of New Imidazole-Triazole-Glycoside Hybrids as Anti-Breast Cancer Candidates. J. Mol. Struct. 2022, 1270, 133942. [Google Scholar] [CrossRef]
- Flefel, E.M.; El-Sofany, W.I.; Awad, H.M.; El-Shahat, M. First Synthesis for Bis-Spirothiazolidine Derivatives as a Novel Heterocyclic Framework and Their Biological Activity. Mini Rev. Med. Chem. 2020, 20, 152–160. [Google Scholar] [CrossRef]
- Flefel, E.M.; El-Sofany, W.I.; El-Shahat, M.; Naqvi, A.; Assirey, E. Synthesis, Molecular Docking and in Vitro Screening of Some Newly Synthesized Triazolopyridine, Pyridotriazine and Pyridine–Pyrazole Hybrid Derivatives. Molecules 2018, 23, 2548. [Google Scholar] [CrossRef]
- Scarselli, M.; Camilli, L.; Castrucci, P.; Nanni, F.; Del Gobbo, S.; Gautron, E.; Lefrant, S.; De Crescenzi, M. In Situ Formation of Noble Metal Nanoparticles on Multiwalled Carbon Nanotubes and Its Implication in Metal–Nanotube Interactions. Carbon N. Y. 2012, 50, 875–884. [Google Scholar] [CrossRef]
- Larrude, D.G.; Maia da Costa, M.E.H.; Monteiro, F.H.; Pinto, A.L.; Freire, F.L. Characterization of Phosphorus-Doped Multiwalled Carbon Nanotubes. J. Appl. Phys. 2012, 111, 064315. [Google Scholar] [CrossRef]
- Castle, A.B.; Gracia-Espino, E.; Nieto-Delgado, C.; Terrones, H.; Terrones, M.; Hussain, S. Hydroxyl-Functionalized and N-Doped Multiwalled Carbon Nanotubes Decorated with Silver Nanoparticles Preserve Cellular Function. ACS Nano 2011, 5, 2458–2466. [Google Scholar] [CrossRef]
- Yu, A.; Wang, Q.; Yong, J.; Mahon, P.J.; Malherbe, F.; Wang, F.; Zhang, H.; Wang, J. Silver Nanoparticle–Carbon Nanotube Hybrid Films: Preparation and Electrochemical Sensing. Electrochim. Acta 2012, 74, 111–116. [Google Scholar] [CrossRef]
- Ding, X.; Su, Y.; Wang, C.; Zhang, F.; Chen, K.; Wang, Y.; Li, M.; Wang, W. Synergistic Suppression of Tumor Angiogenesis by the Co-Delivering of Vascular Endothelial Growth Factor Targeted SiRNA and Candesartan Mediated by Functionalized Carbon Nanovectors. ACS Appl. Mater. Interfaces 2017, 9, 23353–23369. [Google Scholar] [CrossRef] [PubMed]
- Saleem, J.; Wang, L.; Chen, C. Carbon-based Nanomaterials for Cancer Therapy via Targeting Tumor Microenvironment. Adv. Healthc. Mater. 2018, 7, 1800525. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Jaffar Al-Mulla, E.A.; Ibrahim, N.A.; Shabanzadeh, P.; Rustaiyan, A.; Abdollahi, Y.; Bagheri, S.; Abdolmohammadi, S.; Usman, M.S. Green Biosynthesis of Silver Nanoparticles Using Callicarpa Maingayi Stem Bark Extraction. Molecules 2012, 17, 8506–8517. [Google Scholar] [CrossRef] [PubMed]
- Shameli, K.; Ahmad, M.B.; Al-Mulla, E.A.J.; Shabanzadeh, P.; Bagheri, S. Antibacterial Effect of Silver Nanoparticles on Talc Composites. Res. Chem. Intermed. 2015, 41, 251–263. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Escorcia-Díaz, D.; García-Mora, S.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. Advancements in Nanoparticle Deposition Techniques for Diverse Substrates: A Review. Nanomaterials 2023, 13, 2586. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio) Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Abdi, Y.; Mohajerzadeh, S. Silver Nanoparticles within Vertically Aligned Multi-Wall Carbon Nanotubes with Open Tips for Antibacterial Purposes. J. Mater. Chem. 2011, 21, 387–393. [Google Scholar] [CrossRef]
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef]
