Utilization of the Drug–Polymer Solid Dispersion Obtained by Ball Milling as a Taste Masking Method in the Development of Orodispersible Minitablets with Hydrocortisone in Pediatric Doses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Tableting Blends and ODMTs
2.2.1. Ball Milling Process
2.2.2. Granulation Process
2.2.3. Compression Process
2.3. Method Validation
2.4. Quality Assessment of Tableting Blends and ODMTs
2.4.1. Flow Properties of Tableting Blends
2.4.2. Uniformity of Weight and Thickness
2.4.3. Mechanical Properties
2.4.4. Drug Content
2.4.5. Disintegration Time Assessment
Human Taste Panel
Petri Dish
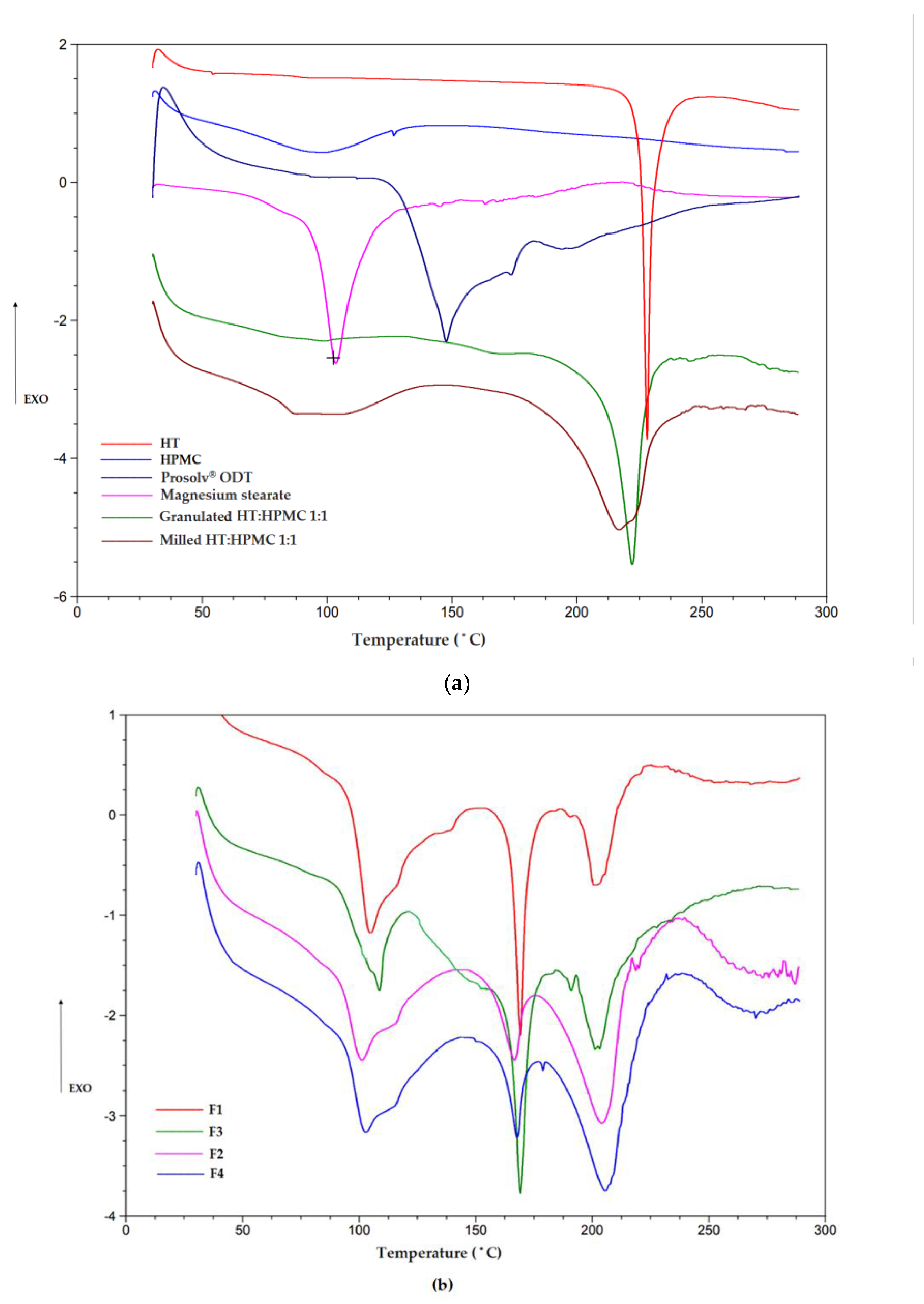
2.4.6. Differential Scanning Calorimetry (DSC)
2.4.7. FTIR Analysis
2.5. Evaluation of Taste-Masking Effectiveness
2.5.1. In Vitro HT Release
2.5.2. In Vivo Evaluation with Healthy Volunteers
2.5.3. Electronic Tongue—Sensor Array Fabrication and ODMTs Measurements
2.6. Bioequivalence Studies
2.6.1. Sampling to Evaluate HT Concentration; HPLC Analysis
2.6.2. Statistical Analysis
3. Results and Discussion
3.1. Pharmaceutical Evaluation of Obtained ODMTs
3.2. Taste-Masking Assessment
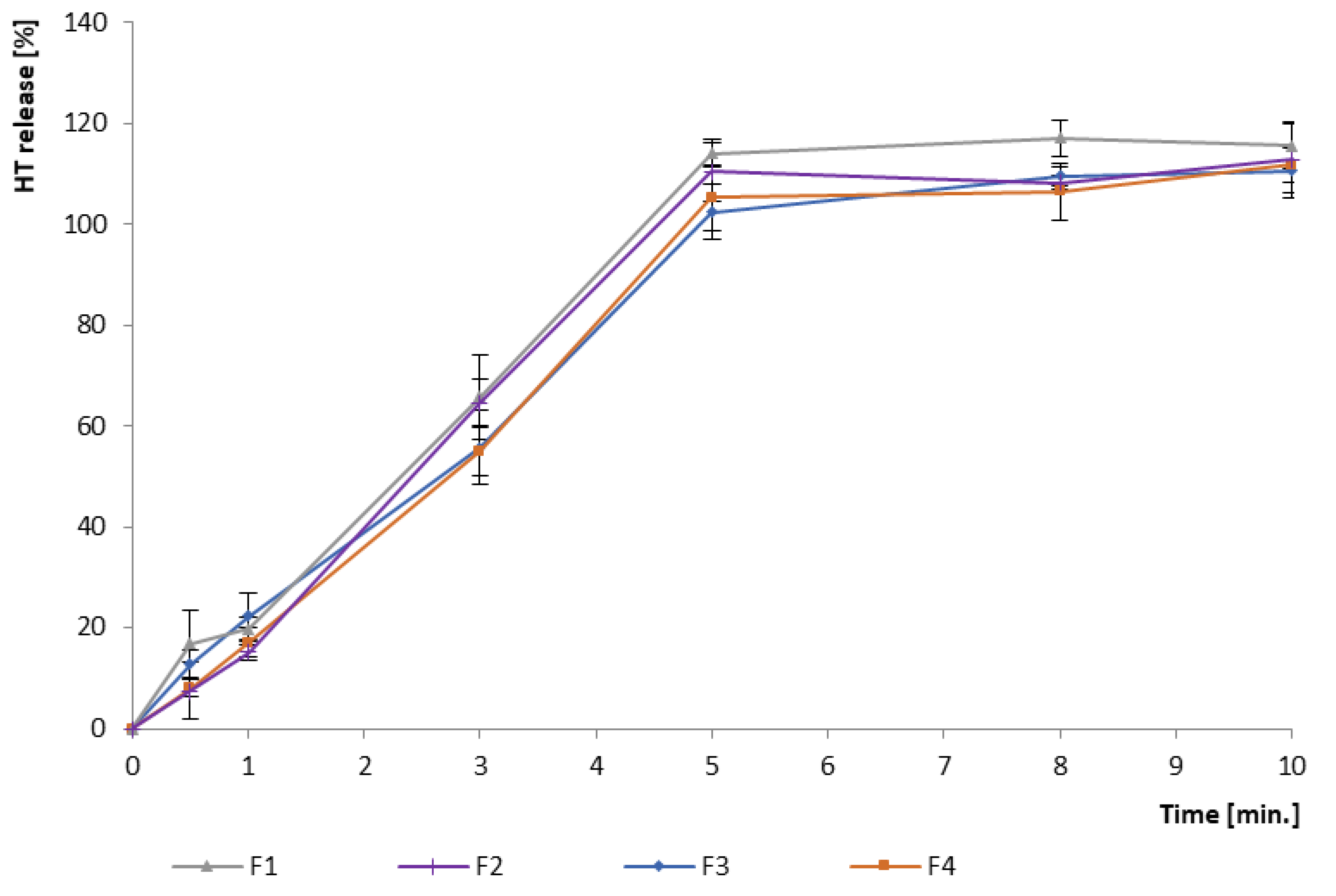
