Design of Novel TRPA1 Agonists Based on Structure of Natural Vasodilator Carvacrol—In Vitro and In Silico Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Experiments
2.1.1. Drugs and Solutions
2.1.2. Tissue Preparation
2.1.3. Experimental Protocols
2.1.4. Ethical Approval
2.1.5. Statistical Analysis
2.2. In Silico Experiments
2.2.1. 3D-QSAR
2.2.2. ADMET Prediction
2.2.3. Molecular Docking
2.2.4. Molecular Dynamic Simulation
3. Results and Discussion
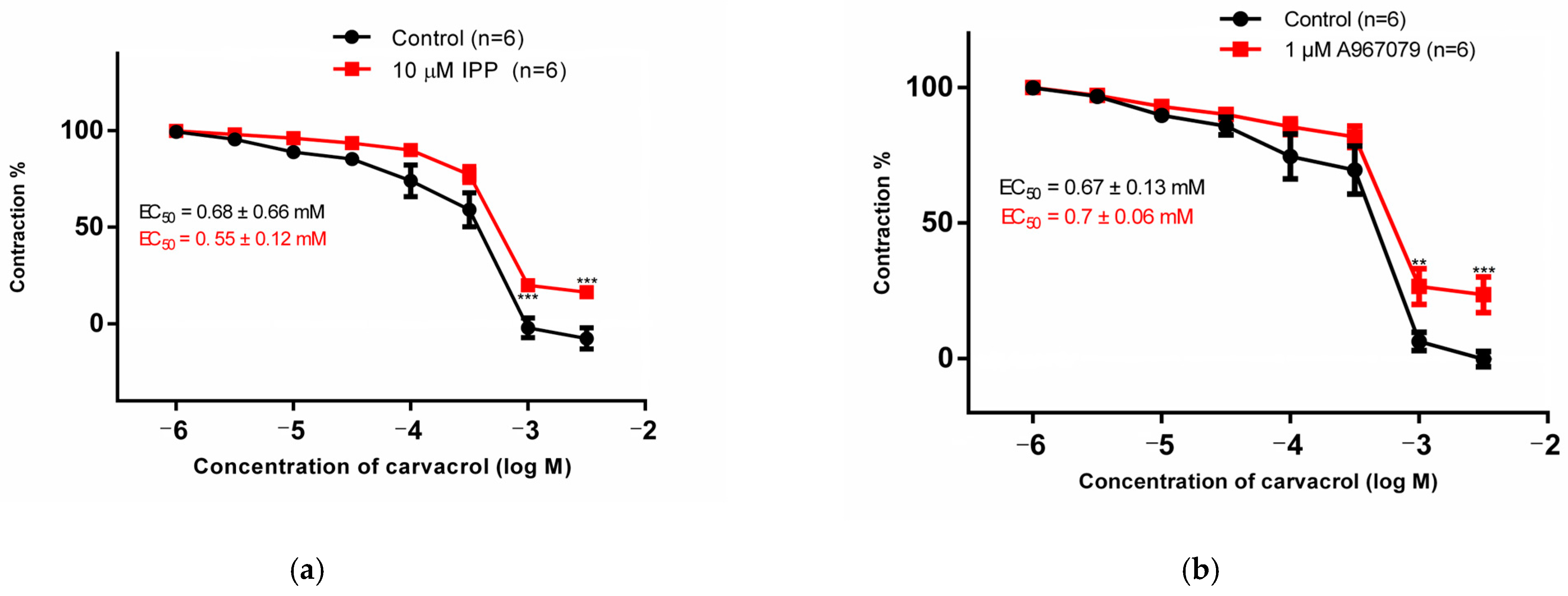
3.1. Organ Bath
3.2. 3D-QSAR
3.3. ADMET Properties
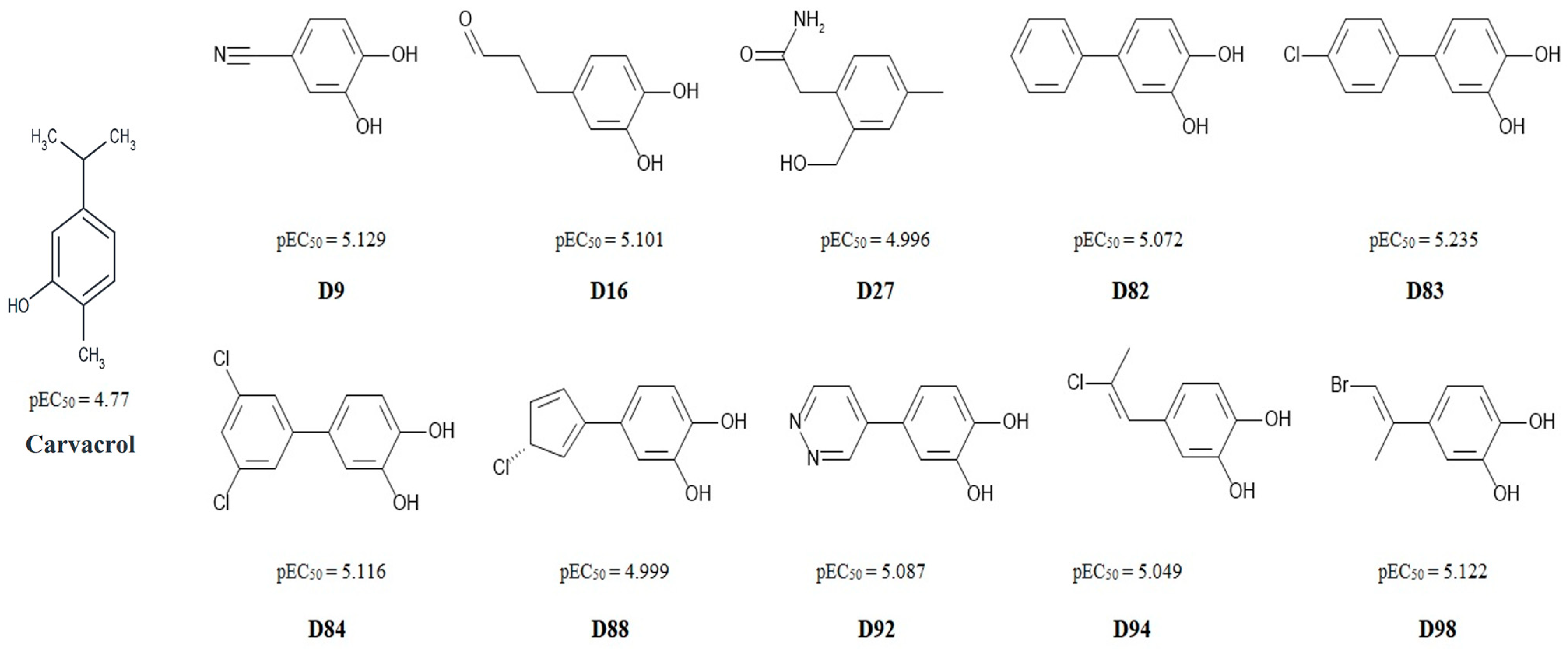
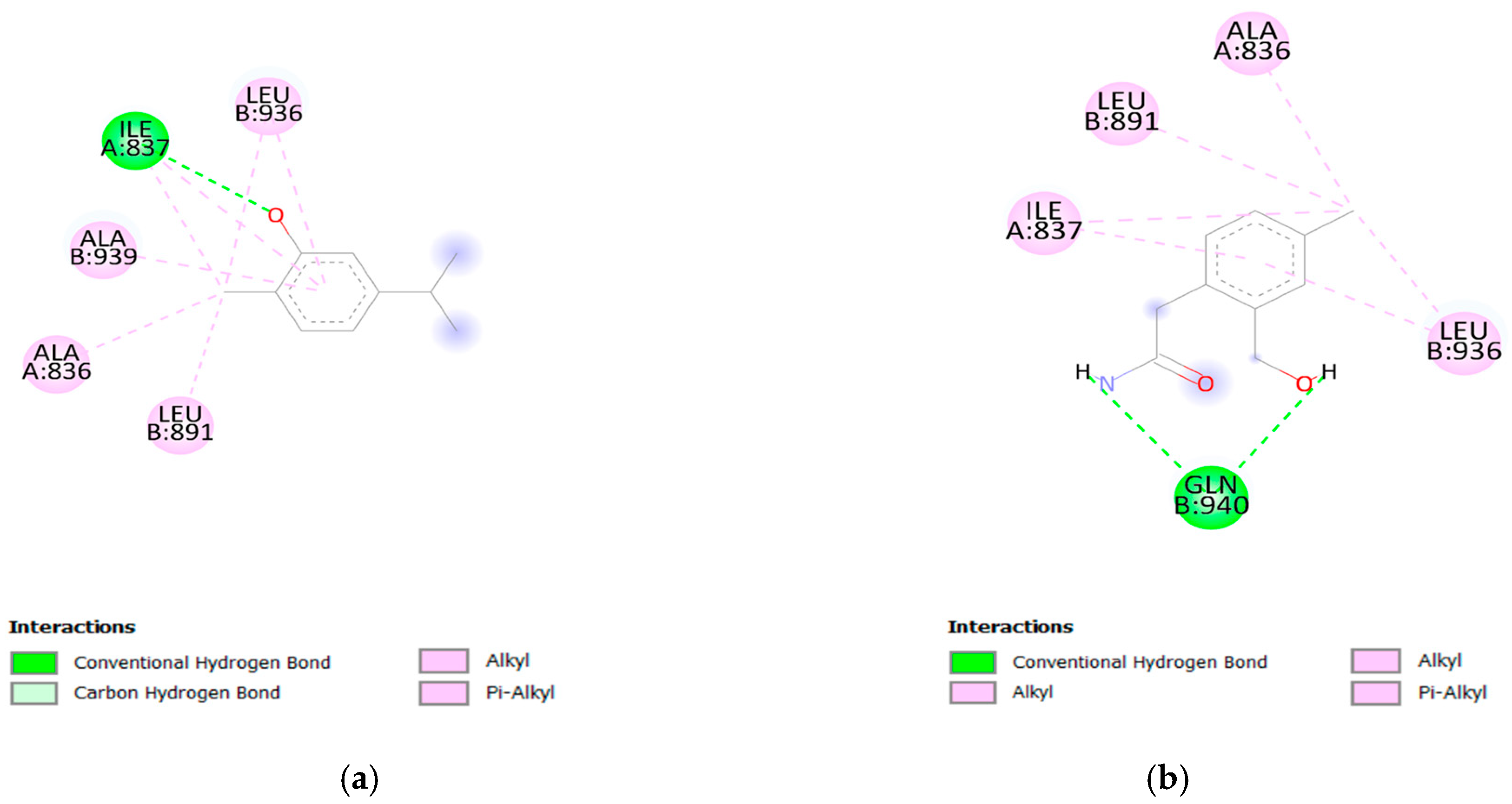
3.4. Molecular Docking
3.5. Molecular Dynamic Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Tackling, G.; Borhade, M.B. Hypertensive Heart Disease; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539800/ (accessed on 20 March 2024).
- Wang, J.; Feng, B.; Yang, X.; Liu, W.; Xiong, X. Chinese herbal medicine for the treatment of prehypertension. Evid. Based Complement. Altern. Med. 2013, 2013, 493521. [Google Scholar] [CrossRef] [PubMed]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Berkay, Y.Y.; Antika, G.; Salehi, B.; Tumer, T.B.; Kulandaisamy, V.C.; Das, G.; Patra, J.K.; Karazhan, N.; Akram, M.; et al. Phytochemical constituents, biological activities, and health-promoting effects of the genus Origanum. Phytother. Res. 2021, 35, 95–121. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Suručić, R.; Kundaković, T.; Lakušić, B.; Drakul, D.; Milovanović, S.R.; Kovačević, N. Variations in Chemical Composition, Vasorelaxant and Angiotensin I-Converting Enzyme Inhibitory Activities of Essential Oil from Aerial Parts of Seseli pallasii Besser (Apiaceae). Chem. Biodivers. 2017, 14, e1600407. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Y.; Kutlay, O.; Ari, S.; Duman, S.; Uzuner, K.; Aydin, S. Hypotensive effects of carvacrol on the blood pressure of normotensive rats. Planta Med. 2007, 73, 1365–1371. [Google Scholar] [CrossRef]
