Analysis of Survival Modification by Furosemide Use in a Cohort of Hospitalized COVID-19 Patients with Severe or Critical Disease in Mexico: Due to Its Chemical Structure, Furosemide Is More than Just a Diuretic
Abstract
1. Introduction
2. Materials and Methods
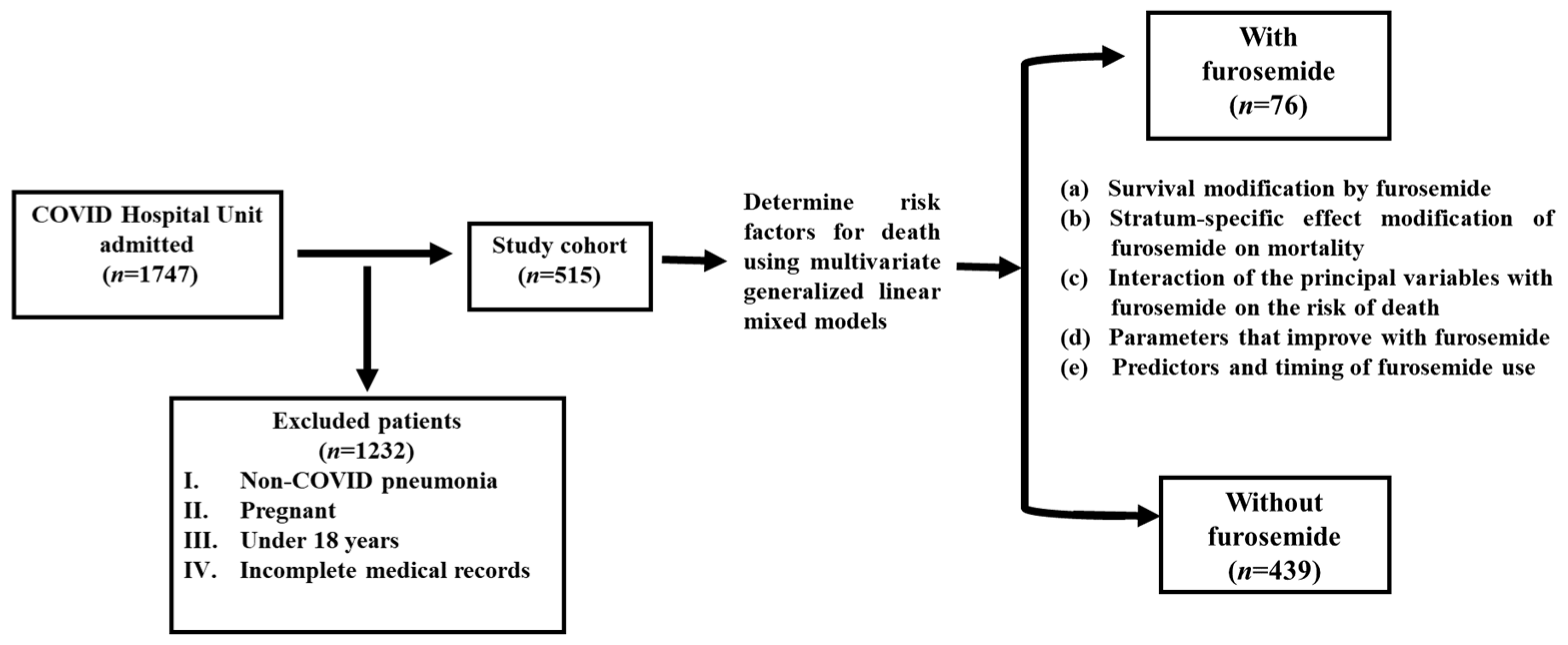
2.1. Study Subjects
2.2. Measures and Follow-Up
2.3. Sample Size
2.4. Statistical Analysis
2.5. Structure–Activity Analysis
3. Results
3.1. Patient Characteristics and Generalities
3.2. Our Approach to Analyzing the Stratum-Specific Effect Modification of Furosemide on Mortality
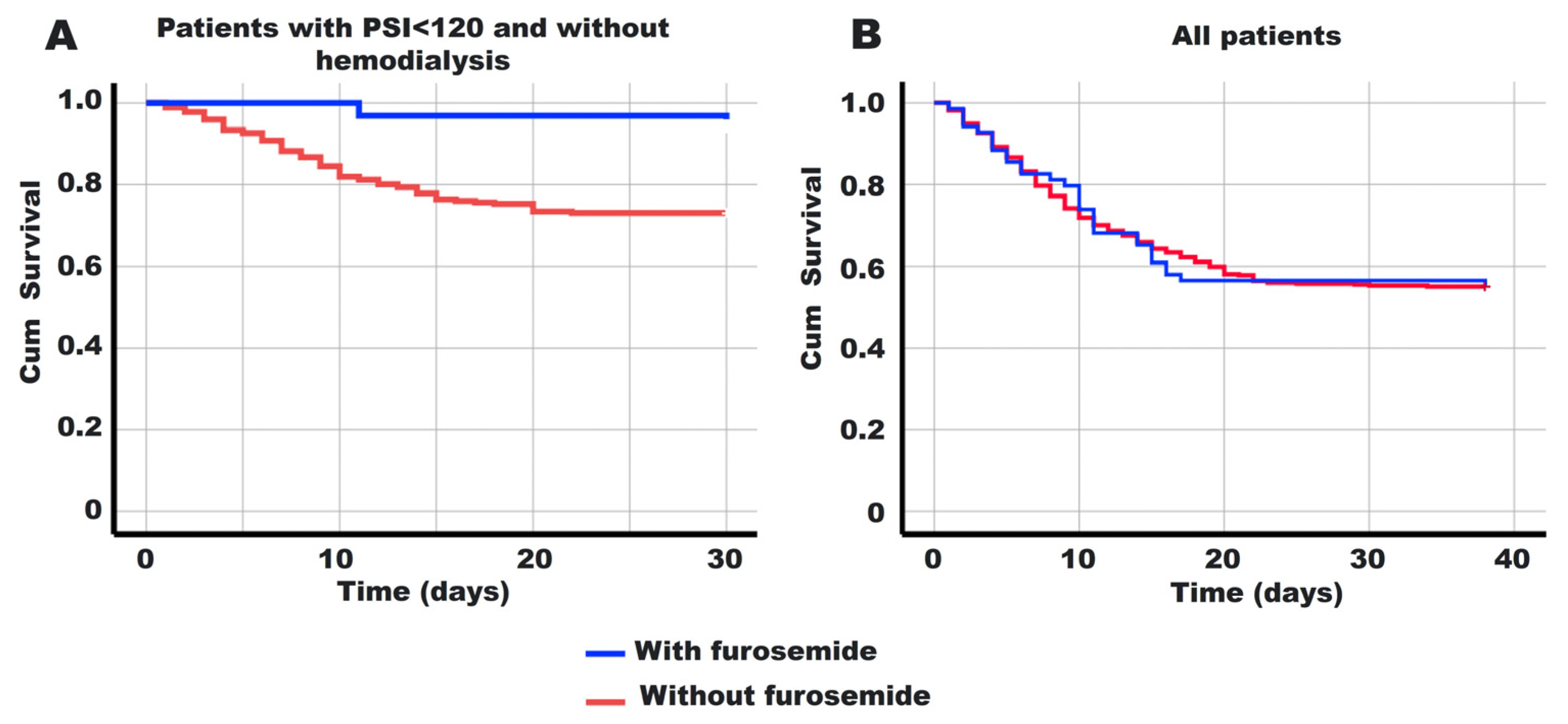
3.3. Analysis of Survival Modification by Furosemide
3.4. Oximetry, One of the Parameters That Improves with Furosemide
3.5. Predictors and Timing of Furosemide Use
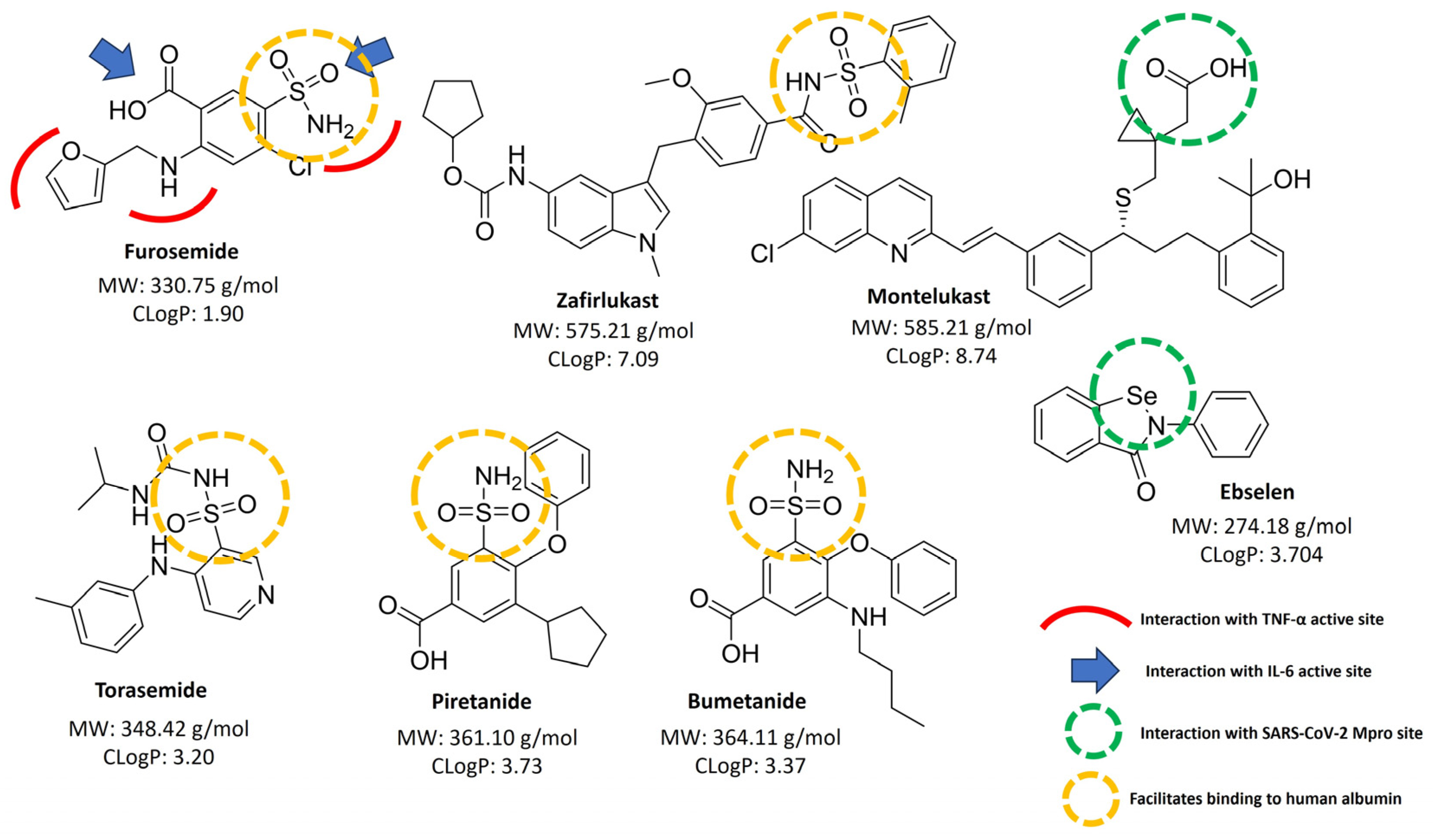
3.6. Structure Relationship Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- World Health Organization (WHO). World Health Organization 2023 Data. Who.Int, WHO Coronavirus (COVID-19) Dashboard > about [Dashboard]. Available online: https://data.who.int/dashboards/covid19/about (accessed on 28 April 2024).
