Use of Drug Sensitisers to Improve Therapeutic Index in Cancer
Abstract
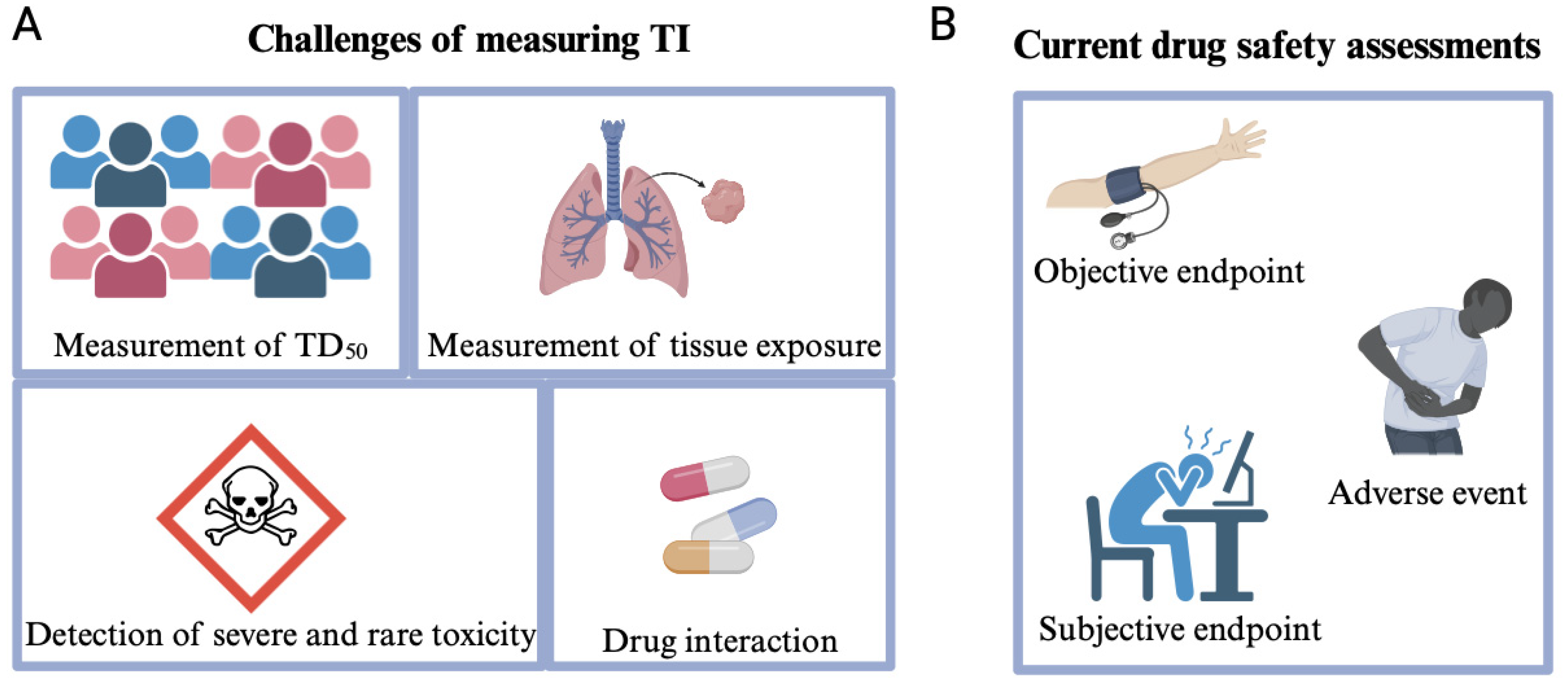
1. Therapeutic Index and Its Use in the Pharmaceutical Sector
2. Strategies to Improve Therapeutic Index in Cancer Treatment
2.1. Modification of Drug Delivery System
2.2. Administration of Multiple Anti-Cancerous Drugs
2.2.1. Combinations of Drugs Targeting Different Pathways
2.2.2. Modification of Drug Delivery into a Multi-Agent Delivery System
2.2.3. Combination of Different Therapies
2.2.4. Drug Interactions and Polypharmacy in Cancer
Pharmacokinetic Interactions
Pharmacodynamic Interactions
Other Interactions
2.3. Drug Repurposing
2.4. Application of Drug Sensitising Agents
2.4.1. Piperine
2.4.2. Amifostine
- (1)
- Radiation therapy: The oxidation tension and haemoglobin saturation caused by radiation therapy induce the oxidation of WR-1065 and HIF activation in normal tissues, activating the cytoprotection [185].
- (2)
- Chemotherapy: Several clinical studies suggest that amifostine does not interfere with the antineoplastic efficacy of different chemotherapeutic agents. The main function of amifostine is to protect the normal tissues under high doses of cisplatin in melanoma patients [185]. In addition, another study also points out that amifostine could further prolong the half-life of platinum-based chemotherapy which could increase the exposure of tumours to chemotherapeutic agents [186].
2.4.3. Tariquidar
2.4.4. Binary Weapon
| Drug Sensitiser | Co-Treated Drug | Description | Current Clinical Status | Functions | Effect on TI | Refs. |
|---|---|---|---|---|---|---|
| Piperine | (1) Rapamycin | (1) Activate the autophagy pathway. | (1) FDA-approved | Bioavailability enhancers | ED50 ⬇️ | [180,193] |
| (2) Nevirapine | (2) Inhibit CYP450 and UDP glucuronyl transferase. | (2) Not approved due to limited clinical trials; few ongoing clinical trials | [194] | |||
| (3) 5-FU | (3) Shorten the half-life of the drug. | (3) Under clinical investigation | [195] | |||
| Amifostine | Cisplatin | Cytoprotective agent used in chemotherapy. | FDA-approved, and it is used in clinic with cisplatin | Toxicity reducers | TD50 ⬆️ | [196,197] |
| Tariquidar | Docetaxel, Paclitaxel | Inhibit P-gp efflux transporter to enhance drug efficacy. | Under clinical investigation | Efflux inhibitors | ED50 ⬇️ | [187] |
| Binary weapon | Gemcitabine | Enhances the gemcitabine toxicity to pancreatic cancer cells by co-treatment but non-toxic by sole treatment. | Laboratory evidence | Enhance anti-cancer drug efficacy and specific to cancer cells | ED50 ⬇️ | [191] |
| Bovine lactoferrin | Cisplatin | Sensitise Cis-anti-neoplastic potency. | No clinical data | Enhance drug efficacy, immunomodulators | ED50 ⬇️ | [198] |
| Fedratinib | Vincristine | Fedratinib is a JAK2 inhibitor that sensitises P-gp-overexpressing drug-resistant cancer cells. | FDA-approved | Inhibits P-gp activity, inducing cytotoxicity and apoptosis in drug-resistant cancer cells | ED50 ⬇️ | [199] |
| MG132 | Idarubicin | Inhibit NF-κB regulations. | Under clinical investigation | Inhibition of NF-κB induces apoptosis of leukaemic stem cells and leaves normal cells viable | ED50 ⬇️ | [200,201] |
| Urolithin A (UroA)/UAS03 (UroA analogue) | 5-FU | Inhibit cancer cell viability, proliferation, and invasion in colon cancer cells. | Laboratory evidence | Enhance drug efficiency and inhibit the expression of related efflux transporters | ED50 ⬇️ | [202] |
2.5. Educating Patients
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Dowden, H.; Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov. 2019, 18, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 2016, 15, 817–818. [Google Scholar] [CrossRef]
- Takebe, T.; Imai, R.; Ono, S. The current status of drug discovery and development as originated in United States academia: The influence of industrial and academic collaboration on drug discovery and development. Clin. Transl. Sci. 2018, 11, 597–606. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Watkins, P.B. Drug safety sciences and the bottleneck in drug development. Clin. Pharmacol. Ther. 2011, 89, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Ray, A. Beyond debacle and debate: Developing solutions in drug safety. Nat. Rev. Drug Discov. 2009, 8, 775–779. [Google Scholar] [CrossRef] [PubMed]
- de Boisferon, M.H.; Benzaid, I.; Chatel, E.M.D.; Hoffmann, N.; Littlefield, B. Development of a panel of breast cancer patient-derived xenograft models (PDX) with estrogen independence and/or acquired resistance to endocrine treatment. Cancer Res. 2020, 80, 5281. [Google Scholar] [CrossRef]
- O’Donovan, B.; Rodgers, R.M.; Cox, A.R.; Krska, J. ‘You feel like you haven’t got any control’: A qualitative study of side effects from medicines. J. Patient Saf. Risk Manag. 2019, 24, 13–24. [Google Scholar] [CrossRef]
- Rudmann, D.G. On-target and off-target-based toxicologic effects. Toxicol. Pathol. 2013, 41, 310–314. [Google Scholar] [CrossRef]
- Lackey, L.; Thompson, G.; Eggers, S. FDA’s Benefit–Risk Framework for Human Drugs and Biologics: Role in Benefit–Risk Assessment and Analysis of Use for Drug Approvals. Ther. Innov. Regul. Sci. 2021, 55, 170–179. [Google Scholar] [CrossRef]
- Juhaeri, J. Benefit–risk evaluation: The past, present and future. Ther. Adv. Drug Saf. 2019, 10, 2042098619871180. [Google Scholar] [CrossRef]
- Smith, M.Y.; Benattia, I.; Strauss, C.; Bloss, L.; Jiang, Q. Structured benefit-risk assessment across the product lifecycle: Practical considerations. Ther. Innov. Regul. Sci. 2017, 51, 501–508. [Google Scholar] [CrossRef]
- Smith, M.Y.; van Til, J.; DiSantostefano, R.L.; Hauber, A.B.; Marsh, K. Quantitative Benefit-Risk Assessment: State of the Practice Within Industry. Ther. Innov. Regul. Sci. 2021, 55, 415–425. [Google Scholar] [CrossRef]
- Tamargo, J.; Le Heuzey, J.Y.; Mabo, P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur. J. Clin. Pharmacol. 2015, 71, 549–567. [Google Scholar] [CrossRef]
- Muller, P.Y.; Milton, M.N. The determination and interpretation of the therapeutic index in drug development. Nat. Rev. Drug Discov. 2012, 11, 751–761. [Google Scholar] [CrossRef]
- Wang, E.; Cesano, A.; Butterfield, L.H.; Marincola, F. Improving the therapeutic index in adoptive cell therapy: Key factors that impact efficacy. J. Immunother. Cancer 2020, 8, e001619. [Google Scholar] [CrossRef]
- Blix, H.S.; Viktil, K.K.; Moger, T.A.; Reikvam, A. Drugs with narrow therapeutic index as indicators in the risk management of hospitalised patients. Pharm Pr. 2010, 8, 50–55. [Google Scholar] [CrossRef]
- Tyson, R.J.; Park, C.C.; Powell, J.R.; Patterson, J.H.; Weiner, D.; Watkins, P.B.; Gonzalez, D. Precision Dosing Priority Criteria: Drug, Disease, and Patient Population Variables. Front. Pharmacol. 2020, 11, 420. [Google Scholar] [CrossRef]