- Gonçalves, T.A.F.; Lima, V.S.; de Almeida, A.J.P.O.; de Arruda, A.V.; Veras, A.C.M.F.; Lima, T.T.; Soares, E.M.C.; Santos, A.C.D.; Vasconcelos, M.E.C.; de Almeida Feitosa, M.S.; et al. Carvacrol Improves Vascular Function in Hypertensive Animals by Modulating Endothelial Progenitor Cells. Nutrients 2023, 15, 3032. [Google Scholar] [CrossRef]
- Dias, C.J.; Costa, H.A.; Alves Dias-Filho, C.A.; Ferreira, A.C.; Rodrigues, B.; Irigoyen, M.C.; Romão Borges, A.C.; de Andadre Martins, V.; Branco Vidal, F.C.; Ribeiro, R.M.; et al. Carvacrol reduces blood pressure, arterial responsiveness and increases expression of MAS receptors in spontaneously hypertensive rats. Eur. J. Pharmacol. 2022, 917, 174717. [Google Scholar] [CrossRef] [PubMed]
- Đukanović, Đ.; Bojić, M.G.; Marinković, S.; Trailović, S.; Stojiljković, M.P.; Škrbić, R. Vasorelaxant effect of monoterpene carvacrol on isolated human umbilical artery. Can. J. Physiol. Pharmacol. 2022, 100, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E.; Runnels, L.W.; Strübing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Andrei, S.R.; Sinharoy, P.; Bratz, I.N.; Damron, D.S. TRPA1 is functionally co-expressed with TRPV1 in cardiac muscle: Co-localization at z-discs, costameres and intercalated discs. Channels 2016, 10, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Francis, M.; Solodushko, V.; Earley, S.; Taylor, M.S. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation 2013, 20, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Kaudimba, K.K.; Guo, S.; Zhang, S.; Liu, T.; Chen, P.; Wang, R. Transient Receptor Potential Ankyrin Type-1 Channels as a Potential Target for the Treatment of Cardiovascular Diseases. Front. Physiol. 