- CONACYT (Consejo Nacional de Ciencia y Tecnología); CentroGeo (Centro de Investigación en Ciencias de Información Geoespacial); GeoInt (Laboratorio Nacional de GeoInteligencia). DataLab COVID-19 Tablero México. Available online: https://datos.covid-19.conacyt.mx/ (accessed on 19 March 2023).
- Niknam, Z.; Jafari, A.; Golchin, A.; Danesh Pouya, F.; Nemati, M.; Rezaei-Tavirani, M.; Rasmi, Y. Potential Therapeutic Options for COVID-19: An Update on Current Evidence. Eur. J. Med. Res. 2022, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.A.; Thakur, N.; Celedón, J.C. Lessons Learned from Health Disparities in Coronavirus Disease-2019 in the United States. Clin. Chest Med. 2023, 44, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, A.; Villar, L.; Wang, Z.; Doyle, L.M.; Meek, A.; Reed, M.; Barden, C.; Weaver, D.F. Is Inhaled Furosemide a Potential Therapeutic for COVID-19? Am. J. Med. Sci. 2020, 360, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Brierley, S.; Gandhi, M.J.; Cohen, M.A.; Moschella, P.C.; Declan, A.B.L. Repurposing Therapeutics for Potential Treatment of SARS-CoV-2: A Review. Viruses 2020, 12, 705. [Google Scholar] [CrossRef] [PubMed]
- Subsecretaría de Salud (SSA); Subsecretaría de Integración y Desarrollo del Sector Salud. Propuesta de Medicamentos Para El Tratamiento de COVID-19; Secretaría de Salud: Ciudad de México, Mexico, 2020.
