Recent Progress of Oral Functional Nanomaterials for Intestinal Microbiota Regulation
Abstract
1. Introduction
2. Nanomaterials as Drug Carriers to Assist Microbiota Regulation
2.1. Nanomaterials as Carriers for Sustained Release of Microbiota-Modulating Drugs
2.2. Nanomaterials as Carriers for Controlled Release of Microbiota-Modulating Drugs
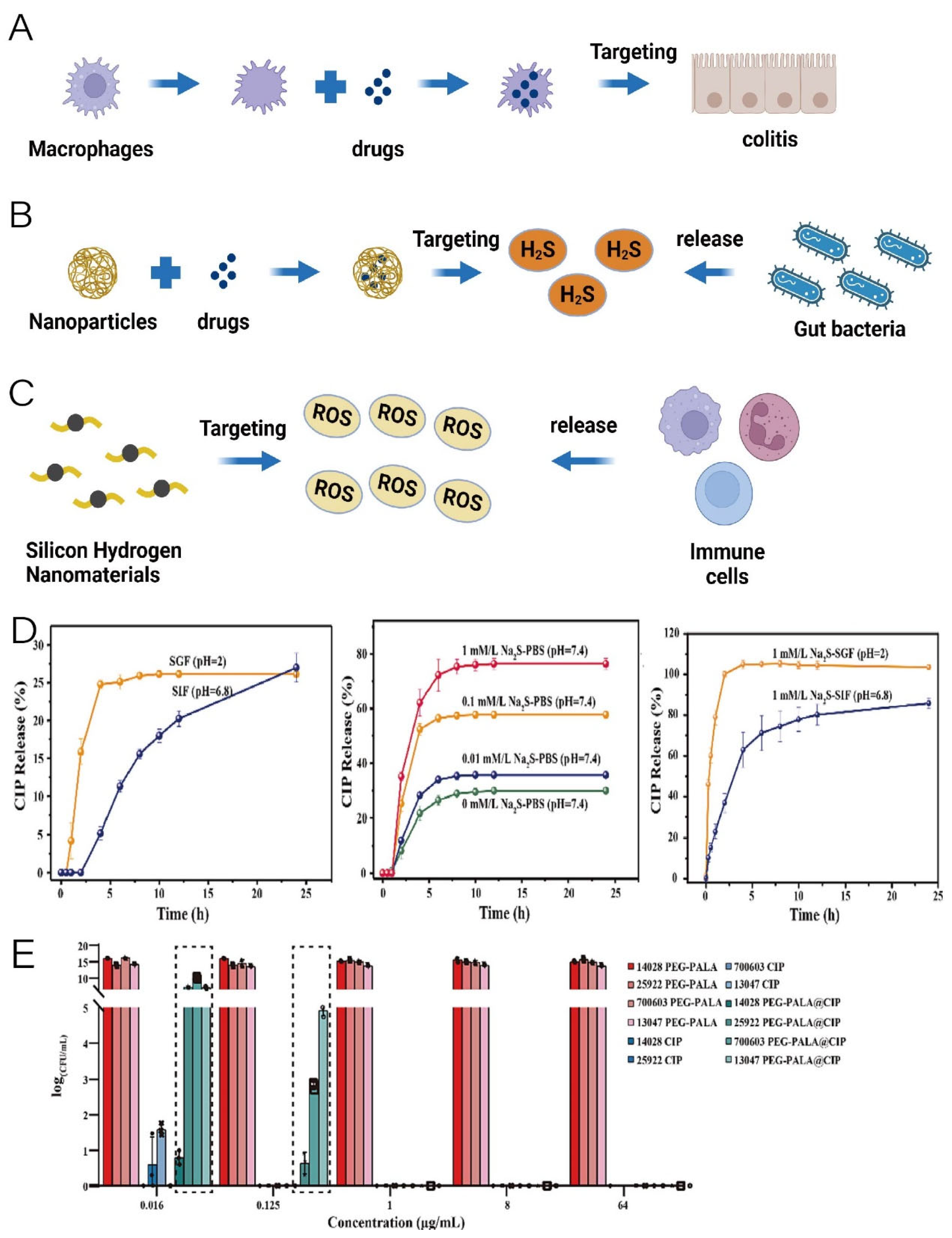
2.3. Nanomaterials Assisting in Targeted Microbiota-Modulating Drug Delivery
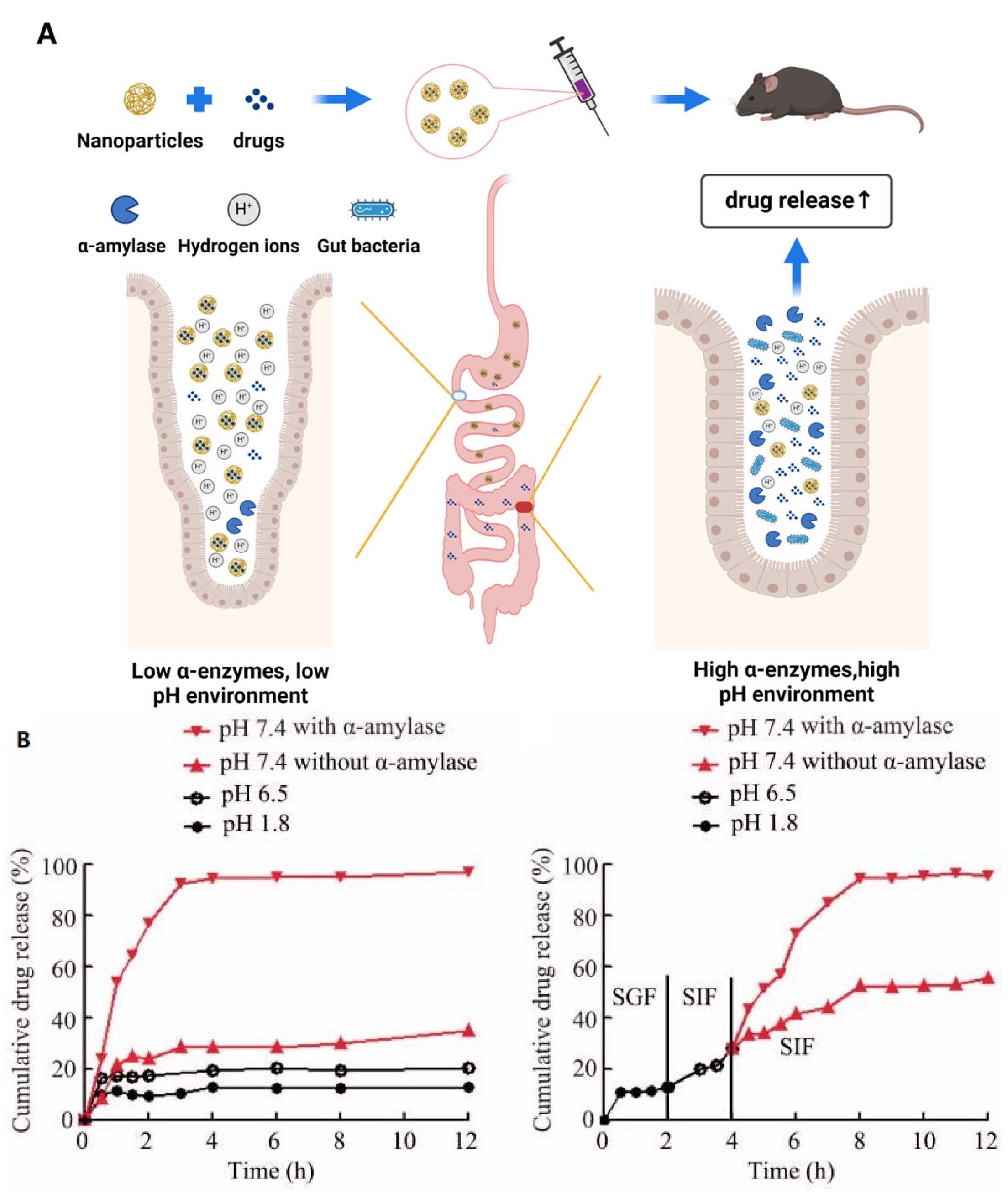
| Target | Material | Drugs | Therapeutic Effects | References |
|---|---|---|---|---|
| Sustained release | Nanoparticles based on Hohenbuehelia serotina polysaccharides and mucin | Polyphenols from Malus baccata (Linn.) Borkh |
| [24] |
| Self-assembled starch nanoparticles derived from quinoa, maize, and waxy maize starches | Chrysin and rutin (natural polyphenols) |
| [26] | |
| Nanoparticles based on bovine serum albumin and Hohenbuehelia serotina polysaccharides | Polyphenols isolated from the shells of Juglans regia L. |
| [27] | |
| Nanostructured lipid carriers and nanostructured lipid carriers imbedded microcapsule | 6-gingerol (polyphenols) |
| [28] | |
| A bioadhesive liquid coacervate based on bidentate hydrogen bonding-driven nanoparticle assembly | A water-soluble sodium phosphate salt of dexamethasone |
| [29] | |
| Controlled release | Low molecular weight chitosan and unsaturated alginate resulting nanoparticles | Curcumin–cyclodextrin inclusion complex |
| [32] |
| A novel nitroreductase labile peptidic hydrogel based on supramolecular self-assembly | Escherichia coli Nissle 1917 (probiotic) |
| [34] | |
| Targeted delivery | Encapsulation of nanocomplexes formed by the coupling of polydopamine nanoparticles with mCRAMP (mouse cathelicidin-related antimicrobial peptide) using macrophage membrane | mCRAMP (an antimicrobial peptide) |
| [35] |
| The PEGylated poly (α lipoic acid) (PEG-PALA) copolymer nanoparticles | Ciprofloxacin |
| [36] | |
| Fe@Fe3O4 nanoparticles | Ginsenoside Rg3 |
| [37] | |
| Rhamnolipid | Fullerene |
| [21] | |
| Highly cross-linked polyphosphazene nanodrug developed by copolymerisation of curcumin and acid-sensitive units (4-hydroxy-benzoic acid (4-hydroxy-benzylidene)-hydrazide) with hexachlorotripolyphosph-onitrile | Curcumin | Downregulate several critical inflammatory cytokines (TNF-α, IL-1β, and IL-8) | [38] |
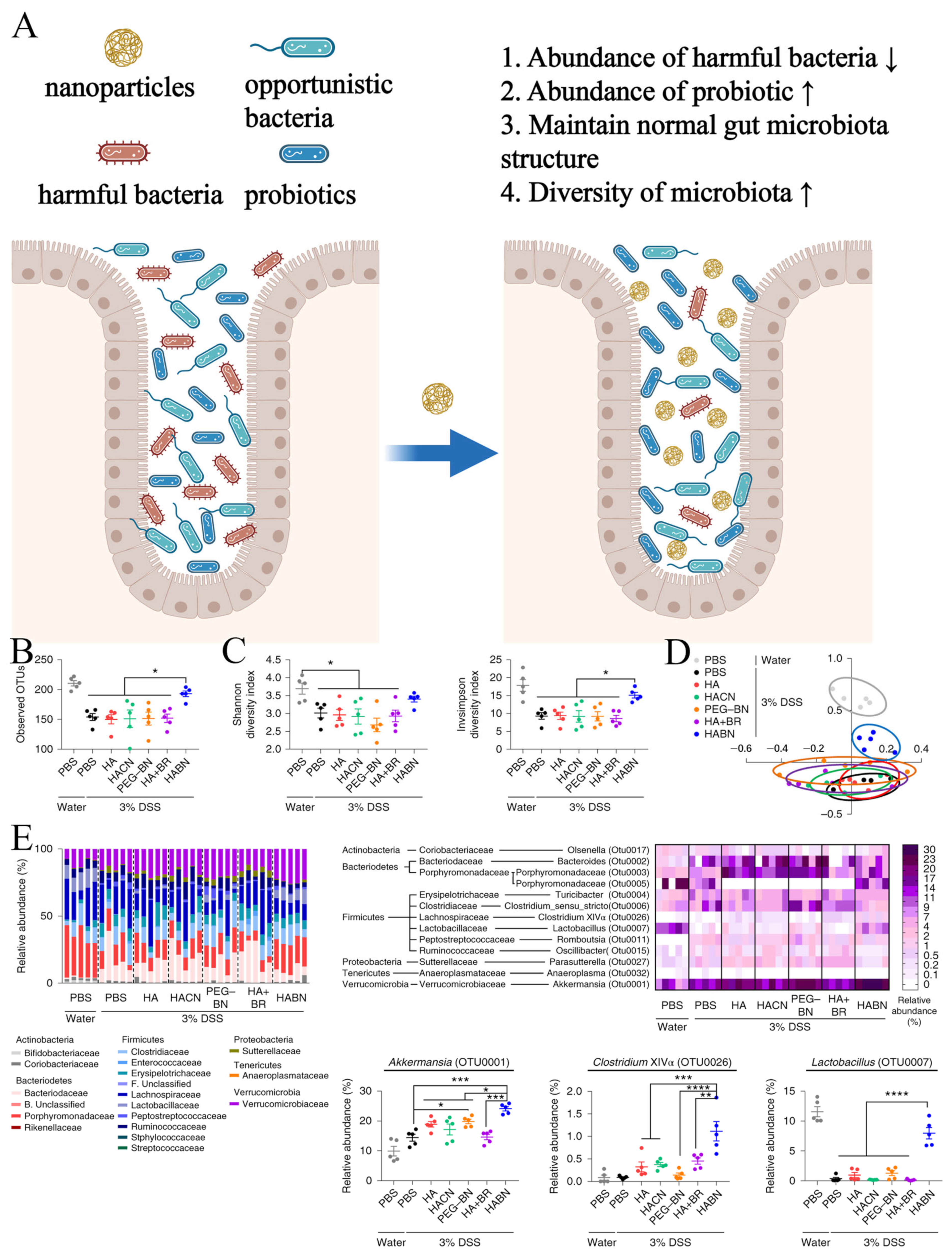
2.4. Protective Effect of Nanomaterials on Gut Microbiota
3. Nanomaterials Regulate Intestinal Microbiota
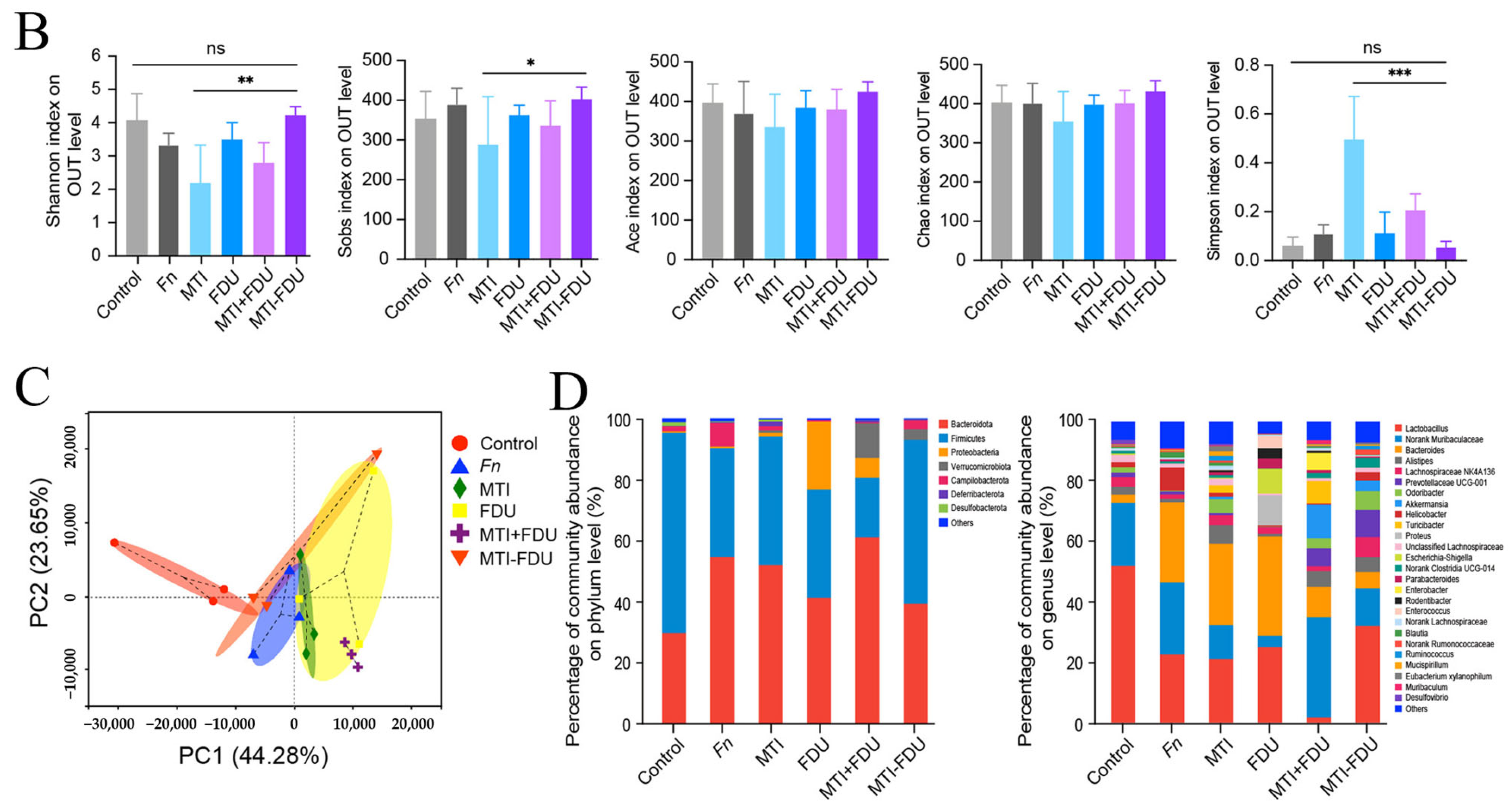
4. The Dual-Edged Sword Effect of Nanomaterials on Gut Microbiota
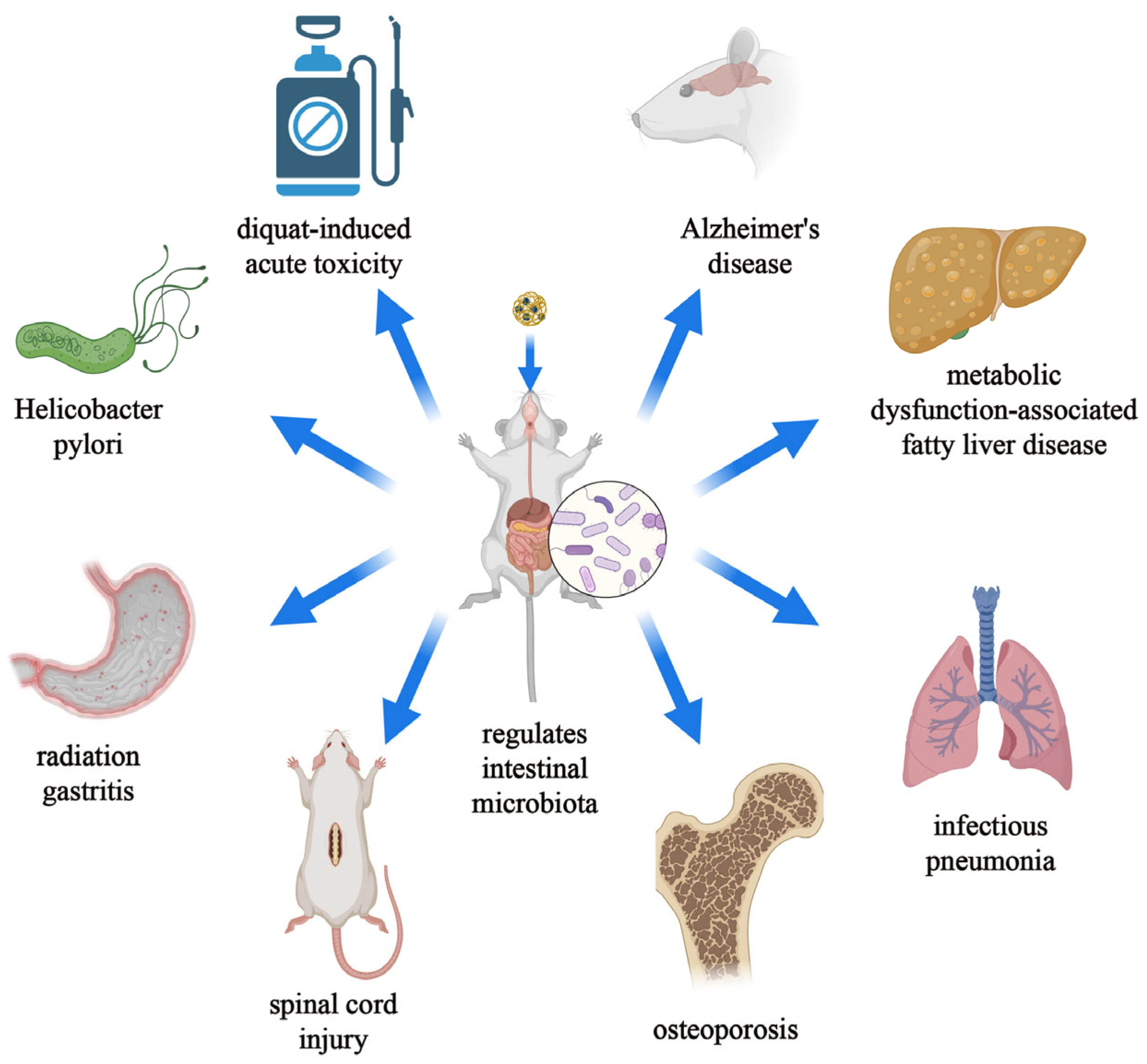
5. Nanomaterials Treat Diseases by Regulating the Intestinal Microbiota
5.1. Gastrointestinal Tract
5.1.1. Immune Diseases
5.1.2. Cancer
5.1.3. Other Disease
5.2. Extra-Intestinal Gastrointestinal Tract
5.2.1. Immune-Related Diseases
5.2.2. Metabolic-Related Diseases
6. Discussion and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, D.V.; Zalomova, L.V.; Zagainova, A.V.; Makarov, V.V.; Mezhevikina, L.M.; Fesenko, E.E.; Yudin, S.M. Cryopreservation of the Human Gut Microbiota: Current State and Perspectives. Int. J. Med. Microbiol. 2019, 309, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Giron, M.C.; Qesari, M.; Porzionato, A.; Caputi, V.; Zoppellaro, C.; Banzato, S.; Grillo, A.R.; Spagnol, L.; De Caro, R.; et al. Toll-Like Receptor 2 Regulates Intestinal Inflammation by Controlling Integrity of the Enteric Nervous System. Gastroenterology 2013, 145, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; De Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Ronan, V.; Yeasin, R.; Claud, E.C. Childhood Development and the Microbiome—The Intestinal Microbiota in Maintenance of Health and Development of Disease During Childhood Development. Gastroenterology 2021, 160, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and Human Aging: Probiotic and Prebiotic Potentials in Longevity, Skin Health and Cellular Senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The Gut-Bone Axis: How Bacterial Metabolites Bridge the Distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, M.E.; et al. The Gut Ecosystem and Immune Tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef]
- Hong, M.; Li, Z.; Liu, H.; Zheng, S.; Zhang, F.; Zhu, J.; Shi, H.; Ye, H.; Chou, Z.; Gao, L.; et al. Fusobacterium Nucleatum Aggravates Rheumatoid Arthritis through FadA-Containing Outer Membrane Vesicles. Cell Host Microbe 2023, 31, 798–810.e7. [Google Scholar] [CrossRef]