2020, 11, 836. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, D.; Ye, J.; Wang, M.; Liu, J.; Jiang, H.; Xu, Y.; Zhang, J.; Chen, J.; Wan, J. The TRPA1 Channel in the Cardiovascular System: Promising Features and Challenges. Front. Pharmacol. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Lu, Y.; Piplani, H.; McAllister, S.L.; Hurt, C.M.; Gross, E.R. Transient Receptor Potential Ankyrin 1 Activation within the Cardiac Myocyte Limits Ischemia-reperfusion Injury in Rodents. Anesthesiology 2016, 125, 1171–1180. [Google Scholar] [CrossRef]
- Petitjean, H.; Héberlé, E.; Hilfiger, L.; Łapieś, O.; Rodrigue, G.; Charlet, A. TRP channels and monoterpenes: Past and current leads on analgesic properties. Front. Mol. Neurosci. 2022, 15, 945450. [Google Scholar] [CrossRef]
- Mihara, S.; Shibamoto, T. The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies. Allergy Asthma Clin. Immunol. 2015, 11, 11. [Google Scholar] [CrossRef]
- Đukanović, Đ.; Gajić, M.; Škrbić, R. Time-dependent and force-dependent vasoreactivity of isolated human umbilical arteries. Scr. Med. 2020, 51, 134–140. [Google Scholar] [CrossRef]
- Gajić Bojić, M.; Đukanović, Đ.; Marinković, S.; Jovičić, S.; Stojiljković, M.P.; Djuric, D.M.; Škrbić, R. Methodological challenges in using human umbilical artery as a model for in vitro studies. Exp. Physiol. 2023, 108, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Xie, Z.; Lorkiewicz, P.; Srivastava, S.; Bhatnagar, A.; Conklin, D.J. Endothelial-dependent relaxation of α-pinene and two metabolites, myrtenol and verbenol, in isolated murine blood vessels. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, 1446–1460. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain 2011, 152, 1156–1164. [Google Scholar] [CrossRef]
