Mitochondria-Targeted Liposomes for Drug Delivery to Tumor Mitochondria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
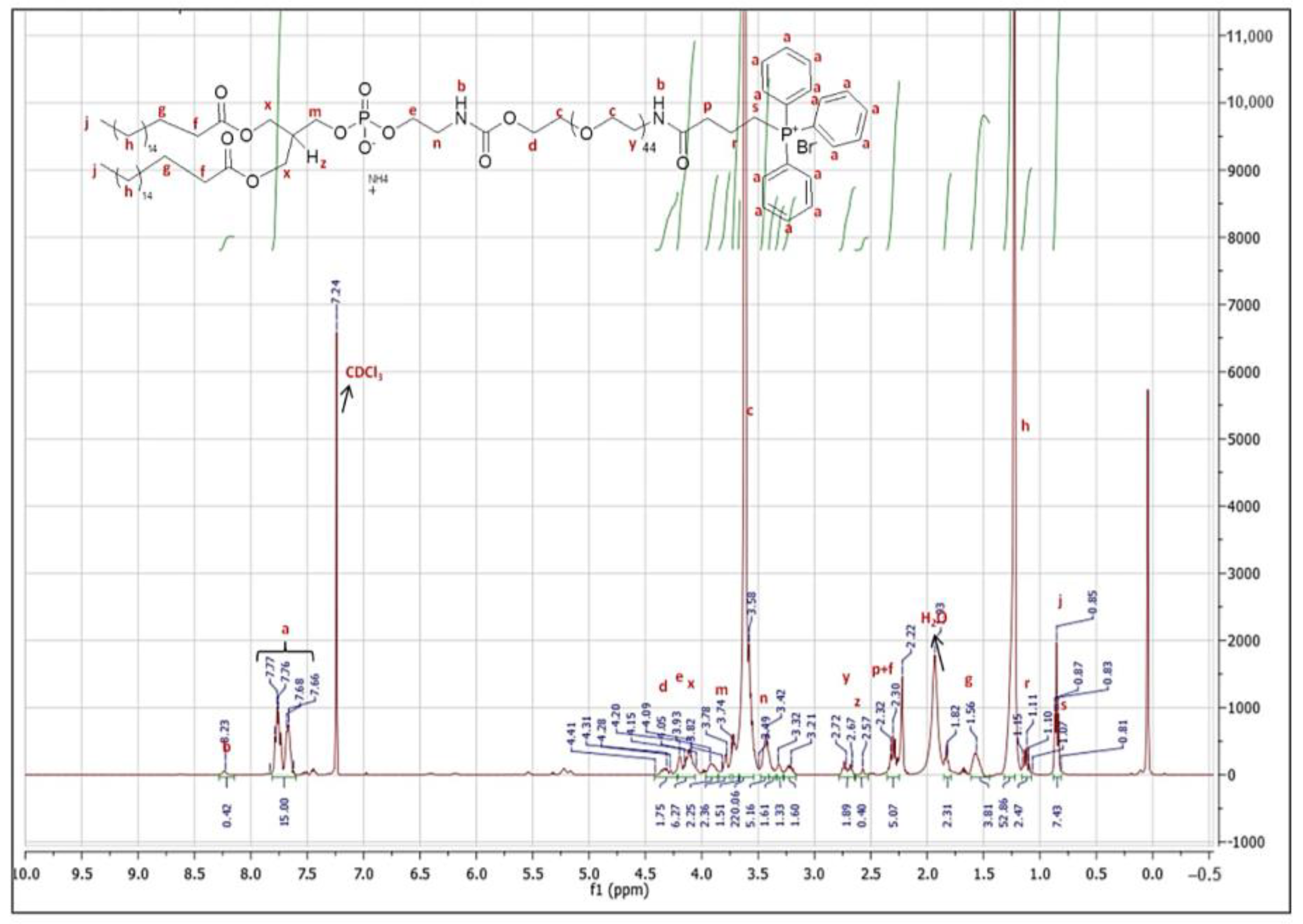
2.2. Synthesis and Characterization of DSPE-PEG-TPP Polymer
2.3. Preparation of Liposomes
2.4. Characterization of Liposomes
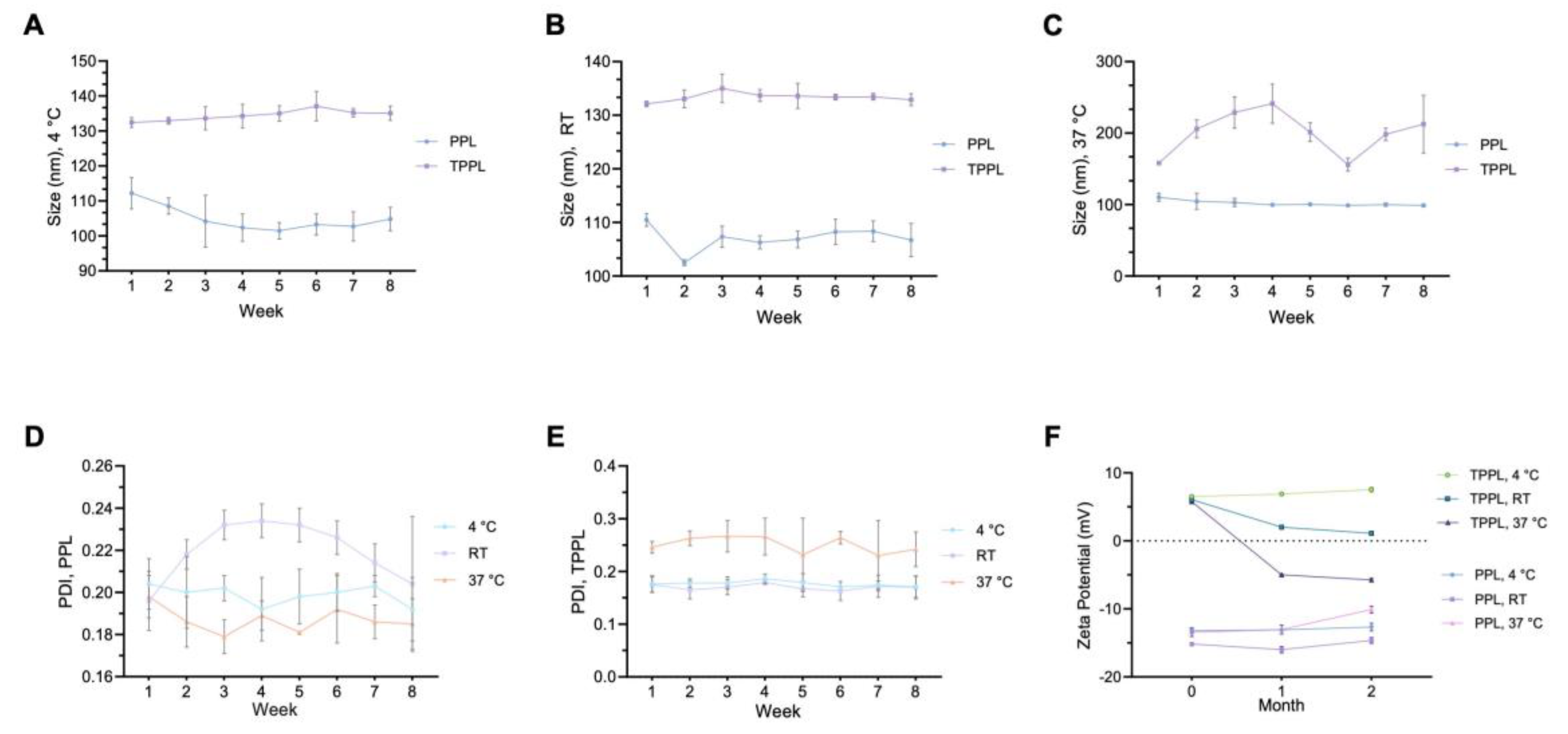
2.5. Stability of Liposomes
2.6. Encapsulation of Liposomes
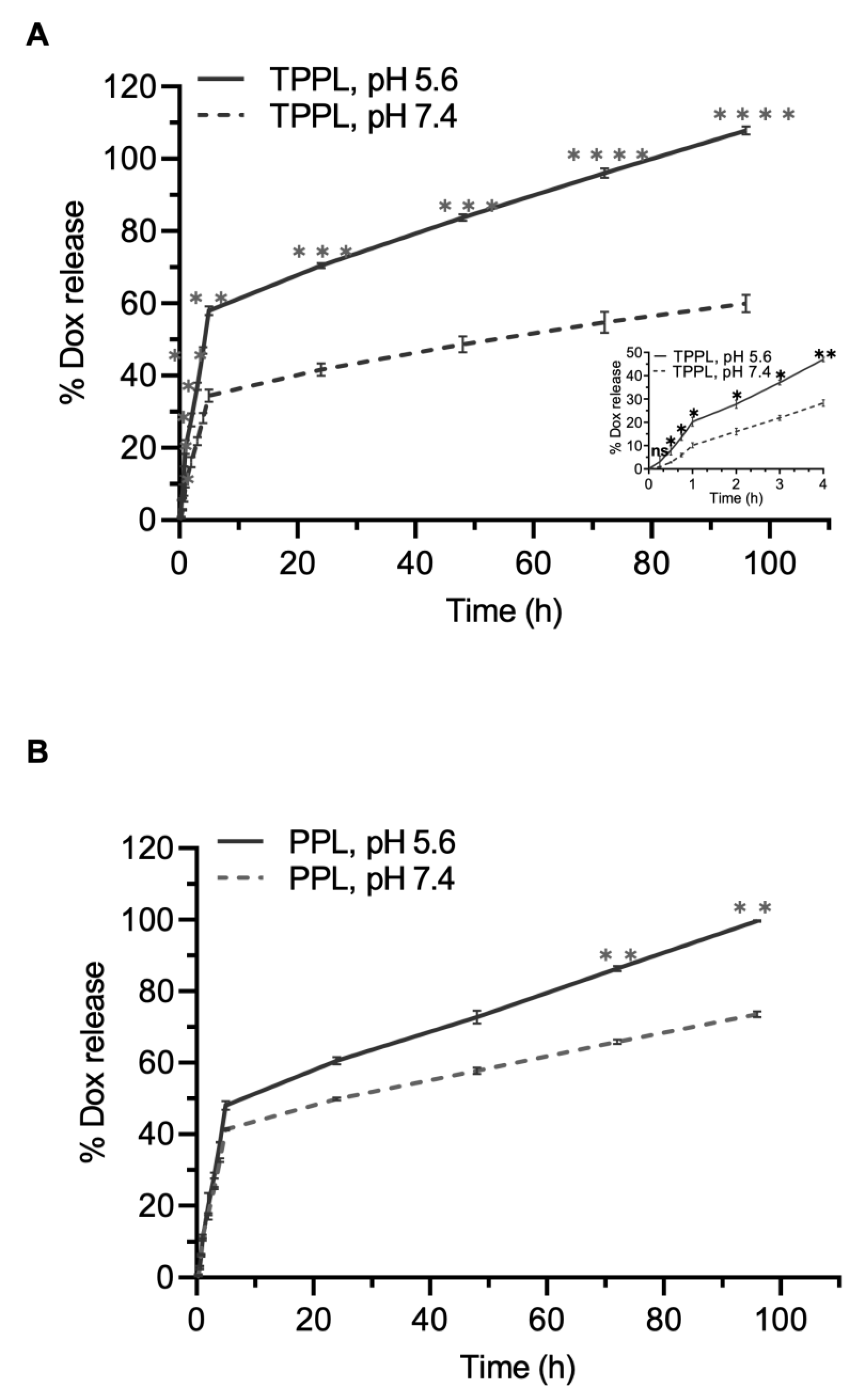
2.7. In Vitro Drug Release
2.8. Cellular and Mitochondrial Uptake of Liposomes
2.9. In Vitro Cytotoxicity of Empty and Dox-Loaded Liposomes
2.10. Reactive Oxygen Species (ROS) Measurements
2.11. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of DSPE-PEG-TPP Polymer Conjugate
3.2. Characterization of Empty and Dox-Loaded Liposomes
3.3. Stability of Empty PPLs and TPPLs
3.4. Determination of Encapsulation Efficiency
3.5. Drug Release
3.6. Cellular Uptake and Cytotoxicity of Empty PPLs and TPPLs
3.7. Cellular Uptake of Dox-Loaded Liposomes
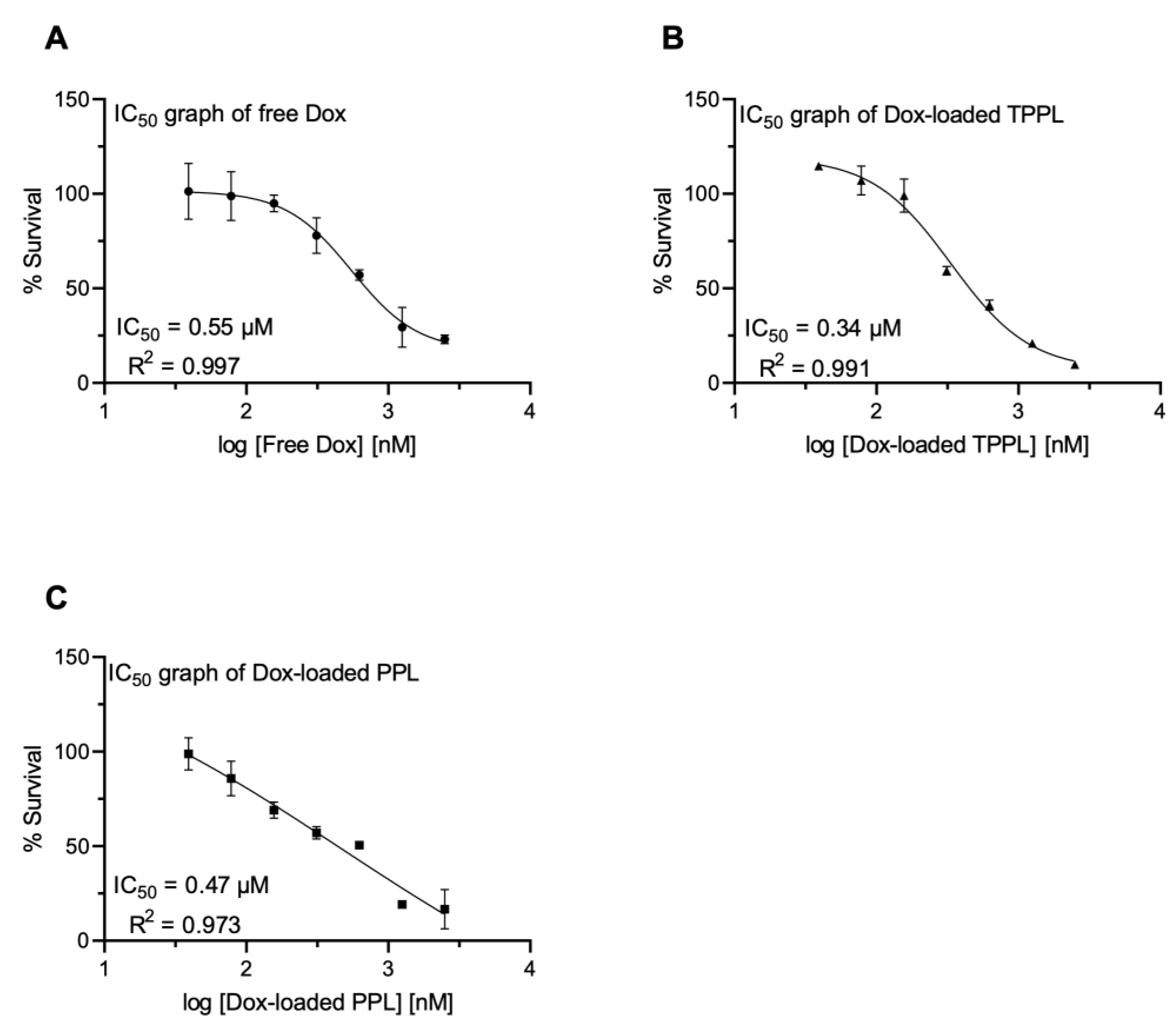
3.8. Cytotoxicity of Dox-Loaded TPPLs and PPLs
3.9. ROS Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baxter-Holland, M.; Dass, C.R. Doxorubicin, mesenchymal stem cell toxicity and antitumour activity: Implications for clinical use. J. Pharm. Pharmacol. 2018, 70, 320–327. [Google Scholar] [CrossRef]
- Guven, C.; Sevgiler, Y.; Taskin, E. Mitochondrial Dysfunction Associated with Doxorubicin. In Mitochondrial Diseases; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.B.; Leung, K.T.; Poon, E.N. Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2022, 23, 1912. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.R.; Tulumello, D.V.; Riganti, C.; Liyanage, S.U.; Schimmer, A.D.; Kelley, S.O. Mitochondrial Targeting of Doxorubicin Eliminates Nuclear Effects Associated with Cardiotoxicity. ACS Chem. Biol. 2015, 10, 2007–2015. [Google Scholar] [CrossRef]