- Antushevich, H. Fecal Microbiota Transplantation in Disease Therapy. Clin. Chim. Acta 2020, 503, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal Microbiota Transplantation: Review and Update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current State and Challenges in Developing Oral Vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Laffleur, F.; Mayer, A.H. Oral Nanoparticulate Drug Delivery Systems for the Treatment of Intestinal Bowel Disease and Colorectal Cancer. Expert Opin. Drug Deliv. 2023, 20, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Rehman, A.; Yu, S.; De Andino, N.M. Brain Fogginess, Gas and Bloating: A Link between SIBO, Probiotics and Metabolic Acidosis. Clin. Transl. Gastroenterol. 2018, 9, e162. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging Issues in Probiotic Safety: 2023 Perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef] [PubMed]
- Nabavi-Rad, A.; Sadeghi, A.; Asadzadeh Aghdaei, H.; Yadegar, A.; Smith, S.M.; Zali, M.R. The Double-Edged Sword of Probiotic Supplementation on Gut Microbiota Structure in Helicobacter Pylori Management. Gut Microbes 2022, 14, 2108655. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, J.; Landfester, K.; Mailänder, V. The Challenges of Oral Drug Delivery via Nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Xia, Y.; Hong, L.; Zheng, J.; Lu, Z.; Zhang, Q.; Chen, S.; Pang, Z.; Li, L.; Qiao, S.; Wang, Q.; et al. Ulcerative Colitis Alleviation of Colon-Specific Delivered Rhamnolipid/Fullerene Nanocomposites via Dual Modulation in Oxidative Stress and Intestinal Microbiome. J. Mater. Chem. B 2023, 11, 5882–5897. [Google Scholar] [CrossRef]
- Guerra, P.R.; Ajalloueian, F.; Wei, S.; Kristensen, K.A.; Bahl, M.I.; Boisen, A.; Licht, T.R. Delivery of Streptomycin to the Rat Colon by Use of Electrospun Nanofibers. Sci. Rep. 2022, 12, 21503. [Google Scholar] [CrossRef] [PubMed]
- Cassini, C.; Zatti, P.H.; Angeli, V.W.; Branco, C.S.; Salvador, M. Mutual Effects of Free and Nanoencapsulated Phenolic Compoundson Human Microbiota. Curr. Med. Chem. 2022, 29, 3160–3178. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; An, S.; Zhu, H.; Li, X.; Gao, D. Malus Baccata (Linn.) Borkh Polyphenols-Loaded Nanoparticles Ameliorate Intestinal Health by Modulating Intestinal Function and Gut Microbiota. Int. J. Biol. Macromol. 2023, 252, 126233. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Remanan, M.K.; Zhu, F. Encapsulation of Chrysin and Rutin Using Self-Assembled Nanoparticles of Debranched Quinoa, Maize, and Waxy Maize Starches. Carbohydr. Polym. 2024, 337, 122118. [Google Scholar] [CrossRef]
- Li, X.; Feng, R.; Zhou, P.; Wang, L.; Luo, Z.; An, S. Construction and Characterization of Juglans Regia L. Polyphenols Nanoparticles Based on Bovine Serum Albumin and Hohenbuehelia Serotina Polysaccharides, and Their Gastrointestinal Digestion and Colonic Fermentation in Vitro. Food Funct. 2021, 12, 10397–10410. [Google Scholar] [CrossRef]
- Tian, W.; Wang, H.; Zhu, Y.; Wang, Q.; Song, M.; Cao, Y.; Xiao, J. Intervention Effects of Delivery Vehicles on the Therapeutic Efficacy of 6-Gingerol on Colitis. J. Controlled Release 2022, 349, 51–66. [Google Scholar] [CrossRef]
- Zhao, P.; Xia, X.; Xu, X.; Leung, K.K.C.; Rai, A.; Deng, Y.; Yang, B.; Lai, H.; Peng, X.; Shi, P.; et al. Nanoparticle-Assembled Bioadhesive Coacervate Coating with Prolonged Gastrointestinal Retention for Inflammatory Bowel Disease Therapy. Nat. Commun. 2021, 12, 7162. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Marabada, D.; Li, J.; Wei, S.; Huang, Q.; Wang, Z. Cyclodextrin Based Nanoparticles for Smart Drug Delivery in Colorectal Cancer. Chem. Biol. Drug Des. 2023, 102, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, M.; Wu, Y.; Jung, S.; Li, D.; He, N.; Lee, M. An Efficient Enzyme-Triggered Controlled Release System for Colon-Targeted Oral Delivery to Combat Dextran Sodium Sulfate (DSS)-Induced Colitis in Mice. Drug Deliv. 2021, 28, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Dharmaprakash, V.; Nighot, P.; Guo, S.; Nighot, M.; Do, T.; Ma, T.Y. Bifidobacterium Bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. Int. J. Mol. Sci. 2021, 22, 8070. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, P.; Wu, C.; Yao, Q.; Cha, R.; Gao, Y. Reductase-Labile Peptidic Supramolecular Hydrogels Aided in Oral Delivery of Probiotics. ACS Appl. Mater. Interfaces 2023, 15, 31214–31223. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Wang, K.; Li, J.; Li, Y.; Zhu, H.; Lu, M.; Zhang, Y.; Fan, Q.; Han, L.; Wang, K.; et al. ROS Scavenging and Inflammation-Directed Polydopamine Nanoparticles Regulate Gut Immunity and Flora Therapy in Inflammatory Bowel Disease. Acta Biomater. 