3.2.1. In Vitro Drug Release
3.2.2. In Vivo Human Taste Panel
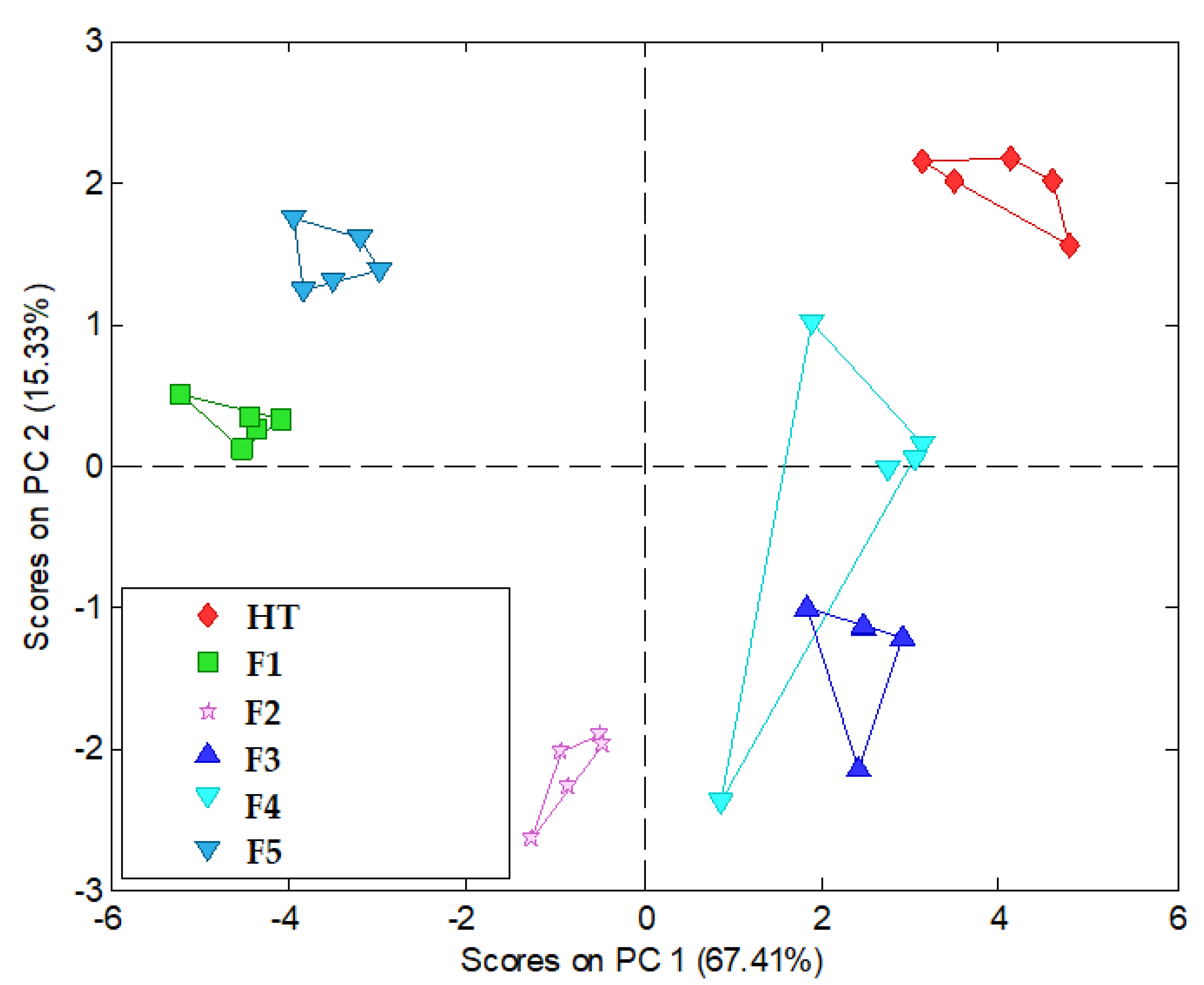
3.2.3. In Vitro Electronic Tongue Utilization
3.3. Bioequivalence Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stegemann, S. Patient centric drug product design in modern drug delivery as an opportunity to increase safety and effectiveness. Expert. Opin. Drug Deliv. 2018, 15, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Timpe, C.; Stegemann, S.; Barrett, A.; Mujumdar, S. Challenges and opportunities to include patient-centric product design in industrial medicines development to improve therapeutic goals. Br. J. Clin. Pharmacol. 2020, 86, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Orlando, V.; De Rosa, G.; Minghetti, P.; Musazzi, U.M.; Cahir, C.; Kurczewska-Michalak, M.; Kardas, P.; Costa, E.; Lobo, J.M.S.; et al. Patient centric pharmaceutical drug product design—The impact on medication adherence. Pharmaceutics 2020, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation. Pharmaceutical Development Q8 (R2). 2009. Available online: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed on 19 May 2024).
