Harnessing Potential of ω-3 Polyunsaturated Fatty Acid with Nanotechnology for Enhanced Breast Cancer Therapy: A Comprehensive Investigation into ALA-Based Liposomal PTX Delivery
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Reagent
2.2. Pre-Formulation Study
2.3. Liposome Preparation and Optimization
2.3.1. Design of Experiment
2.3.2. Preparation of Liposomes
2.4. Characterization of Liposomes
2.4.1. PS, PDI, and Zeta Potential
2.4.2. Compatibility Studies Using FTIR
2.4.3. Entrapment Efficiency
2.4.4. In-Vitro Drug Release
2.4.5. Scanning Electron Microscopy
2.4.6. Transmission Electron Microscopy
2.4.7. Accelerated Stability Study
2.5. In-Vitro Evaluation
2.5.1. Cell Culture
2.5.2. Cytotoxicity Assay
2.5.3. Cellular Uptake by Confocal Microscopy
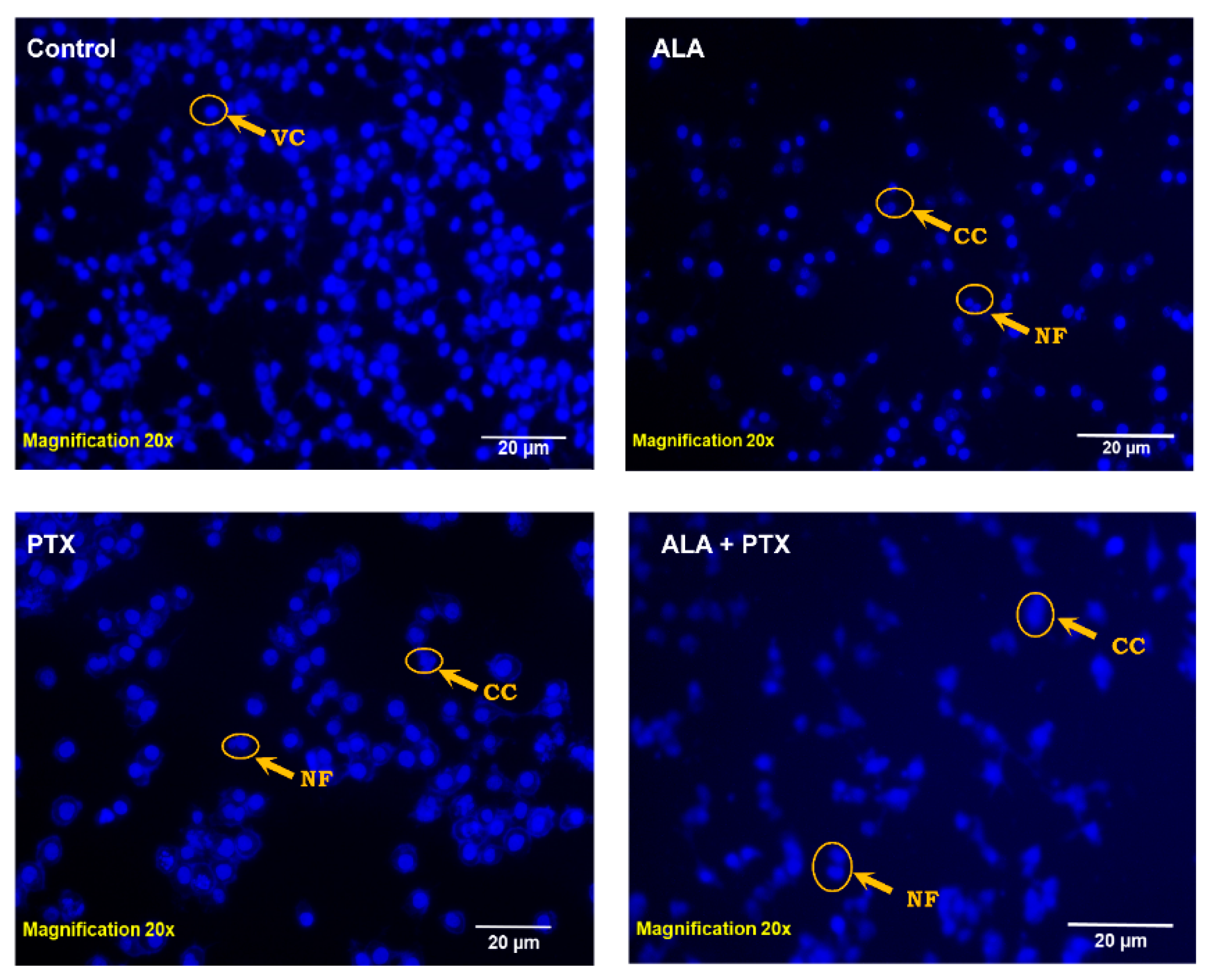
2.5.4. DAPI Staining
2.5.5. AO/EtBr Staining
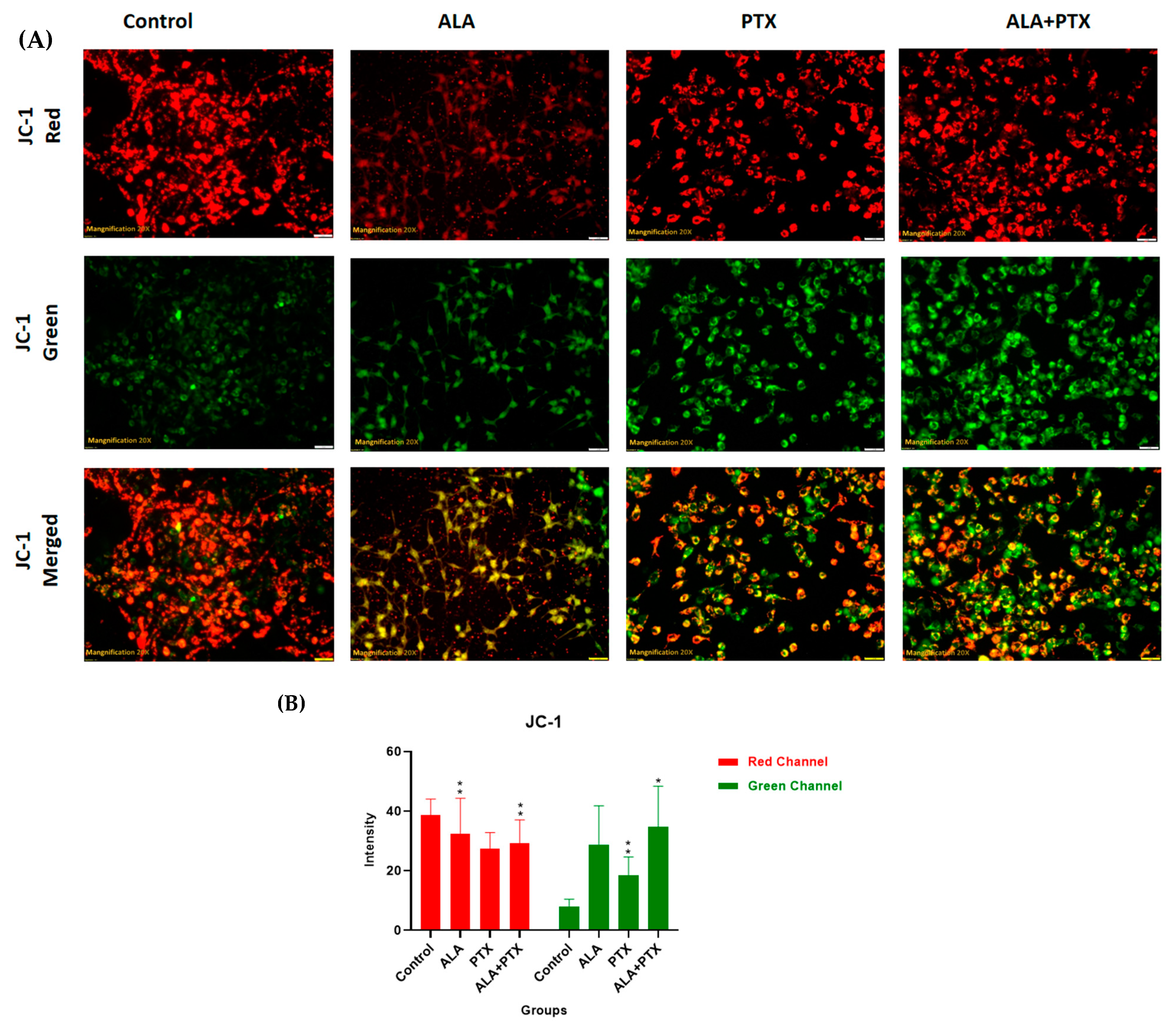
2.5.6. JC-1 Staining
2.5.7. Statistical Analysis
3. Results
3.1. Pre-Formulation Study and DoE
3.2. In- Vitro Characterization
3.2.1. Particle Size and PDI, Zeta Potential, and Entrapment Efficiency
3.2.2. FT-IR
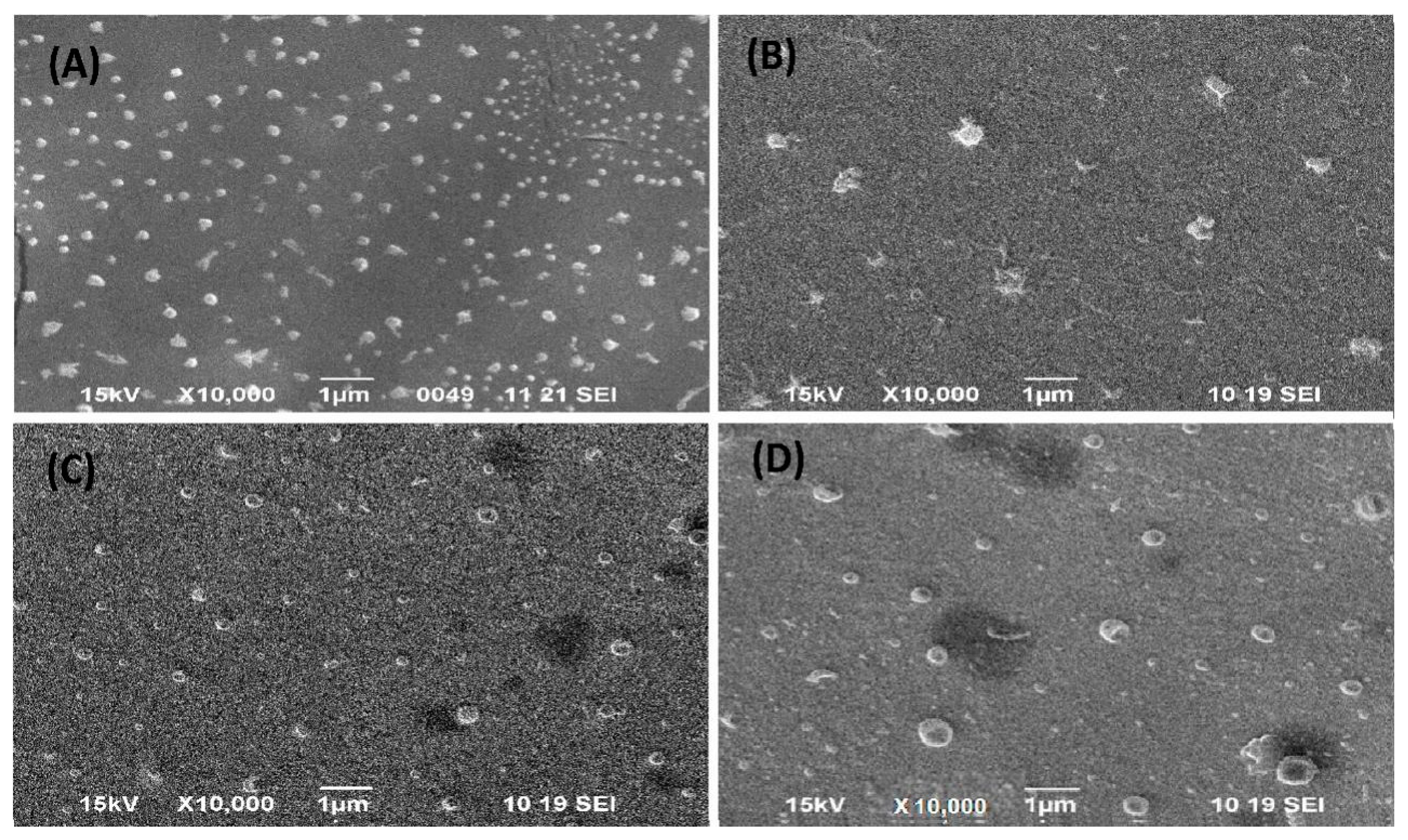
3.2.3. Surface Morphology
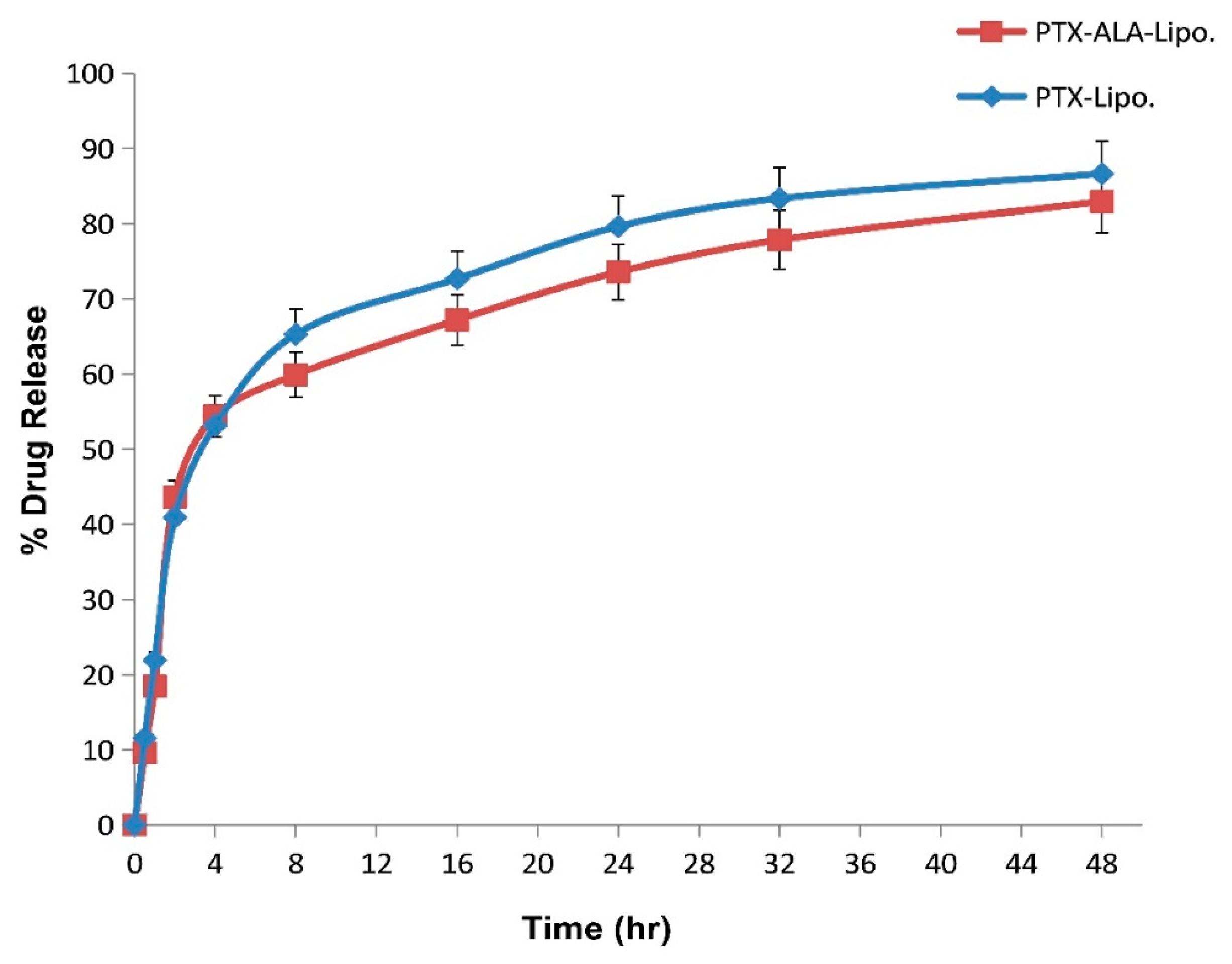
3.2.4. In Vitro Drug Release
3.2.5. Stability Study
3.3. In Vitro Cell Culture Study
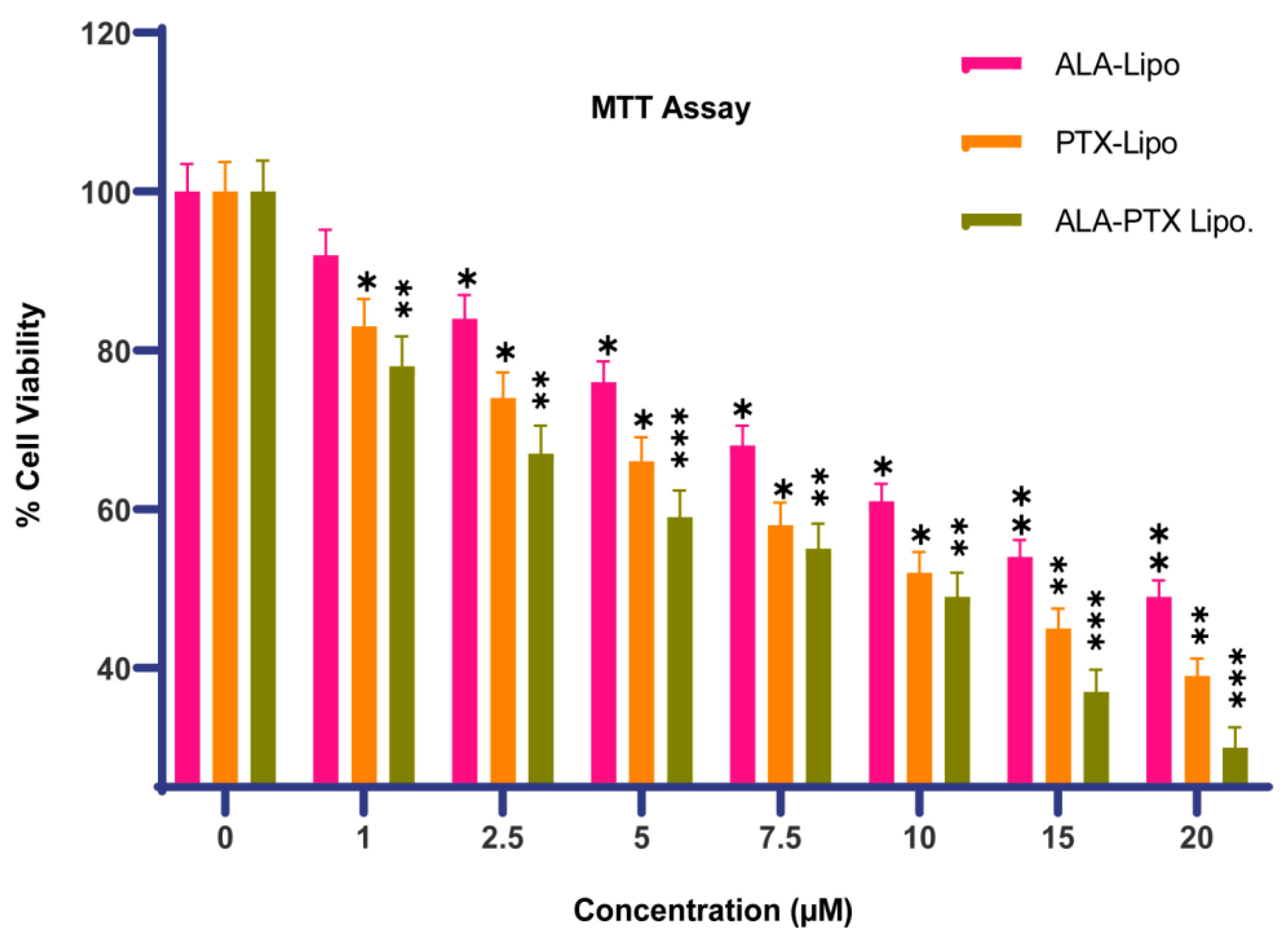
3.3.1. Cytotoxicity Assay