2023, 161, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, X.W.; Zang, T.; Ren, H.; Liang, D.S.; Bai, S.C.; Li, C.; Liao, X.P.; Liu, Y.H.; Zhang, C.; et al. H2S Responsive PEGylated Poly (Lipoic Acid) with Ciprofloxacin for Targeted Therapy of Salmonella. J. Controlled Release 2022, 351, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, X.; Hong, L.; Zhao, X.; Cui, G.; Li, A.; Liu, Y.; Zhou, L.; Sun, R.; Shen, S.; et al. Nanoparticle Conjugation of Ginsenoside Rg3 Inhibits Hepatocellular Carcinoma Development and Metastasis. Small 2020, 16, 1905233. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jing, X.; Jiang, W.; Li, M.; Liu, K.; Teng, M.; Ma, Y.; Wang, D.; Meng, L.; Zhang, Y.; et al. Polyphosphazene Nanodrugs for Targeting Delivery and Inflammation Responsive Release of Curcumin to Treat Acute Lung Injury by Effectively Inhibiting Cytokine Storms. Colloids Surf. B Biointerfaces 2023, 229, 113446. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Gong, F.; Wu, J.; Tang, W.; Liao, F.; Han, Z.; Pei, Z.; Lei, H.; Wang, L.; Shao, M.; et al. Orally Administered Silicon Hydrogen Nanomaterials as Target Therapy to Treat Intestinal Diseases. ACS Nano 2023, 17, 21539–21552. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Q.; Tao, W.; Jiang, W.; Elinav, E.; Wang, Y.; Zhu, S. Glucosylated Nanoparticles for the Oral Delivery of Antibiotics to the Proximal Small Intestine Protect Mice from Gut Dysbiosis. Nat. Biomed. Eng. 2022, 6, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, X.; Yang, B.; Yuan, W.; Huang, W.; Wu, G.; Ma, J. Synergistic Target of Intratumoral Microbiome and Tumor by Metronidazole–Fluorouridine Nanoparticles. ACS Nano 2023, 17, 7335–7351. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sugihara, K.; Gillilland, M.G.; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic Acid–Bilirubin Nanomedicine for Targeted Modulation of Dysregulated Intestinal Barrier, Microbiome and Immune Responses in Colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Dong, X.; Pan, P.; Chen, K.W.; Fan, J.X.; Cheng, S.X.; Zhang, X.Z. PhageGuided Modulation of the Gut Microbiota of Mouse Models of Colorectal Cancer Augments Their Responses to Chemotherapy. Nat. Biomed. Eng. 2019, 3, 717–728. [Google Scholar] [CrossRef]
- Qiao, L.; Dou, X.; Song, X.; Chang, J.; Pi, S.; Zhang, X.; Zhu, L.; Zeng, X.; Xu, C. Protective Effect of Biogenic Selenium Nanoparticles against Diquat-Induced Acute Toxicity via Regulation of Gut Microbiota and Its Metabolites. Food Chem. Toxicol. 2022, 170, 113480. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Sun, Y.; Yang, T.; Tan, L.; Lv, P.; Xu, Q.; Tao, G.; Qin, S.; Lu, X.; He, Q. Nanocapsule-Mediated Sustained H2 Release in the Gut Ameliorates Metabolic Dysfunction-Associated Fatty Liver Disease. Biomaterials 2021, 276, 121030. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Iohara, D.; Michihara, A.; Ifuku, S.; Azuma, K.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Hirayama, F.; Anraku, M. Effects of Surface-Deacetylated Chitin Nanofibers on Non-Alcoholic Steatohepatitis Model Rats and Their Gut Microbiota. Int. J. Biol. Macromol. 2020, 164, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yu, S.; Shi, C.; Gu, J.; Shao, Y.; Chen, Q.; Li, Y.; Mezzenga, R. Amyloid–Polyphenol Hybrid Nanofilaments Mitigate Colitis and Regulate Gut Microbial Dysbiosis. ACS Nano 2020, 14, 2760–2776. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Aβ Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef]
- Xu, J.; Xu, J.; Shi, T.; Zhang, Y.; Chen, F.; Yang, C.; Guo, X.; Liu, G.; Shao, D.; Leong, K.W.; et al. Probiotic-Inspired Nanomedicine Restores Intestinal Homeostasis in Colitis by Regulating Redox Balance, Immune Responses, and the Gut Microbiome. Adv. Mater. 2023, 35, 2207890. [Google Scholar] [CrossRef]
- Li, L.; Cui, H.; Li, T.; Qi, J.; Chen, H.; Gao, F.; Tian, X.; Mu, Y.; He, R.; Lv, S.; et al. Synergistic Effect of Berberine-Based Chinese Medicine Assembled Nanostructures on Diarrhea-Predominant Irritable Bowel Syndrome In Vivo. Front. Pharmacol. 2020, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xu, H.; Liang, Y.; Xu, J.; Yue, N.; Zhang, Y.; Tian, C.; Yao, J.; Wang, L.; Nie, Y.; et al. Edible Exosome-like Nanoparticles from Portulaca Oleracea L Mitigate DSS-Induced Colitis via Facilitating Double-Positive CD4+CD8+T Cells Expansion. J. Nanobiotechnol. 2023, 21, 309. [Google Scholar] [CrossRef]
- Ma, L.; Lyu, W.; Song, Y.; Chen, K.; Lv, L.; Yang, H.; Wang, W.; Xiao, Y. Anti-Inflammatory Effect of Clostridium Butyricum -Derived Extracellular Vesicles in Ulcerative Colitis: Impact on Host microRNAs Expressions and Gut Microbiome Profiles. Mol. Nutr. Food Res. 2023, 67, 2200884. [Google Scholar] [CrossRef]