- Thabet, Y.; Klingmann, V.; Breitkreutz, J. Drug formulations: Standards and novel strategies for drug administration in pediatrics. J. Clin. Pharmacol. 2018, S10, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Malkawi, W.A.; AlRafayah, E.; AlHazabreh, M.; AbuLaila, S.; Al-Ghananeem, A.M. Formulation challenges and strategies to develop pediatric dosage forms. Children 2022, 9, 488. [Google Scholar] [CrossRef]
- Zuccari, G.; Alfei, S.; Marimpietri, D.; Iurilli, V.; Barabino, P.; Marchitto, L. Mini-Tablets: A valid strategy to combine efficacy and safety in pediatrics. Pharmaceuticals 2022, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.; Clapham, D.; Krysiak, K.; Batchelor, H.; Field, P.; Caivano, G.; Pertile, M.; Nunn, A.; Tuleu, C. Making medicines baby size: The challenges in bridging the formulation gap in neonatal medicine. Int. J. Mol. Sci. 2019, 20, 2688. [Google Scholar] [CrossRef] [PubMed]
- WHO. Development of paediatric medicines: Points to consider in formulation. WHO Tech. Rep. Series. 2012, 970, 1–22. Available online: https://cdn.who.int/media/docs/default-source/medicines/norms-and-standards/guidelines/trs970/annex5trs-70.pdf?sfvrsn=699cdb68_8&download=true (accessed on 19 May 2024).