- Habet, S. Narrow Therapeutic Index drugs: Clinical pharmacology perspective. J. Pharm. Pharmacol. 2021, 73, 1285–1291. [Google Scholar] [CrossRef]
- Cheifetz, A. Overview of Therapeutic Drug Monitoring of Biologic Agents in Patients With Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2017, 13, 556–559. [Google Scholar]
- Zylbersztajn, B.; Barraza, M.; Torres, J.P.; Morales, J. Therapeutic monitoring of antimicrobial agents in pediatrics. Review based on Latin American experiences. Rev. Chil. Infectol. 2018, 35, 22–28. [Google Scholar] [CrossRef]
- Stanley, T.H. Anesthesia for the 21st century. Bayl. Univ. Med. Cent. Proc. 2000, 13, 7–10. [Google Scholar] [CrossRef]
- Filozof, C.; Chow, S.C.; Dimick-Santos, L.; Chen, Y.F.; Williams, R.N.; Goldstein, B.J.; Sanyal, A. Clinical endpoints and adaptive clinical trials in precirrhotic nonalcoholic steatohepatitis: Facilitating development approaches for an emerging epidemic. Hepatol. Commun. 2017, 1, 577–585. [Google Scholar] [CrossRef]
- de Cacqueray, N.; Boujaafar, S.; Bille, E.; Moulin, F.; Gana, I.; Benaboud, S.; Hirt, D.; Beranger, A.; Toubiana, J.; Renolleau, S.; et al. Therapeutic Drug Monitoring of Antibiotics in Critically Ill Children: An Observational Study in a Pediatric Intensive Care Unit. Ther. Drug Monit. 2022, 44, 319–327. [Google Scholar] [CrossRef]
- Bottino, D.C.; Patel, M.; Kadakia, E.; Zhou, J.; Patel, C.; Neuwirth, R.; Iartchouk, N.; Brake, R.; Venkatakrishnan, K.; Chakravarty, A. Dose Optimization for Anticancer Drug Combinations: Maximizing Therapeutic Index via Clinical Exposure-Toxicity/Preclinical Exposure-Efficacy Modeling. Clin. Cancer Res. 2019, 25, 6633–6643. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yuan, D.; Wu, Y.; Cao, Y. Pharmacokinetics and pharmacodynamics modeling and simulation systems to support the development and regulation of liposomal drugs. Pharmaceutics 2019, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Gummadi, A.C.; Guddati, A.K. Genetic polymorphisms in pharmaceuticals and chemotherapy. World J. Oncol. 2021, 12, 149. [Google Scholar] [CrossRef]
- Capulli, A.K.; MacQueen, L.A.; O’Connor, B.B.; Dauth, S.; Parker, K.K. Acute pergolide exposure stiffens engineered valve interstitial cell tissues and reduces contractility in vitro. Cardiovasc. Pathol. 2016, 25, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.E.; Zimmerman, K.O.; Gonzalez, D.; Melloni, C.; Guptill, J.T.; Hill, K.D.; Wu, H.; Cohen-Wolkowiez, M. A Systematic Literature Review Approach to Estimate the Therapeutic Index of Selected Immunosuppressant Drugs After Renal Transplantation. Ther. Drug Monit. 2017, 39, 13–20. [Google Scholar] [CrossRef]
- Schmelas, C.; Grimm, D. Split Cas9, Not Hairs—Advancing the Therapeutic Index of CRISPR Technology. Biotechnol. J. 2018, 13, e1700432. [Google Scholar] [CrossRef]
- Hartl, D.; de Luca, V.; Kostikova, A.; Laramie, J.; Kennedy, S.; Ferrero, E.; Siegel, R.; Fink, M.; Ahmed, S.; Millholland, J.; et al. Translational precision medicine: An industry perspective. J. Transl. Med. 2021, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef]
- De Maria Marchiano, R.; Di Sante, G.; Piro, G.; Carbone, C.; Tortora, G.; Boldrini, L.; Pietragalla, A.; Daniele, G.; Tredicine, M.; Cesario, A.; et al. Translational Research in the Era of Precision Medicine: Where We Are and Where We Will Go. J. Pers. Med. 2021, 11, 216. [Google Scholar] [CrossRef]
- Stalidzans, E.; Zanin, M.; Tieri, P.; Castiglione, F.; Polster, A.; Scheiner, S.; Pahle, J.; Stres, B.; List, M.; Baumbach, J. Mechanistic modeling and multiscale applications for precision medicine: Theory and practice. Netw. Syst. Med. 2020, 3, 36–56. [Google Scholar] [CrossRef]
- Faulkner, E.; Holtorf, A.-P.; Liu, C.Y.; Lin, H.; Biltaj, E.; Brixner, D.; Barr, C.; Oberg, J.; Shandhu, G.; Siebert, U. Being precise about precision medicine: What should value frameworks incorporate to address precision medicine? A report of the personalized precision medicine special interest group. Value Health 2020, 23, 529–539. [Google Scholar] [CrossRef]
- Naithani, N.; Sinha, S.; Misra, P.; Vasudevan, B.; Sahu, R. Precision medicine: Concept and tools. Med. J. Armed Forces India 2021, 77, 249–257. [Google Scholar] [CrossRef]
- MacEachern, S.J.; Forkert, N.D. Machine learning for precision medicine. Genome 2021, 64, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Suwinski, P.; Ong, C.; Ling, M.H.; Poh, Y.M.; Khan, A.M.; Ong, H.S. Advancing personalized medicine through the application of whole exome sequencing and big data analytics. Front. Genet. 2019, 10, 49. [Google Scholar] [CrossRef]
- Hassan, M.; Awan, F.M.; Naz, A.; deAndres-Galiana, E.J.; Alvarez, O.; Cernea, A.; Fernandez-Brillet, L.; Fernandez-Martinez, J.L.; Kloczkowski, A. Innovations in Genomics and Big Data Analytics for Personalized Medicine and Health Care: A Review. Int. J. Mol. Sci. 2022, 23, 4645. [Google Scholar] [CrossRef]
- Sisodiya, S.M. Precision medicine and therapies of the future. Epilepsia 2021, 62 (Suppl. S2), S90–S105. [Google Scholar] [CrossRef]
- Lander, E.S. Cutting the Gordian helix—Regulating genomic testing in the era of precision medicine. N. Engl. J. Med. 2015, 372, 1185–1186. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Komuro, I. Precision medicine for heart failure based on molecular mechanisms: The 2019 ISHR Research Achievement Award Lecture. J. Mol. Cell. Cardiol. 2021, 152, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Marquet, P.; Cattaneo, D.; Svinarov, D.; Bergan, S.; Walson, P.; Vinks, A.A.; Shipkova, M. Therapeutic Drug Monitoring in the Era of Precision Medicine: Achievements, Gaps, and Perspectives-An Interview in Honor of Professor Charles Pippenger. Ther. Drug Monit. 2021, 43, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Edsjö, A.; Holmquist, L.; Geoerger, B.; Nowak, F.; Gomon, G.; Alix-Panabières, C.; Staaf, J.; Ploeger, C.; Lassen, U.; Le Tourneau, C. Precision cancer medicine: Concepts, current practice, and future developments. J. Intern. Med. 2023, 294, 455–481. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. Half of the UK population can expect a diagnosis of cancer. BMJ 2015, 350, h614. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Ormiston-Smith, N.; Sasieni, P.D. Trends in the lifetime risk of developing cancer in Great Britain: Comparison of risk for those born from 1930 to 1960. Br. J. Cancer 2015, 112, 943–947. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Pote, M.S.; Gacche, R.N. ATP-binding cassette efflux transporters and MDR in cancer. Drug Discov. Today 2023, 28, 103537. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updat. 2021, 59, 100796. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Stock, C.M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Dong, S.; Zhao, S.C.; Liu, K.; Tan, Y.; Jiang, X.; Assaraf, Y.G.; Qin, B.; Chen, Z.S.; Zou, C. Novel nanomedicines to overcome cancer multidrug resistance. Drug Resist. Updat. 2021, 58, 100777. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Song, D.; Xu, M.; Liebmen, M. New strategies for targeting drug combinations to overcome mutation-driven drug resistance. Semin. Cancer Biol. 2017, 42, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Thorat, S.S.; Gujar, K.N.; Karale, C.K. Bioenhancers from mother nature: An overview. Future J. Pharm. Sci. 2023, 9, 20. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Meganathan, P.; Tan, H.L.; Dahlan, N.A.; Ooi, L.-T.; Neerooa, B.N.H.M.; Essa, R.Z.; Shameli, K.; Teow, S.-Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels 2021, 7, 153. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Abribat, T. The rise and rise of drug delivery. Nat. Rev. Drug Discov. 2005, 4, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Jain, S.; Datta, M. Montmorillonite-alginate microspheres as a delivery vehicle for oral extended release of venlafaxine hydrochloride. J. Drug Deliv. Sci. Technol. 2016, 33, 149–156. [Google Scholar] [CrossRef]