- Chandrarathna, H.P.S.U.; Liyanage, T.D.; Edirisinghe, S.L.; Dananjaya, S.H.S.; Thulshan, E.H.T.; Nikapitiya, C.; Oh, C.; Kang, D.H.; De Zoysa, M. Marine Microalgae, Spirulina Maxima-Derived Modified Pectin and Modified Pectin Nanoparticles Modulate the Gut Microbiota and Trigger Immune Responses in Mice. Mar. Drugs 2020, 18, 175. [Google Scholar] [CrossRef]
- Bantun, F.; Singh, R.; Alkhanani, M.F.; Almalki, A.H.; Alshammary, F.; Khan, S.; Haque, S.; Srivastava, M. Gut Microbiome Interactions with Graphene Based Nanomaterials: Challenges and Opportunities. Sci. Total Environ. 2022, 830, 154789. [Google Scholar] [CrossRef]
- Yong, C.; Valiyaveettil, S.; Tang, B. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Bazeli, J.; Banikazemi, Z.; Hamblin, M.R.; Sharafati Chaleshtori, R. Could Probiotics Protect against Human Toxicity Caused by Polystyrene Nanoplastics and Microplastics? Front. Nutr. 2023, 10, 1186724. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Chen, Q.; Su, S.; Zhuang, J.; Qiao, D. Polystyrene Micro- and Nanoparticles Exposure Induced Anxiety-like Behaviors, Gut Microbiota Dysbiosis and Metabolism Disorder in Adult Mice. Ecotoxicol. Environ. Saf. 2023, 259, 115000. [Google Scholar] [CrossRef]
- Scarcello, E.; Sofranko, A.; Wahle, T.; Schins, R.P.F. Neurotoxicity of Engineered Nanomaterials: Testing Considerations. Front. Public Health 2022, 10, 904544. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.G.; Chiu, M.; Taurino, G.; Bergamaschi, E.; Turroni, F.; Mancabelli, L.; Longhi, G.; Ventura, M.; Bussolati, O. Amorphous Silica Nanoparticles and the Human Gut Microbiota: A Relationship with Multiple Implications. J. Nanobiotechnol. 2024, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Ghebretatios, M.; Schaly, S.; Prakash, S. Nanoparticles in the Food Industry and Their Impact on Human Gut Microbiome and Diseases. Int. J. Mol. Sci. 2021, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.; Hahn, D.; Ossig, R.; Ritz, S.; Sauer, L.; Buesen, R.; Rehm, S.; Wohlleben, W.; Groeters, S.; Strauss, V.; et al. Gut Microbiome and Plasma Metabolome Changes in Rats after Oral Gavage of Nanoparticles: Sensitive Indicators of Possible Adverse Health Effects. Part. Fibre Toxicol. 2022, 19, 21. [Google Scholar] [CrossRef]
- Meier, M.J.; Nguyen, K.C.; Crosthwait, J.; Kawata, A.; Rigden, M.; Leingartner, K.; Wong, A.; Holloway, A.; Shwed, P.S.; Beaudette, L.; et al. Low Dose Antibiotic Ingestion Potentiates Systemic and Microbiome Changes Induced by Silver Nanoparticles. NanoImpact 2021, 23, 100343. [Google Scholar] [CrossRef]
- Muhammad, A.; He, J.; Yu, T.; Sun, C.; Shi, D.; Jiang, Y.; Xianyu, Y.; Shao, Y. Dietary exposure of copper and zinc oxides nanoparticles affect the fitness, enzyme activity, and microbial community of the model insect, silkworm Bombyx mori. Sci. Total Environ. 2022, 813, 152608. [Google Scholar] [CrossRef]
- Yan, J.; Chen, Q.; Tian, L.; Li, K.; Lai, W.; Bian, L.; Han, J.; Jia, R.; Liu, X.; Xi, Z. Intestinal Toxicity of Micro- and Nano-Particles of Foodborne Titanium Dioxide in Juvenile Mice: Disorders of Gut Microbiota–Host Co-Metabolites and Intestinal Barrier Damage. Sci. Total Environ. 2022, 821, 153279. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Evariste, L.; Houdeau, E. Dysregulation along the Gut Microbiota-Immune System Axis after Oral Exposure to Titanium Dioxide Nanoparticles: A Possible Environmental Factor Promoting Obesity-Related Metabolic Disorders. Environ. Pollut. 2023, 330, 121795. [Google Scholar] [CrossRef]
- Zhang, T.; Li, D.; Zhu, X.; Zhang, M.; Guo, J.; Chen, J. Nano-Al2O3 Particles Affect Gut Microbiome and Resistome in an in Vitro Simulator of the Human Colon Microbial Ecosystem. J. Hazard. Mater. 2022, 439, 129513. [Google Scholar] [CrossRef]
- Askri, D.; Ouni, S.; Galai, S.; Chovelon, B.; Arnaud, J.; Sturm, N.; Lehmann, S.G.; Sakly, M.; Amara, S.; Sève, M. Nanoparticles in Foods? A Multiscale Physiopathological Investigation of Iron Oxide Nanoparticle Effects on Rats after an Acute Oral Exposure: Trace Element Biodistribution and Cognitive Capacities. Food Chem. Toxicol. 2019, 127, 173–181. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Yadav, B.; Rosen, L.; Nagpal, R.; Yadav, H.; Yadav, J.S. Crosstalk between Gut Microbiota and Lung Inflammation in Murine Toxicity Models of Respiratory Exposure or Co-Exposure to Carbon Nanotube Particles and Cigarette Smoke Extract. Toxicol. Appl. Pharmacol. 2022, 447, 116066. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, D.V.; Jacobsen, N.R.; Andersen, M.H.G.; Connell, S.P.; Barfod, K.K.; Thomsen, M.B.; Miller, M.R.; Duffin, R.; Lykkesfeldt, J.; Vogel, U.; et al. Cardiovascular Health Effects of Oral and Pulmonary Exposure to Multi-Walled Carbon Nanotubes in ApoE-Deficient Mice. Toxicology 2016, 371, 29–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Liu, Y.; Chen, X.; Wang, C.; Chen, X.; Liu, W.; Huang, K.; Chen, H.; Yang, J. Multi-Walled Carbon Nanotubes Exacerbate Doxorubicin-Induced Cardiotoxicity by Altering Gut Microbiota and Pulmonary and Colonic Macrophage Phenotype in Mice. Toxicology 2020, 435, 152410. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Hong, S.Y.; Wang, H.M.; Shi, Y.; Wang, P.; Wang, X.J.; Jiang, Q.Y.; Yang, K.D.; Chen, W.; Xu, X.L. The Subacute Toxicity and Underlying Mechanisms of Biomimetic Mesoporous Polydopamine Nanoparticles. Part. Fibre Toxicol. 2023, 20, 38. [Google Scholar] [CrossRef]
- Chaplin, A.; Gao, H.; Asase, C.; Rengasamy, P.; Park, B.; Skander, D.; Bebek, G.; Rajagopalan, S.; Maiseyeu, A. Systemically-Delivered Biodegradable PLGA Alters Gut Microbiota and Induces Transcriptomic Reprogramming in the Liver in an Obesity Mouse Model. Sci. Rep. 2020, 10, 13786. [Google Scholar] [CrossRef]
- Lin, X.; Xie, H.; Zhang, Y.; Tian, X.; Cui, L.; Shi, N.; Wang, L.; Zhao, J.; An, L.; Wang, J.; et al. The Toxicity of Nano Polyethylene Terephthalate to Mice: Intestinal Obstruction, Growth Retardant, Gut Microbiota Dysbiosis and Lipid Metabolism Disorders. Food Chem. Toxicol. 2023, 172, 113585. [Google Scholar] [CrossRef]
- Aniwan, S.; Santiago, P.; Loftus, E.V.; Park, S.H. The Epidemiology of Inflammatory Bowel Disease in Asia and Asian Immigrants to Western Countries. United Eur. Gastroenterol. J. 2022, 10, 1063–1076. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, X.; Xia, W.; Li, X.; Wang, S.; Ye, S.; Tian, L.; Zhao, L.; Ai, F.; Shen, Z.; et al. Evolving Trends and Burden of Inflammatory Bowel Disease in Asia, 1990–2019: A Comprehensive Analysis Based on the Global Burden of Disease Study. J. Epidemiol. Glob. Health 2023, 13, 725–739. [Google Scholar] [CrossRef]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.Y.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-First Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Liu, J.; Di, B.; Xu, L. Recent Advances in the Treatment of IBD: Targets, Mechanisms and Related Therapies. Cytokine Growth Factor Rev. 2023, 71–72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome—Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Shalon, D.; Culver, R.N.; Grembi, J.A.; Folz, J.; Treit, P.V.; Shi, H.; Rosenberger, F.A.; Dethlefsen, L.; Meng, X.; Yaffe, E.; et al. Profiling the Human Intestinal Environment under Physiological Conditions. Nature 2023, 617, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cao, G.; Luo, C.; Tan, D.; Vong, C.T.; Xu, Y.; Wang, S.; Lu, H.; Wang, Y.; Jing, W. Emerging Pharmacotherapy for Inflammatory Bowel Diseases. Pharmacol. Res. 2022, 178, 106146. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, S.; Moayedi, S.; Mohajeri, S.; Yadegar, A.; Haririan, I. Targeting Pathophysiological Changes Using Biomaterials-Based Drug Delivery Systems: A Key to Managing Inflammatory Bowel Disease. Front. Pharmacol. 2022, 13, 1045575. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Chen, Y.; Liu, X.; Tian, S.; Zhao, L.; Bai, Y.; Wang, H.; Lin, J.; Jiang, D.; Lei, Z.; et al. Targeted Computed Tomography Visualization and Healing of Inflammatory Bowel Disease by Orally Delivered Bacterial-Flagella-Inspired Polydiiododiacetylene Nanofibers. ACS Nano 2023, 17, 3873–3888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Hua, Z.; Xing, S.; Li, J.; Fei, S.; Tan, M. ROS-Triggered Self-Disintegrating and pH-Responsive Astaxanthin Nanoparticles for Regulating the Intestinal Barrier and Colitis. Biomaterials 2023, 292, 121937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Q.; Shan, J.; Xing, J.; Liu, X.; Ma, Y.; Qian, H.; Chen, X.; Wang, X.; Wu, L.M.; et al. Multifunctional Two-Dimensional Bi2Se3 Nanodiscs for Anti-Inflammatory Therapy of Inflammatory Bowel Diseases. Acta Biomater. 2023, 160, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Alkushi, A.G.; Elazab, S.T.; Abdelfattah-Hassan, A.; Mahfouz, H.; Salem, G.A.; Sheraiba, N.I.; Mohamed, E.A.A.; Attia, M.S.; El-Shetry, E.S.; Saleh, A.A.; et al. Multi-Strain-Probiotic-Loaded Nanoparticles Reduced Colon Inflammation and Orchestrated the Expressions of Tight Junction, NLRP3 Inflammasome and Caspase-1 Genes in DSS-Induced Colitis Model. Pharmaceutics 2022, 14, 1183. [Google Scholar] [CrossRef]