- Van der Zanden, T.M.; Mooij, M.G.; Vet, N.J.; Neubert, A.; Rascher, W.; Lagler, F.B.; Male, C.; Grytli, H.; Halvorsen, T.; de Hoog, M.; et al. Benefit-risk assessment of off-label drug use in children: The Bravo framework. Clin. Pharm. Therap. 2021, 110, 952–965. [Google Scholar] [CrossRef]
- Petkova, V.; Georgieva, D.; Dimitrov, M.; Nikolova, I. Off-label prescribing in pediatric population—Literature review for 2012–2022. Pharmaceutics 2023, 15, 2652. [Google Scholar] [CrossRef]
- Al-Rayess, H.; Fleissner, K.; Jaber, M.; Brundage, R.C.; Sarafoglou, K. Manipulation of hydrocortisone tablets leads to iatrogenic cushing syndrome in a 6-year-old girl with CAH. J. Endocr. Soc. 2020, 4, bvaa091. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, A.B.; Khan, M.A.; Gupta, A.; Faustino, P.J. Dose uniformity of scored and unscored tablets: Application of the FDA tablet scoring guidance for industry. PDA J. Pharm. Sci. Technol. 2016, 70, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Rumondor, A.; Zhu, W.; Colace, T.; Marota, M.; Mora, J.; Liu, Z.; Li, Y. The development of minitablets for a pediatric dosage form for a combination therapy. J. Pharm. Sci. 2021, 109, 3590–3597. [Google Scholar] [CrossRef]
- Harrisa, D.; Hermansb, E.; Kleinc, S.; Hattlerd, L.; Walshe, J. Age-appropriate solid oral formulations for pediatric applications with a focus on multiparticulates and minitablets: Summary of September 2019 EuPFI workshop. Eur. J. Pharm. Biopharm. 2020, 153, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, E.; Brako, F.; Scarpa, M.; Lupo, M.; Bonifazi, D.; Pignataro, V.; Cavallo, M.; Cullufe, O.; Enache, C.; Nafria, B.; et al. Children’s preferences for oral dosage forms and their involvement in formulation research via EPTRI (European Paediatric Translational Research Infrastructure). Pharmaceutics 2021, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Singaraju, A.B.; Vinjamuri, B.P.; Ternik, R.; Stagner, W.C. Current state of minitablet product design: A review. J. Pharm. Sci. 2024, 113, 1123–1154. [Google Scholar] [CrossRef]
- Page, S.; Rode, T.; Breitkreutz, J.; Wagner-Hattler, L. Minitablets current use and future opportunities—An APV course on manufacturing, packaging, characterization and use of minitablets. Eur. J. Pharm.Biopharm. 2024, 199, 114294. [Google Scholar] [CrossRef]
- WHO. Innovative Delivery Systems for Paediatric Medicines: Technology Landscape; World Health Organization: Geneva, Switzerland, 2020; Available online: https://iris.who.int/bitstream/handle/10665/348336/9789240008182-eng.pdf?sequence=1 (accessed on 19 May 2024).
- Cilurzo, F.; Musazzi, U.M.; Franze, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: Biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef]
- Freerks, L.; Sommerfeldt, J.; Löper, P.C.; Klein, S. Safe, swallowable and palatable paediatric mini-tablet formulations for a WHO model list of essential medicines for children compound—A promising starting point for future PUMA applications. Eur. J. Pharm. Biopharm. 2020, 156, 11–19. [Google Scholar] [CrossRef]
- Salunke, S.; Liu, F.; Batchelor, H.; Walsh, J.; Turner, R.; Ju, T.R.; Tuleu, C. European Paediatric Formulation Initiative (EuPFI). AAPS Pharm. Sci. Tech. 2017, 18, 257–262. [Google Scholar] [CrossRef]
- Comoglu, T.; Ozyilmaz, E.D. Orally disintegrating tablets and orally disintegrating mini tablets—Novel dosage forms for pediatric use. Pharm. Dev. Technol. 2019, 24, 902–914. [Google Scholar] [CrossRef]
- Ortega, C.A.; Favier, L.S.; Cianchino, V.A.; Cifuente, D.A. New orodispersible mini tablets of enalapril maleate by direct compression for pediatric patients. CDD 2020, 17, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Meissner, T.; Mayatepek, E.; Wargenau, M.; Breitkreutz, J.; Bosse, H.M.; Klingmann, V. Acceptability of small-sized oblong tablets in comparison to syrup and mini-tablets in infants and toddlers: A randomized controlled trial. Eur. J. Pharm. Biopharm. 2021, 166, 126–134. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hida, N.; Kamiya, T.; Yamazaki, T.; Murayama, N.; Kuroiwa, M.; Kurata, N.; Ishikawa, Y.; Yamashita, S.; Nakamura, H.; et al. Comparative acceptability of mini-tablets, fine granules, and liquid formulations in young children: An exploratory randomised crossover study. J. Drug Deliv. Sci. Technol. 2022, 70, 103154. [Google Scholar] [CrossRef]