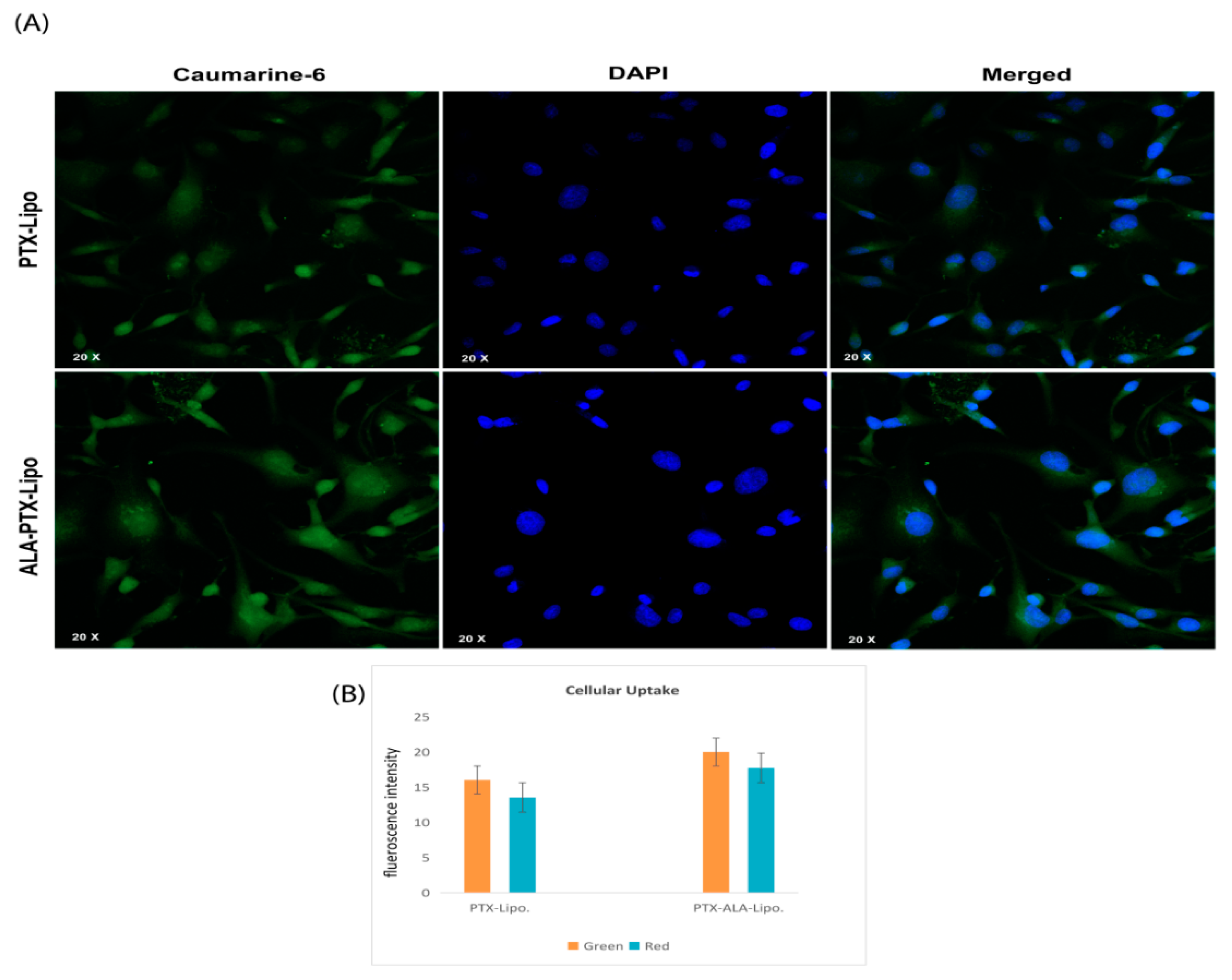
3.3.2. Cellular Uptake
3.3.3. DAPI Staining
3.3.4. AO/EtBr Staining
3.3.5. JC-1 Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavez, J.D.; Keller, A.; Zhou, B.; Tian, R.; Bruce, J.E. Cellular interactome dynamics during paclitaxel treatment. Cell Rep. 2019, 29, 2371–2383.E5. [Google Scholar] [PubMed]
- Iacoviello, L.; Bonaccio, M.; de Gaetano, G.; Donati, M.B. Epidemiology of breast cancer, a paradigm of the “common soil” hypothesis. Semin. Cancer Biol. 2021, 72, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Jafari, S.M. Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydr. Polym. 2021, 272, 118464. [Google Scholar] [CrossRef] [PubMed]
- Mansara, P.P.; Deshpande, R.A.; Vaidya, M.M.; Kaul-Ghanekar, R. Differential ratios of omega fatty acids (AA/EPA+ DHA) modulate growth, lipid peroxidation and expression of tumor regulatory MARBPs in breast cancer cell lines MCF7 and MDA-MB-231. PLoS ONE 2015, 10, e0136542. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Ten Hagen, T.L. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.A.; Bhonde, R.R. Omega-3 polyunsaturated fatty acids promote angiogenesis in placenta derived mesenchymal stromal cells. Pharmacol. Res. 2018, 132, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. “Cell membrane theory of senescence” and the role of