- MarvinSketch 5.5.1.0; ChemAxon: Budapest, Hungary, 2011; Available online: https://www.chemaxon.com (accessed on 20 January 2024).
- ChemBio3D Ultra 7.0.0; CambridgeSoft Corporation: Cambridge, MA, USA, 2001; Available online: http://www.cambridgesoft.com (accessed on 24 January 2024).
- Pentacle 1.0.7; Molecular Discovery Ltd.: Perugia, Italy, 2015; Available online: http://www.moldiscovery.com (accessed on 6 February 2024).
- Pastor, M.; Cruciani, G.; McLay, I.; Pickett, S.; Clementi, S. GRid-INdependent descriptors (GRIND): A novel class of alignment-independent three-dimensional molecular descriptors. J. Med. Chem. 2000, 43, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Gramatica, P. On the development and validation of QSAR models. Methods Mol. Biol. 2013, 930, 499–526. [Google Scholar] [CrossRef]
- Tropsha, A. Best Practices for QSAR Model Development, Validation, and Exploitation. Mol. Inform. 2010, 29, 476–488. [Google Scholar] [CrossRef]
- Pratim, R.P.; Paul, S.; Mitra, I.; Roy, K. On two novel parameters for validation of predictive QSAR models. Molecules 2009, 14, 1660–1701. [Google Scholar] [CrossRef]
- Roy, K.; Kar, S.; Ambure, P. On a simple approach for determining applicability domain of QSAR models. Chemom. Intell. Lab. Syst. 2015, 145, 22–29. [Google Scholar] [CrossRef]
- ADM. ET Predictor (TM) Version 11.0, 64-bit Edition (Cloud Access); Simulations Plus Inc.: West Lancaster, CA, USA, 2023; Available online: https://www.simulations-plus.com/software/admetpredictor/ (accessed on 4 March 2024).
- Protein Data Bank. Available online: http://www.rcsb.org/ (accessed on 16 January 2024).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Discovery Studio Visualizer 4.1.0.14169; Accelrys Inc.: San Diego, CA, USA, 2014; Available online: http://accelrys.com (accessed on 14 March 2024).