- Beckwith, M.C.; Feddema, S.S.; Barton, R.G.; Graves, C. A guide to drug therapy in patients with enteral feeding tubes: Dosage form selection and administration methods. Hosp. Pharm. 2004, 39, 225–237. [Google Scholar] [CrossRef]
- Bruno, M.C.; Cristiano, M.C.; Celia, C.; d’Avanzo, N.; Mancuso, A.; Paolino, D.; Wolfram, J.; Fresta, M. Injectable drug delivery systems for osteoarthritis and rheumatoid arthritis. ACS Nano 2022, 16, 19665–19690. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.P.; Nguyen, M.K.; Pi, B.S.; Kim, M.S.; Chae, S.Y.; Lee, K.C.; Kim, B.S.; Kim, S.W.; Lee, D.S. Functionalized injectable hydrogels for controlled insulin delivery. Biomaterials 2008, 29, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Cicero, T.J.; Ellis, M.S.; Kasper, Z.A. Relative preferences in the abuse of immediate-release versus extended-release opioids in a sample of treatment-seeking opioid abusers. Pharmacoepidemiol. Drug Saf. 2017, 26, 56–62. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Roy, S.; Sen, C.K.; Guan, J. Injectable, highly flexible, and thermosensitive hydrogels capable of delivering superoxide dismutase. Biomacromolecules 2009, 10, 3306–3316. [Google Scholar] [CrossRef]
- Walters, K. Transdermal drug delivery. In Routes of Drug Administration: Topics in Pharmacy; Wright: Bristol, UK, 2013; Volume 2, p. 78. [Google Scholar]
- Østergaard, J.; Meng-Lund, E.; Larsen, S.W.; Larsen, C.; Petersson, K.; Lenke, J.; Jensen, H. Real-time UV imaging of nicotine release from transdermal patch. Pharm. Res. 2010, 27, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.N. Transdermal Drug Delivery: Approaches and Significance; Taylor & Francis: New York, NY, USA, 2012; pp. 1–2. [Google Scholar]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef]
- Nayak, K.; Choudhari, M.V.; Bagul, S.; Chavan, T.A.; Misra, M. Ocular drug delivery systems. In Drug Delivery Devices and Therapeutic Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 515–566. [Google Scholar]
- Nelson, H.S. Inhalation devices, delivery systems, and patient technique. Ann. Allergy Asthma Immunol. 2016, 117, 606–612. [Google Scholar] [CrossRef]
- Sorino, C.; Negri, S.; Spanevello, A.; Visca, D.; Scichilone, N. Inhalation therapy devices for the treatment of obstructive lung diseases: The history of inhalers towards the ideal inhaler. Eur. J. Intern. Med. 2020, 75, 15–18. [Google Scholar] [CrossRef]
- Rajgor, N.; Patel, M.; Bhaskar, V. Implantable Drug Delivery Systems: An Overview. Syst. Rev. Pharm. 2011, 2, 473–511. [Google Scholar] [CrossRef]
- Yasin, M.N.; Svirskis, D.; Seyfoddin, A.; Rupenthal, I.D. Implants for drug delivery to the posterior segment of the eye: A focus on stimuli-responsive and tunable release systems. J. Control. Release 2014, 196, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, M.J.; Banakar, U.V. Implantable drug delivery. Adv. Control. Deliv. Drugs 2022, 8, 21–58. [Google Scholar]
- Arslan, F.B.; Ozturk, K.; Calis, S. Antibody-mediated drug delivery. Int. J. Pharm. 2021, 596, 120268. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.M.; Devine, M.; DeRemer, D. Brentuximab vedotin: An anti-CD30 antibody–drug conjugate. Am. J. Health-Syst. Pharm. 2013, 70, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.T.; Chen, Y.; Marhoul, J.; Jacobson, F. Statistical modeling of the drug load distribution on trastuzumab emtansine (Kadcyla), a lysine-linked antibody drug conjugate. Bioconjugate Chem. 2014, 25, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.A.; Landín, M.; Aquino, R.P. Technologies and formulation design of polysaccharide-based hydrogels for drug delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T. The principles of cancer treatment—Changes in chemotherapy. Gan Kagaku Ryoho 2002, 29, 1263–1278. [Google Scholar]
- Espinosa, E.; Zamora, P.; Feliu, J.; Gonzalez Baron, M. Classification of anticancer drugs—A new system based on therapeutic targets. Cancer Treat. Rev. 2003, 29, 515–523. [Google Scholar] [CrossRef] [PubMed]
- McClean, S.; Costelloe, C.; Denny, W.A.; Searcey, M.; Wakelin, L.P. Sequence selectivity, cross-linking efficiency and cytotoxicity of DNA-targeted 4-anilinoquinoline aniline mustards. Anticancer. Drug Des. 1999, 14, 187–204. [Google Scholar]
- Lajous, H.; Lelievre, B.; Vauleon, E.; Lecomte, P.; Garcion, E. Rethinking Alkylating(-Like) Agents for Solid Tumor Management. Trends Pharmacol. Sci. 2019, 40, 342–357. [Google Scholar] [CrossRef]
- Kaye, S.B. New antimetabolites in cancer chemotherapy and their clinical impact. Br. J. Cancer 1998, 78 (Suppl. S3), 1–7. [Google Scholar] [CrossRef]
- Rassy, E.; Rached, L.; Pistilli, B. Antibody drug conjugates targeting HER2: Clinical development in metastatic breast cancer. Breast 2022, 66, 217–226. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Burgess, D.J.; Doles, J.; Zender, L.; Xue, W.; Ma, B.; McCombie, W.R.; Hannon, G.J.; Lowe, S.W.; Hemann, M.T. Topoisomerase levels determine chemotherapy response in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 9053–9058. [Google Scholar] [CrossRef]
- Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Mukherjee, S. Cancer therapy using antibiotics. J. Cancer Ther. 2015, 6, 849. [Google Scholar] [CrossRef]
- Madabhavi, I.; Modi, G.; Patel, A.; Anand, A.; Panchal, H.; Parikh, S. Pulmonary toxicity following bleomycin use: A single-center experience. J. Cancer Res. Ther. 2017, 13, 466–470. [Google Scholar] [CrossRef]
- Yan, V.C.; Butterfield, H.E.; Poral, A.H.; Yan, M.J.; Yang, K.L.; Pham, C.D.; Muller, F.L. Why Great Mitotic Inhibitors Make Poor Cancer Drugs. Trends Cancer 2020, 6, 924–941. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Luangmonkong, T.; Mangmool, S.; Kurose, H. Therapeutic targets for the treatment of cardiac fibrosis and cancer: Focusing on TGF-β signaling. Front. Cardiovasc. Med. 2020, 7, 34. [Google Scholar] [CrossRef]