- Mitsui, N.; Hida, N.; Kamiya, T.; Yamazaki, T.; Miyazaki, K.; Saito, K.; Saito, J.; Yamatani, A.; Ishikawa, Y.; Nakamura, H.; et al. Swallowability of minitablets among children aged 6–23 months: An exploratory, randomized crossover study. Pharmaceutics 2022, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fitaihi, R.; Abukhamees, S.; Abdelhakim, H. Formulation and characterisation of carbamazepine orodispersible 3D-printed mini-tablets for Paediatric Use. Pharmaceutics 2023, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Alalaiwe, A.; Alsenaidy, M.A.; Almalki, Z.S.; Fayed, M.H. Development and optimization of sildenafil orodispersible mini-tablets (ODMTs) for treatment of pediatric pulmonary hypertension using response surface methodology. Pharmaceutics 2023, 15, 923. [Google Scholar] [CrossRef]
- Hejduk, A.; Teżyk, M.; Jakubowska, E.; Krüger, K.; Lulek, J. Implementing the design of experiments (DoE) concept into the development phase of orodispersible minitablets (ODMTs) containing melatonin. AAPS Pharm. Sci. Tech. 2022, 23, 60. [Google Scholar] [CrossRef]
- Protopapa, C.H.; Siamidi, A.; Kolipala, S.S.; Jungueira, L.A.; Douroumis, D.; Vlachou, M. In vitro profile of hydrocortisone release from three-dimensionally printed paediatric mini-tablets. Pharmaceutics 2024, 16, 385. [Google Scholar] [CrossRef]
- Protopapa, C.H.; Bya, L.-A.; Goebel, J.; Servais, A.-C.; Lechanteur, A.; Evrard, B. Development of 3D printed mini-waffle shapes containing hydrocortisone for children’s personalized medicines. Int. J. Pharm. 2023, 642, 123131. [Google Scholar] [CrossRef]
- Wasilewska, K.; Skibińska-Ciosek, P.; Lenik, J.; Srcic, S.; Basa, A.; Winnicka, K. Utilization of ethylcellulose microparticles with rupatadine fumarate in designing orodispersible minitablets with taste masking effect. Materials 2020, 13, 2715. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.M.; Stepien, K.; Krishan, A. Glucocorticoid replacement regimens in the treatment of 21-hydroxylase deficiency congenital adrenal hyperplasia. Cochrane Database Syst. Rev. 2020, 3, CD012517. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.S.; Spencer, S.P.; Geffner, M.E.; Gourgari, E.; Lahoti, A.; Kamboi, M.; Stanley, T.L.; Uli, N.K.; Wicklow, B.A.; Sarafoglou, K. Emergency management of adrenal insufficiency in children: Advocating for treatment options in outpatient and field settings. J. Investig. Med. 2019, 68, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kirkgoz, T.; Guran, T. Primary adrenal insufficiency in children: Diagnosis and management. Best. Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Braune, K.; Whitaker, M.J.; Wiegand, S.; Krude, H.; Porter, J.; Digweed, D.; Voet, B.; Ross, R.J.M.; Blankenstein, O. A Prospective study of children sged 0–8 Years with CAH and adrenal insufficiency treated with hydrocortisone granules. JCEM 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Hydrocortisone Dosage Regimen. Available online: https://www.medscape.co.uk/drug/hydrocortisone-oral-standard-release-69049-69049 (accessed on 19 May 2024).
- Saimbi, S.; Madden, V.; Stirling, H.; Yahyouche, A.; Batchelor, H. Comparison of hydrocortisone 10 mg tablets: Tablet hardness optimised for adult use has negative consequences for paediatric use. Arch. Dis. Child. 2016, 101, e2–e3. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Webb, E.A.; Kerr, S.; Davies, J.; Stirling, H.; Batchelor, H. How close is the dose? Manipulation of 10 mg hydrocortisone tablets to provide appropriate doses to children. Int. J. Pharm. 2018, 545, 57–63. [Google Scholar] [CrossRef]
- Peak, M.; Madathilethu, J.; Ford, J.; Prescott, R.; Blair, J.C.; Roberts, M. Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. BMJPO 2018, 2, e000198. [Google Scholar] [CrossRef]
- Neumann, U.; Burau, D.; Spielmann, S.; Whitaker, M.J.; Ross, R.J.M.; Kloft, C.H. Quality of compounded hydrocortisone capsules used in the treatment of children. Eur. J. Endocrinol. 2017, 177, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Alkindi® Sprinkle. Available online: https://ec.europa.eu/health/documents/community-register/2021/20210519151899/anx_151899_pl.pdf (accessed on 19 May 2024).