- Taha, M.O.; Habash, M.; Al-Hadidi, Z.; Al-Bakri, A.; Younis, K.; Sisan, S. Docking-based comparative intermolecular contacts analysis as new 3-D QSAR concept for validating docking studies and in silico screening: NMT and GP inhibitors as case studies. J. Chem. Inf. Model. 2011, 51, 647–669. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Dunbrack, R.L., Jr.; Hooft, R.W.; Krieger, B. Assignment of protonation states in proteins and ligands: Combining pKa prediction with hydrogen bonding network optimization. Methods Mol. Biol. 2012, 819, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Lorente, I.; Ocete, M.A.; Zarzuelo, A.; Cabo, M.M.; Jimenez, J. Bioactivity of the essential oil of Bupleurum fruticosum. J. Nat. Prod. 1989, 52, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Gonçalves, Á.; Ferreira-da-Silva, F.W.; de Holanda-Angelin-Alves, C.M.; Cardoso-Teixeira, A.C.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. 1,8-Cineole blocks voltage-gated L-type calcium channels in tracheal smooth muscle. Pflug. Arch. 2018, 470, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Evaristo Rodrigues da Silva, R.; de Alencar, S.A.; Pereira-de-Morais, L.; de Sousa Almeida, N.; Iriti, M.; Kerntopf, M.R.; Menezes, I.R.A.; Coutinho, H.D.M.; Barbosa, R. Relaxant Effect of Monoterpene (-)-Carveol on Isolated Human Umbilical Cord Arteries and the Involvement of Ion Channels. Molecules 2020, 25, 2681. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Chericoni, S.; Martelli, A.; Flamini, G.; Breschi, M.C.; Calderone, V. Voltage-operated potassium (Kv) channels contribute to endothelium-dependent vasorelaxation of carvacrol on rat aorta. J. Pharm. Pharmacol. 2016, 68, 1177–1183. [Google Scholar] [CrossRef]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef]
- Jin, L.; Jagatheesan, G.; Guo, L.; Nystoriak, M.; Malovichko, M.; Lorkiewicz, P.; Bhatnagar, A.; Srivastava, S.; Conklin, D.J. Formaldehyde Induces Mesenteric Artery Relaxation via a Sensitive Transient Receptor Potential Ankyrin-1 (TRPA1) and Endothelium-Dependent Mechanism: Potential Role in Postprandial Hyperemia. Front. Physiol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, L.; Zhang, M.; Wang, J.; Rong, S.; Lu, W.; Dong, H. Zinc pyrithione induces endothelium-dependent hyperpolarization-mediated mesenteric vasorelaxation in healthy and colitic mice. Biochem. Pharmacol. 2023, 217, 115828. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Lorkiewicz, P.; Xie, Z.; Bhatnagar, A.; Srivastava, S.; Conklin, D.J. Acrolein but not its metabolite, 3-Hydroxypropylmercapturic acid (3HPMA), activates vascular transient receptor potential Ankyrin-1 (TRPA1): Physiological to toxicological implications. Toxicol. Appl. Pharmacol. 