- Yamaoka, T.; Kusumoto, S.; Ando, K.; Ohba, M.; Ohmori, T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3491. [Google Scholar] [CrossRef]
- Alves, R.; Gonçalves, A.C.; Rutella, S.; Almeida, A.M.; De Las Rivas, J.; Trougakos, I.P.; Sarmento Ribeiro, A.B. Resistance to tyrosine kinase inhibitors in chronic myeloid leukemia—From molecular mechanisms to clinical relevance. Cancers 2021, 13, 4820. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Kelloff, G. Seeing into cells: The promise of in vivo molecular imaging in oncology. EMBO Rep. 2005, 6, 292–296. [Google Scholar] [CrossRef]
- Hevener, K.; Verstak, T.A.; Lutat, K.E.; Riggsbee, D.L.; Mooney, J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B 2018, 8, 844–861. [Google Scholar] [CrossRef]
- Talukdar, A.; Kundu, B.; Sarkar, D.; Goon, S.; Mondal, M.A. Topoisomerase I inhibitors: Challenges, progress and the road ahead. Eur. J. Med. Chem. 2022, 236, 114304. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Dietrich, J.; Rao, K.; Pastorino, S.; Kesari, S. Corticosteroids in brain cancer patients: Benefits and pitfalls. Expert. Rev. Clin. Pharmacol. 2011, 4, 233–242. [Google Scholar] [CrossRef]
- Goodman, R.S.; Johnson, D.B.; Balko, J.M. Corticosteroids and Cancer Immunotherapy. Clin. Cancer Res. 2023, 29, 2580–2587. [Google Scholar] [CrossRef]
- van Weelden, W.J.; Massuger, L.; Enitec; Pijnenborg, J.M.A.; Romano, A. Anti-estrogen Treatment in Endometrial Cancer: A Systematic Review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef]
- Mallick, S.; Benson, R.; Julka, P.K. Breast cancer prevention with anti-estrogens: Review of the current evidence and future directions. Breast Cancer 2016, 23, 170–177. [Google Scholar] [CrossRef]
- Gu, R.; Jia, W.; Zeng, Y.; Rao, N.; Hu, Y.; Li, S.; Wu, J.; Jin, L.; Chen, L.; Long, M.; et al. A comparison of survival outcomes and side effects of toremifene or tamoxifen therapy in premenopausal estrogen and progesterone receptor positive breast cancer patients: A retrospective cohort study. BMC Cancer 2012, 12, 161. [Google Scholar] [CrossRef]
- Lin, T.H.; Lee, S.O.; Niu, Y.; Xu, D.; Liang, L.; Li, L.; Yeh, S.D.; Fujimoto, N.; Yeh, S.; Chang, C. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/Enzalutamide versus anti-androgen receptor ASC-J9(R) Lead to promotion versus suppression of prostate cancer metastasis. J. Biol. Chem. 2013, 288, 19359–19369. [Google Scholar] [CrossRef]
- Obayemi, J.; Salifu, A.; Eluu, S.; Uzonwanne, V.; Jusu, S.; Nwazojie, C.; Onyekanne, C.; Ojelabi, O.; Payne, L.; Moore, C.M. LHRH-conjugated drugs as targeted therapeutic agents for the specific targeting and localized treatment of triple negative breast cancer. Sci. Rep. 2020, 10, 8212. [Google Scholar] [CrossRef]
- Ghosh, N.; Chaki, R.; Mandal, V.; Mandal, S.C. COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. 2010, 62, 233–244. [Google Scholar] [CrossRef]
- Sood, A.; Lang, D.K.; Kaur, R.; Saini, B.; Arora, S. Relevance of Aromatase Inhibitors in Breast Cancer Treatment. Curr. Top. Med. Chem. 2021, 21, 1319–1336. [Google Scholar] [CrossRef]
- Dyavar, S.R.; Singh, R.; Emani, R.; Pawar, G.P.; Chaudhari, V.D.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V.; Salunke, D.B. Role of toll-like receptor 7/8 pathways in regulation of interferon response and inflammatory mediators during SARS-CoV2 infection and potential therapeutic options. Biomed. Pharmacother. 2021, 141, 111794. [Google Scholar] [CrossRef]
- Amaria, R.N.; Reuben, A.; Cooper, Z.A.; Wargo, J.A. Update on use of aldesleukin for treatment of high-risk metastatic melanoma. Immunotargets Ther. 2015, 4, 79–89. [Google Scholar] [CrossRef]
- Wei, X.X.; Fong, L.; Small, E.J. Prostate cancer immunotherapy with sipuleucel-T: Current standards and future directions. Expert Rev. Vaccines 2015, 14, 1529–1541. [Google Scholar] [CrossRef]
- Lawler, S.E.; Chiocca, E.A. Oncolytic virus-mediated immunotherapy: A combinatorial approach for cancer treatment. J. Clin. Oncol. 2015, 33, 2812–2814. [Google Scholar] [CrossRef]
- Ralph, S.J.; Low, P.; Dong, L.; Lawen, A.; Neuzil, J. Mitocans: Mitochondrial targeted anti-cancer drugs as improved therapies and related patent documents. Recent Pat. Anti-Cancer Drug Discov. 2006, 1, 327–346. [Google Scholar] [CrossRef]
- Nikravesh, H.; Khodayar, M.J.; Behmanesh, B.; Mahdavinia, M.; Teimoori, A.; Alboghobeish, S.; Zeidooni, L. The combined effect of dichloroacetate and 3-bromopyruvate on glucose metabolism in colorectal cancer cell line, HT-29; the mitochondrial pathway apoptosis. BMC Cancer 2021, 21, 903. [Google Scholar] [CrossRef]
- Mani, S.; Swargiary, G.; Singh, K.K. Natural Agents Targeting Mitochondria in Cancer. Int. J. Mol. Sci. 2020, 21, 6992. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Hu, Y.; Huang, P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by β-phenethyl isothiocyanate: Mechanisms for anti-leukemia activity. Antioxid. Redox Signal. 2011, 15, 2911–2921. [Google Scholar] [CrossRef]
- D’Souza, G.G.; Wagle, M.A.; Saxena, V.; Shah, A. Approaches for targeting mitochondria in cancer therapy. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 689–696. [Google Scholar] [CrossRef]
- Guo, L.; Shestov, A.A.; Worth, A.J.; Nath, K.; Nelson, D.S.; Leeper, D.B.; Glickson, J.D.; Blair, I.A. Inhibition of mitochondrial complex II by the anticancer agent lonidamine. J. Biol. Chem. 2016, 291, 42–57. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Xiao, Y.; Fu, B.; Qin, Z. TPP-based mitocans: A potent strategy for anticancer drug design. RSC Med. Chem. 2020, 11, 858–875. [Google Scholar] [CrossRef]
- Dong, L.; Gopalan, V.; Holland, O.; Neuzil, J. Mitocans revisited: Mitochondrial targeting as efficient anti-cancer therapy. Int. J. Mol. Sci. 2020, 21, 7941. [Google Scholar] [CrossRef]
- Koya, K.; Li, Y.; Wang, H.; Ukai, T.; Tatsuta, N.; Kawakami, M.; Shishido, T.; Chen, L.B. MKT-077, a novel rhodacyanine dye in clinical trials, exhibits anticarcinoma activity in preclinical studies based on selective mitochondrial accumulation. Cancer Res. 1996, 56, 538–543. [Google Scholar] [PubMed]
- Heise, N.V.; Hoenke, S.; Serbian, I.; Csuk, R. An improved partial synthesis of corosolic acid and its conversion to highly cytotoxic mitocans. Eur. J. Med. Chem. Rep. 2022, 6, 100073. [Google Scholar] [CrossRef]
- Neuzil, J.; Dong, L.-F.; Rohlena, J.; Truksa, J.; Ralph, S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion 2013, 13, 199–208. [Google Scholar] [CrossRef]
- Chen, G.; Wang, F.; Trachootham, D.; Huang, P. Preferential killing of cancer cells with mitochondrial dysfunction by natural compounds. Mitochondrion 2010, 10, 614–625. [Google Scholar] [CrossRef]