- Forough, A.S.; Lau, E.T.; Steadman, K.J.; Cichero, J.A.; Kyle, G.J.; Santos, J.M.S.; Nissen, L.M. A spoonful of sugar helps the medicine go down? A review of strategies for making pills easier to swallow. Patient Prefer. Adherence 2018, 12, 1337–1346. [Google Scholar] [CrossRef]
- Pein, M.; Preis, M.; Eckert, C.; Kiene, F.E. Taste-masking assessment of solid oral dosage forms—A critical review. Int. J. Pharm. 2014, 465, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Faisal, W.; Farag, F.; Abdellatif, A.A.H.; Abbas, A. Taste masking approaches for medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Thoke, S.; Amol, G.; Rahul, D.; Puranik, P.; Yogesh, S. Review on: Taste masking approaches and evaluation of taste masking. Int. J. Pharm. Sci. 2012, 4, 1895–1907. [Google Scholar]
- Ahmed, K.K.; Kassab, H.J.; Al Ramahi, I.J.; Alwan, Z.S. Taste masking of steroids for oral formulations. J. Pharm. Sci. 2023, 20, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, H.; Tian, B.; Gou, J.; Yao, Q.; Tao, X.; He, H.; Zhang, Y.; Tang, X.; Cai, C. Preparation and characterization of azithromycin—Aerosil 200 solid dispersions with enhanced physical stability. Pharm. Nanotechnol. 2015, 486, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Al-kasmi, B.; Alsirawan, M.H.D.B.; Paradkar, A.; Nattouf, A.-H.; El-Zein, H. Aqueous and pH dependent coacervation method for taste masking of paracetamol via amorphous solid dispersion formation. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shahoud, S.; Abdlwahed, W.; Hammad, T. Development a taste-masked acetaminophen solid dispersions using Eudragit® E100 polymer by solvent evaporation technique. J. Res. Pharm. 2023, 27, 1998–2006. [Google Scholar] [CrossRef]
- Zhao, M.; You, D.; Yin, J.; Sun, W.; Yin, T.; Gou, J.; Zhang, Y.; Wang, Y.; He, H.; Tang, X. Quaternary enteric solid dispersion prepared by hot-melt extrusion to mask the bitter taste and enhance drug stability. Int. J. Pharm. 2021, 597, 120279. [Google Scholar] [CrossRef]
- Loh, Z.H.; Samanta, A.K.; Heng, P.W.S. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 255–274. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose—A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA; Washington, DC, USA, 2009. [Google Scholar]
- FDA, HPMC. Available online: https://www.fda.gov/search?s=hypromellose&sort_bef_combine=rel_DESC (accessed on 19 May 2024).
- Mistry, P.; Stirling, H.; Callens, C.; Hodson, J.; Batchelor, H. Evaluation of patient-reported outcome measurements as a reliable tool to measure acceptability of the taste of paediatric medicines in an inpatient paediatric population. BMJ Open 2018, 8, e021961. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, A.H.; Soto, J.; Ernest, T.; Tuleu, C. Non-human tools for the evaluation of bitter taste in the design and development of medicines: A systematic review. Drug Discov. Today 2016, 21, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Łabańska, M.; Ciosek-Skibińska, P.; Wróblewski, W. Critical evaluation of laboratory potentiometric electronic tongues for pharmaceutical analysis—An overview. Sensors 2019, 19, 5376. [Google Scholar] [CrossRef]
- Hydrocortisone-SF. Available online: https://www.sunfarm.pl/library/2020/09/09/159964896157.pdf (accessed on 19 May 2024).
- Council of Europe. European Pharmacopoeia, 11th ed.; Council of Europe: Strasburg, France, 2023. [Google Scholar]
- Pistone, S.; Goycoolea, F.M.; Young, A.; Smistad, G.; Hiorth, M. Formulation of polysaccharide-based nanoparticles for local administration into the oral cavity. Eur. J. Pharm. Sci. 2017, 96, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Pytko-Polończyk, J.J.; Jakubik, A.; Przeklasa-Bierowiec, A.; Muszyńska, B. Artificial saliva and its use in biological experiments. J. Physiol. Pharmacol. 2017, 68, 807–813. [Google Scholar]
- Chikukwa, M.T.R.; Wesoly, M.; Korzeniowska, A.; Ciosek-Skibinska, P.; Walker, R.B.; Khamanga, S.M.M. Assessment of taste masking of captopril by ion-exchange resins using electronic gustatory system. Pharm. Dev. Technol. 2020, 25, 281–289. [Google Scholar] [CrossRef]
- Wasilewska, K.; Szekalska, M.; Ciosek-Skibinska, P.; Lenik, J.; Basa, A.; Jacyna, J.; Markuszewski, M.; Winnicka, K. Ethylcellulose in organic solution or aqueous dispersion form in designing taste-masked microparticles by the spray drying technique with a model bitter drug: Rupatadine fumarate. Polymers 2019, 11, 522. [Google Scholar] [CrossRef]
- Derendorf, H.; Möllmann, H.; Barth, J.; Möllmann, C.; Tunn, S.; Krieg, M. Pharmacokinetics and oral bioavailability of hydrocortisone. J. Clin. Pharmacol. 1991, 31, 473–476. [Google Scholar] [CrossRef]
- Buning, J.W.; Touw, D.J.; Brummelman, P.; Dullaart, R.P.F.; Van den Berg, G.; van der Klauw, M.M.; Kamp, J.; Wolffenbuttel, H.R.B.; Van Beek, A.P. Pharmacokinetics of oral hydrocortisone—Results and implications from a randomized controlled trial. Metabolism 2017, 71, 7–16. [Google Scholar] [CrossRef]
- Johnson, T.N.; Whitaker, M.J.; Keevil, B.; Ross, R.J. Bioavailability of oral hydrocortisone corrected for binding proteins and measured by LC-MS/MS using serum cortisol and salivary cortisone. J. Bioequivalence Bioavailab. 2018, 10, 001–003. [Google Scholar] [CrossRef] [PubMed]
- Bowles, B.J.; Dziemidowicz, K.; Lopez, F.L.; Orlu, M.; Tuleu, C.; Edwards, A.J.; Ernest, T.B. Co-processed excipients for dispersible tablets—Part 1: Manufacturability. AAPS Pharm. Sci. Tech. 2018, 19, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Vimal, V.V.; Aarathi, T.S.; Soumya, B.J. Superdisintegrants in fast disintegrating drug delivery systems: A brief review. Int. J. Pharm. 2013, 3, 380–385. [Google Scholar] [CrossRef]
- STEP Database. Available online: https://step-db.ucl.ac.uk/eupfi/appDirectLink.do?appFlag=login (accessed on 19 May 2024).