- Kil, J.; Lobarinas, E.; Spankovich, C.; Griffiths, S.K.; Antonelli, P.J.; Lynch, E.D.; Le Prell, C.G. Safety and Efficacy of Ebselen for the Prevention of Noise-Induced Hearing Loss: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet 2017, 390, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Haritha, C.V.; Sharun, K.; Jose, B. Ebselen, a New Candidate Therapeutic against SARS-CoV-2. Int. J. Surg. 2020, 84, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Cokljat, M.; Cruz, C.V.; Carrara, V.I.; Puttaraksa, K.; Capriglioni, C.; Insaurralde, S.M.; Rousseau-Portalis, M.; Roldan, A.; Watson, J.A.; Tarning, J.; et al. Comparison of WHO versus National COVID-19 Therapeutic Guidelines across the World: Not Exactly a Perfect Match. BMJ Glob. Health 2024, 9, e014188. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.M.; Patel, R.; Siddiqui, A.H. Furosemide; StatPearls Publishing: Petersburg, FL, USA, 2023. [Google Scholar]
- Wang, Z.; Wang, Y.; Vilekar, P.; Yang, S.-P.; Gupta, M.; Oh, M.I.; Meek, A.; Doyle, L.; Villar, L.; Brennecke, A.; et al. Small Molecule Therapeutics for COVID-19: Repurposing of Inhaled Furosemide. PeerJ 2020, 8, e9533. [Google Scholar] [CrossRef]
- Yuengsrigul, A.; Chin, T.W.; Nussbaum, E. Immunosuppressive and Cytotoxic Effects of Furosemide on Human Peripheral Blood Mononuclear Cells. Ann. Allergy Asthma Immunol. 1999, 83, 559–566. [Google Scholar] [CrossRef]
- Prandota, J. Furosemide: Progress in Understanding Its Diuretic, Anti-Inflammatory, and Bronchodilating Mechanism of Action, and Use in the Treatment of Respiratory Tract Diseases. Am. J. Ther. 2002, 9, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Sidhwani, S.K.; Mirza, T.; Khatoon, A.; Shaikh, F.; Khan, R.; Shaikh, O.A.; Nashwan, A.J. Inflammatory Markers and COVID-19 Disease Progression. J. Infect. Public Health 2023, 16, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Guragai, N.; Vasudev, R.; Hosein, K.; Habib, H.; Patel, B.; Kaur, P.; Patel, B.; Santana, M.; Elkattawy, S.; Noori, M.A.M.; et al. Does Baseline Diuretics Use Affect Prognosis in Patients With COVID-19? Cureus 2021, 13, e15573. [Google Scholar] [CrossRef] [PubMed]
- Pengo, M.; Stefanini, G.; Pivato, C.; Soranna, D.; Zambra, G.; Zambon, A.; Torlasco, C.; Bilo, G.; Condorelli, G.; Parati, G. In-hospital diuretic use is associated with worse outcome in patients with COVID-19. J. Hypertens. 2021, 39, e38. [Google Scholar] [CrossRef]
- Oliveira, F.M.S.; Caetano, M.M.M.; de Godoy, A.R.V.; de Oliveira, L.L.; de Melo Mambrini, J.V.; Rezende, M.S.; Fantini, M.P.R.; De Oliveira Mendes, T.A.; Medeiros, N.I.; Guimarães, H.C.; et al. Retrospective Cohort Study to Evaluate the Continuous Use of Anticholesterolemics and Diuretics in Patients with COVID-19. Front. Med. 2024, 10, 1252556. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, V.; Zakynthinos, G.E.; Mantzarlis, K.; Makris, D. Increased Mortality among Hypertensive COVID-19 Patients: Pay a Closer Look on Diuretics in Mechanically Ventilated Patients. Heart Lung 2020, 49, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Li, C.; Xu, L.; Che, L.; Wang, Y.; Yang, C.; Zhang, N.; Liu, Z.; Zhao, L.; Zhou, B.; et al. Hospitalized Patients Received Furosemide Undergoing Acute Kidney Injury: The Risk and Prediction Tool. Eur. J. Med. Res. 2023, 28, 312. [Google Scholar] [CrossRef] [PubMed]
- Gobierno de México Guía Clínica Para El Tratamiento de La COVID-19 En México; Consenso Interinstitucional: Ciudad de México, Mexico, 2021.
- Miranda-Novales, M.G.; Villasís-Keever, M.Á. El Protocolo de Investigación VIII. La Ética de La Investigación En Seres Humanos. Rev. Alerg. Mex. 2019, 66, 115–122. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Varol, Y.; Hakoglu, B.; Kadri Cirak, A.; Polat, G.; Komurcuoglu, B.; Akkol, B.; Atasoy, C.; Bayramic, E.; Balci, G.; Ataman, S.; et al. The Impact of Charlson Comorbidity Index on Mortality from SARS-CoV-2 Virus Infection and A Novel COVID-19 Mortality Index: CoLACD. Int. J. Clin. Pract. 2021, 75, e13858. [Google Scholar] [CrossRef]
- National Center for Health Statistics National Center for Health Statistics (NCHS) C for DC and P (CDC): Glossary. Available online: https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm (accessed on 5 February 2024).