- Saad, F.; Al-Shaikh, T.M.; Zouidi, F.; Taher, M.A.; Saidi, S.A.; Hamden, K. Betalain-Enriched Beetroots Exhibit Antiulcer and Anti-Inflammatory Potentials. J. Food. Process. Pres. 2023, 2023, 9522830. [Google Scholar] [CrossRef]
- Abdelkader Saidi, S.; Al-Shaikh, T.M.; Hamden, K. Evaluation of Gastroprotective Effect of Betalain-Rich Ethanol Extract from Opuntia Stricta Var. Dillenii Employing an In Vivo Rat Model. J. Food Qual. 2023, 2023, 2215454. [Google Scholar] [CrossRef]
- Maes, M.L.; Fixen, D.R.; Linnebur, S.A. Adverse Effects of Proton-Pump Inhibitor Use in Older Adults: A Review of the Evidence. Ther. Adv. Drug Saf. 2017, 8, 273–297. [Google Scholar] [CrossRef]
- Herszényi, L.; Bakucz, T.; Barabás, L.; Tulassay, Z. Pharmacological Approach to Gastric Acid Suppression: Past, Present, and Future. Dig. Dis. 2020, 38, 104–111. [Google Scholar] [CrossRef]
- El-Sofany, W.I.; Flefel, E.M.; Darwesh, O.M.; El-Shahat, M. Boosting the Antimicrobial Performance Based on New Fused Spirothiazolidine Framework Analogs. J. Iran. Chem. Soc. 2022, 19, 4223–4236. [Google Scholar] [CrossRef]
- Flefel, E.M.; Sayed, H.H.; Hashem, A.I.; Shalaby, E.A.; El-Sofany, W.; Abdel-Megeid, F.M.E. Pharmacological Evaluation of Some Novel Synthesized Compounds Derived from Spiro (Cyclohexane-1, 2′-Thiazolidines). Med. Chem. Res. 2014, 23, 2515–2527. [Google Scholar] [CrossRef]
- Azzam, E.M.S.; Sami, R.M.; Alenezi, K.M.; Haque, A.; El Moll, H.; Soury, R.A.; Ismail, A.R. Inhibition of Sulfate-Reducing Bacteria by Para-amino-N-((1-Alkylpyridin-1-Ium Bromide)-4-Yl) Benzamide Surfactants and Surfactant-Coated Silver Nanoparticles. J. Surfactants Deterg. 2022, 25, 125–131. [Google Scholar] [CrossRef]
- Azzam, E.M.S.; Fathy, N.A.; El-Khouly, S.M.; Sami, R.M. Enhancement the Photocatalytic Degradation of Methylene Blue Dye Using Fabricated CNTs/TiO2/AgNPs/Surfactant Nanocomposites. J. Water Process Eng. 2019, 28, 311–321. [Google Scholar] [CrossRef]
- Ofusori, A.E.; Moodley, R.; Jonnalagadda, S.B. Antiulcerogenic Effects of Celosia Trigyna Plant Extracts on Ethanol-Induced Gastric Ulcer in Adult Wistar Rats. J. Tradit. Complement. Med. 2020, 10, 586–593. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of Cutaneous Inflammation: Estimation of Neutrophil Content with an Enzyme Marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Nagaya, H.; Satoh, H.; Maki, Y. Actions of Antisecretory Agents on Proton Transport in Hog Gastric Microsomes. Biochem. Pharmacol. 1987, 36, 513–519. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. [30] Microsomal Lipid Peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. ISBN 0076-6879. [Google Scholar]
- Dingeon, B.; Ferry, J.P.; Roullet, A. Automatic Assay of Blood Sugar by Trinder’s Method. Annales de Biologie Clinique 1975, 33, 3–13. [Google Scholar] [PubMed]
- Jorgensen, J.H.; Swenson, J.M.; Tenover, F.C.; Ferraro, M.J.; Hindler, J.A.; Murray, P.R. Development of Interpretive Criteria and Quality Control Limits for Broth Microdilution and Disk Diffusion Antimicrobial Susceptibility Testing of Streptococcus Pneumoniae. J. Clin. Microbiol. 1994, 32, 2448–2459. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.M.S.; Elsofany, W.I.; Abdulaziz, F.; AlGhamdi, H.A.; Al Alhareth, A.Y. Ecofriendly Elimination of Ni (II) Using Fabricated Nanocomposite Based on Chitosan/Silver Nanoparticles/Carbon Nanotubes. Polymers 2023, 15, 2759. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. An Overview of Applications of Silver-Based Polymer Nano Composite as Biomaterials. In Polymer Nanocomposites Based on Silver Nanoparticles. Engineering Materials; Springer: Cham, Switzerland, 2021; pp. 213–246. [Google Scholar]