bioactive lipids in aging, and aging associated diseases and their therapeutic implications. Biomolecules 2021, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Garg, A.; Aggarwal, D. Paclitaxel and its formulations. Int. J. Pharm. 2002, 235, 179–192. [Google Scholar] [CrossRef]
- Yadav, R.K.; Singh, M.; Roy, S.; Ansari, M.N.; Saeedan, A.S.; Kaithwas, G. Modulation of oxidative stress response by flaxseed oil: Role of lipid peroxidation and underlying mechanisms. Prostaglandins Other Lipid Mediat. 2018, 135, 21–26. [Google Scholar] [CrossRef]
- Singh, M.; Kaithwas, G. Alpha-Linolenic Acid Mediated Stabilization of Hif-1-Alpha and Downregulation Fasn to Inhibit Palmitic Acid Synthesis and Activation of Mitochondrial Apoptosis for Mammary Gland Chemoprevention. J. Cancer Res. Ther. 2017, 13, S150. [Google Scholar]
- Weissman, S.A.; Anderson, N.G. Design of experiments (DoE) and process optimization. A review of recent publications. Org. Process Res. Dev. 2015, 19, 1605–1633. [Google Scholar] [CrossRef]
- Kushwah, V.; Jain, D.K.; Agrawal, A.K.; Jain, S. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Colloids Surf. B Biointerfaces 2018, 172, 213–223. [Google Scholar] [CrossRef]
- Jurić Simčić, A.; Erak, I.; Cetina Čižmek, B.; Hafner, A.; Filipović-Grčić, J. Selection of Excipients for the Preparation of Vancomycin-Loaded Poly (D, L-lactide-co-glycolide) Microparticles with Extended Release by Emulsion Spray Drying. Pharmaceutics 2023, 15, 2438. [Google Scholar] [CrossRef]