- Mendoza-Hernandez, M.A.; Guzman-Esquivel, J.; Ramos-Rojas, M.A.; Santillan-Luna, V.V.; Sanchez-Ramirez, C.A.; Hernandez-Fuentes, G.A.; Diaz-Martinez, J.; Melnikov, V.; Rojas-Larios, F.; Martinez-Fierro, M.L.; et al. Differences in the Evolution of Clinical, Biochemical, and Hematological Indicators in Hospitalized Patients with COVID-19 According to Their Vaccination Scheme: A Cohort Study in One of the World’s Highest Hospital Mortality Populations. Vaccines 2024, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lu, J.; Liu, D.; He, Y. Characteristics and Risk Factors of Secondary Bacterial Infections in COVID-19 Patients. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e156. [Google Scholar] [CrossRef] [PubMed]
- Muscedere, J.; Maslove, D.M.; Barden, C.J.; Weaver, D.F.; Boyd, J.G.; Sibley, S.; Boyd, T.; Rewa, O.; Albert, M.; Roussos, M.; et al. Nebulized Furosemide for Pulmonary Inflammation in Intubated Patients With COVID-19: A Phase 2 Randomized Controlled Double-Blind Study. Crit. Care Explor. 2024, 6, e1045. [Google Scholar] [CrossRef] [PubMed]
- Jaffa, M.A.; Gebregziabher, M.; Luttrell, D.K.; Luttrell, L.M.; Jaffa, A.A. Multivariate Generalized Linear Mixed Models with Random Intercepts to Analyze Cardiovascular Risk Markers in Type-1 Diabetic Patients. J. Appl. Stat. 2016, 43, 1447–1464. [Google Scholar] [CrossRef]
- Loza, A.; Wong-Chew, R.M.; Jiménez-Corona, M.-E.; Zárate, S.; López, S.; Ciria, R.; Palomares, D.; García-López, R.; Iša, P.; Taboada, B.; et al. Two-Year Follow-up of the COVID-19 Pandemic in Mexico. Front. Public Health 2023, 10, 1050673. [Google Scholar] [CrossRef]
- Naimi, A.I.; Whitcomb, B.W. Estimating Risk Ratios and Risk Differences Using Regression. Am. J. Epidemiol. 2020, 189, 508–510. [Google Scholar] [CrossRef]
- ClinCalc.com » Statistics » Post-Hoc Power Calculator Post-Hoc Power Calculator. Evaluate Statistical Power of an Existing Study. Available online: https://clincalc.com/stats/Power.aspx (accessed on 28 April 2023).
- Levine, M.; Ensom, M.H. Post Hoc Power Analysis: An Idea Whose Time Has Passed? Pharmacotherapy 2001, 21, 405–409. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics/Bernard Rosner, 7th ed.; Brooks/Cole, Cengage Learning: Boston, MA, USA, 2011; Volume 1. [Google Scholar]
- Durdagi, S.; Avsar, T.; Orhan, M.D.; Serhatli, M.; Balcioglu, B.K.; Ozturk, H.U.; Kayabolen, A.; Cetin, Y.; Aydinlik, S.; Bagci-Onder, T.; et al. The Neutralization Effect of Montelukast on SARS-CoV-2 Is Shown by Multiscale in Silico Simulations and Combined in Vitro Studies. Mol. Ther. 2022, 30, 963–974. [Google Scholar] [CrossRef]
- Lazniewski, M.; Dermawan, D.; Hidayat, S.; Muchtaridi, M.; Dawson, W.K.; Plewczynski, D. Drug Repurposing for Identification of Potential Spike Inhibitors for SARS-CoV-2 Using Molecular Docking and Molecular Dynamics Simulations. Methods 2022, 203, 498–510. [Google Scholar] [CrossRef]
- ChemDraw 3D. PerkinElmer Informatics PerkinElmer Informatics; Version 20.0; PerkinElmer Inc.: Shelton, CT, USA, 2020. [Google Scholar]
- VanderWeele, T.J. On the Distinction Between Interaction and Effect Modification. Epidemiology 2009, 20, 863–871. [Google Scholar] [CrossRef]
- Hart, J.E.; Puett, R.C.; Rexrode, K.M.; Albert, C.M.; Laden, F. Effect Modification of Long-Term Air Pollution Exposures and the Risk of Incident Cardiovascular Disease in US Women. J. Am. Heart Assoc. 2015, 4, e002301. [Google Scholar] [CrossRef] [PubMed]
- Arce, C.M.; Rhee, J.J.; Cheung, K.L.; Hedlin, H.; Kapphahn, K.; Franceschini, N.; Kalil, R.S.; Martin, L.W.; Qi, L.; Shara, N.M.; et al. Kidney Function and Cardiovascular Events in Postmenopausal Women: The Impact of Race and Ethnicity in the Women’s Health Initiative. Am. J. Kidney Dis. 2016, 67, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-Y.; Liu, J.C.; Bell, M.L. Temperature-Related Mortality: A Systematic Review and Investigation of Effect Modifiers. Environ. Res. Lett. 2019, 14, 073004. [Google Scholar] [CrossRef]
- Lunt, M.; Solomon, D.; Rothman, K.; Glynn, R.; Hyrich, K.; Symmons, D.P.M.; Sturmer, T. Different Methods of Balancing Covariates Leading to Different Effect Estimates in the Presence of Effect Modification. Am. J. Epidemiol. 2009, 169, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.E.; Pearce, N.; Cummins, S. Neighborhood Environment and Socioeconomic Inequalities in Cancer Admissions: A Prospective Study Using UK Biobank and Linked Hospital Records. Cancer Causes Control 2022, 33, 1431–1444. [Google Scholar] [CrossRef]