- Xu, J.; Shamul, J.G.; Kwizera, E.A.; He, X. Recent Advancements in Mitochondria-Targeted Nanoparticle Drug Delivery for Cancer Therapy. Nanomaterials 2022, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Jeena, M.T.; Kim, S.; Jin, S.; Ryu, J.H. Recent Progress in Mitochondria-Targeted Drug and Drug-Free Agents for Cancer Therapy. Cancers 2019, 12, 4. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hamblin, M.R. Mitochondria-targeted nanoparticles (mitoNANO): An emerging therapeutic shortcut for cancer. Biomater. Biosyst. 2021, 3, 100023. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.R.; Tulumello, D.V.; Kelley, S.O. Targeted delivery of doxorubicin to mitochondria. ACS Chem. Biol. 2013, 8, 1389–1395. [Google Scholar] [CrossRef]
- Cui, H.; Huan, M.L.; Ye, W.L.; Liu, D.Z.; Teng, Z.H.; Mei, Q.B.; Zhou, S.Y. Mitochondria and Nucleus Dual Delivery System To Overcome DOX Resistance. Mol. Pharm. 2017, 14, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Vakili, M.R.; Soleymani Abyaneh, H.; Molavi, O.; Lai, R.; Lavasanifar, A. Mitochondrial delivery of doxorubicin via triphenylphosphine modification for overcoming drug resistance in MDA-MB-435/DOX cells. Mol. Pharm. 2014, 11, 2640–2649. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yu, X.; Shen, Y.; Shi, Y.; Su, C.; Zhao, L. Triphenyl Phosphine-Functionalized Chitosan Nanoparticles Enhanced Antitumor Efficiency Through Targeted Delivery of Doxorubicin to Mitochondria. Nanoscale Res. Lett. 2017, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Choi, Y.S.; Kim, Y.G.; Yoon, K.; Nurunnabi, M.; Li, L.; Lee, E.; Kang, H.C.; Huh, K.M. Bioreducible Poly(ethylene glycol)-Triphenylphosphonium Conjugate as a Bioactivable Mitochondria-Targeting Nanocarrier. Biomacromolecules 2017, 18, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.N.; Guo, N.N.; Guo, W.W.; Huang-Fu, M.Y.; Vakili, M.R.; Chen, J.J.; Xu, W.H.; Wei, Q.C.; Han, M.; Lavasanifar, A.; et al. Delivery of mitochondriotropic doxorubicin derivatives using self-assembling hyaluronic acid nanocarriers in doxorubicin-resistant breast cancer. Acta Pharmacol. Sin. 2018, 39, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Du, S.; Ni, J.; Zhou, J.; Yao, J. Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite nanoparticles for enhancing therapeutic efficacy of doxorubicin. Biomaterials 2016, 94, 70–83. [Google Scholar] [CrossRef]
- Biswas, S.; Dodwadkar, N.S.; Deshpande, P.P.; Torchilin, V.P. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J. Control Release 2012, 159, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Cui, F.D.; Choi, M.K.; Lin, H.; Chung, S.J.; Shim, C.K.; Kim, D.D. Liposome formulation of paclitaxel with enhanced solubility and stability. Drug Deliv. 2007, 14, 301–308. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, W.Y.; Ma, X.; Ju, R.J.; Li, X.Y.; Li, N.; Sun, M.G.; Shi, J.F.; Zhang, C.X.; Lu, W.L. The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials 2013, 34, 3626–3638. [Google Scholar] [CrossRef]