- Ling, Y.; Huang, Y. Preparation and release efficiency of poly (lactic-co-glycolic) acid nanoparticles for drug loaded paclitaxel. In Proceedings of the 7th Asian-Pacific Conference on Medical and Biological Engineering: APCMBE 2008, Beijing, China, 22–25 April 2008; pp. 514–517. [Google Scholar]
- Liu, Y.; Liu, D.; Zhu, L.; Gan, Q.; Le, X. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Res. Int. 2015, 74, 97–105. [Google Scholar] [CrossRef]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surf. B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef]
- Li, R.; Deng, L.; Cai, Z.; Zhang, S.; Wang, K.; Li, L.; Ding, S.; Zhou, C. Liposomes coated with thiolated chitosan as drug carriers of curcumin. Mater. Sci. Eng. C 2017, 80, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Gupta, A.; Singh, P.; Gupta, C.; Sadhu, V.; Reddy, K.R. Synthesis and characterization of celecoxib loaded PEGylated liposome nanoparticles for biomedical applications. Nano-Struct. Nano-Objects 2019, 18, 100288. [Google Scholar] [CrossRef]
- Perrie, Y.; Mohammed, A.U.; Vangala, A.; McNeil, S.E. Environmental scanning electron microscopy offers real-time morphological analysis of liposomes and niosomes. J. Liposome Res. 2007, 17, 27–37. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Zhang, H.; Yang, Y.; Xie, X.-Y.; Yang, Y.-F.; Li, Z.; Li, Y.; Gong, W.; Yu, F.-L.; Yang, Z.; et al. Preparation, characterization, and efficacy of thermosensitive liposomes containing paclitaxel. Drug Deliv. 2016, 23, 1222–1231. [Google Scholar] [CrossRef]
- Bhalerao, S.; Raje Harshal, A. Preparation, optimization, characterization, and stability studies of salicylic acid liposomes. Drug Dev. Ind. Pharm. 2003, 29, 451–467. [Google Scholar] [CrossRef]
- Arunasree, K.M. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Sharma, S.; Khan, I.; Gothwal, A.; Sharma, A.K.; Singh, Y.; Chourasia, M.K.; Kumar, V. Enhanced apoptotic and anticancer potential of paclitaxel loaded biodegradable nanoparticles based on chitosan. Int. J. Biol. Macromol. 2017, 98, 810–819. [Google Scholar] [CrossRef]
- de Castro, C.E.; Ribeiro, C.A.; Alavarse, A.C.; Albuquerque, L.J.; da Silva, M.C.; Jäger, E.; Surman, F.; Schmidt, V.; Giacomelli, C.; Giacomelli, F.C. Nanoparticle–cell interactions: Surface chemistry effects on the cellular uptake of biocompatible block copolymer assemblies. Langmuir 2018, 34, 2180–2188. [Google Scholar] [CrossRef]
- Suganya, K.S.U.; Govindaraju, K.; Prabhu, D.; Arulvasu, C.; Karthick, V.; Changmai, N.J.A.S.S. Anti-proliferative effect of biogenic gold nanoparticles against breast cancer cell lines (MDA-MB-231 & MCF-7). Appl. Surf. Sci. 2016, 371, 415–424. [Google Scholar]