- Carvalho, T. Personalized anti-cancer vaccine combining mRNA and immunotherapy tested in melanoma trial. Nat. Med. 2023, 29, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Rybakova, Y.; Kowalski, P.S.; Huang, Y.; Gonzalez, J.T.; Heartlein, M.W.; DeRosa, F.; Delcassian, D.; Anderson, D.G. mRNA Delivery for Therapeutic Anti-HER2 Antibody Expression In Vivo. Mol. Ther. 2019, 27, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Soundara Rajan, T.; Gugliandolo, A.; Bramanti, P.; Mazzon, E. In Vitro-Transcribed mRNA Chimeric Antigen Receptor T Cell (IVT mRNA CAR T) Therapy in Hematologic and Solid Tumor Management: A Preclinical Update. Int. J. Mol. Sci. 2020, 21, 6514. [Google Scholar] [CrossRef] [PubMed]
- Wijdeven, R.H.; Pang, B.; Assaraf, Y.G.; Neefjes, J. Old drugs, novel ways out: Drug resistance toward cytotoxic chemotherapeutics. Drug Resist. Updat. 2016, 28, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.D. Combination Therapy using Co-Encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in vivo. Sci. Rep. 2016, 6, 22390. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chaudhai, R.; Zhang, S. Polypharmacology in Drug Development: A Minireview of Current Technologies. ChemMedChem 2016, 11, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Frei, E., III; Karon, M.; Levin, R.H.; Freireich, E.J.; Taylor, R.J.; Hananian, J.; Selawry, O.; Holland, J.F.; Hoogstraten, B.; Wolman, I.J.; et al. The effectiveness of combinations of antileukemic agents in inducing and maintaining remission in children with acute leukemia. Blood 1965, 26, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.M. Editorial: Novel Combination Therapies for the Treatment of Solid Cancers. Front. Oncol. 2021, 11, 708943. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef]
- Schein, C.H. Repurposing approved drugs on the pathway to novel therapies. Med. Res. Rev. 2020, 40, 586–605. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Subramaniam, V.; Allimuthu, D. Editorial: Adopting drug repurposing to overcome drug resistance in cancer. Front. Cell Dev. Biol. 2023, 11, 1191682. [Google Scholar] [CrossRef]
- Ercan, G.; Ilbar Tartar, R.; Solmaz, A.; Gulcicek, O.B.; Karagulle, O.O.; Meric, S.; Cayoren, H.; Kusaslan, R.; Kemik, A.; Gokceoglu Kayali, D.; et al. Potent therapeutic effects of ruscogenin on gastric ulcer established by acetic acid. Asian J. Surg. 2020, 43, 405–416. [Google Scholar] [CrossRef]
- Rodrigues, J.P.B.; Fernandes, A.; Dias, M.I.; Pereira, C.; Pires, T.; Calhelha, R.C.; Carvalho, A.M.; Ferreira, I.; Barros, L. Phenolic Compounds and Bioactive Properties of Ruscus aculeatus L. (Asparagaceae): The Pharmacological Potential of an Underexploited Subshrub. Molecules 2021, 26, 1882. [Google Scholar] [CrossRef]
- Hua, H.; Zhu, Y.; Song, Y.H. Ruscogenin suppressed the hepatocellular carcinoma metastasis via PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 2018, 101, 115–122. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. A review on pharmaceutically important medical plant: Plumbago zeylanica. J. Ayurvedic Herb. Med. 2017, 3, 225–228. [Google Scholar] [CrossRef]
- Aziz, M.H.; Dreckschmidt, N.E.; Verma, A.K. Plumbagin, a medicinal plant–derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008, 68, 9024–9032. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; You, W.; Zhou, F.; Guo, Z.; Qian, K.; Xiao, Y.; Wang, X. Suppressive effects of plumbagin on the growth of human bladder cancer cells via PI3K/AKT/mTOR signaling pathways and EMT. Cancer Cell Int. 2020, 20, 520. [Google Scholar] [CrossRef]
- Chen, S.M.; Feng, J.N.; Zhao, C.K.; Yao, L.C.; Wang, L.X.; Meng, L.; Cai, S.Q.; Liu, C.Y.; Qu, L.K.; Jia, Y.X.; et al. A multi-targeting natural product, aiphanol, inhibits tumor growth and metastasis. Am. J. Cancer Res. 2022, 12, 4930–4953. [Google Scholar]
- Méndez-Lucio, O.; Naveja, J.J.; Vite-Caritino, H.; Prieto-Martínez, F.D.; Medina-Franco, J.L. One drug for multiple targets: A computational perspective. J. Mex. Chem. Soc. 2016, 60, 168–181. [Google Scholar] [CrossRef]
- Aw, M.S.; Addai-Mensah, J.; Losic, D. A multi-drug delivery system with sequential release using titania nanotube arrays. Chem. Commun. 2012, 48, 3348–3350. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kolbuk, D.; Sajkiewicz, P. Multifluid electrospinning for multi-drug delivery systems: Pros and cons, challenges, and future directions. Biomater. Sci. 2022, 11, 37–61. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Yang, H. Drug delivery systems for differential release in combination therapy. Expert Opin. Drug Deliv. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Davies, L.E.; Spiers, G.; Kingston, A.; Todd, A.; Adamson, J.; Hanratty, B. Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J. Am. Med. Dir. Assoc. 2020, 21, 181–187. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef]
- Sun, L.; Mi, K.; Hou, Y.; Hui, T.; Zhang, L.; Tao, Y.; Liu, Z.; Huang, L. Pharmacokinetic and Pharmacodynamic Drug-Drug Interactions: Research Methods and Applications. Metabolites 2023, 13, 897. [Google Scholar] [CrossRef]
- Viscoli, C.; Castagnola, E.; Machetti, M. Antifungal treatment in patients with cancer. J. Intern. Med. 1997, 242, 89–94. [Google Scholar] [CrossRef]
- Nikulin, S.; Tonevitsky, E.; Poloznikov, A. Effect of ketoconazole on the transport and metabolism of drugs in the human liver cell model. Russ. Chem. Bull. 2017, 66, 150–155. [Google Scholar] [CrossRef]
- Svanström, H.; Lund, M.; Melbye, M.; Pasternak, B. Concomitant use of low-dose methotrexate and NSAIDs and the risk of serious adverse events among patients with rheumatoid arthritis. Pharmacoepidemiol. Drug Saf. 2018, 27, 885–893. [Google Scholar] [CrossRef]
- Lee, C.-H.; Shen, M.-C.; Tsai, M.-J.; Chang, J.-S.; Huang, Y.-B.; Yang, Y.-H.; Hsieh, K.-P. Proton pump inhibitors reduce the survival of advanced lung cancer patients with therapy of gefitinib or erlotinib. Sci. Rep. 2022, 12, 7002. [Google Scholar] [CrossRef]
- Frye, R.F.; Fitzgerald, S.M.; Lagattuta, T.F.; Hruska, M.W.; Egorin, M.J. Effect of St John’s wort on imatinib mesylate pharmacokinetics. Clin. Pharmacol. Ther. 2004, 76, 323–329. [Google Scholar] [CrossRef]
- Whitman, A.; Erdeljac, P.; Jones, C.; Pillarella, N.; Nightingale, G. Managing Polypharmacy in Older Adults with Cancer Across Different Healthcare Settings. Drug Heal. Patient Saf. 2021, 13, 101–116. [Google Scholar] [CrossRef]
- Khezrian, M.; McNeil, C.J.; Murray, A.D.; Myint, P.K. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther. Adv. Drug Saf. 2020, 11, 2042098620933741. [Google Scholar] [CrossRef]
- Goh, I.; Lai, O.; Chew, L. Prevalence and risk of polypharmacy among elderly cancer patients receiving chemotherapy in ambulatory oncology setting. Curr. Oncol. Rep. 2018, 20, 38. [Google Scholar] [CrossRef]
- Couderc, A.L.; Boisseranc, C.; Rey, D.; Nouguerede, E.; Greillier, L.; Barlesi, F.; Duffaud, F.; Deville, J.L.; Honore, S.; Villani, P.; et al. Medication Reconciliation Associated with Comprehensive Geriatric Assessment in Older Patients with Cancer: ChimioAge Study. Clin. Interv. Aging 2020, 15, 1587–1598. [Google Scholar] [CrossRef]
