Potential Wound Healing and Anti-Melanogenic Activities in Skin Cells of Aralia elata (Miq.) Seem. Flower Essential Oil and Its Chemical Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Aralia elata (Miq.) Seem. Flower Absolute-Type Essential Oil
2.3. Compounds Analysis in Aralia elata (Miq.) Seem. Flower Absolute-Type Essential Oil
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Proliferation Assay
2.7. Cell Migration Assay
2.8. Collagen Synthesis Assay
2.9. Western Blotting
2.10. Melanin Content Assay
2.11. Tyrosinase Activity Analysis
2.12. Analysis
3. Results and Discussion
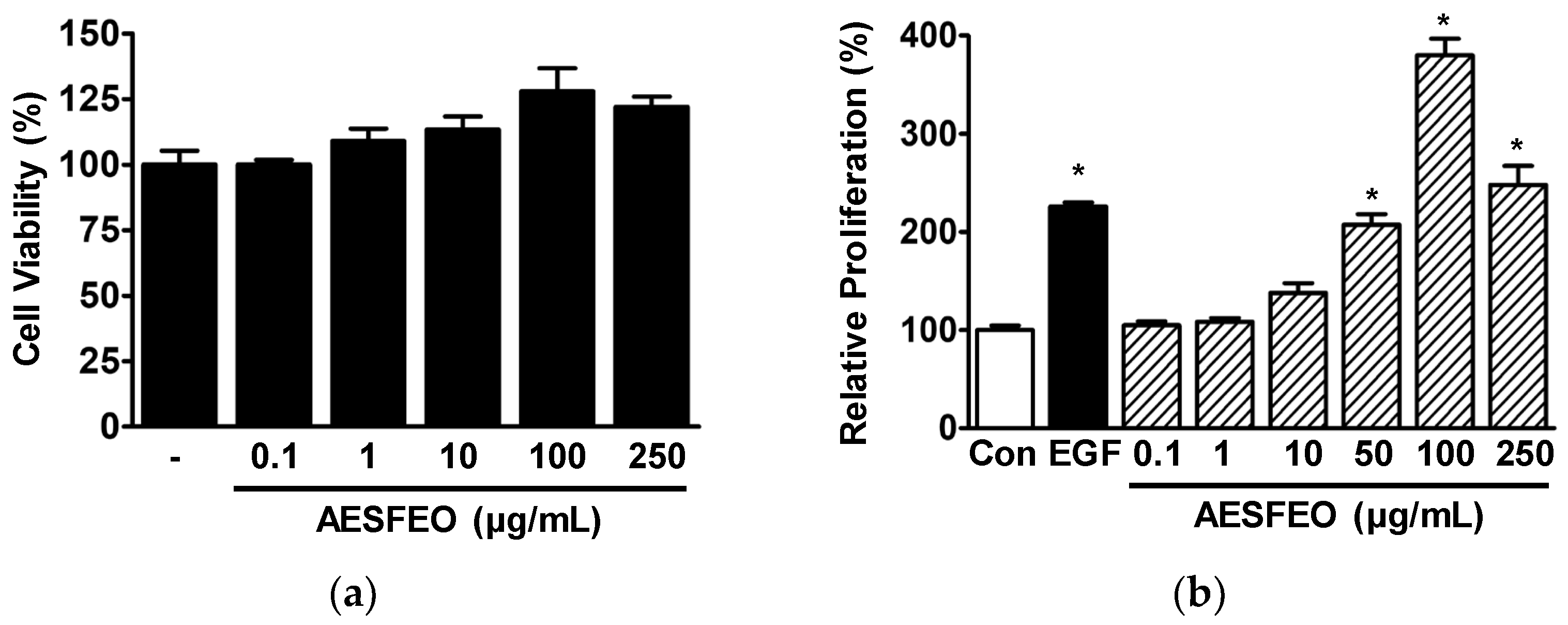
3.1. Proliferation and Migration of AESFEO-Treated HaCaT Cells
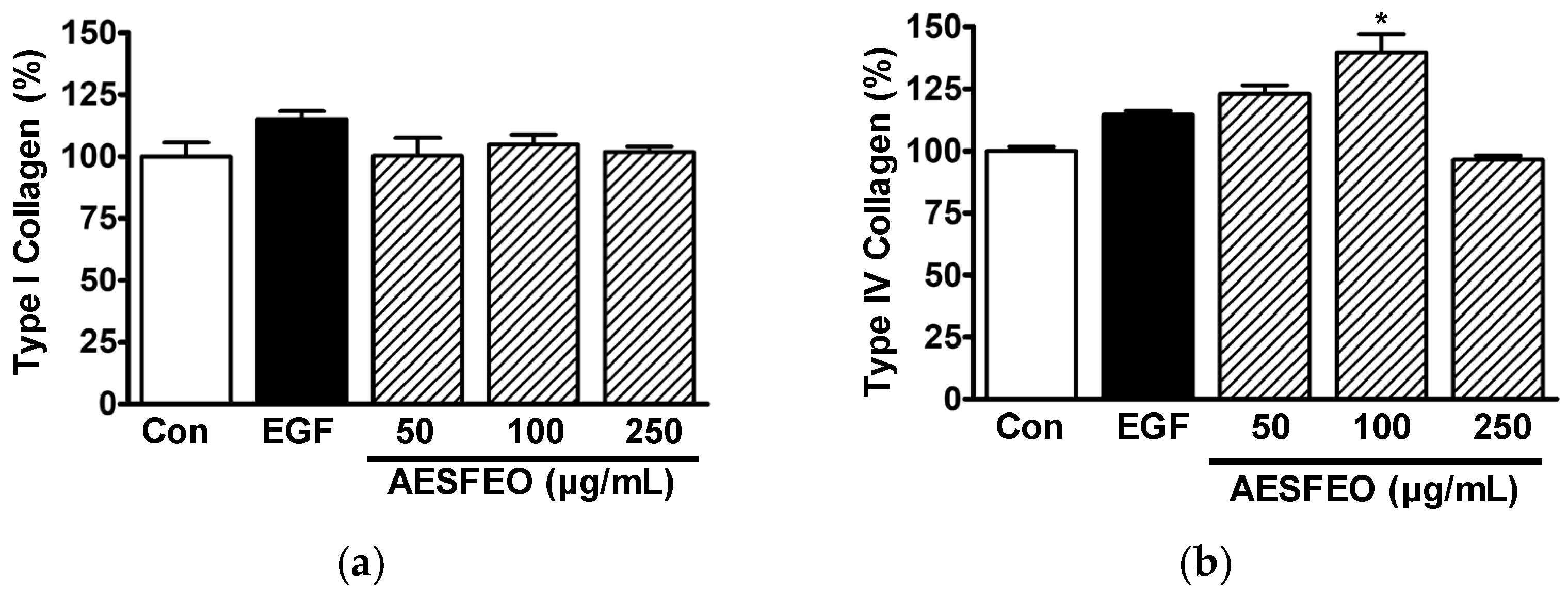
3.2. Influence of AESFEO on Collagen Synthesis in HaCaT Cells
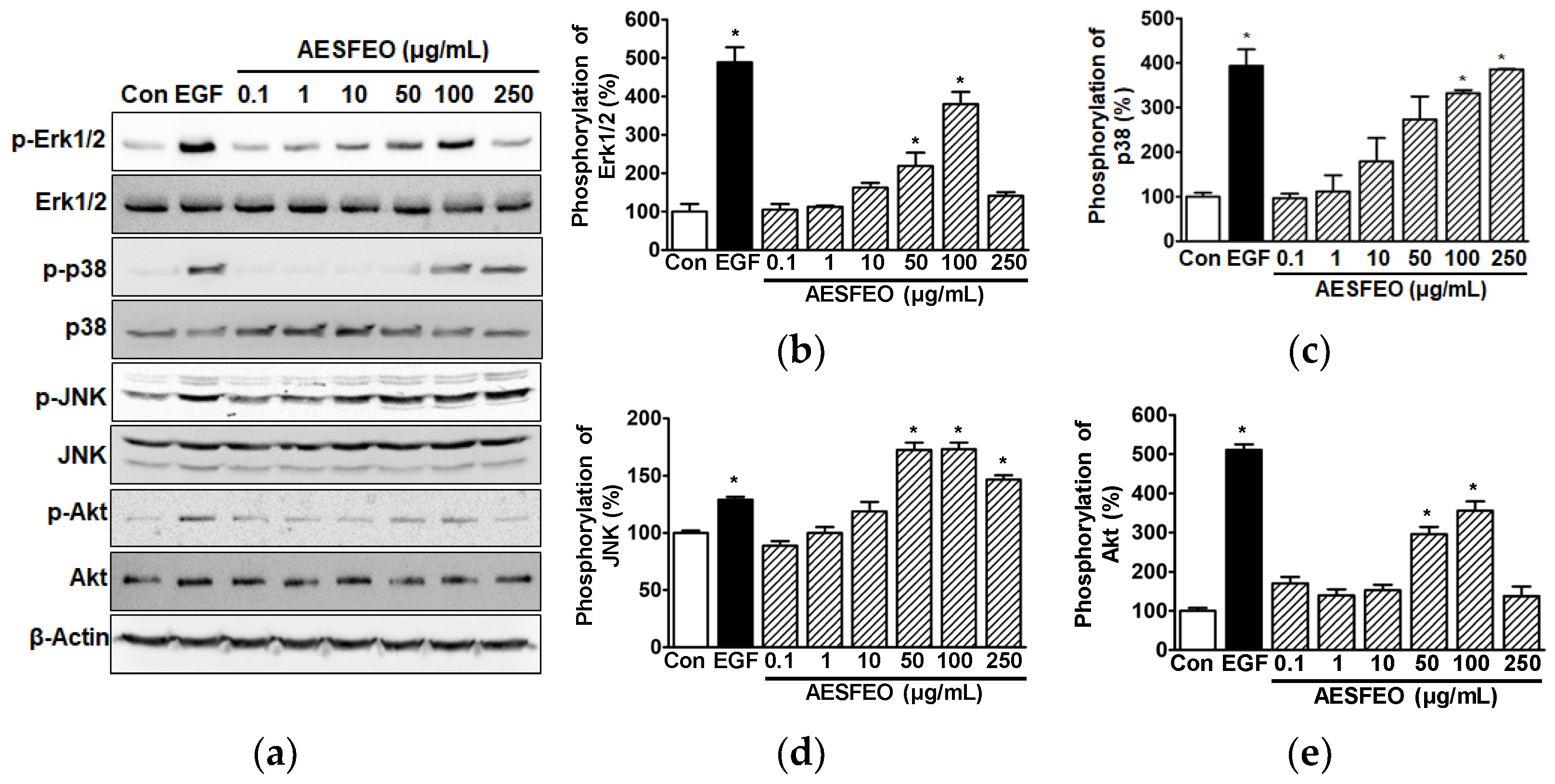
3.3. AESFEO Induced Changes in Intracellular Signal Kinases in HaCaT Cells
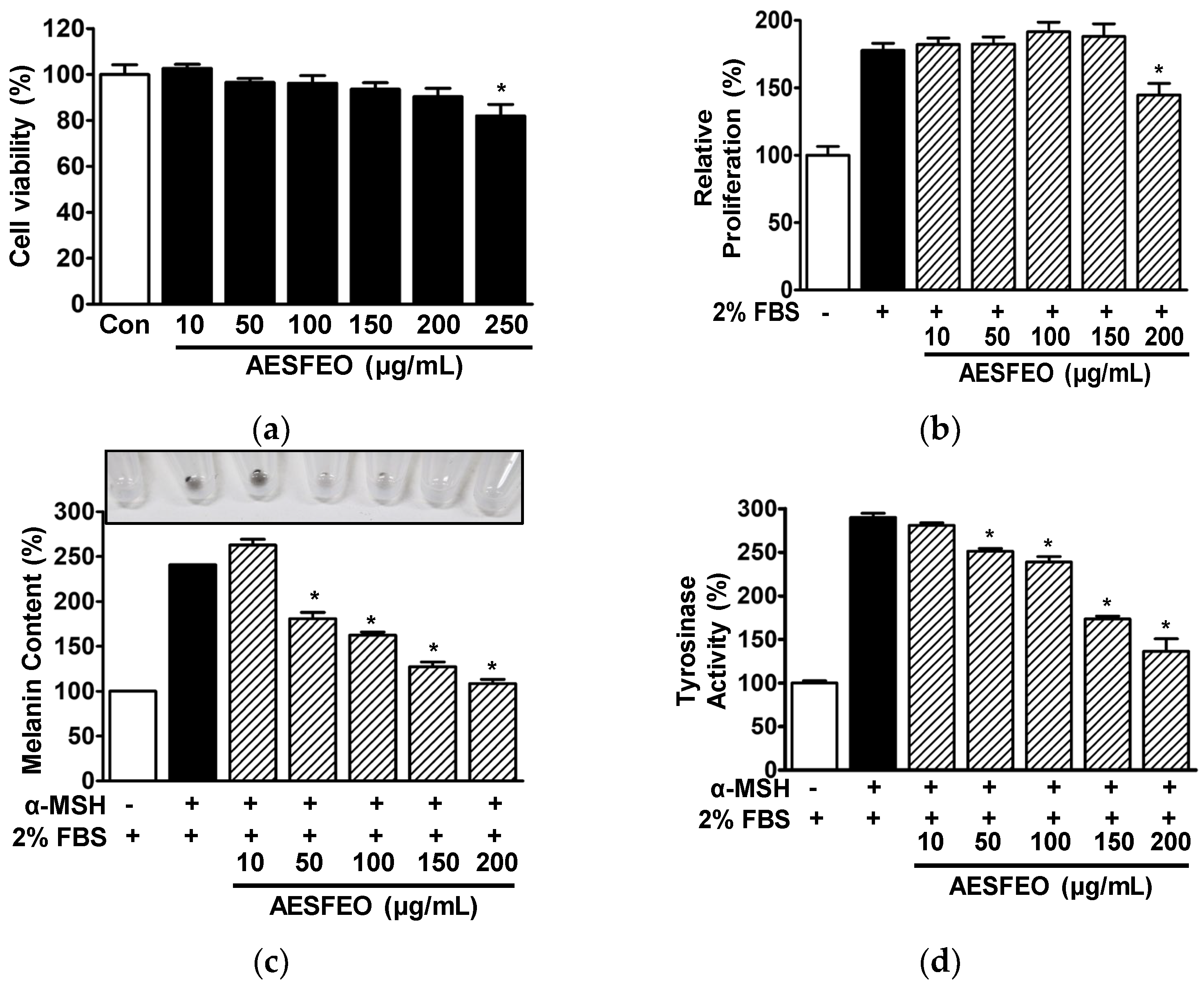
3.4. Cell Proliferation, Melanin Synthesis, and Tyrosinase Activity in AESFEO-Treated B16BL6 Cells
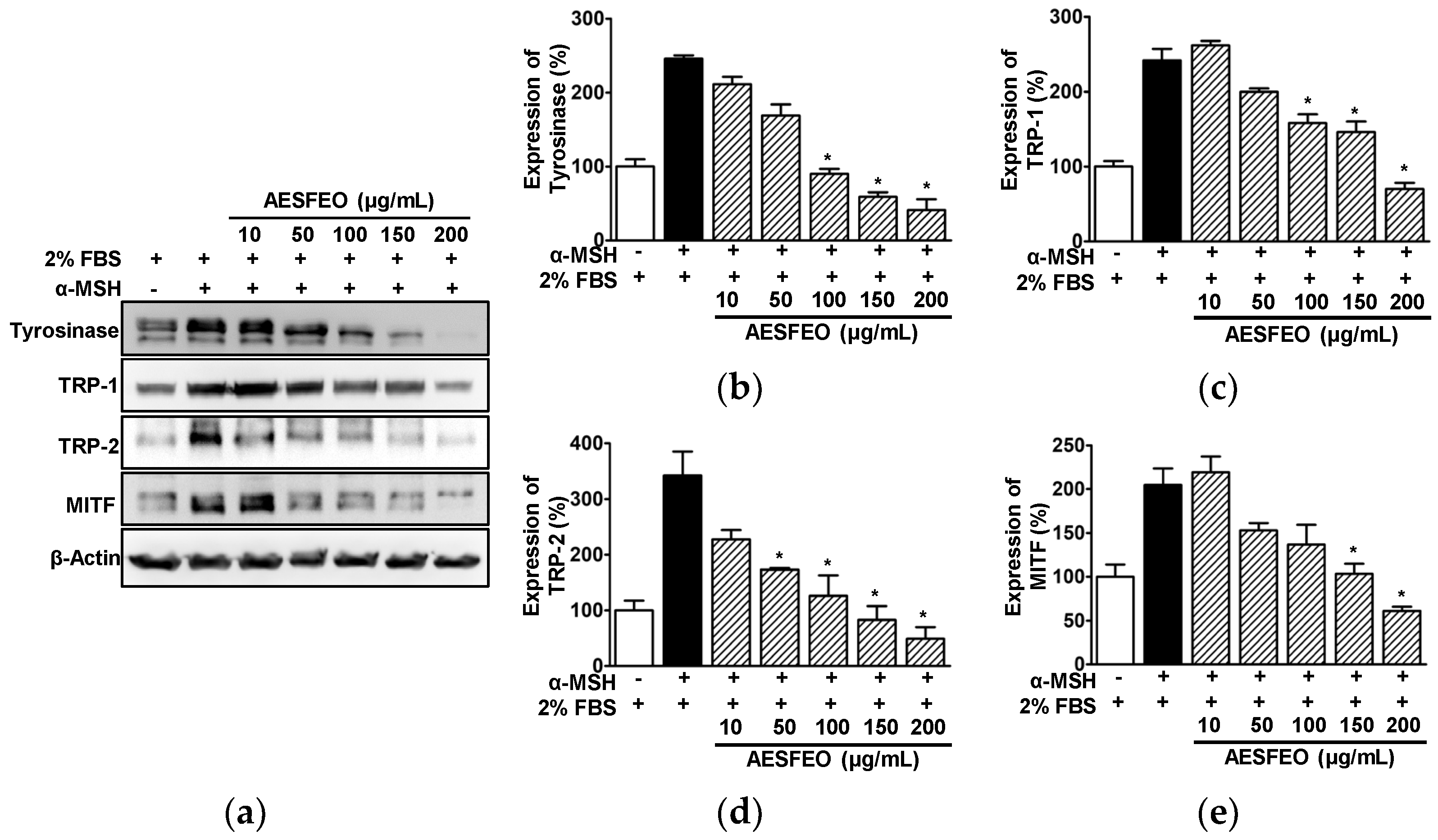
3.5. Effect of AESFEO on Melanogenesis Regulation-Related Proteins in B16BL6 Cells
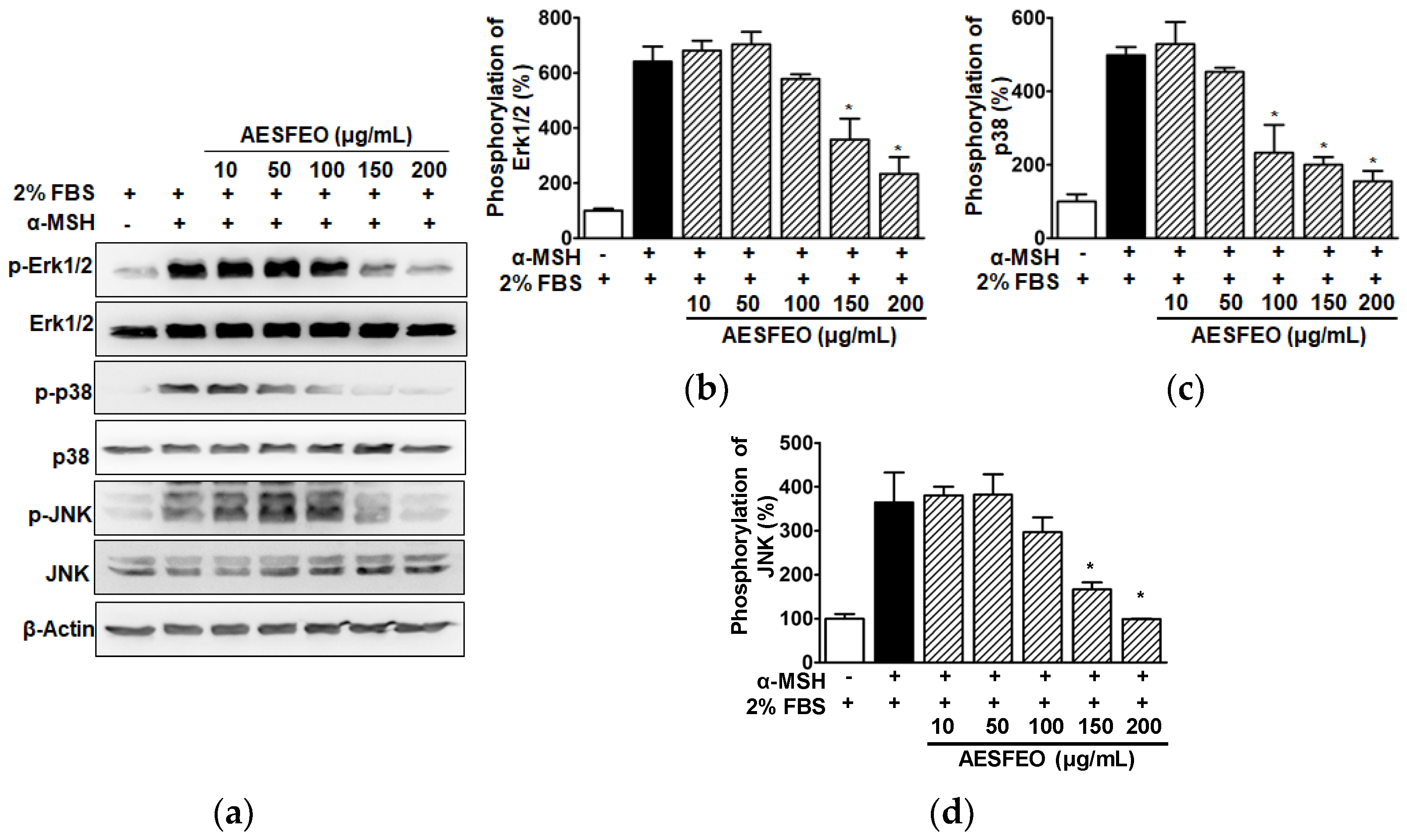
3.6. MAPK Phosphorylations in AESFEO-Treated B16BL6 Cells
3.7. Chemical Composition of AESFEO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The therapeutic wound healing bioactivities of various medicinal plants. Life 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.D.; Oreffo, R.O.; Healy, E.; Thurner, P.J.; Man, Y.H. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 2013, 28, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Gazel, A.; Nijhawan, R.I.; Walsh, R.; Blumenberg, M. Transcriptional profiling defines the roles of ERK and p38 kinases in epidermal keratinocytes. J. Cell Physiol. 2008, 215, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Fu, L.; Li, X.; Zhang, Y.; Li, Z.; Wu, X.; Li, Y.; Bai, X.; Hu, D. Loss of CAR promotes migration and proliferation of HaCaT cells, and accelerates wound healing in rats via Src-p38 MAPK pathway. Sci. Rep. 2016, 6, 19735–19741. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, J.Q.; Zheng, Z.; Zhang, J.; Wang, S.Y.; Han, S.C.; Zhou, Q.; Guan, H.; Li, C.; Su, L.L.; et al. Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways. Cell Tissue Res. 2016, 365, 85–99. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Zhang, Q.; Wang, W.; Zhang, D.; Jia, J.; Lv, Y.; Yuan, H.; Song, H.; Xiang, F.; et al. Microtubule-associated protein 4 phosphorylation regulates epidermal keratinocyte migration and proliferation. Int. J. Biol. Sci. 2019, 15, 1962–1976. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, E.A. Extracellular matrix and keratinocyte migration. Clin. Exp. Dermatol. 2001, 26, 525–530. [Google Scholar] [CrossRef]
- Seo, W.Y.; Kim, J.H.; Baek, D.S.; Kim, S.J.; Kang, S.; Yang, W.S.; Song, J.A.; Lee, M.S.; Kim, S.; Kim, Y.S. Production of recombinant human procollagen type I C-terminal propeptide and establishment of a sandwich ELISA for quantification. Sci. Rep. 2017, 7, 15946. [Google Scholar] [CrossRef]
- Tang, L.; Sierra, J.O.; Kelly, R.; Kirsner, R.S.; Li, J. Wool-derived keratin stimulates human keratinocyte migration and types IV and VII collagen expression. Exp. Dermatol. 2012, 21, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Lu, J.; Ding, Y.; Jiang, L.; Hu, S.; Chen, J.; Zeng, Q. The role and mechanism of Asian medicinal plants in treating skin pigmentary disorders. J. Ethnopharmacol. 2019, 245, 112173. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.H. Inhibitors of melanogenesis: An updated review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Huh, Y.; Lim, K.M. Anti-pigmentary natural compounds and their mode of action. Int. J. Mol. Sci. 2021, 22, 6206. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant extracts as skin care and therapeutic agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Insaf, A.; Parveen, R.; Gautam, G.; Samal, M.; Zahiruddin, S.; Ahmad, S. A comprehensive study to explore tyrosinase inhibitory medicinal plants and respective phytochemicals for hyperpigmentation; molecular approach and future perspectives. Curr. Pharm. Biotechnol. 2023, 24, 780–813. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhou, X.; Ma, J.; Li, T.; Zhang, X.; Li, J.; Xueyanfu. A review on a medicinal and edible plant: Aralia elata (Miq.) Seem. Mini Rev. Med. Chem. 2021, 21, 2567–2583. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, C.S. Suppressive effects on the biosynthesis of inflammatory mediators by Aralia elata extract fractions in macrophage cells. Environ. Toxicol. Pharmacol. 2009, 28, 333–341. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Lim, H.Y.; Kiem, P.V.; Minh, C.V.; Thu, V.K.; Tai, B.H.; Quang, T.H.; Song, S.B.; Kim, Y.H. Oleanane-type triterpene saponins from the bark of Aralia elata and their NF-κB inhibition and PPAR activation signal pathway. Bioorg. Med. Chem. Lett. 2011, 21, 6143–6147. [Google Scholar] [CrossRef]
- Tomatsu, M.; Ohnishi-Kameyama, M.; Shibamoto, N. Aralin, a new cytotoxic protein from Aralia elata, inducing apoptosis in human cancer cells. Cancer Lett. 2003, 199, 19–25. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zeng, L.; Chen, P. Research progress in pharmacological effects of Aralia elata. Zhejiang Da Xue Xue Bao Yi Xue Ban 2023, 52, 616–626. [Google Scholar] [CrossRef]
- Kim, N.Y.; Won, K.J.; Kim, H.B.; Kim, D.Y.; Kim, M.J.; Won, Y.R.; Lee, H.M. Chemical composition of Salix koreensis Anderss flower absolute and its skin wound healing activities in vitro. Plants 2022, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Kovats, E. Gas chromatographic characterization of organic substances in the retention index system. Adv. Chromatogr. 1965, 1, 229–247. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Hwang, D.I.; Won, K.J.; Kim, D.Y.; Kim, H.B.; Li, Y.; Lee, H.M. Chemical composition of Patrinia scabiosifolia flower absolute and its migratory and proliferative activities in human keratinocytes. Chem. Biodivers. 2019, 16, e1900252. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar]
- Han, H.; Hyun, C.G. Syringetin promotes melanogenesis in B16F10 cells. Int. J. Mol. Sci. 2023, 24, 9960. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in wound healing: A comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- DiPersio, C.M.; Zheng, R.; Kenney, J.; Van De Water, L. Integrin-mediated regulation of epidermal wound functions. Cell Tissue Res. 2016, 365, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.M.; Schulze, K.; Bergman, W.; Welle, M.; Roosje, P.; Müller, E.J. The keratinocyte in epidermal renewal and defence. Vet. Dermatol. 2009, 20, 515–532. [Google Scholar] [CrossRef] [PubMed]