- Kogermann, K.; Lass, J.; Nellis, G.; Metsvaht, T.; Lutsar, I. Age-appropriate formulations including pharmaceutical excipients in neonatal medicines. CPD 2018, 23, 5779–5789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yin, D.; Rowe, J.; Badawy, S.; Nikfar, F.; Pandey, P. Understanding the factors that control the quality of mini-tablet compression: Flow, particle size, and tooling dimension. J. Pharm. Sci. 2018, 107, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- FDA, CDER. Guidance for industry—Orally disintegrating tablets. 2008. Available online: https://www.fda.gov/downloads/Drugs/Guidances/ucm070578.pdf (accessed on 19 May 2024).
- Ali, H.S.; Khan, S.; York, P.; Shah, S.M.; Khan, J.; Hussain, Z.; Khan, B.A. A stable hydrocortisone nanosuspension for improved dissolution: Preparation, characterization and in vitro evaluation. Pak. J. Pharm. Sci. 2017, 30, 1635–1643. [Google Scholar]
- Pubchem. Hydrocortisone Melting Point. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydrocortisone (accessed on 19 May 2024).
- Pubchem. Mannitol Melting Point. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Mannitol#section=Boiling-Point (accessed on 19 May 2024).
- Pubchem. Magnesium Stearate Melting Point. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Magnesium-Stearate (accessed on 19 May 2024).
- Manivannan, P.; Kumar, R.T.; Periyanayagasamy, V. Spectral analysis of hydrocortisone. J. Chem. Pharm. Sci. 2009, 2, 1–5. [Google Scholar]
- European Medicines Agency (EMA): Guidelines on the Investigation of Bioequivalence, London 2010. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 19 May 2024).



| Composition [%] | |||||
| Formulation | F1 (0.5 mg) | F2(1.0 mg) | F3 (0.5 mg) | F4 (1.0 mg) | F5 |
| Ball-Milled, Granulated | Granulated | Placebo | |||
| Ingredients | |||||
| HT:HPMC 1:1 | 15 | 30 | 15 | 30 | - |
| Prosolv ODT® | 80 | 65 | 80 | 65 | 95 |
| Magnesium stearate | 5 | ||||
| Method Validation Parameters | HT/Salvia Imitating Fluid | HT/Phase | HT/Blood Rat Plasma |
|---|---|---|---|
| Range | 5–100 µg/mL | 5–100 µg/mL | 10–200 ng/mL |
| Linearity | p = 34.13C + 20.91 | p = 40.29C − 33.62 | p = 33.51C + 0.61 |
| R2 | 0.999 | 0.999 | 0.999 |
| Intra-day precision (n = 9) | |||
| RSD | 0.03 | 0.04 | 0.04 |
| CV % | 2.67 | 4.48 | 3.96 |
| Accuracy | |||
| Recovery % | 97.49–99.57 | 98.27–102.04 | 95.33–99.94 |
| Inter-day precision (n = 18) | |||
| RSD | 0.06 | 0.03 | 0.04 |
| CV % | 5.76 | 3.45 | 4.39 |
| Accuracy | |||
| Recovery % | 98.14–103.96 | 95.33–99.94 | 99.86–101.10 |
| No | ISE ID* | Polymer | Plasticizer * | Lipophilic salt | Ionophore |
|---|---|---|---|---|---|
| 1 | CS | PVC, 66 mg | o-NPOE, 132 mg | KTFPB, 2.0 mg | - |
| 2 | CS | DOS, 132 mg | KTpClPB, 2.0 mg | - | |
| 3 | CS | o-NPOE, 132 mg | KTpClPB, 2.0 mg | - | |
| 4 | AS | PVC, 65 mg | o-NPOE, 128 mg | TDMAC, 7.0 mg | - |
| 5 | AS | DOS, 128 mg | TDMAC, 7.0 mg | - | |
| 6 | AS | o-NPOE, 128 mg | TDAC, 7.0 mg | - | |
| 7 | AM | PVC, 54 mg | DOS, 136 mg | - | Amine ionophore I, 10 mg |
| 8 | CS-AS | PVC, 64 mg | o-NPOE, 130 mg | TDMA-TCPB, 6 mg | - |
| Group Number | Group Description | Group Size (Number of Rats) | Formulation | Dose [mg] | |