- Chopra, D.; Rehan, H.S.; Sharma, V.; Mishra, R. Chemotherapy-induced adverse drug reactions in oncology patients: A prospective observational survey. Indian. J. Med. Paediatr. Oncol. 2016, 37, 42–46. [Google Scholar] [CrossRef]
- Alwhaibi, M.; AlRuthia, Y.; Alhawassi, T.M.; Almalag, H.; Alsalloum, H.; Balkhi, B. Polypharmacy and comorbidities among ambulatory cancer patients: A cross-sectional retrospective study. J. Oncol. Pharm. Pract. 2020, 26, 1052–1059. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell Ther. 2016, 4, 3. [Google Scholar] [CrossRef]
- Munger, M.A. Polypharmacy and combination therapy in the management of hypertension in elderly patients with co-morbid diabetes mellitus. Drugs Aging 2010, 27, 871–883. [Google Scholar] [CrossRef]
- Mittal, N.; Mittal, R. Repurposing old molecules for new indications: Defining pillars of success from lessons in the past. Eur. J. Pharmacol. 2021, 912, 174569. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.M.; Kauppi, D.; Schonfeld, J. Therapeutic drug repurposing, repositioning and rescue. Drug Discov. 2015, 16, 57–63. [Google Scholar]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Gartner, F.; Vale, N. Drug combination and repurposing for cancer therapy: The example of breast cancer. Heliyon 2021, 7, e05948. [Google Scholar] [CrossRef] [PubMed]
- Park, K. A review of computational drug repurposing. Transl. Clin. Pharmacol. 2019, 27, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Che, C.; Jin, B.; Zhang, N.; Su, C.; Wang, F. Knowledge-driven drug repurposing using a comprehensive drug knowledge graph. Health Inform. J. 2020, 26, 2737–2750. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleem, J.; Granet, R.; Ramakrishnan, S.; Ciancetta, N.A.; Saveson, C.; Gessner, C.; Zhou, Q. Knowledge Graph-Based Approaches to Drug Repurposing for COVID-19. J. Chem. Inf. Model. 2021, 61, 4058–4067. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.M.; Ravi Kumar, B.V.V.; Sruti, J.; Mahapatra, M.K.; Banik, B.K.; Borah, P. Drug Repurposing Strategy (DRS): Emerging Approach to Identify Potential Therapeutics for Treatment of Novel Coronavirus Infection. Front. Mol. Biosci. 2021, 8, 628144. [Google Scholar] [CrossRef]
- Truong, M.; Monahan, L.G.; Carter, D.A.; Charles, I.G. Repurposing drugs to fast-track therapeutic agents for the treatment of cryptococcosis. PeerJ 2018, 6, e4761. [Google Scholar] [CrossRef]
- Barrett, T.; Suzek, T.O.; Troup, D.B.; Wilhite, S.E.; Ngau, W.-C.; Ledoux, P.; Rudnev, D.; Lash, A.E.; Fujibuchi, W.; Edgar, R. NCBI GEO: Mining millions of expression profiles—Database and tools. Nucleic Acids Res. 2005, 33, D562–D566. [Google Scholar] [CrossRef]
- Zador, Z.; King, A.T.; Geifman, N. New drug candidates for treatment of atypical meningiomas: An integrated approach using gene expression signatures for drug repurposing. PLoS ONE 2018, 13, e0194701. [Google Scholar] [CrossRef]
- Xu, H.; Aldrich, M.C.; Chen, Q.; Liu, H.; Peterson, N.B.; Dai, Q.; Levy, M.; Shah, A.; Han, X.; Ruan, X.; et al. Validating drug repurposing signals using electronic health records: A case study of metformin associated with reduced cancer mortality. J. Am. Med. Inf. Assoc. 2015, 22, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Morsi, D.; Salem, M.L.; Ibrahim, H.; Osman, G.; Mohamed, A.; Nofal, A. Synergistic and chemosensitizing effects of bovine lactoferrin or muramyl dipeptide in Ehrlich solid tumor-bearing mice treated with cisplatin. Int. J. Cancer Biomed. Res. 2021, 5, 75–94. [Google Scholar]
- Brahmachari, G. Discovery and Development of Neuroprotective Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Tatiraju, D.V.; Bagade, V.B.; Karambelkar, P.J.; Jadhav, V.M.; Kadam, V. Natural bioenhancers: An overview. J. Pharmacogn. Phytochem. 2013, 2, 55–60. [Google Scholar]
- Tasneen, R.; Mortensen, D.S.; Converse, P.J.; Urbanowski, M.E.; Upton, A.; Fotouhi, N.; Nuermberger, E.; Hawryluk, N. Dual mTORC1/mTORC2 inhibition as a host-directed therapeutic target in pathologically distinct mouse models of tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e0025321. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.S.; Oh, D.H.; Choi, H.M.; Sur, B.J.; Lim, S.J.; Kim, J.Y.; Yang, H.I.; Yoo, M.C.; Hahm, D.H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef]
- Therizols, G.; Bash-Imam, Z.; Panthu, B.; Machon, C.; Vincent, A.; Ripoll, J.; Nait-Slimane, S.; Chalabi-Dchar, M.; Gaucherot, A.; Garcia, M. Alteration of ribosome function upon 5-fluorouracil treatment favors cancer cell drug-tolerance. Nat. Commun. 2022, 13, 173. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Sivalingam, N. Mechanism of 5-fluorouracil induced resistance and role of piperine and curcumin as chemo-sensitizers in colon cancer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Taylor, C.; Wang, L.; List, A.; Fernandes, D.; Paine-Murrieta, G.; Johnson, C.; Capizzi, R. Amifostine protects normal tissues from paclitaxel toxicity while cytotoxicity against tumour cells is maintained. Eur. J. Cancer 1997, 33, 1693–1698. [Google Scholar] [CrossRef]
- Koukourakis, M.I. Amifostine in clinical oncology: Current use and future applications. Anticancer. Drugs 2002, 13, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Ekborn, A.; Hansson, J.; Ehrsson, H.; Eksborg, S.; Wallin, I.; Wagenius, G.; Laurell, G. High-dose Cisplatin with amifostine: Ototoxicity and pharmacokinetics. Laryngoscope 2004, 114, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Bates, S.E. Tariquidar (XR9576): A P-glycoprotein drug efflux pump inhibitor. Expert Rev. Anticancer Ther. 2007, 7, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Matzneller, P.; Kussmann, M.; Eberl, S.; Maier-Salamon, A.; Jäger, W.; Bauer, M.; Langer, O.; Zeitlinger, M.; Poeppl, W. Pharmacokinetics of the P-gp inhibitor tariquidar in rats after intravenous, oral, and intraperitoneal administration. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, T.H.; Kim, B.D.; Kim, H.K.; Lyu, M.J.; Jung, H.M.; Goo, Y.T.; Kang, M.J.; Lee, S.; Choi, Y.W. Co-administration of tariquidar using functionalized nanostructured lipid carriers overcomes resistance to docetaxel in multidrug resistant MCF7/ADR cells. J. Drug Deliv. Sci. Technol. 2022, 71, 103323. [Google Scholar] [CrossRef]
- Patel, N.R.; Rathi, A.; Mongayt, D.; Torchilin, V.P. Reversal of multidrug resistance by co-delivery of tariquidar (XR9576) and paclitaxel using long-circulating liposomes. Int. J. Pharm. 2011, 416, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Grixti, J.M.; O’Hagan, S.; Day, P.J.; Kell, D.B. Enhancing drug efficacy and therapeutic index through cheminformatics-based selection of small molecule binary weapons that improve transporter-mediated targeting: A cytotoxicity system based on gemcitabine. Front. Pharmacol. 2017, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Rollando, R.; Maulada, F.; Afthoni, M.H.; Monica, E.; Yuniati, Y.; Nugraha, A.T. Screening Carica Papaya Compounds as an Antimalarial Agent: In Silico Study. Trop. J. Nat. Prod. Res. 2023, 7, 2895–2903. [Google Scholar]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; V Anil Kumar, N.; Salehi, B.; C Cho, W.; Sharifi-Rad, J. Piperine-a major principle of black pepper: A review of its bioactivity and studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Kasibhatta, R.; Naidu, M. Influence of piperine on the pharmacokinetics of nevirapine under fasting conditions: A randomised, crossover, placebo-controlled study. Drugs R. D 2007, 8, 383–391. [Google Scholar] [CrossRef]