- Karak, N. Silver Nanomaterials and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–89. [Google Scholar]
- Ghosh, P.; Bairagi, D.; Hazra, N.; Jana, S.; Banerjee, A. Carbon-Dot-Decorated Silver and Gold Nanocomposites for Antibacterial Activity and Degradation of Organic Dyes. ACS Appl. Nano Mater. 2023, 6, 18100–18112. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Trzcińska-Wencel, J.; Wypij, M.; Bonde, S.; Yadav, A.; Kratošová, G.; Golińska, P. Biogenic Silver Nanoparticles: What We Know and What Do We Need to Know? Nanomaterials 2021, 11, 2901. [Google Scholar] [CrossRef]
- Espíndola, C.; Correa, A.J.; López-López, M.; López-Cornejo, P.; Bernal, E.; Lebrón, J.A.; Ostos, F.J.; Benhnia, M.R.-E.-I.; Moyá, M.L. Single-and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics 2022, 14, 2806. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Liu, B.; Li, B.; Chai, J.; Zhang, L.; Jiao, L.; Li, D.; Yu, Z.; Ren, F.; Shi, X. Enhanced Transport of Shape and Rigidity-Tuned α-Lactalbumin Nanotubes across Intestinal Mucus and Cellular Barriers. Nano Lett. 2020, 20, 1352–1361. [Google Scholar] [CrossRef]
- Homayun, B.; Choi, H.-J. Halloysite Nanotube-Embedded Microparticles for Intestine-Targeted Co-Delivery of Biopharmaceuticals. Int. J. Pharm. 2020, 579, 119152. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent Advancements in the Development of Heterocyclic Anti-Inflammatory Agents. Eur. J. Med. Chem. 2020, 200, 112438. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Malini, K.V.; Rao, B.S.; Sarojini, B.K.; Kumari, N.S. Synthesis of Some New 2, 4-Disubstituted Thiazoles as Possible Antibacterial and Anti-Inflammatory Agents. Eur. J. Med. Chem. 2003, 38, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, H.I.; Dhake, A.S.; Surana, A.R. Synthesis and Screening of Thiosemicarbazide-Dithiocarbamate Conjugates for Antioxidant and Anticancer Activities. Bioorg. Chem. 2022, 124, 105832. [Google Scholar]
- Nair, N.; Majeed, J.; Pandey, P.K.; Sweety, R.; Thakur, R. Antioxidant Potential of Pyrimidine Derivatives against Oxidative Stress. Indian J. Pharm. Sci. 2022, 84, 14–26. [Google Scholar] [CrossRef]
- Aldosary, S.K.; El-Rahman, S.N.A.; Al-Jameel, S.S.; Alromihi, N.M. Antioxidant and Antimicrobial Activities of Thymus vulgaris Essential Oil Contained and Synthesis Thymus (Vulgaris) Silver Nanoparticles. Braz. J. Biol. 2021, 83, e244675. [Google Scholar] [CrossRef] [PubMed]
- Nyaki, H.Y.; Mahmoodi, N.O. Synthesis and Characterization of Derivatives Including Thiazolidine-2, 4-Dione/1-H-Imidazole and Evaluation of Antimicrobial, Antioxidant, and Cytotoxic Properties of New Synthetic Heterocyclic Compounds. Res. Chem. Intermed. 2021, 47, 4129–4155. [Google Scholar] [CrossRef]
- Ahn, E.-Y.; Jin, H.; Park, Y. Assessing the Antioxidant, Cytotoxic, Apoptotic and Wound Healing Properties of Silver Nanoparticles Green-Synthesized by Plant Extracts. Mater. Sci. Eng. C 2019, 101, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.F.; Santos, A.M.; Oliveira, A.M.S.; Nascimento Junior, J.A.C.; Frank, L.A.; Santana Souza, M.T.D.; Camargo, E.A.; Serafini, M.R. Pharmaceuticals Agents for Preventing NSAID-Induced Gastric Ulcers: A Patent Review. Expert Rev. Clin. Pharmacol. 2021, 14, 677–686. [Google Scholar] [CrossRef]