- Alkushi, A.G.; Abdelfattah-Hassan, A.; Eldoumani, H.; Elazab, S.T.; Mohamed, S.A.M.; Metwally, A.S.; El-Shetry, E.S.; Saleh, A.A.; ElSawy, N.A.; Ibrahim, D. Probiotics-Loaded Nanoparticles Attenuated Colon Inflammation, Oxidative Stress, and Apoptosis in Colitis. Sci. Rep. 2022, 12, 5116. [Google Scholar] [CrossRef]
- Li, J.; Hou, W.; Lin, S.; Wang, L.; Pan, C.; Wu, F.; Liu, J. Polydopamine Nanoparticle-Mediated Dopaminergic Immunoregulation in Colitis. Adv. Sci. 2022, 9, 2104006. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular Vesicles and Nanoparticles: Emerging Complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Hill, A.F. Extracellular Vesicles and Neurodegenerative Diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Xu, J.; Xu, H.; Zhu, M.; Liang, Y.; Zhang, Y.; Tian, C.; Nie, Y.; Shi, R.; et al. Extracellular Vesicles: The Next Generation Theranostic Nanomedicine for Inflammatory Bowel Disease. Int. J. Nanomed. 2022, 17, 3893–3911. [Google Scholar] [CrossRef]
- Kim, S.H.; Keum, B.; Kwak, S.; Byun, J.; Shin, J.M.; Kim, T.H. Therapeutic Applications of Extracellular Vesicles in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 745. [Google Scholar] [CrossRef] [PubMed]
- Olovo, C.V.; Wiredu Ocansey, D.K.; Ji, Y.; Huang, X.; Xu, M. Bacterial Membrane Vesicles in the Pathogenesis and Treatment of Inflammatory Bowel Disease. Gut Microbes 2024, 16, 2341670. [Google Scholar] [CrossRef]
- Gul, L.; Modos, D.; Fonseca, S.; Madgwick, M.; Thomas, J.P.; Sudhakar, P.; Booth, C.; Stentz, R.; Carding, S.R.; Korcsmaros, T. Extracellular Vesicles Produced by the Human Commensal Gut Bacterium Bacteroides Thetaiotaomicron Affect Host Immune Pathways in a Cell-type Specific Manner That Are Altered in Inflammatory Bowel Disease. J. Extracell. Vesicles 2022, 11, e12189. [Google Scholar] [CrossRef]
- Wei, Z.; Hang, S.; Wiredu Ocansey, D.K.; Zhang, Z.; Wang, B.; Zhang, X.; Mao, F. Human Umbilical Cord Mesenchymal Stem Cells Derived Exosome Shuttling Mir-129-5p Attenuates Inflammatory Bowel Disease by Inhibiting Ferroptosis. J. Nanobiotechnol. 2023, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral Administration of Turmeric-Derived Exosome-like Nanovesicles with Anti-Inflammatory and pro-Resolving Bioactions for Murine Colitis Therapy. J. Nanobiotechnol. 2022, 20, 206. [Google Scholar] [CrossRef]
- Sriwastva, M.K.; Deng, Z.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like Nanoparticles from Mulberry Bark Prevent DSS-induced Colitis via the AhR/COPS8 Pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Zhang, Y.; Belaid, M.; Luo, X.; Daci, A.; Limani, R.; Mantaj, J.; Zilbauer, M.; Nayak, K.; Vllasaliu, D. Probing Milk Extracellular Vesicles for Intestinal Delivery of RNA Therapies. J. Nanobiotechnol. 2023, 21, 406. [Google Scholar] [CrossRef]
- Garbati, P.; Picco, C.; Magrassi, R.; Signorello, P.; Cacopardo, L.; Dalla Serra, M.; Faticato, M.G.; De Luca, M.; Balestra, F.; Scavo, M.P.; et al. Targeting the Gut: A Systematic Review of Specific Drug Nanocarriers. Pharmaceutics 2024, 16, 431. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent Advances in Extracellular Vesicles for Therapeutic Cargo Delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
- Guo, J.; Wang, F.; Hu, Y.; Luo, Y.; Wei, Y.; Xu, K.; Zhang, H.; Liu, H.; Bo, L.; Lv, S.; et al. Exosome-Based Bone-Targeting Drug Delivery Alleviates Impaired Osteoblastic Bone Formation and Bone Loss in Inflammatory Bowel Diseases. Cell Rep. Med. 2023, 4, 100881. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, B.W.; Sun, R.; Li, X.; Wu, D.; Hu, J.N. Colon-Targeting Angelica Sinensis Polysaccharide Nanoparticles with Dual Responsiveness for Alleviation of Ulcerative Colitis. ACS Appl. Mater. Interfaces 2023, 15, 26298–26315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.; Liu, R.; Zhao, M.; Ding, X.; Zhang, T.; He, R.; Zhu, S.; Dong, X.; Xie, J.; et al. Adhesive Ergothioneine Hyaluronate Gel Protects against Radiation Gastroenteritis by Alleviating Apoptosis, Inflammation, and Gut Microbiota Dysbiosis. ACS Appl. Mater. Interfaces 2023, 15, 19833–19846. [Google Scholar] [CrossRef]
- Deng, L.; Zeng, H.; Hu, X.; Xiao, M.; He, D.; Zhang, Y.; Jin, Y.; Hu, Y.; Zhu, Y.; Gong, L.; et al. Se@Albumin Nanoparticles Ameliorate Intestinal Mucositis Caused by Cisplatin via Gut Microbiota-Targeted Regulation. Nanoscale 2021, 13, 11250–11261. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The Microbiome and Human Cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Chervin, C.S.; Gajewski, T.F. Cancer and the Microbiome—Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology 2021, 160, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dai, X.; Zhou, C.C.; Li, K.; Zhang, Y.; Lou, X.Y.; Zhu, Y.M.; Sun, Y.L.; Peng, B.X.; Cui, W. Integrated Analysis of the Faecal Metagenome and Serum Metabolome Reveals the Role of Gut Microbiome-Associated Metabolites in the Detection of Colorectal Cancer and Adenoma. Gut 2022, 71, 1315–1325. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aggarwal, P.; Costa, R.G.F.; Cole, A.M.; Trinchieri, G. Targeting the Gut Microbiota for Cancer Therapy. Nat. Rev. Cancer 2022, 22, 703–722. [Google Scholar] [CrossRef] [PubMed]