- Rastogi, S.; Ansari, M.N.; Saeedan, A.S.; Singh, S.K.; Mukerjee, A.; Kaithwas, G. Novel furan chalcone modulates PHD-2 induction to impart antineoplastic effect in mammary gland carcinoma. J. Biochem. Mol. Toxicol. 2024, 38, e23679. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Singh, M.; Rawat, A.; Kumar, D.; Kaithwas, G. Mitochondrial apoptosis and curtailment of hypoxia-inducible factor-1α/fatty acid synthase: A dual edge perspective of gamma linolenic acid in ER+ mammary gland cancer. Cell Biochem. Funct. 2020, 38, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Decuzzi, P.; Cristofanilli, M.; Sakamoto, J.H.; Tasciotti, E.; Robertson, F.M.; Ferrari, M. Nanotechnology for breast cancer therapy. Biomed. Microdevices 2009, 11, 49–63. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-lnduced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Ten Tije, A.J.; Verweij, J.; Loos, W.J.; Sparreboom, A. Pharmacological effects of formulation vehicles: Implications for cancer chemotherapy. Clin. Pharmacokinet. 2003, 42, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Sparreboom, A.; Van Zuylen, L.; Brouwer, E.; Loos, W.J.; De Bruijn, P.; Gelderblom, H.; Pillay, M.; Nooter, K.; Stoter, G.; Verweij, J. Cremophor EL-mediated alteration of paclitaxel distribution in human blood: Clinical pharmacokinetic implications. Cancer Res. 1999, 59, 1454–1457. [Google Scholar] [PubMed]