2021, 426, 115647. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Kodji, X.; Abdul-Kader, N.; Heads, R.; Fernandes, E.S.; Bevan, S.; Brain, S.D. TRPA1 activation leads to neurogenic vasodilatation: Involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br. J. Pharmacol. 2016, 173, 2419–2433. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Gonzales, A.L.; Crnich, R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ. Res. 2009, 104, 987–994. [Google Scholar] [CrossRef]
- Bamps, D.; Blockeel, A.J.; Dreesen, E.; Marynissen, H.; Laenen, J.; Van Hecken, A.; Wilke, A.; Shahabi, S.; Johnson, K.W.; Collins, E.C.; et al. TRPA1 Antagonist LY3526318 Inhibits the Cinnamaldehyde-Evoked Dermal Blood Flow Increase: Translational Proof of Pharmacology. Clin. Pharmacol. Ther. 2023, 114, 1093–1103. [Google Scholar] [CrossRef]
- Pozsgai, G.; Bodkin, J.V.; Graepel, R.; Bevan, S.; Andersson, D.A.; Brain, S.D. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc. Res. 2010, 87, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Mihai, D.P.; Nitulescu, G.M.; Ion, G.N.D.; Ciotu, C.I.; Chirita, C.; Negres, S. Computational Drug Repurposing Algorithm Targeting TRPA1 Calcium Channel as a Potential Therapeutic Solution for Multiple Sclerosis. Pharmaceutics 2019, 11, 446. [Google Scholar] [CrossRef]
- Prampolini, G.; Campetella, M.; Ferretti, A. Solvent effects on catechol’s binding affinity: Investigating the role of the intra-molecular hydrogen bond through a multi-level computational approach. Phys. Chem. Chem. Phys. 2023, 18, 2523–2536. [Google Scholar] [CrossRef]
- Callear, S.K.; Johnston, A.; McLain, S.E.; Imberti, S. Conformation and interactions of dopamine hydrochloride in solution. J. Chem. Phys. 2015, 142, 014502. [Google Scholar] [CrossRef]
- Niu, C.; Sun, X.; Hu, F.; Tang, X.; Wang, K. Molecular determinants for the chemical activation of the warmth-sensitive TRPV3 channel by the natural monoterpenoid carvacrol. J. Biol. Chem. 2022, 298, 101706. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 525, 552. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Reese, R.; Vu, S.; Rougé, L.; Shields, S.D.; Kakiuchi-Kiyota, S.; Chen, H.; Johnson, K.; Shi, Y.P.; Chernov-Rogan, T.; et al. A Non-covalent Ligand Reveals Biased Agonism of the TRPA1 Ion Channel. Neuron 2021, 109, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Arnittali, M.; Rissanou, A.N.; Harmandaris, V. Structure of biomolecules through molecular dynamics simulations. Procedia Comput. Sci. 2019, 156, 69–78. [Google Scholar] [CrossRef]
- Mukaiyama, M.; Usui, T.; Nagumo, Y. Non-electrophilic TRPA1 agonists, menthol, carvacrol and clotrimazole, open epithelial tight junctions via TRPA1 activation. J. Biochem. 2020, 168, 407–415. [Google Scholar] [CrossRef]