- Bezerra, D.P.; de Castro, F.O.; Alves, A.P.; Pessoa, C.; de Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; de Alencar, N.M.; Mesquita, R.O.; et al. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J. Appl. Toxicol. 2008, 28, 156–163. [Google Scholar] [CrossRef]
- Culy, C.R.; Spencer, C.M. Amifostine: An update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs 2001, 61, 641–684. [Google Scholar] [CrossRef]
- King, M.; Joseph, S.; Albert, A.; Thomas, T.V.; Nittala, M.R.; Woods, W.C.; Vijayakumar, S.; Packianathan, S. Use of amifostine for cytoprotection during radiation therapy: A review. Oncology 2020, 98, 61–80. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Mohamed, A.H.; Salem, M.L.; Osman, G.Y.; Morsi, D.S. Anti-neoplastic and immunomodulatory potency of co-treatment based on bovine lactoferrin and/or muramyl dipeptide in tumor-bearing mice. Toxicol. Res. 2020, 9, 137–147. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, J.S.; Lee, J.S.; Park, J.H.; Kim, H.S.; Yoon, S. JAK2 Inhibitor, Fedratinib, Inhibits P-gp Activity and Co-Treatment Induces Cytotoxicity in Antimitotic Drug-Treated P-gp Overexpressing Resistant KBV20C Cancer Cells. Int. J. Mol. Sci. 2022, 23, 4597. [Google Scholar] [CrossRef]
- Guzman, M.L.; Swiderski, C.F.; Howard, D.S.; Grimes, B.A.; Rossi, R.M.; Szilvassy, S.J.; Jordan, C.T. Preferential induction of apoptosis for primary human leukemic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 16220–16225. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.d.C.; Costa, R.G.; Silva, S.L.; Dias, I.R.; Dias, R.B.; Bezerra, D.P. Cell signaling pathways as molecular targets to eliminate AML stem cells. Crit. Rev. Oncol. /Hematol. 2021, 160, 103277. [Google Scholar] [CrossRef]
- Ghosh, S.; Singh, R.; Vanwinkle, Z.M.; Guo, H.; Vemula, P.K.; Goel, A.; Haribabu, B.; Jala, V.R. Microbial metabolite restricts 5-fluorouracil-resistant colonic tumor progression by sensitizing drug transporters via regulation of FOXO3-FOXM1 axis. Theranostics 2022, 12, 5574–5595. [Google Scholar] [CrossRef]
- Wilhelmsen, N.C.; Eriksson, T. Medication adherence interventions and outcomes: An overview of systematic reviews. Eur. J. Hosp. Pharm. 2019, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Granger, B.B.; Swedberg, K.; Ekman, I.; Granger, C.B.; Olofsson, B.; McMurray, J.J.; Yusuf, S.; Michelson, E.L.; Pfeffer, M.A.; CHARM investigators. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: Double-blind, randomised, controlled clinical trial. Lancet 2005, 366, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Tjelle, K.; Opstad, H.B.; Solem, S.; Launes, G.; Hansen, B.; Kvale, G.; Hagen, K. Treatment adherence as predictor of outcome in concentrated exposure treatment for obsessive-compulsive disorder. Front. Psychiatry 2021, 12, 667167. [Google Scholar] [CrossRef]
- Baryakova, T.H.; Pogostin, B.H.; Langer, R.; McHugh, K.J. Overcoming barriers to patient adherence: The case for developing innovative drug delivery systems. Nat. Rev. Drug Discov. 2023, 22, 387–409. [Google Scholar] [CrossRef]
- Isaac, M.; Holvey, C. Transdermal patches: The emerging mode of drug delivery system in psychiatry. Ther. Adv. Psychopharmacol. 2012, 2, 255–263. [Google Scholar] [CrossRef]
- Badwan, B.A.; Liaropoulos, G.; Kyrodimos, E.; Skaltsas, D.; Tsirigos, A.; Gorgoulis, V.G. Machine learning approaches to predict drug efficacy and toxicity in oncology. Cell Rep. Methods 2023, 3, 100413. [Google Scholar] [CrossRef]


| Criteria | Equation | Pros and Cons |
|---|---|---|
| Half-Maximal Effective Dose (ED50) | ED50 = Dose at which the drug produces a half-maximal effect | Reflects the relationship between therapeutic effect and acute toxicity but not chronic toxicity and allergenicity. |
| Lethal Dose (LD50) | LD50 = Dose that is lethal to 50% of the test population | Can evaluate the acute toxicity but does not reflect the chronic toxicity and carcinogenicity. LD50 may vary due to varying testing conditions. |
| Therapeutic Index (TI) | TI = LD50/ED50 = TD50/ED50 | Widely used safety index but is not applicable to very rare or idiosyncratic adverse drug reactions. |
| Maximum Tolerated Dose (MTD) | The highest dose of a drug that can be administered without unacceptable toxicity | Usually used to assess chemotherapy drugs. |
| Therapeutic Window (TW) | TW = minimum toxic concentration (MTC)/minimum effective concentration (MEC) | Related to TI and is more flexible but lacks formal definition. |
| Margin of Safety (MoS)/Certain safety factor (CSF) | MoS/CSF = TD1/ED99 | Patients will not be exposed to high risks. Can be seldom achieved in clinic. |
| Routes of Drug Administration | Working Model | Advantages | Disadvantages | Current Drugs | Refs. |
|---|---|---|---|---|---|
| Oral drug delivery | 1. Small molecule delivery 2. Patient self-administration | 1. Sustained and easy administration 2. Major method to establish patient compliance 3. Large surface area for mucosal layer attachment | 1. Need to pass through GI system (multiple barriers) 2. Slow absorption 3. Degradation problems | 1. Venlafaxine hydrochloride 2. Diltiazem 3. Indomethacin 4. Heparin | [59,60,61] |
| Injectable drug delivery | 1. Protein and peptide delivery 2. Intravenous (IV), intramuscular (IM), intranasal (IN) 3. Induce immune response mechanisms | 1. The highest bioavailability and the fastest effect 2. Acute and emergency responses | 1. Needle-related pain, wounds, phobia | 1. Glucagon-like peptide-1 2. Insulin 3. Superoxide dismutase 4. Hydrocodone (Vicodin) | [62,63,64,65] |
| Transdermal patch drug delivery | 1. From skin layers to the blood circulatory system | 1. Direct treatment away from GI system 2. Can maintain sustained drug level 3. Readily administered | 1. Lower drug absorption level | 1. Nitroglycerin 2. Nicotine 3. Scopolamine 4. Clonidine 5. Fentanyl 6. Testosterone | [66,67,68] |
| Ocular drug delivery | 1. Deliver drugs to eyes against disorders related to vision 2. Formation: eye drop, eye implant | 1. Easy administration and preparation 2. High patient convenience and compliance | 1. Poor bioavailability 2. Low retention time 3. Side effect caused by high-frequency administration 4. Instability for dissolved drugs | 1. Ocusert® Pilo-20 2. Pilo-40 Ocular system | [69,70] |