- Xu, M.-Q.; Hao, Y.-L.; Wang, J.-R.; Li, Z.-Y.; Li, H.; Feng, Z.-H.; Wang, H.; Wang, J.-W.; Zhang, X. Antitumor activity of α-linolenic acid-paclitaxel conjugate nanoparticles: In vitro and in vivo. Int. J. Nanomed. 2021, 16, 7269–7281. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Xing, J.Z.; Huang, M.; Zhang, X.; Chen, J. Nanoformulation of paclitaxel to enhance cancer therapy. J. Biomater. Appl. 2013, 28, 298–307. [Google Scholar] [CrossRef]



| Formulation | Particle Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) |
|---|---|---|---|---|
| Placebo | 133.43 ± 3.95 | 0.39 ± 0.02 | −15.00 ± 5.76 | - |
| PTX-lipo | 162.80 ± 9.20 | 0.41 ± 0.05 | −22.40 ± 4.80 | 84.70 ± 0.05 |
| ALA-lipo | 154.67 ± 8.70 | 0.32 ± 0.02 | −22.00 ± 7.80 | - |
| ALA-PTX-lipo | 206.30 ± 9.96 | 0.35 ± 0.04 | −20.80 ± 4.34 | 80.58 ± 0.07 |
| Liposome | Initial | After 12 Weeks | ||||
|---|---|---|---|---|---|---|
| PS (nm) | ZP | 8 °C | 37 °C | |||
| PS (nm) | ZP (mV) | PS (nm) | ZP (mV) | |||
| ALA-Lipo | 154.7 ± 8.7 | −22.0 ± 4.8 | 200.6 ± 6.3 | −20.4 ± 5.3 | 187.2 ± 5.8 | −16.8 ± 3.7 |
| PTX- Lipo | 162.5 ± 9.2 | −22.4 ± 7.8 | 215.6 ± 16.7 | −18.9 ± 3.7 | 227.8 ± 3.2 | −21.2 ± 7.5 |