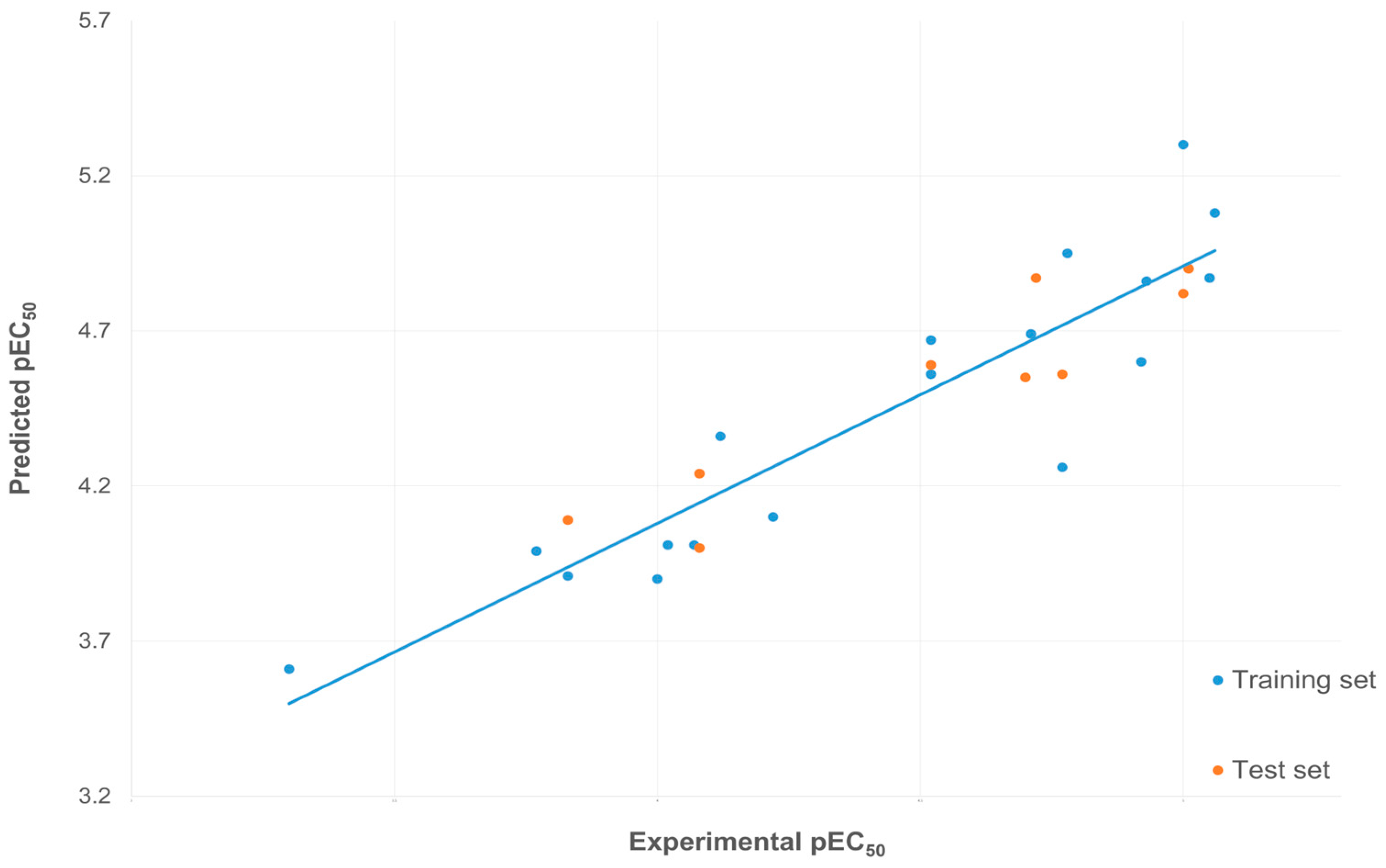


| Training Set | |||||
| Compounds | Experimental pEC50 | Predicted pEC50 | Compounds | Experimental pEC50 | Predicted pEC50 |
| L1 | 4.77 | 4.26 | L12 | 4.52 | 4.59 |
| L2 | 5.05 | 4.87 | L13 | 4.07 | 4.01 |
| L3 | 5.00 | 5.30 | L14 | 3.30 | 3.61 |
| L4 | 4.02 | 4.01 | L15 | 4.71 | 4.69 |
| L5 | 3.77 | 3.99 | L16 | 3.83 | 3.91 |
| L6 | 4.22 | 4.10 | L17 | 4.52 | 4.56 |
| L7 | 4.93 | 4.86 | L18 | 4.78 | 4.95 |
| L8 | 4.00 | 3.90 | L19 | 5.00 | 4.82 |
| L9 | 5.06 | 5.08 | L20 | 4.12 | 4.36 |
| L10 | 4.92 | 4.60 | R2 = 0.83 | ||
| L11 | 4.52 | 4.67 | Q2 = 0.59 | ||
| Test Set | |||||
| Compounds | Experimental pEC50 | PredictedpEC50 | Compounds | Experimental pEC50 | Predicted pEC50 |
| L21 | 5.01 | 4.90 | L27 | 4.72 | 4.87 |
| L22 | 4.70 | 4.55 | L28 | 4.08 | 4.00 |
| L23 | 4.08 | 4.24 | L29 | 4.52 | 4.59 |
| L24 | 3.83 | 4.09 | = 0.84226 RMSEP = 0.16145 | ||
| L25 | 4.77 | 4.56 | = 0.75732 = 0.61483 | ||
| L26 | 5.00 | 4.82 | = 0.68608 = 0.14249 | ||
| Variable | Node Pair | Distance [Å] | Impact | Description |
|---|---|---|---|---|
| 450 | N1–TIP | 10.8–11.2 | + | Distance between HBA and steric region on the benzene ring |
| 397 | O–TIP | 8.4–8.8 | − | Distance between HBD and steric region on the benzene ring |
| 350 | O–N1 | 8.4–8.8 | + | Distance between HBD and HBA on the benzene ring |
| 106 | N1–N1 | 4.8–5.2 | + | Distance between two HBAs on the benzene ring |
| 115 | N1–N1 | 8.4–8.8 | + | Distance between two HBAs on the benzene ring |
| 51 | O–O | 1.6–2.0 | + | Distance between two HBDs on the benzene ring |
| 60 | O–O | 5.2–5.6 | + | Distance between two HBDs on the benzene ring |
| 68 | O–O | 8.4–8.8 | + | Distance between two HBDs on the benzene ring |
| 388 | O–TIP | 4.8–5.2 | − | Distance between HBD and the steric region on the benzene ring |
| 406 | O–TIP | 12.0–12.4 | − | Distance between HBD and the steric region on the benzene ring |
| Compound | LogBBB | S+PrUnbnd | S+Pgp | RuleOf5 | CYP_Risk | CYP_Code | TOX_MUT_Risk | TOX_MUT_Code | TOX_Risk | TOX_Code | ADMET_Risk | ADMET_Code | SynthDiff |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carvacrol | 0.26 | 18.3 | No | 0 | 2.7 | 1A2; 2C9; CL | 0 | / | 0 | / | 2.7 | 1A2; 2C9; CL | 1.49 |
| D9 | −0.45 | 32.2 | Yes | 0 | 0 | / | 0.6 | m_97 | 1 | HEPX | 1 | HEPX | 1.86 |
| D16 | −0.22 | 50.0 | Yes | 0 | 1 | CL | 0.6 | S_97 | 1 | HEPX | 2 | HEPX; CL | 2.09 |
| D27 | −0.66 | 70.4 | Yes | 0 | 0 | / | 0 | / | 0 | / | 0 | / | 1.82 |
| D82 | 0.16 | 7.9 | No | 0 | 1 | CL | 0.6 | m_97 | 0 | / | 1 | CL | 1.39 |