- Boby, N.; Abbas, M.A.; Lee, E.-B.; Im, Z.-E.; Hsu, W.H.; Park, S.-C. Protective Effect of Pyrus Ussuriensis Maxim. Extract against Ethanol-Induced Gastritis in Rats. Antioxidants 2021, 10, 439. [Google Scholar] [CrossRef]
- Shareef, S.H.; Al-Medhtiy, M.H.; Ibrahim, I.A.A.; Alzahrani, A.R.; Jabbar, A.A.; Galali, Y.; Agha, N.F.S.; Aziz, P.Y.; Thabit, M.A.; Agha, D.N.F. Gastroprophylactic Effects of P-Cymene in Ethanol-Induced Gastric Ulcer in Rats. Processes 2022, 10, 1314. [Google Scholar] [CrossRef]
- Cerqua, I.; Musella, S.; Peltner, L.K.; D’Avino, D.; Di Sarno, V.; Granato, E.; Vestuto, V.; Di Matteo, R.; Pace, S.; Ciaglia, T. Discovery and Optimization of Indoline-Based Compounds as Dual 5-LOX/Seh Inhibitors: In Vitro and in Vivo Anti-Inflammatory Characterization. J. Med. Chem. 2022, 65, 14456–14480. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-S.; Lin, S.-C.; Chu, C.-H.; Chang, Y.-K.; Zhang, X.; Lin, C.-C.; Tung, Y.-T. The Gastroprotective Effect of Naringenin against Ethanol-Induced Gastric Ulcers in Mice through Inhibiting Oxidative and Inflammatory Responses. Int. J. Mol. Sci. 2021, 22, 11985. [Google Scholar] [CrossRef]








| Con | Ag@Py-ST-NO2@CNT | Ag@Py-ST-Br@CNT | |
|---|---|---|---|
| TC (g/L) | 0.96 ± 0.09 | 1.01 ± 0.13 | 0.98 ± 0.08 |
| LDL-C (g/L) | 0.46 ± 0.02 | 0.51 ± 0.08 | 0.48 ± 0.11 |
| GOT | 31 ± 3.4 | 34 ± 2.5 | 33 ± 1.9 |
| GPT | 15 ± 1.7 | 18 ± 1.3 | 17 ± 2.1 |
| Glycemia (g/L) | 0.97 ± 0.13 | 0.99 ± 0.35 | 0.96 ± 0.51 |
| Creat (mg/L) | 11.3 ± 1.1 | 12.9 ± 1.8 | 12.1 ± 1.3 |
| Urea (g/L) | 0.75 ± 0.17 | 0.81 ± 0.1 | 0.79 ± 0.17 |
| Sample Code Microorganisms | Py-ST-X | Ag@Py-ST-X | Ag@Py-ST-X@CNT | Ketoconazole | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X=Cl | X=NO2 | X=Br | X=Cl | X=NO2 | X=Br | X=Cl | X=NO2 | X=Br | ||
| A. fumigatus (RCMB 002008) | 15 | NA | NA | NA | NA | NA | NA | NA | 22 | 17 |
| C. albicans RCMB 005003 | 11 | NA | 12 | 12 | NA | NA | 16 | NA | 18 | 20 |
| Microorganisms | Py-ST-X | Ag@Py-ST-X | Ag@Py-ST-X@CNT | Gentamycin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X=Cl | X=NO2 | X=Br | X=Cl | X=NO2 | X=Br | X=Cl | X=NO2 | X=Br | ||
| S. aureus (ATCC 25923) | 11 | 9 | 9 | 12 | NA | NA | 16 | NA | 15 | 24 |
| E. coli ATCC 25922 | NA | NA | NA | 12 | 9 | NA | 17 | NA | 18 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sofany, W.I.; Azzam, E.M.S.; Latif, S.; Hamden, K. Spirothiazolidine-Derivative on Silver Nanoparticles and Carbon Nanotubes: Evaluation of Antibacterial, Anti-Fungal, Anti-Inflammatory, Antioxidant and Gastroprotective Activities. Pharmaceutics 2024, 16, 901. https://doi.org/10.3390/pharmaceutics16070901
El-Sofany WI, Azzam EMS, Latif S, Hamden K. Spirothiazolidine-Derivative on Silver Nanoparticles and Carbon Nanotubes: Evaluation of Antibacterial, Anti-Fungal, Anti-Inflammatory, Antioxidant and Gastroprotective Activities. Pharmaceutics. 2024; 16(7):901. https://doi.org/10.3390/pharmaceutics16070901
Chicago/Turabian StyleEl-Sofany, Walaa I., Eid. M. S. Azzam, Salman Latif, and Khaled Hamden. 2024. "Spirothiazolidine-Derivative on Silver Nanoparticles and Carbon Nanotubes: Evaluation of Antibacterial, Anti-Fungal, Anti-Inflammatory, Antioxidant and Gastroprotective Activities" Pharmaceutics 16, no. 7: 901. https://doi.org/10.3390/pharmaceutics16070901