| ALA-PTA-Lipo | 206.0 ± 9.9 | −20.8 ± 4.3 | 288.4 ± 15.2 | −16.2 ± 1.9 | 317.6 ± 4.0 | −19.2 ± 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Kumar, A.; Kumar, D.; Yadav, S.; Shrivastava, N.K.; Singh, J.; Sonkar, A.B.; Verma, P.; Arya, D.K.; Kaithwas, G.; et al. Harnessing Potential of ω-3 Polyunsaturated Fatty Acid with Nanotechnology for Enhanced Breast Cancer Therapy: A Comprehensive Investigation into ALA-Based Liposomal PTX Delivery. Pharmaceutics 2024, 16, 913. https://doi.org/10.3390/pharmaceutics16070913
Kumar R, Kumar A, Kumar D, Yadav S, Shrivastava NK, Singh J, Sonkar AB, Verma P, Arya DK, Kaithwas G, et al. Harnessing Potential of ω-3 Polyunsaturated Fatty Acid with Nanotechnology for Enhanced Breast Cancer Therapy: A Comprehensive Investigation into ALA-Based Liposomal PTX Delivery. Pharmaceutics. 2024; 16(7):913. https://doi.org/10.3390/pharmaceutics16070913
Chicago/Turabian StyleKumar, Rohit, Anurag Kumar, Dharmendra Kumar, Sneha Yadav, Neeraj Kumar Shrivastava, Jyoti Singh, Archana Bharti Sonkar, Pratibha Verma, Dilip Kumar Arya, Gaurav Kaithwas, and et al. 2024. "Harnessing Potential of ω-3 Polyunsaturated Fatty Acid with Nanotechnology for Enhanced Breast Cancer Therapy: A Comprehensive Investigation into ALA-Based Liposomal PTX Delivery" Pharmaceutics 16, no. 7: 913. https://doi.org/10.3390/pharmaceutics16070913