- El-Serafi, A.T.; El-Serafi, I.; Steinvall, I.; Sjöberg, F.; Elmasry, M. A systematic review of keratinocyte secretions: A regenerative perspective. Int. J. Mol. Sci. 2022, 23, 7934. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascré, G.; Simons, B.D.; Blanpain, C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Yoon, S.W.; Lee, S.J.; Park, J.H.; Yoon, M.S.; Kim, B.; Lee, H.M. Potential skin regeneration activity and chemical composition of absolute from Pueraria thunbergiana flower. Nat. Prod. Commun. 2015, 10, 2009–2012. [Google Scholar]
- Park, S.M.; Won, K.J.; Hwang, D.I.; Kim, D.Y.; Kim, H.B.; Li, Y.; Lee, H.M. Potential beneficial effects of Digitaria ciliaris flower absolute on the wound healing-linked activities of fibroblasts and keratinocytes. Planta Med. 2020, 86, 348–355. [Google Scholar] [CrossRef]
- Kang, J.; Chen, W.; Xia, J.; Li, Y.; Yang, B.; Chen, B.; Sun, W.; Song, X.; Xiang, W.; Wang, X.; et al. Extracellular matrix secreted by senescent fibroblasts induced by UVB promotes cell proliferation in HaCaT cells through PI3K/AKT and ERK signaling pathways. Int. J. Mol. Med. 2008, 21, 777–784. [Google Scholar] [CrossRef][Green Version]
- Huang, C.; Jacobson, K.; Schaller, M.D. MAP kinases and cell migration. J. Cell Sci. 2004, 117 Pt 20, 4619–4628. [Google Scholar] [CrossRef]
- Kim, H.E.; Cho, H.; Ishihara, A.; Kim, B.; Kim, O. Cell proliferation and migration mechanism of caffeoylserotonin and serotonin via serotonin 2B receptor in human keratinocyte HaCaT cells. BMB Rep. 2018, 51, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, Y.K.; Eun, H.C.; Cho, K.H.; Chung, J.H. All-trans retinoic acid antagonizes UV-induced VEGF production and angiogenesis via the inhibition of ERK activation in human skin keratinocytes. J. Investing. Dermatol. 2006, 126, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F. PI3K/Akt: Getting it right matters. Oncogene 2008, 27, 6473–6488. [Google Scholar] [CrossRef] [PubMed]