- Guzman-Villanueva, D.; Mendiola, M.R.; Nguyen, H.X.; Weissig, V. Influence of triphenylphosphonium (TPP) cation hydrophobization with phospholipids on cellular toxicity and mitochondrial selectivity. SOJ Pharm. Pharm. Sci. 2015, 2, 1–9. [Google Scholar]
- Nam, J.H.; Kim, S.Y.; Seong, H. Investigation on Physicochemical Characteristics of a Nanoliposome-Based System for Dual Drug Delivery. Nanoscale Res. Lett. 2018, 13, 101. [Google Scholar] [CrossRef]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des. Devel Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Vila-Caballer, M.; Codolo, G.; Munari, F.; Malfanti, A.; Fassan, M.; Rugge, M.; Balasso, A.; de Bernard, M.; Salmaso, S. A pH-sensitive stearoyl-PEG-poly(methacryloyl sulfadimethoxine)-decorated liposome system for protein delivery: An application for bladder cancer treatment. J. Control Release 2016, 238, 31–42. [Google Scholar] [CrossRef]
- Somuncu, B.; Ekmekcioglu, A.; Antmen, F.M.; Ertuzun, T.; Deniz, E.; Keskin, N.; Park, J.; Yazici, I.E.; Simsek, B.; Erman, B.; et al. Targeting mitochondrial DNA polymerase gamma for selective inhibition of MLH1 deficient colon cancer growth. PLoS ONE 2022, 17, e0268391. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; I Okeke, C.; Hu, Z.-B.; Xu, J. DSPE-PEG: A distinctive component in drug delivery system. Curr. Pharm. Des. 2015, 21, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, S.; Simon, J.; Passlick, D.; Frey, M.L.; Wagner, M.; Mailander, V.; Crespy, D.; Landfester, K. Brush Conformation of Polyethylene Glycol Determines the Stealth Effect of Nanocarriers in the Low Protein Adsorption Regime. Nano Lett. 2021, 21, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Angali, K.A.; Dorkoosh, F.A.; Handali, S. Transferrin targeted liposomal 5-fluorouracil induced apoptosis via mitochondria signaling pathway in cancer cells. Life Sci. 2018, 194, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Handali, S.; Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Kouchak, M.; Amini, M.; Angali, K.A.; Saremy, S.; Dorkoosh, F.A. A novel 5-Fluorouracil targeted delivery to colon cancer using folic acid conjugated liposomes. Biomed. Pharmacother. 2018, 108, 1259–1273. [Google Scholar] [CrossRef]
- Boddapati, S.V.; D’Souza, G.G.; Erdogan, S.; Torchilin, V.P.; Weissig, V. Organelle-targeted nanocarriers: Specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett. 2008, 8, 2559–2563. [Google Scholar] [CrossRef] [PubMed]
- Battogtokh, G.; Cho, Y.Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondrial-Targeting Anticancer Agent Conjugates and Nanocarrier Systems for Cancer Treatment. Front. Pharmacol. 2018, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Andra, V.; Pammi, S.V.N.; Bhatraju, L.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef]
- Soema, P.C.; Willems, G.J.; Jiskoot, W.; Amorij, J.P.; Kersten, G.F. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015, 94, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Monnier, C.A.; Thévenaz, D.C.; Balog, S.; Fiore, G.L.; Vanhecke, D.; Rothen-Rutishauser, B.; Petri-Fink, A. A guide to investigating colloidal nanoparticles by cryogenic transmission electron microscopy: Pitfalls and benefits. AIMS Biophys. 2015, 2, 245–258. [Google Scholar] [CrossRef]
- Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. BioMed Res. Int. 2014, 2014, 424239. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Ghizdareanu, A.I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.A. Coating Materials to Increase the Stability of Liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Muppidi, K.; Pumerantz, A.S.; Wang, J.; Betageri, G. Development and stability studies of novel liposomal vancomycin formulations. ISRN Pharm. 2012, 2012, 636743. [Google Scholar] [CrossRef]
- Ball, R.L.; Bajaj, P.; Whitehead, K.A. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2017, 12, 305–315. [Google Scholar] [CrossRef]
- Kang, J.H.; Ko, Y.T. Enhanced Subcellular Trafficking of Resveratrol Using Mitochondriotropic Liposomes in Cancer Cells. Pharmaceutics 2019, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Wang, Z.; Hao, S.; He, H.; Wan, Y.; Zhu, C.; Sun, L.P.; Cheng, G.; Zheng, S.Y. Mitochondria-Targeting Polydopamine Nanoparticles to Deliver Doxorubicin for Overcoming Drug Resistance. ACS Appl. Mater. Interfaces 2017, 9, 16793–16802. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Feng, D.; Lu, J.; Cui, X.; Wang, Y.; Wang, Q.; Zhang, L. Application Prospects of Triphenylphosphine-Based Mitochondria-Targeted Cancer Therapy. Cancers 2023, 15, 666–688. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Qin, Z.; Shen, C.; Gong, H.L.; He, Z.Y. Multifunctional Mitochondria-Targeting Nanosystems for Enhanced Anticancer Efficacy. Front. Bioeng. Biotechnol. 2021, 9, 786621. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, J.; Li, X.; Pan, S.; Li, J.; Yang, C.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. School of Pharmacy, Mitochondria-targeted delivery of doxorubicin to enhance antitumor activity with HER -2 peptide-mediated multifunctional pH-sensitive DQAsomes. Int. J. Nanomed. 2018, 13, 4209–4226. [Google Scholar] [CrossRef] [PubMed]