| D83 | 0.37 | 4.4 | No | 0 | 1 | CL | 0.6 | m_97 | 0 | / | 2.8 | Kow; fu; CL | 1.52 |
| D84 | 0.52 | 3.5 | No | 0 | 1 | CL | 0.6 | m_97 | 0 | / | 4 | Kow; Sw; fu; CL | 1.97 |
| D88 | 0.14 | 8.9 | Yes | 0 | 1 | CL | 0.6 | NIHS | 1 | HEPX | 2 | HEPX; CL | 3.92 |
| D92 | −0.26 | 25.9 | No | 0 | 0.3 | CL | 1.2 | m_97; NIHS | 3 | Xm; HEPX; MUT | 3.3 | Xm; HEPX; MUT; CL | 2.41 |
| D94 | 0.22 | 13.7 | No | 0 | 1 | CL | 0.6 | m_97 | 1 | HEPX | 2 | HEPX; CL | 2.46 |
| D98 | 0.22 | 15.9 | Yes | 0 | 1.7 | 2C19; CL | 0.6 | m_97 | 1 | HEPX | 2.7 | HEPX; 2C19; CL | 2.60 |
| Compound | Predicte dpEC50 | Bind Energy [kcal/mol] | Interacting Residue * |
|---|---|---|---|
| Carvacrol | 4.770 § | −5.2 | Ala (B:939); Leu (B:936); Leu (B:891); Ala (A:836); Ile (A:837) |
| D9 | 5.129 | −5.0 | Leu (B:936); Ile (A:837) |
| D16 | 5.101 | −5.4 | Ser (B:943); Ala (B:939); Phe (A:841); Gln (B:940) |
| D27 | 4.996 | −6.0 | Ala (A:836); Leu (B:891); Ile (A:837); Leu (B:936); Phe (A:841); Gln (B:940) |
| D82 | 5.072 | −6.3 | Ser (B:943); Ile (A:837); Leu (B:936); Tyr (A:840); Phe (A:841) |
| D83 | 5.235 | −6.0 | Ser (B:943); Phe (A:841); Gln (B:940); Phe (B:947); Met (A:844); Leu (A:807); Ile (A:803); Ala (B:939) |
| D84 | 5.116 | −5.9 | Phe (A:841) |
| D88 | 4.999 | −5.7 | Phe (A:841); Ile (A:837); Leu (B:936); Ala (A:836); Tyr (A:840); Ala (B:939) |
| D92 | 5.087 | −5.8 | Ala (B:939); Leu (B:936); Ile (A:837); Ser (B:887) |
| D94 | 5.049 | −5.4 | Ile (A:837); Leu (B:936); Phe (A:841); Tyr (A:840) |
| D98 | 5.122 | −5.2 | Ile (A:837); Leu (B:936) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đukanović, Đ.; Suručić, R.; Bojić, M.G.; Trailović, S.M.; Škrbić, R.; Gagić, Ž. Design of Novel TRPA1 Agonists Based on Structure of Natural Vasodilator Carvacrol—In Vitro and In Silico Studies. Pharmaceutics 2024, 16, 951. https://doi.org/10.3390/pharmaceutics16070951
Đukanović Đ, Suručić R, Bojić MG, Trailović SM, Škrbić R, Gagić Ž. Design of Novel TRPA1 Agonists Based on Structure of Natural Vasodilator Carvacrol—In Vitro and In Silico Studies. Pharmaceutics. 2024; 16(7):951. https://doi.org/10.3390/pharmaceutics16070951
Chicago/Turabian StyleĐukanović, Đorđe, Relja Suručić, Milica Gajić Bojić, Saša M. Trailović, Ranko Škrbić, and Žarko Gagić. 2024. "Design of Novel TRPA1 Agonists Based on Structure of Natural Vasodilator Carvacrol—In Vitro and In Silico Studies" Pharmaceutics 16, no. 7: 951. https://doi.org/10.3390/pharmaceutics16070951
APA StyleĐukanović, Đ., Suručić, R., Bojić, M. G., Trailović, S. M., Škrbić, R., & Gagić, Ž. (2024). Design of Novel TRPA1 Agonists Based on Structure of Natural Vasodilator Carvacrol—In Vitro and In Silico Studies. Pharmaceutics, 16(7), 951. https://doi.org/10.3390/pharmaceutics16070951