- Schüppel, M.; Kürschner, U.; Kleuser, U.; Schäfer-Korting, M.; Kleuser, B. Sphingosine 1-phosphate restrains insulin-mediated keratinocyte proliferation via inhibition of Akt through the S1P2 receptor subtype. J. Investing. Dermatol. 2008, 128, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Wu, Y.; Yang, T.; Yang, K.; Wang, R.; Yang, J.; Guo, H. Wnt5a inhibits the proliferation and melanogenesis of melanocytes. Int. J. Med. Sci. 2013, 10, 699–706. [Google Scholar] [CrossRef]
- Maeda, K.; Yokokawa, Y.; Hatao, M.; Naganuma, M.; Tomita, Y. Comparison of the melanogenesis in human black and light brown melanocytes. J. Dermatol. Sci. 1997, 14, 199–206. [Google Scholar] [CrossRef]
- Makino, E.T.; Mehta, R.C.; Banga, A.; Jain, P.; Sigler, M.L.; Sonti, S. Evaluation of a hydroquinone-free skin brightening product using in vitro inhibition of melanogenesis and clinical reduction of ultraviolet-induced hyperpigmentation. J. Drugs Dermatol. 2013, 12, s16–s20. [Google Scholar]
- Kameyama, K.; Sakai, C.; Kuge, S.; Nishiyama, S.; Tomita, Y.; Ito, S.; Wakamatsu, K.; Hearing, V.J. The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment. Cell Res. 1995, 8, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2019, 9, 1703480. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.H.; Choi, J.H.; Kim, D.H.; Chung, M.J.; Lim, M.H. Ligularia fischeri ethanol extract: An inhibitor of alpha-melanocyte-stimulating hormone-stimulated melanogenesis in B16F10 melanoma cells. J. Cosmet. Dermatol. 2023, 22, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, M.; Song, N.; Sun, S.; Choi, J.; Park, K. Antioxidant and anti-melanogenesis effects of colloidal gold Camellia sinensis L. extracts. Molecules 2022, 27, 5593. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Kim, M.S.; Choi, Y.H.; Oren, M.; Park, E.K.; Kim, G.Y. Flumequine-mediated upregulation of p38 MAPK and JNK results in melanogenesis in B16F10 cells and zebrafish larvae. Biomolecules 2019, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hyun, C. Acenocoumarol, an anticoagulant drug, prevents melanogenesis in B16F10 melanoma cells. Pharmaceuticals 2023, 16, 604. [Google Scholar] [CrossRef]