- Groenwold, R.H.; Rovers, M.M.; Lubsen, J.; van der Heijden, G.J. Subgroup Effects despite Homogeneous Heterogeneity Test Results. BMC Med. Res. Methodol. 2010, 10, 43. [Google Scholar] [CrossRef]
- Carter, A.R.; Harrison, S.; Gill, D.; Davey Smith, G.; Taylor, A.E.; Howe, L.D.; Davies, N.M. Educational Attainment as a Modifier for the Effect of Polygenic Scores for Cardiovascular Risk Factors: Cross-Sectional and Prospective Analysis of UK Biobank. Int. J. Epidemiol. 2022, 51, 885–897. [Google Scholar] [CrossRef]
- dos Santos, S.I. Cancer Epidemiology: Principles and Methods, 1st ed.; International Agency for Research on Cancer: Lyon, France, 1999; Volume 1. [Google Scholar]
- Berger, T.A.; Berger, B.K.; Kogelman, K. Supercritical Fluid Chromatography for Chiral Analysis and Semi-Preparative Purification. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Thun-Hohenstein, S.T.D.; Suits, T.F.; Malla, T.R.; Tumber, A.; Brewitz, L.; Choudhry, H.; Salah, E.; Schofield, C.J. Structure-Activity Studies Reveal Scope for Optimisation of Ebselen-Type Inhibition of SARS-CoV-2 Main Protease. ChemMedChem 2022, 17, e202100582. [Google Scholar] [CrossRef]
- de Munnik, M.; Lohans, C.T.; Lang, P.A.; Langley, G.W.; Malla, T.R.; Tumber, A.; Schofield, C.J.; Brem, J. Targeting the Mycobacterium Tuberculosis Transpeptidase Ldt Mt2 with Cysteine-Reactive Inhibitors Including Ebselen. Chem. Commun. 2019, 55, 10214–10217. [Google Scholar] [CrossRef]
- Amporndanai, K.; Meng, X.; Shang, W.; Jin, Z.; Rogers, M.; Zhao, Y.; Rao, Z.; Liu, Z.-J.; Yang, H.; Zhang, L.; et al. Inhibition Mechanism of SARS-CoV-2 Main Protease by Ebselen and Its Derivatives. Nat. Commun. 2021, 12, 3061. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Burka, L.T. Chemical and Enzymatic Oxidation of Furosemide: Formation of Pyridinium Salts. Chem. Res. Toxicol. 2007, 20, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Alegría-Baños, J.A.; Rosas-Alvarado, M.A.; Jiménez-López, J.C.; Juárez-Muciño, M.; Méndez-Celis, C.A.; Enríquez-De Los Santos, S.T.; Valdez-Vázquez, R.R.; Prada-Ortega, D. Sociodemographic, Clinical and Laboratory Characteristics and Risk Factors for Mortality of Hospitalized COVID-19 Patients at Alternate Care Site: A Latin American Experience. Ann. Med. 2023, 55, 2224049. [Google Scholar] [CrossRef]
- Macedo, A.; Gonçalves, N.; Febra, C. COVID-19 Fatality Rates in Hospitalized Patients: Systematic Review and Meta-Analysis. Ann. Epidemiol. 2021, 57, 14–21. [Google Scholar] [CrossRef]
- Mikami, T.; Ishii, M.; Yamamoto, N.; Marume, K.; Nakai, M.; Ogata, S.; Kaichi, R.; Ikebe, S.; Mori, T.; Komaki, S.; et al. Association of Early Administration of Furosemide with Improved Oxygenation in Patients with Acute Heart Failure. ESC Heart Fail. 2021, 8, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Bharath Kumar Reddy, K.R.; Basavaraja, G.V.; Shivananda. Furosemide Infusion in Children with Dengue Fever and Hypoxemia. Indian. Pediatr. 2014, 51, 303–305. [Google Scholar] [CrossRef]
- Santos, J.L.F.; Zanardi, P.; Alo, V.; Rodriguez, M.; Magdaleno, F.; De Langhe, V.; Dos Santos, V.; Murialdo, G.; Villoldo, A.; Coria, M.; et al. Pulmonary Edema in COVID-19 Treated with Furosemide and Negative Fluid Balance (NEGBAL): A Different and Promising Approach. J. Clin. Med. 2021, 10, 5599. [Google Scholar] [CrossRef]
- Osawa, E.A.; Cutuli, S.L.; Bitker, L.; Canet, E.; Cioccari, L.; Iguchi, N.; Lankadeva, Y.R.; Eastwood, G.M.; Evans, R.G.; May, C.N.; et al. Effect of Furosemide on Urinary Oxygenation in Patients with Septic Shock. Blood Purif. 2019, 48, 336–345. [Google Scholar] [CrossRef]
- Russotto, V.; Bellani, G.; Foti, G. Respiratory Mechanics in Patients with Acute Respiratory Distress Syndrome. Ann. Transl. Med. 2018, 6, 382. [Google Scholar] [CrossRef] [PubMed]
- Michard, F.; Shelley, K. Should We Monitor Pulsus Paradoxus via Pulse Oximetry in Patients with COVID-19 and Acute Respiratory Failure? Am. J. Respir. Crit. Care Med. 2020, 202, 770–771. [Google Scholar] [CrossRef]
- Mohammed, A.; Madkour, M.; Hassanien, H. Furosemide: Would It Help to Improve the Lungs as Evaluated by Sonography and Compliance during Aortic Coarctation Surgery. Ann. Card. Anaesth. 2019, 22, 254. [Google Scholar] [CrossRef]
- Kulkarni, M.; Slain, K.N.; Rotta, A.T.; Shein, S.L. The Effects of Furosemide on Oxygenation in Mechanically Ventilated Children with Bronchiolitis. J. Pediatr. Intensive Care 2020, 09, 087–091. [Google Scholar] [CrossRef]