|---|---|---|---|---|---|
| Subgroups Size | |||||
| I | Study group, F1; orally administration | 30 | A 6 B 6 C 6 D 6 E 6 | F1 | 0.5 |
| II | Study group, F2; orally administration | 30 | F2 | 1.0 | |
| III | Referential product A; orally administration | 30 | Reference A | 1.0 | |
| IV | Referential product B; orally administration | 30 | Reference B | 1.0 | |
| Total: | 120 | ||||
| Powder Mixture | Angle of Response [°] | Hausner Ratio | Carr’s Index [%] |
|---|---|---|---|
| Physical mixture: HT, Prosolv® ODT, magnesium stearate | 42 (passable) | 1.28 (passasble) | 24.65 (passable) |
| Tableting mass: HT:HPMC (1:1) after granulation, Prosolv® ODT, magnesium stearate | 28.57 (excellent) | 1.09 (excellent) | 7.86 (excellent) |
| Parameter | Formulation | |
|---|---|---|
| F1 | F2 | |
| Weight [mg] | 7.7 ± 0.2 | 7.8 ± 0.2 |
| Thickness [mm] | 2.0 ± 0.1 | 2.1 ± 0.1 |
| Hardness [N] | 10.5 ± 1.2 | 5.3 ± 0.9 |
| Friability [%] | 0.8 | 0.9 |
| Drug content [mg] | 0.52 ± 0.1 | 1.1 ± 0.1 |
| Formulation | ||||||
|---|---|---|---|---|---|---|
| Volunteer | F5 | HT 2.5 mg | F1 | F2 | F3 | F4 |
| 1 | 0 | 3 | 1 | 2 | 2 | 2 |
| 2 | 0 | 3 | 2 | 2 | 3 | 3 |
| 3 | 0 | 3 | 1 | 2 | 2 | 3 |
| 4 | 0 | 3 | 0 | 1 | 2 | 2 |
| 5 | 0 | 3 | 0 | 1 | 2 | 2 |
| 6 | 0 | 3 | 1 | 2 | 2 | 3 |
| 7 | 0 | 3 | 1 | 2 | 3 | 2 |
| 8 | 0 | 3 | 0 | 1 | 2 | 2 |
| Parameters | F1 | F2 | Ref. A | Ref. B |
|---|---|---|---|---|
| Cmax [ng/mL] | 50.6 ± 2.5 | 81 ± 2.6 | 85.6 ± 2.1 | 85.7 ± 1.6 |
| tmax [h] | 2 | 2 | 2 | 2 |
| AUC (0–8) | 245 ± 2 | 409 ± 10 | 412 ± 13 | 429 ± 7 |
| AUC (0→∞) | 292 ± 12 | 505 ± 26 | 470 ± 25 | 476 ± 18 |
| AUC (0–8)/AUC (0→∞) | 0.81 ± 0.03 | 0.81 ± 0.03 | 0.88 ± 0.03 | 0.9 ± 0.02 |
| Λz | 0.27 ± 0.03 | 0.25 ± 0.02 | 0.31 ± 0.03 | 0.36 ± 0.04 |
| T0.5 [h] | 2.6 ± 0.3 | 2.8 ± 0.3 | 2.2 ±. 0.3 | 2.0 ± 0.2 |
| EBARef.A [%] | 59 | 99 | - | - |
| EBARef.B [%] | 57 | 95 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofimiuk, M.; Olechno, K.; Trofimiuk, E.; Czajkowska-Kośnik, A.; Ciosek-Skibińska, P.; Głowacz, K.; Lenik, J.; Basa, A.; Car, H.; Winnicka, K. Utilization of the Drug–Polymer Solid Dispersion Obtained by Ball Milling as a Taste Masking Method in the Development of Orodispersible Minitablets with Hydrocortisone in Pediatric Doses. Pharmaceutics 2024, 16, 1041. https://doi.org/10.3390/pharmaceutics16081041
Trofimiuk M, Olechno K, Trofimiuk E, Czajkowska-Kośnik A, Ciosek-Skibińska P, Głowacz K, Lenik J, Basa A, Car H, Winnicka K. Utilization of the Drug–Polymer Solid Dispersion Obtained by Ball Milling as a Taste Masking Method in the Development of Orodispersible Minitablets with Hydrocortisone in Pediatric Doses. Pharmaceutics. 2024; 16(8):1041. https://doi.org/10.3390/pharmaceutics16081041
Chicago/Turabian StyleTrofimiuk, Monika, Katarzyna Olechno, Emil Trofimiuk, Anna Czajkowska-Kośnik, Patrycja Ciosek-Skibińska, Klaudia Głowacz, Joanna Lenik, Anna Basa, Halina Car, and Katarzyna Winnicka. 2024. "Utilization of the Drug–Polymer Solid Dispersion Obtained by Ball Milling as a Taste Masking Method in the Development of Orodispersible Minitablets with Hydrocortisone in Pediatric Doses" Pharmaceutics 16, no. 8: 1041. https://doi.org/10.3390/pharmaceutics16081041