- Gubernatorova, E.O.; Gorshkova, E.A.; Bondareva, M.A.; Podosokorskaya, O.A.; Sheynova, A.D.; Yakovleva, A.S.; Bonch-Osmolovskaya, E.A.; Nedospasov, S.A.; Kruglov, A.A.; Drutskaya, M.S. Akkermansia Muciniphila-Friend or Foe in Colorectal Cancer? Front. Immunol. 2023, 14, 1303795. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, N.; Li, Q.; Chu, E.S.H.; Lau, H.C.H.; Fong, W.; Liu, W.; Liang, C.; Nakatsu, G.; Su, A.C.Y.; Coker, O.O.; et al. Lactobacillus Gallinarum Modulates the Gut Microbiota and Produces Anti-Cancer Metabolites to Protect against Colorectal Tumourigenesis. Gut 2022, 71, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Su, H.; Liu, D.; Wong, C.C.; Shang, H.; Fang, Y.; Zeng, X.; Chen, H.; Li, Y.; Huang, Z.; et al. Traditional Medicine Pien Tze Huang Suppresses Colorectal Tumorigenesis Through Restoring Gut Microbiota and Metabolites. Gastroenterology 2023, 165, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng Polysaccharides Alter the Gut Microbiota and Kynurenine/Tryptophan Ratio, Potentiating the Antitumour Effect of Antiprogrammed Cell Death 1/Programmed Cell Death Ligand 1 (Anti-PD-1/PD-L1) Immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, H.; Qi, J.; Ma, K.; Luo, Y.; Weng, L. Interactions of Nanomaterials with Gut Microbiota and Their Applications in Cancer Therapy. Sensors 2023, 23, 4428. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Zhu, R.; Zhu, X.; Yan, W.; Li, Y.; Zhai, Y.; Wu, T.; Huang, X.; Yin, Q.; Li, Y. Combining Gut Microbiota Modulation and Chemotherapy by Capecitabine-Loaded Prebiotic Nanoparticle Improves Colorectal Cancer Therapy. Nat. Commun. 2023, 14, 4746. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Pan, P.; Zheng, D.W.; Bao, P.; Zeng, X.; Zhang, X.Z. Bioinorganic Hybrid Bacteriophage for Modulation of Intestinal Microbiota to Remodel Tumor-Immune Microenvironment against Colorectal Cancer. Sci. Adv. 2020, 6, eaba1590. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cha, R.; Hao, W.; Du, R.; Zhang, P.; Hu, Y.; Jiang, X. Nanocrystalline Cellulose Cures Constipation via Gut Microbiota Metabolism. ACS Nano 2022, 16, 16481–16496. [Google Scholar] [CrossRef]
- Duan, T.; Wang, X.; Dong, X.; Wang, C.; Wang, L.; Yang, X.; Li, T. Broccoli-Derived Exosome-like Nanoparticles Alleviate Loperamide-Induced Constipation, in Correlation with Regulation on Gut Microbiota and Tryptophan Metabolism. J. Agric. Food Chem. 2023, 71, 16568–16580. [Google Scholar] [CrossRef]
- Zhi, X.; Liu, Y.; Lin, L.; Yang, M.; Zhang, L.; Zhang, L.; Liu, Y.; Alfranca, G.; Ma, L.; Zhang, Q.; et al. Oral pH Sensitive GNS@ab Nanoprobes for Targeted Therapy of Helicobacter Pylori without Disturbance Gut Microbiome. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102019. [Google Scholar] [CrossRef] [PubMed]
- Arifa, R.D.N.; De Paula, T.P.; Lima, R.L.; Brito, C.B.; Andrade, M.E.R.; Cardoso, V.N.; Pinheiro, M.V.B.; Ladeira, L.O.; Krambrock, K.; Teixeira, M.M.; et al. Anti-Inflammatory and Antioxidant Effects of the Nanocomposite Fullerol Decrease the Severity of Intestinal Inflammation Induced by Gut Ischemia and Reperfusion. Eur. J. Pharmacol. 2021, 898, 173984. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bai, M.; Xu, K.; Zhou, J.; Zhang, X.; Yu, R.; Huang, R.; Yin, Y. Effects of Different Concentrations of Coated Nano Zinc Oxide Material on Fecal Bacterial Composition and Intestinal Barrier in Weaned Piglets. J. Sci. Food Agric. 2021, 101, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, J.; Sun, M.; Chen, H.; Shi, X.; You, F.; Qiao, S. Polymeric Nanohybrids Engineered by Chitosan Nanoparticles and Antimicrobial Peptides as Novel Antimicrobials in Food Biopreservatives: Risk Assessment and Anti-Foodborne Pathogen Escherichia Coli O157:H7 Infection by Immune Regulation. J. Agric. Food Chem. 2022, 70, 12535–12549. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Lu, F.; Du, X.; Zhang, M.; Zhang, Y.; Ma, Y.; Zhao, Y.; Huang, H.; Kang, Z. Green Carbon Dots Derived from Atractylodes Macrocephala: A Potential Nanodrug for Treating Alcoholic Gastric Ulcer. Colloids Surf. B Biointerfaces 2023, 230, 113492. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zheng, Y.; Zhang, Y.; Li, Z.; Cui, Z.; Jiang, H.; Zhu, S.; Wu, S. Ultrasmall Cortex Moutan Nanoclusters for the Therapy of Pneumonia and Colitis. Adv. Healthc. Mater. 2023, 12, 2300402. [Google Scholar] [CrossRef]