| Formulation | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| Empty PPL | 117.51 ± 2.82 | 0.19 ± 0.02 | −13.20 ± 0.30 |
| Empty TPPL | 135.76 ± 4.29 | 0.15 ± 0.01 | 6.07 ± 0.17 |
| Dox-PPL | 109.66 ± 2.09 | 0.20 ± 0.01 | −23.63 ± 0.21 |
| Dox-TPPL | 126.50 ± 1.29 | 0.18 ± 0.02 | 9.97 ± 0.90 |
| RhB-PPL | 147.89 ± 1.31 | 0.15 ± 0.03 | −14.97 ± 0.31 |
| RhB-TPPL | 157.65 ± 0.18 | 0.18 ± 0.03 | 6.80 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekmekcioglu, A.; Gok, O.; Oz-Arslan, D.; Erdal, M.S.; Yagan Uzuner, Y.; Muftuoglu, M. Mitochondria-Targeted Liposomes for Drug Delivery to Tumor Mitochondria. Pharmaceutics 2024, 16, 950. https://doi.org/10.3390/pharmaceutics16070950
Ekmekcioglu A, Gok O, Oz-Arslan D, Erdal MS, Yagan Uzuner Y, Muftuoglu M. Mitochondria-Targeted Liposomes for Drug Delivery to Tumor Mitochondria. Pharmaceutics. 2024; 16(7):950. https://doi.org/10.3390/pharmaceutics16070950
Chicago/Turabian StyleEkmekcioglu, Aysegul, Ozgul Gok, Devrim Oz-Arslan, Meryem Sedef Erdal, Yasemin Yagan Uzuner, and Meltem Muftuoglu. 2024. "Mitochondria-Targeted Liposomes for Drug Delivery to Tumor Mitochondria" Pharmaceutics 16, no. 7: 950. https://doi.org/10.3390/pharmaceutics16070950
APA StyleEkmekcioglu, A., Gok, O., Oz-Arslan, D., Erdal, M. S., Yagan Uzuner, Y., & Muftuoglu, M. (2024). Mitochondria-Targeted Liposomes for Drug Delivery to Tumor Mitochondria. Pharmaceutics, 16(7), 950. https://doi.org/10.3390/pharmaceutics16070950