| Pulmonary drug delivery | 1. Inhalation of drugs via nebulisers or inhalers 2. Drugs that target the lungs | 1. Rapid effects 2. High therapeutics due to large surface of lungs | 1. Drug irritation to the lung 2. Limited drug dissolution 3. High drug clearance | 1. Nebulisers 2. Pressurised metered dose inhalers (pMDIs) 3. Soft-mist inhalers 4. Dry powder inhalers (DPIs) | [71,72] |
| Implantable drug delivery | 1. A reservoir surrounded by polymers or drug–polymer mixture 2. Passive delivery: use diffusion, osmosis, or gradients to control drug release 3. Active delivery: activate a pump to release drugs | 1. Reduce dosing frequency 2. Increase patient compliance | 1. Lack of systemic treatment 2. Undegradable, need a removal process | 1. Vitrasert 2. Norplant® | [73,74,75] |
| Antibody drug conjugate delivery | 1. Combination of clonal antibodies and drugs 2. Conjugation between target sites and antibodies | 1. Highly toxic drug delivery (high specificity, low off-targets) | 1. Poor tumour penetration (ex: hypoxic area) 2. Side effects in the non-target sites 3. Undesirable immune response (ex: Fc interaction) | 1. Brentuximab vedotin 2. Trastuzumab emtansine (Kadcyla) | [76,77,78] |
| Polysaccharide-based hydrogel drug delivery | 1. Use natural polymers to build beads 2. Suitable for peptides, proteins, DNA, and RNA | 1. High biocompatibility and biodegradability 2. Cost efficiency 3. Ease of surface modification 4. Low toxicity 5. Rapid drug release | 1. The quantity and homogeneity of drugs are limited (hydrophobic drugs) 2. Unable to bind with hard tissues (bone) 3. Difficult to sterilise | 1. Poly (ethylene glycol)-diacrylate (PEGDA) | [56,79] |
| Classification of Anti-Cancer Drugs | Principle | Subtypes | Examples | Ref. |
|---|---|---|---|---|
| Chemotherapy | Interfere with tumour cell cycle, cell proliferation, and replication | (1) Alkylating agents | (1) Cyclophosphamide, chlormethine | [82,83] |
| (2) Anti-metabolites | (2) 5-FU, 6-mercaptopurine, gemcitabine | [84] | ||
| (3) Anti-tumour antibodies | (3) Atezolizumab, trastuzumab-deruxtecan | [85,86] | ||
| (4) Topoisomerase inhibitors | (4) TOPI: camptothecin TOPII: doxorubicin | [87] | ||
| (5) Tubulin-binding drugs | (5) Microtubule-stabilising: taxanes Microtubule-destabilising: vincristine | [88] | ||
| (6) Antibiotics | (6) Bleomycin, daunorubicin, doxorubicin | [89,90] | ||
| (7) Mitosis inhibitors | (7) Alisertib, ispinesib, GSK461364 | [91] | ||
| Targeted therapy | Target specific proteins or genes related to cancer growth | (1) Receptor tyrosine kinase inhibitors | (1) Erlotinib, gefitnib, lapatnib, afatinib | [92,93] |
| (2) Intracellular tyrosine inhibitors | (2) Imatnib, nilotnib, everlimus | [92,94] | ||
| (3) DNA/RNA synthesis inhibitors | (3) Capecitabine, oxaliplatin | [95] | ||
| (4) Topoisomerase I inhibitors | (4) Irinotecan, belotecan, topotecan | [96,97] | ||
| (5) Proteasome inhibitors | (5) Bortzomib, ixazomib, carfilzomib | [98] | ||
| Hormonal therapy | Inhibit tumour growth dependent on hormones | (1) Steroids | (1) Dexamethasone, methylprednisolone | [99,100] |
| (2) Anti-estrogens | (2) Tamoxifen, raloxifene, toremifene | [101,102,103] | ||
| (3) Anti-androgens | (3) Bicalutamide, enzalutamide | [104] | ||
| (4) LHRH conjugated drugs | (4) LHRH-paclitaxel, LHRH-prodigiosin | [105] | ||
| (5) Anti-aromatase agents | (5) Exemestane, anastrozole, | [106,107] | ||
| Immunotherapy | Induce anti-tumour responses from the immune system | (1) Interferon | (1) IFNα-1a, IFNα-1b | [108] |
| (2) Interleukin 2 | (2) Aldesleukin | [109] | ||
| (3) Vaccines | (3) Sipuleucel-T | [110] | ||
| (4) Oncolytic virus therapy | (4) T-VEC | [111] | ||
| Others | (1) Disrupt energy production, essential cellular processes in mitochondria (2) Induce apoptosis pathways | Mitochondria-targeted anti-cancer drugs (mitocans) | ||
| (1) Hexokinase inhibitors | (1) 2-deoxyglucose, 3-bromopyruvate | [112,113] | ||
| (2) Bcl-2/Bcl-xL mimetics | (2) Antimycin A, Gossypol, ABT-263 | [114] | ||
| (3) Thiol redox inhibitors | (3) Dichloroacetate, isothiocyanates | [115] | ||
| (4) VDAC/ANT targeting drugs | (4) CD437, lonidamine | [116,117] | ||
| (5) Electron transport chain targeting drugs | (5) Tamoxifen, MitoVES | [118,119] | ||
| (6) Lipophilic cations targeting inner membrane | (6) MKT-077, Rhodamine-123 | [120,121] | ||
| (7) Drug targeting TCA cycle | (7) DCA, 3-bromopyruvate | [122] | ||
| (8) Drug targeting mtDNA | (8) Vitamin K3, Mito VES | [123] | ||
| Induce cells to produce specific proteins | mRNA drugs | |||
| (1) Vaccines | (1) mRNA-4157, pembrolizumab | [124] | ||
| (2) Antibodies | (2) Anti-HER2 | [125] | ||
| (3) Antigen receptors | (3) Chimeric antigen receptor T cell therapy | [126] |
| Strategy | Concept | Pros and Cons | Examples | Refs. |
|---|---|---|---|---|
| Knowledge-based repurposing | Use the properties of drugs to predict the possibility of treating diseases: (1) Target-based repurposing (2) Pathway-based repurposing (3) Target-based repurposing | Pros: 1. Large scale prediction 2. Precise prediction 3. Time-efficient 4. Cost-effective Cons: 1. Only positive data can be found in knowledge database | 1. CAS biomedical knowledge graph for COVID-19 2. L-type calcium channel blockers for cryptococcosis | [170,171,172,173] |
| Signature-based repurposing | A method to discover new off-targets or pathways. Genetic and molecular mechanisms are highly involved in the analysis. | Pros: 1. Identify new mechanisms of drugs Cons: 1. Only provide data for preliminary analysis | 1. New candidates for atypical meningioma 2. New candidates for inflammatory bowel disease | [174,175] |
| Phenotype-based repurposing | A method for systemic approaches to detect human diseases, multiple independent screens for similar compounds and their potential for repurposing. | Pros: 1. Can predict extra adverse events Cons: 1. Might lead to compounds with poor pharmacokinetics | 1. Electronic health records (EHRs) define the use of metformin in cancer treatment 2. Killing ability towards Toxoplasma by Pimozide and tamoxifen | [167,176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-S.; Jin, E.; Day, P.J. Use of Drug Sensitisers to Improve Therapeutic Index in Cancer. Pharmaceutics 2024, 16, 928. https://doi.org/10.3390/pharmaceutics16070928
Chen Y-S, Jin E, Day PJ. Use of Drug Sensitisers to Improve Therapeutic Index in Cancer. Pharmaceutics. 2024; 16(7):928. https://doi.org/10.3390/pharmaceutics16070928
Chicago/Turabian StyleChen, Yu-Shan, Enhui Jin, and Philip J. Day. 2024. "Use of Drug Sensitisers to Improve Therapeutic Index in Cancer" Pharmaceutics 16, no. 7: 928. https://doi.org/10.3390/pharmaceutics16070928
APA StyleChen, Y.-S., Jin, E., & Day, P. J. (2024). Use of Drug Sensitisers to Improve Therapeutic Index in Cancer. Pharmaceutics, 16(7), 928. https://doi.org/10.3390/pharmaceutics16070928