- Ng, L.T.; Lin, L.T.; Chen, C.L.; Chen, H.W.; Wu, S.J.; Lin, C.C. Anti-melanogenic effects of δ-tocotrienol are associated with tyrosinase-related proteins and MAPK signaling pathway in B16 melanoma cells. Phytomedicine 2014, 21, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hemesath, T.J.; Takemoto, C.M.; Horstmann, M.A.; Wells, A.G.; Price, E.R.; Fisher, D.Z.; Fisher, D.E. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000, 14, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Hwang, E.S.; Lee, J.E.; Kim, S.Y.; Kwon, S.B.; Park, K.C. Sphingosine1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell Sci. 2003, 116, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.R.; Zhang, J.A.; Zhang, Q.; Permatasari, F.; Xu, Y.; Wu, D.; Yin, Z.Q.; Luo, D. Palmitic acid induces production of proinflammatory cytokines interleukin-6, interleukin-1β, and tumor necrosis factor-α via a NF-κB-dependent mechanism in HaCaT keratinocytes. Mediat. Inflamm. 2013, 2013, 530429. [Google Scholar] [CrossRef] [PubMed]
- Dudau, M.; Codrici, E.; Tarcomnicu, I.; Mihai, S.; Popescu, I.D.; Albulescu, L.; Constantin, N.; Cucolea, I.; Costache, T.; Rambu, D.; et al. A fatty acid fraction purified from Sea Buckthorn seed oil has regenerative properties on normal skin cells. Front. Pharmacol. 2021, 12, 737571. [Google Scholar] [CrossRef] [PubMed]
- Biskup, E.; Gołębiowski, M.; Gniadecki, R.; Stepnowski, P.; Łojkowska, E. Triterpenoid α-amyrin stimulates proliferation of human keratinocytes but does not protect them against UVB damage. Acta Biochim. Pol. 2012, 59, 255–260. [Google Scholar] [CrossRef] [PubMed]
- McAnally, J.A.; Jung, M.; Mo, H. Farnesyl-O-acetylhydroquinone and geranyl-O-acetylhydroquinone suppress the proliferation of murine B16 melanoma cells, human prostate and colon adenocarcinoma cells, human lung carcinoma cells, and human leukemia cells. Cancer Lett. 2003, 202, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Tatman, D.; Jung, M.; Elson, C.E. Farnesyl anthranilate suppresses the growth, in vitro and in vivo, of murine B16 melanomas. Cancer Lett. 2000, 157, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, R.O.; Bustos, S.O.; Gonzalez, J.R.; Soares, C.W.; Barbosa, M.D.; Chammas, R.; Votto, A.P.; Trindade, G.S. Interaction