- Cavaliere, F.; Masieri, S. Furosemide Protective Effect Against Airway Obstruction. Curr. Drug Targets 2002, 3, 197–201. [Google Scholar] [CrossRef]
- Atwi, Z. Effects of Inhaled Furosemide on Dyspnea and Pulmonary Function in People with COPD: A Literature Review. Can. J. Respir. Ther. 2022, 58, 170–174. [Google Scholar] [CrossRef]
- Waskiw-Ford, M.; Wu, A.; Mainra, A.; Marchand, N.; Alhuzaim, A.; Bourbeau, J.; Smith, B.M.; Jensen, D. Effect of Inhaled Nebulized Furosemide (40 and 120 Mg) on Breathlessness during Exercise in the Presence of External Thoracic Restriction in Healthy Men. Front. Physiol. 2018, 9, 86. [Google Scholar] [CrossRef]
- Rijsbergen, M.; Niemeyer-van der Kolk, T.; Hogendoorn, G.; Kouwenhoven, S.; Lemoine, C.; Klaassen, E.S.; de Koning, M.; Beck, S.; Bouwes Bavinck, J.N.; Feiss, G.; et al. A Randomized Controlled Proof-of-concept Trial of Digoxin and Furosemide in Adults with Cutaneous Warts. Br. J. Dermatol. 2019, 180, 1058–1068. [Google Scholar] [CrossRef]
- VOSS, T.G.; GATTI, P.J.; FERMIN, C.D.; GARRY, R.F. Reduction of Human Immunodeficiency Virus Production and Cytopathic Effects by Inhibitors of the Na+/K+/2Cl− Cotransporter. Virology 1996, 219, 291–294. [Google Scholar] [CrossRef][Green Version]
- Visco, V.; Vitale, C.; Rispoli, A.; Izzo, C.; Virtuoso, N.; Ferruzzi, G.J.; Santopietro, M.; Melfi, A.; Rusciano, M.R.; Maglio, A.; et al. Post-COVID-19 Syndrome: Involvement and Interactions between Respiratory, Cardiovascular and Nervous Systems. J. Clin. Med. 2022, 11, 524. [Google Scholar] [CrossRef]
- Baigent, C.; Windecker, S.; Andreini, D.; Arbelo, E.; Barbato, E.; Bartorelli, A.L.; Baumbach, A.; Behr, E.R.; Berti, S.; Bueno, H.; et al. European Society of Cardiology Guidance for the Diagnosis and Management of Cardiovascular Disease during the COVID-19 Pandemic: Part 1—Epidemiology, Pathophysiology, and Diagnosis. Eur. Heart J. 2022, 43, 1033–1058. [Google Scholar] [CrossRef]
- Kirkpatrick, J.N.; Swaminathan, M.; Adedipe, A.; Garcia-Sayan, E.; Hung, J.; Kelly, N.; Kort, S.; Nagueh, S.; Poh, K.K.; Sarwal, A.; et al. American Society of Echocardiography COVID-19 Statement Update: Lessons Learned and Preparation for Future Pandemics. J. Am. Soc. Echocardiogr. 2023, 36, 1127–1139. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Cut, T.G.; Lazureanu, V.E.; Oancea, C.; Marinescu, A.R.; Pescariu, S.A.; Pop, G.N.; Bende, F. Evolution of Echocardiographic Abnormalities Identified in Previously Healthy Individuals Recovering from COVID-19. J. Pers. Med. 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
| Clinical | Furosemide Use | |||
|---|---|---|---|---|
| Characteristic | All | No | Yes | p-Value |
| n = 515 (100%) | n = 439 (100%) | n = 76 (100%) | ||
| ≥60 years | 63.0% | 62.8% | 63.9% | 0.896 |
| Male (%) | 61.9% | 60.9% | 65.3% | 0.515 |
| Diabetes | 43.3% | 39.2% | 66.7% | <0.001 * |
| HBP | 42.3% | 35.8% | 79.2% | <0.001 * |
| BMI | 30.3 ± 6.9 | 30.2 ± 7.2 | 31.6 ± 5.6 | 0.412 |
| Smoking | 7.6% | 7.7% | 7.5% | 0.999 * |
| CKD | 22.4% | 16.1% | 61.1% | <0.001 |
| Heart disease ** | 3.4% | 3.2% | 4.2% | 0.721 |
| Charlson Index | 3.6 ± 2.1 | 3.5 ± 2.1 | 4.5 ± 1.8 | <0.001 * |
| Vaccine (any doses) | 43.9% | 41.5% | 55.6% | 0.029 |
| Vaccine complete | 37.3% | 35.1% | 50.8% | 0.019 |
| Clinical data upon hospital admission | ||||
| PSI | 105 ± 40 | 104 ± 41 | 117 ± 28 | 0.048 |
| Disease phase | 0.006 | |||
| Viral | 38.1% | 36.1% | 50.0% | |
| Pulmonary | 46.2% | 49.0% | 29.2% | |
| Inflammation | 15.8% | 14.9% | 20.8% | |
| Oximetry | 91.0 ± 11.1 | 91.1 ± 10.9 | 90.8 ± 12.1 | 0.855 |
| Neutrophils | 8503 ± 6234 | 7956 ± 5793 | 11,809 ± 7788 | <0.001 |
| Lymphocytes | 923 ± 798 | 931 ± 837 | 880 ± 558 | 0.623 |
| PlateletsX1000 | 257 ± 120 | 255 ± 114 | 272 ± 150 | 0.266 |
| NLR | 13.3 ± 14.1 | 12.6 ± 12.4 | 17.9 ± 21.4 | 0.004 |
| D-Dimer | 1639 ± 3163 | 1581 ± 3112 | 2230 ± 3707 | 0.430 |
| ESR | 29.5 ± 12.1 | 28.9 ± 11.7 | 32.7 ± 14.5 | 0.214 |
| CRP | 16.9 ± 19.6 | 17.1 ± 20.5 | 16.1 ± 11.6 | 0.755 * |
| Ferritin | 737.7 ± 578.5 | 733.5 ± 542.6 | 807.5 ± 796.0 | 0.017 |
| Creatinine | 2.5 ± 3.7 | 2.0 ± 2.9 | 5.6 ± 6.1 | <0.001 |
| Urea | 80.1 ± 67.2 | 70.3 + 58.1 | 137.4 + 87.4 | <0.001 |
| eGFR | 65.8 ± 46.3 | 72.0 ± 46.4 | 30.2 ± 27.2 | <0.001 |
| AST | 62.2 ± 183.5 | 55.8 ± 129.2 | 106.7 ± 388.6 | 0.075 |
| ALT | 47.4 ± 101.1 | 43.4 ± 69.0 | 74.6 + 218.2 | 0.047 |