- Su, X.; Jing, X.; Jiang, W.; Li, M.; Liu, K.; Teng, M.; Wang, D.; Meng, L.; Zhang, Y.; Ji, W. Curcumin-Containing Polyphosphazene Nanodrug for Anti-Inflammation and Nerve Regeneration to Improve Functional Recovery after Spinal Cord Injury. Int. J. Pharm. 2023, 642, 123197. [Google Scholar] [CrossRef]
- Liao, W.; Ni, C.; Ge, R.; Li, Y.; Jiang, S.; Yang, W.; Yan, F. Nel-like Molecule Type 1 Combined with Gold Nanoparticles Modulates Macrophage Polarization, Osteoclastogenesis, and Oral Microbiota in Periodontitis. ACS Appl. Mater. Interfaces 2024, 16, 8442–8458. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, H.; Yang, C.; Du, H.; Wang, H.; Tu, J. Effects of Chitosan Nanoparticle Supplementation on Growth Performance, Humoral Immunity, Gut Microbiota and Immune Responses after Lipopolysaccharide Challenge in Weaned Pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 597–605. [Google Scholar] [CrossRef]
- Yang, L.; Cui, Y.; Liang, H.; Li, Z.; Wang, N.; Wang, Y.; Zheng, G. Multifunctional Selenium Nanoparticles with Different Surface Modifications Ameliorate Neuroinflammation through the Gut Microbiota-NLRP3 Inflammasome-Brain Axis in APP/PS1 Mice. ACS Appl. Mater. Interfaces 2022, 14, 30557–30570. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Amin, M.A.; Mohamed, A.E.A.; Edris, A.E.; Sharaf, O.M.; Mabrok, H.B.; Ramadan, A.A. Basil Essential Oil and Its Nanoemulsion Mitigate Non-Alcoholic Steatohepatitis in Rat Model with Special Reference to Gut Microbiota. J. Oleo Sci. 2020, 69, 913–927. [Google Scholar] [CrossRef]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S.; et al. Garlic Exosome-like Nanoparticles Reverse High-Fat Diet Induced Obesity via the Gut/Brain Axis. Theranostics 2022, 12, 1220–1246. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, C.; Zhang, J.; Sun, M.; Xu, J.; Xu, C.; Kuang, H.; Xu, L. Chiral Nanoparticle-Remodeled Gut Microbiota Alleviates Neurodegeneration via the Gut–Brain Axis. Nat. Aging 2023, 3, 1415–1429. [Google Scholar] [CrossRef]
- Liu, N.; Yang, C.; Liang, X.; Cao, K.; Xie, J.; Luo, Q.; Luo, H. Mesoporous Silica Nanoparticle-Encapsulated Bifidobacterium Attenuates Brain Aβ Burden and Improves Olfactory Dysfunction of APP/PS1 Mice by Nasal Delivery. J. Nanobiotechnol. 2022, 20, 439. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Li, Z.; Wu, X.; Mei, J.; Zheng, G. Brain Targeted Peptide-Functionalized Chitosan Nanoparticles for Resveratrol Delivery: Impact on Insulin Resistance and Gut Microbiota in Obesity-Related Alzheimer’s Disease. Carbohydr. Polym. 2023, 310, 120714. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; Dai, Q.; Tan, J.; Dou, C.; Luo, F. Gold-Nanosphere Mitigates Osteoporosis through Regulating TMAO Metabolism in a Gut Microbiota-Dependent Manner. J. Nanobiotechnol. 2023, 21, 125. [Google Scholar] [CrossRef]
- Gątarek, P.; Kałużna-Czaplińska, J. Trimethylamine N-Oxide (TMAO) in Human Health. EXCLI J. 2021, 20, excli2020–excli3239. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Teng, Y.; Mu, J.; Sriwastva, M.K.; Zhang, L.; Hood, J.L.; Yan, J.; Zhang, X.; Park, J.W.; et al. Ginger Nanoparticles Mediated Induction of Foxa2 Prevents High-Fat Diet-Induced Insulin Resistance. Theranostics 2022, 12, 1388–1403. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Li, S.; Liang, X.; Zhang, Y.; Wang, Y.; Liu, Y. Sustained Oral Intake of Nano-Iron Oxide Perturbs the Gut-Liver Axis. NanoImpact 2023, 30, 100464. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhan, M.; Wen, Y.; Chen, Y.; Zhang, Z.; Wang, S.; Tian, D.; Tian, S. Recent Progress of Oral Functional Nanomaterials for Intestinal Microbiota Regulation. Pharmaceutics 2024, 16, 921. https://doi.org/10.3390/pharmaceutics16070921
Li W, Zhan M, Wen Y, Chen Y, Zhang Z, Wang S, Tian D, Tian S. Recent Progress of Oral Functional Nanomaterials for Intestinal Microbiota Regulation. Pharmaceutics. 2024; 16(7):921. https://doi.org/10.3390/pharmaceutics16070921
Chicago/Turabian StyleLi, Wanneng, Minle Zhan, Yue Wen, Yu Chen, Zhongchao Zhang, Shuhui Wang, Dean Tian, and Sidan Tian. 2024. "Recent Progress of Oral Functional Nanomaterials for Intestinal Microbiota Regulation" Pharmaceutics 16, no. 7: 921. https://doi.org/10.3390/pharmaceutics16070921
APA StyleLi, W., Zhan, M., Wen, Y., Chen, Y., Zhang, Z., Wang, S., Tian, D., & Tian, S. (2024). Recent Progress of Oral Functional Nanomaterials for Intestinal Microbiota Regulation. Pharmaceutics, 16(7), 921. https://doi.org/10.3390/pharmaceutics16070921