between omega 3 PUFA and UVB radiation: Photoprotective effect in normal and tumoral murine melanocytes? J. Photochem. Photobiol. B 2016, 164, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Huang, Y.C.; Tsai, M.L.; Cheng, C.Y.; Liu, L.L.; Yen, Y.W.; Chen, W.L. Inhibition of melanogenesis by β-caryophyllene from lime mint essential oil in mouse B16 melanoma cells. Int. J. Cosmet. Sci. 2015, 37, 550–554. [Google Scholar] [CrossRef]
- Yoon, W.J.; Kim, M.J.; Moon, J.Y.; Kang, H.J.; Kim, G.O.; Lee, N.H.; Hyun, C.G. Effect of palmitoleic acid on melanogenic protein expression in murine b16 melanoma. J. Oleo Sci. 2010, 59, 315–319. [Google Scholar] [CrossRef]
- Ando, H.; Funasaka, Y.; Oka, M.; Ohashi, A.; Furumura, M.; Matsunaga, J.; Matsunaga, N.; Hearing, V.J.; Ichihashi, M. Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids on melanogenesis. J. Lipid Res. 1999, 40, 1312–1316. [Google Scholar] [CrossRef]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef]
- Huh, S.; Kim, Y.S.; Jung, E.; Lim, J.; Jung, K.S.; Kim, M.O.; Lee, J.; Park, D. Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis. Biol. Pharm. Bull. 2010, 33, 1242–1245. [Google Scholar] [CrossRef]
- Huang, H.C.; Wei, C.M.; Siao, J.H.; Tsai, T.C.; Ko, W.P.; Chang, K.J.; Hii, C.H.; Chang, T.M. Supercritical fluid extract of spent coffee grounds attenuates melanogenesis through downregulation of the PKA, PI3K/Akt, and MAPK signaling pathways. Evid. Based Complement. Alternat. Med. 2016, 2016, 5860296. [Google Scholar] [CrossRef]
- Kamei, Y.; Otsuka, Y.; Abe, K. Comparison of the inhibitory effects of vitamin E analogues on melanogenesis in mouse B16 melanoma cells. Cytotechnology 2009, 59, 183–190. [Google Scholar] [CrossRef] [PubMed]
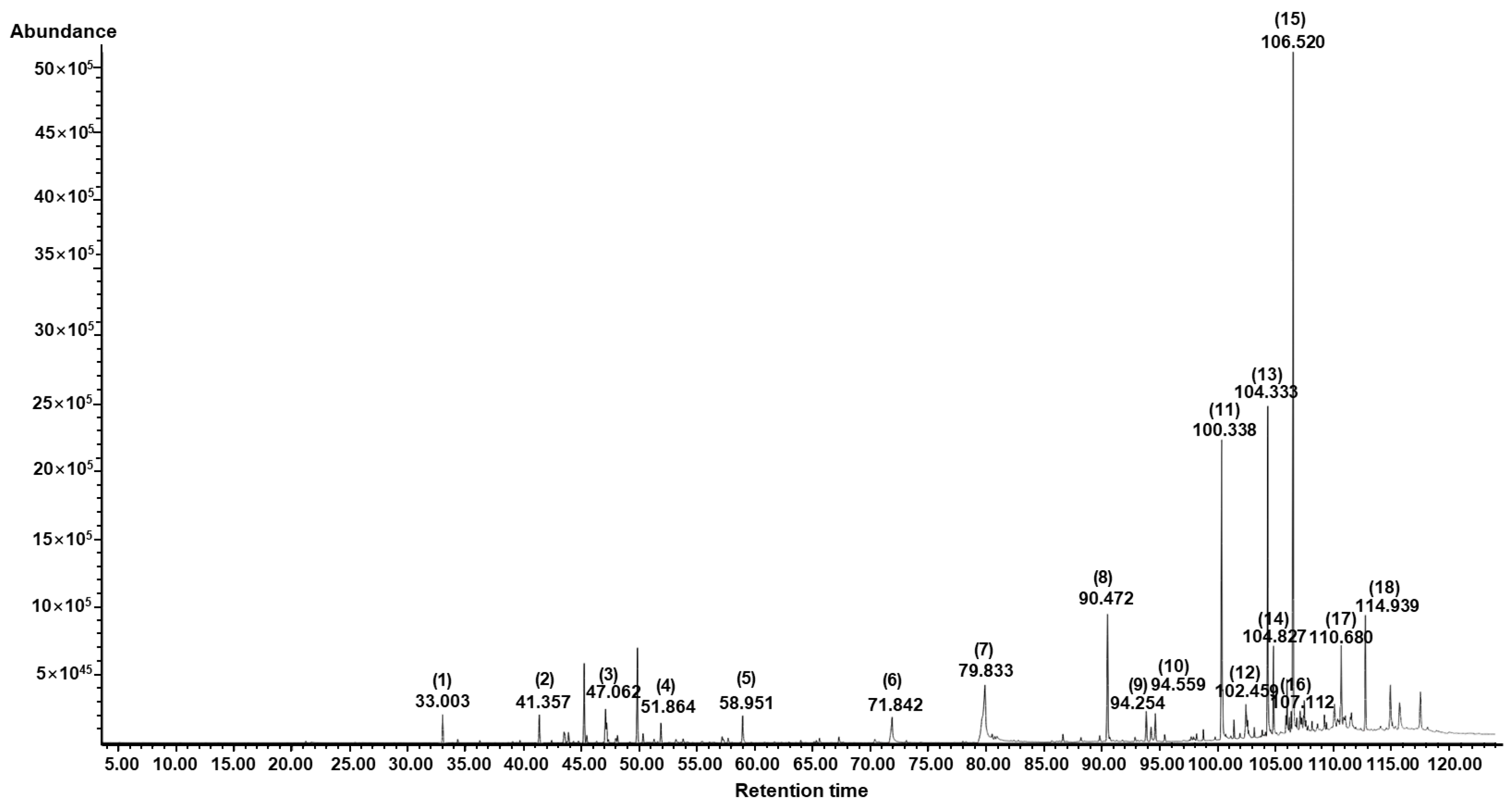
| No | Component Name | RT 1 | RI 2 | Area (%) | CAS No. | |
|---|---|---|---|---|---|---|
| Observed | Literature | |||||
| 1 | l-Bornyl acetate | 33.00 | 1280 | 1280 | 1.37 | 5655-61-8 |
| 2 | Caryophyllene | 41.36 | 1410 | 1418 | 1.42 | 87-44-5 |
| 3 | α-Bisabolene | 47.06 | 1503 | 1547 | 1.78 | 25532-79-0 |
| 4 | β-Bisabolene | 51.86 | 1585 | 1509 | 1.01 | 495-61-4 |