| ALP | 105.4 ± 90.7 | 103.2 ± 95.0 | 127.5 ± 59.2 | 0.189 |
| LDH | 392.9 ± 414.8 | 385.8 ± 396.5 | 446.8 ± 530.6 | 0.294 |
| Glucose | 195.7 ± 144.9 | 191.1 ± 141.7 | 217.5 ± 165.1 | 0.176 |
| INR | 1.14 ± 0.32 | 1.14 ± 0.34 | 1.12 ± 0.15 | 0.715 |
| Main prescribed treatments during hospitalization | ||||
| Anticoagulants | 90.5% | 89.9% | 93.1% | 0.520 |
| Antibiotics | 48.4% | 46.3% | 58.6% | 0.038 |
| Amine support | 8.6% | 8.5% | 9.7% | 0.658 |
| Steroids | 92.9% | 91.0% | 97.2% | 0.990 |
| Mech. Vent. | 31.9% | 31.7% | 33.3% | 0.786 |
| Hemodialysis | 10.3% | 6.0% | 34.7% | <0.001 |
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| Covariate | AdRR | Lower | Upper | p | AdRR | Lower | Upper | p |
| Diabetes | 0.93 | 0.63 | 1.39 | 0.738 | ||||
| HBP | 0.95 | 0.65 | 1.39 | 0.779 | ||||
| Smoking | 3.89 | 2.19 | 6.91 | <0.001 | 2.48 | 1.53 | 4.01 | <0.001 |
| Charlson Ind | 7.37 | 2.03 | 26.79 | 0.002 | 6.86 | 2.46 | 19.11 | <0.001 |
| Vaccine | 0.58 | 0.38 | 0.88 | 0.010 | 0.58 | 0.42 | 0.80 | 0.001 |
| CKD | 1.67 | 0.82 | 3.40 | 0.159 | ||||
| Phase | 1.94 | 1.32 | 2.85 | 0.001 | 1.92 | 1.41 | 2.61 | <0.001 |
| PSI ≥ 120 | 7.11 | 4.92 | 10.27 | <0.001 | 7.89 | 5.82 | 10.70 | <0.001 |
| Neutrophilia | 2.19 | 1.46 | 3.29 | <0.001 | 2.12 | 1.52 | 2.95 | <0.001 |
| NLR | 2.06 | 1.36 | 3.11 | 0.001 | 2.39 | 1.72 | 3.32 | <0.001 |
| Ferritin | 0.90 | 0.63 | 1.29 | 0.574 | ||||
| ALT ≥ 45 UL | 0.60 | 0.39 | 0.92 | 0.018 | 0.71 | 0.50 | 1.00 | 0.049 |
| eGFR ≥ 60 | 0.67 | 0.37 | 1.20 | 0.179 | ||||
| Creatinine | 0.57 | 0.21 | 1.51 | 0.255 | ||||
| Urea > 135 | 1.38 | 0.63 | 3.02 | 0.421 | ||||
| Mech. Vent. | 23.59 | 16.46 | 33.80 | <0.001 | 23.12 | 17.28 | 30.92 | <0.001 |
| Hemodialysis | 1.75 | 0.86 | 3.59 | 0.125 | 1.85 | 1.07 | 3.19 | 0.027 |
| Antibiotic | 1.71 | 1.19 | 2.45 | 0.004 | 1.52 | 1.13 | 2.06 | 0.006 |
| Furosemide | 0.58 | 0.36 | 0.92 | 0.022 | 0.60 | 0.41 | 0.87 | 0.008 |
| Stratum-Specific | Effect of Furosemide | ||||
|---|---|---|---|---|---|
| Present | AdRR | 95% CI Lower–Upper | p | ||
| All patients | 0.60 | 0.41 | 0.87 | 0.008 | |
| Smoking | No | 0.55 | 0.37 | 0.82 | 0.003 |
| Yes | 1.42 | 0.77 | 2.60 | 0.248 | |
| Charlson Index ≥ 8 | No | 0.65 | 0.43 | 0.97 | 0.037 |
| Yes | 0.62 | 0.43 | 0.90 | 0.014 | |
| Vaccine | No | 0.53 | 0.36 | 0.78 | 0.001 |
| Yes | 0.87 | 0.39 | 1.90 | 0.718 | |
| Advanced phase | No | 0.67 | 0.30 | 1.48 | 0.323 |
| Yes | 0.70 | 0.48 | 1.14 | 0.151 | |
| PSI score ≥ 120 | No | 0.25 | 0.14 | 0.46 | <0.001 |
| Yes | 1.76 | 0.96 | 3.24 | 0.069 | |
| Neutro ≥ 9.1 × 103/µL | No | 0.54 | 0.33 | 0.90 | 0.019 |
| Yes | 0.71 | 0.42 | 1.21 | 0.208 | |
| NLR > 14.6 | No | 0.45 | 0.27 | 0.75 | 0.002 |
| Yes | 0.79 | 0.47 | 1.34 | 0.394 | |
| ALT ≥ 45 UL | No | 0.68 | 0.44 | 1.06 | 0.092 |
| Yes | 0.40 | 0.22 | 0.74 | 0.003 | |
| Mech. Vent. | No | 1.03 | 0.52 | 2.04 | 0.092 |
| Yes | 0.45 | 0.29 | 0.70 | <0.001 | |
| Hemodialysis | No | 0.45 | 0.30 | 0.68 | <0.001 |
| Yes | 2.30 | 0.73 | 7.23 | 0.153 | |
| Antibiotic | No | 0.22 | 0.11 | 0.45 | <0.001 |
| Yes | 1.09 | 0.72 | 1.65 | 0.672 | |
| Variables in Interaction | 95% CI | |||||
|---|---|---|---|---|---|---|
| Furosemide | PSI ≥ 120 | Hemodialysis | AdRR | Lower | Upper | p |
| No | No | No | 1.000 a | |||
| Yes | No | No | 0.227 | 0.149 | 0.345 | <0.001 |
| No | Yes | No | 9.382 | 7.671 | 11.474 | <0.001 |
| Yes | Yes | No | 9.326 | 5.571 | 15.613 | <0.001 |
| No | No | Yes | 3.779 | 2.270 | 6.289 | <0.001 |
| Yes | No | Yes | 4.576 | 1.944 | 10.774 | 0.001 |
| No | Yes | Yes | 5.742 | 4.023 | 8.197 | <0.001 |
| Yes | Yes | Yes | 15.996 | 9.775 | 26.177 | <0.001 |
| Furosemide | Death: n (%) | |||
|---|---|---|---|---|
| Use | Total | No | Yes | p * |
| No | 265 (100%) | 197 (74.3%) | 68 (25.7%) | 0.008 |
| Yes | 27 (100%) | 26 (96.3%) | 1 (3.7%) | |
| Clinical | Upon Hospital Admission | Throughout Hospitalization | ||||
|---|---|---|---|---|---|---|
| Characteristic | Furosemide | Furosemide | ||||
| No | Yes | p * | No | Yes | p * | |
| Male | 58.3% | 59.3% | 0.548 | |||
| Age (years) | 60.2 ± 16.4 | 63.9 ± 13.8 | 0.256 | 60.1 ± 15.8 | 63.7 ± 10.7 | 0.001 |
| CKD | 8.3% | 40.7% | <0.001 | NA ** | NA ** | |
| Vaccine | 45.8% | 54.2% | 0.120 | NA ** | NA ** | |
| Mech. Vent. | 1.5% | 3.7% | 0.402 | 2.3% | 11.1% | 0.041 |
| Antibiotic | 4.9% | 3.7% | 0.780 | 42% | 63% | 0.043 |
| PSI | 81.5 ± 24.3 | 95.5 ± 16.2 | 0.004 | 80.4 ± 24.1 | 94.1 ± 16.0 | <0.001 |