| 5 | Farnesol | 58.95 | 1713 | 1713 | 1.60 | 4602-84-0 |
| 6 | Hexadecanoic acid | 71.84 | 1968 | 1984 | 2.87 | 57-10-3 |
| 7 | Linolenic acid | 79.83 | 2142 | 2199 | 11.85 | 463-40-1 |
| 8 | Antioxidant BKF | 90.47 | 2394 | 2398 | 7.51 | 119-47-1 |
| 9 | 1-Octadecene | 94.25 | 2491 | 1800 | 0.98 | 112-88-9 |
| 10 | Pentacosane | 94.60 | 2500 | 2500 | 1.48 | 629-99-2 |
| 11 | Cyclotetracosane | 100.34 | 2697 | 2445 | 14.31 | 297-03-0 |
| 12 | Muscalure | 102.46 | 2798 | 2272 | 2.28 | 27519-02-4 |
| 13 | 1-Docosene | 104.34 | 2902 | 2196 | 14.00 | 1599-67-3 |
| 14 | δ-Tocopherol | 104.83 | 2932 | 2951 | 3.40 | 119-13-1 |
| 15 | γ-Tocopherol | 106.52 | 3040 | 3055 | 26.93 | 7616-22-0 |
| 16 | 9-(β-Chlorostyryl)-10-(phenylethynyl)anthracene | 107.11 | 3080 | - | 1.05 | 80364-59-6 |
| 17 | α-Amyrin | 110.68 | 3330 | 3376 | 3.17 | 638-95-9 |
| 18 | dl-Tetramisole | 114.94 | 3556 | - | 2.99 | 5036-02-2 |
| Total Identified (%) | 100.00 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Won, K.J.; Kim, Y.Y.; Yoo, D.Y.; Lee, H.M. Potential Wound Healing and Anti-Melanogenic Activities in Skin Cells of Aralia elata (Miq.) Seem. Flower Essential Oil and Its Chemical Composition. Pharmaceutics 2024, 16, 1008. https://doi.org/10.3390/pharmaceutics16081008
Kim DY, Won KJ, Kim YY, Yoo DY, Lee HM. Potential Wound Healing and Anti-Melanogenic Activities in Skin Cells of Aralia elata (Miq.) Seem. Flower Essential Oil and Its Chemical Composition. Pharmaceutics. 2024; 16(8):1008. https://doi.org/10.3390/pharmaceutics16081008
Chicago/Turabian StyleKim, Do Yoon, Kyung Jong Won, Yoon Yi Kim, Da Yeon Yoo, and Hwan Myung Lee. 2024. "Potential Wound Healing and Anti-Melanogenic Activities in Skin Cells of Aralia elata (Miq.) Seem. Flower Essential Oil and Its Chemical Composition" Pharmaceutics 16, no. 8: 1008. https://doi.org/10.3390/pharmaceutics16081008
APA StyleKim, D. Y., Won, K. J., Kim, Y. Y., Yoo, D. Y., & Lee, H. M. (2024). Potential Wound Healing and Anti-Melanogenic Activities in Skin Cells of Aralia elata (Miq.) Seem. Flower Essential Oil and Its Chemical Composition. Pharmaceutics, 16(8), 1008. https://doi.org/10.3390/pharmaceutics16081008