| Oximetry | 92.3 ± 10.5 | 93.5 ± 8.9 | 0.578 | 92.6 ± 8.3 | 95.6 ± 3.8 | 0.012 |
| ESR | 29.7 ± 10.4 | 36.5 ± 18.4 | 0.091 | 30.4 ± 10.7 | 28.5 ± 11.4 | 0.282 |
| Neutrophils | 6985 ± 4699 | 10,340 ± 7743 | 0.001 | 7353 ± 5025 | 8145 ± 5409 | 0.065 |
| Lymphocytes | 986 ± 875 | 854 ± 312 | 0.441 | 971 ± 830 | 970 ± 502 | 0.988 |
| NLR | 10.4 ± 9.0 | 13.9 ± 12.5 | 0.068 | 11.4 ± 15.0 | 10.0 ± 7.9 | 0.263 |
| PlateletsX1000 | 255 ± 111 | 227 ± 140 | 0.219 | 302 ± 136 | 277 ± 186 | 0.045 |
| PLR | 817.5 ± 1530.9 | 272.8 ± 161.7 | 0.066 | 938.9 ± 1978.5 | 834.7 ± 2025.8 | 0.535 |
| eGFR | 84.7 ± 45.9 | 37.7 ± 28.1 | <0.001 | 109.6 ± 60.5 | 66.0 ± 44.5 | <0.001 |
| Creatinine | 1.5 ± 2.4 | 3.1 ± 3.1 | <0.001 | 1.2 ± 2.3 | 2.3 ± 2.9 | <0.001 |
| Urea | 52.0 ± 42.4 | 87.6 ± 44.9 | <0.001 | 57.1 ± 42.3 | 85.1 ± 63.4 | <0.001 |
| AST | 45.9 ± 58.8 | 59.8 ± 79.6 | 0.330 | 40.5 ± 51.1 | 45.7 ± 33.5 | 0.328 |
| ALT | 41.6 ± 40.9 | 40.5 ± 49.6 | 0.907 | 49.0 ± 56.8 | 41.2 ± 25.6 | 0.177 |
| ALP | 101.5 ± 100.8 | 153.7 ± 71.3 | 0.071 | 86.3 ± 55.0 | 196.6 ± 184.2 | <0.001 |
| LDH | 366.1 ± 448.6 | 306.1 ± 129.9 | 0.542 | 341.3 ± 186.3 | 248.4 ± 116.5 | <0.001 |
| Glucose | 172.1 ± 110.1 | 205.2 ± 107.0 | 0.145 | 149.0 ± 81.7 | 168.0 ± 102.6 | 0.009 |
| D-Dimer | 771.1 ± 1102.4 | 717.0 ± 314.3 | 0.866 | 1305.9 ± 2262.4 | 1747.2 + 1722.2 | 0.089 |
| CRP | 15.5 ± 20.1 | 16.5 ± 9.6 | 0.852 | 7.3 ± 7.9 | 8.8 ± 5.8 | 0.419 |
| Ferritin | 678.2 ± 506.6 | 477.8 ± 420.3 | 0.171 | 792.9 ± 597.6 | 920.0 ± 778.0 | 0.291 |
| Variable | Group | AUC | SE | 95%CI | Cutoff | p | Difference * | ||
|---|---|---|---|---|---|---|---|---|---|
| AUC | p | ||||||||
| PSI | Lived | 0.587 | 0.034 | 0.520 | 0.655 | 90.5 | 0.011 | 0.082 | 0.039 |
| Died | 0.506 | 0.019 | 0.468 | 0.544 | 141.5 | 0.766 | |||
| Oximetry | Lived | 0.551 | 0.038 | 0.477 | 0.626 | 80.5 | 0.176 | 0.054 | 0.215 |
| Died | 0.498 | 0.021 | 0.457 | 0.539 | 73.5 | 0.910 | |||
| Creatinine | Lived | 0.730 | 0.033 | 0.666 | 0.794 | 2.6 | <0.001 | 0.088 | 0.020 |
| Died | 0.642 | 0.019 | 0.604 | 0.679 | 3.5 | <0.001 | |||
| Urea | Lived | 0.779 | 0.027 | 0.726 | 0.832 | 53.2 | <0.001 | 0.164 | <0.001 |
| Died | 0.614 | 0.022 | 0.572 | 0.657 | 87.5 | <0.001 | |||
| eGFR | Lived | 0.301 | 0.035 | 0.233 | 0.369 | 84.7 | <0.001 | −0.047 | 0.229 |
| Died | 0.348 | 0.019 | 0.312 | 0.385 | 53.7 | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Martinez, J.; Kotzker, W.; Mendoza-Hernandez, M.A.; Gadh, R.S.; Hernandez-Fuentes, G.A.; Bañuelos, A.; Guzmán-Esquivel, J.; Hong, A.; Delgado-Enciso, O.G.; Geyer-Roberts, E.; et al. Analysis of Survival Modification by Furosemide Use in a Cohort of Hospitalized COVID-19 Patients with Severe or Critical Disease in Mexico: Due to Its Chemical Structure, Furosemide Is More than Just a Diuretic. Pharmaceutics 2024, 16, 920. https://doi.org/10.3390/pharmaceutics16070920
Diaz-Martinez J, Kotzker W, Mendoza-Hernandez MA, Gadh RS, Hernandez-Fuentes GA, Bañuelos A, Guzmán-Esquivel J, Hong A, Delgado-Enciso OG, Geyer-Roberts E, et al. Analysis of Survival Modification by Furosemide Use in a Cohort of Hospitalized COVID-19 Patients with Severe or Critical Disease in Mexico: Due to Its Chemical Structure, Furosemide Is More than Just a Diuretic. Pharmaceutics. 2024; 16(7):920. https://doi.org/10.3390/pharmaceutics16070920
Chicago/Turabian StyleDiaz-Martinez, Janet, Wayne Kotzker, Martha A. Mendoza-Hernandez, Rajdeep S. Gadh, Gustavo A. Hernandez-Fuentes, Andrew Bañuelos, José Guzmán-Esquivel, Angelina Hong, Osiris G. Delgado-Enciso, Elizabeth Geyer-Roberts, and et al. 2024. "Analysis of Survival Modification by Furosemide Use in a Cohort of Hospitalized COVID-19 Patients with Severe or Critical Disease in Mexico: Due to Its Chemical Structure, Furosemide Is More than Just a Diuretic" Pharmaceutics 16, no. 7: 920. https://doi.org/10.3390/pharmaceutics16070920
APA StyleDiaz-Martinez, J., Kotzker, W., Mendoza-Hernandez, M. A., Gadh, R. S., Hernandez-Fuentes, G. A., Bañuelos, A., Guzmán-Esquivel, J., Hong, A., Delgado-Enciso, O. G., Geyer-Roberts, E., Martinez-Fierro, M. L., Rodriguez-Sanchez, I. P., Garza-Veloz, I., Canseco-Ávila, L. M., & Delgado-Enciso, I. (2024). Analysis of Survival Modification by Furosemide Use in a Cohort of Hospitalized COVID-19 Patients with Severe or Critical Disease in Mexico: Due to Its Chemical Structure, Furosemide Is More than Just a Diuretic. Pharmaceutics, 16(7), 920. https://doi.org/10.3390/pharmaceutics16070920








