A Physiologically-Based Pharmacokinetic Simulation to Evaluate Approaches to Mitigate Efavirenz-Induced Decrease in Levonorgestrel Exposure with a Contraceptive Implant
Abstract
:1. Introduction
2. Materials and Methods
2.1. PBPK Model
2.2. Reference PK Parameters
2.3. Simulations
2.3.1. Effects of Double-Dose of Levonorgestrel, Lower Dose of Efavirenz or Both
- 1:
- levonorgestrel 150 mg (75 mg × 2 rods) subdermally (control)
- 2:
- levonorgestrel 150 mg subdermally + efavirenz 600 mg orally once daily
- 3:
- levonorgestrel 150 mg subdermally + efavirenz 400 mg orally once daily
- 4:
- levonorgestrel 300 mg (75 mg × 4 rods) subdermally + efavirenz 600 mg orally once daily
- 5:
- levonorgestrel 300 mg subdermally + efavirenz 400 mg orally once daily
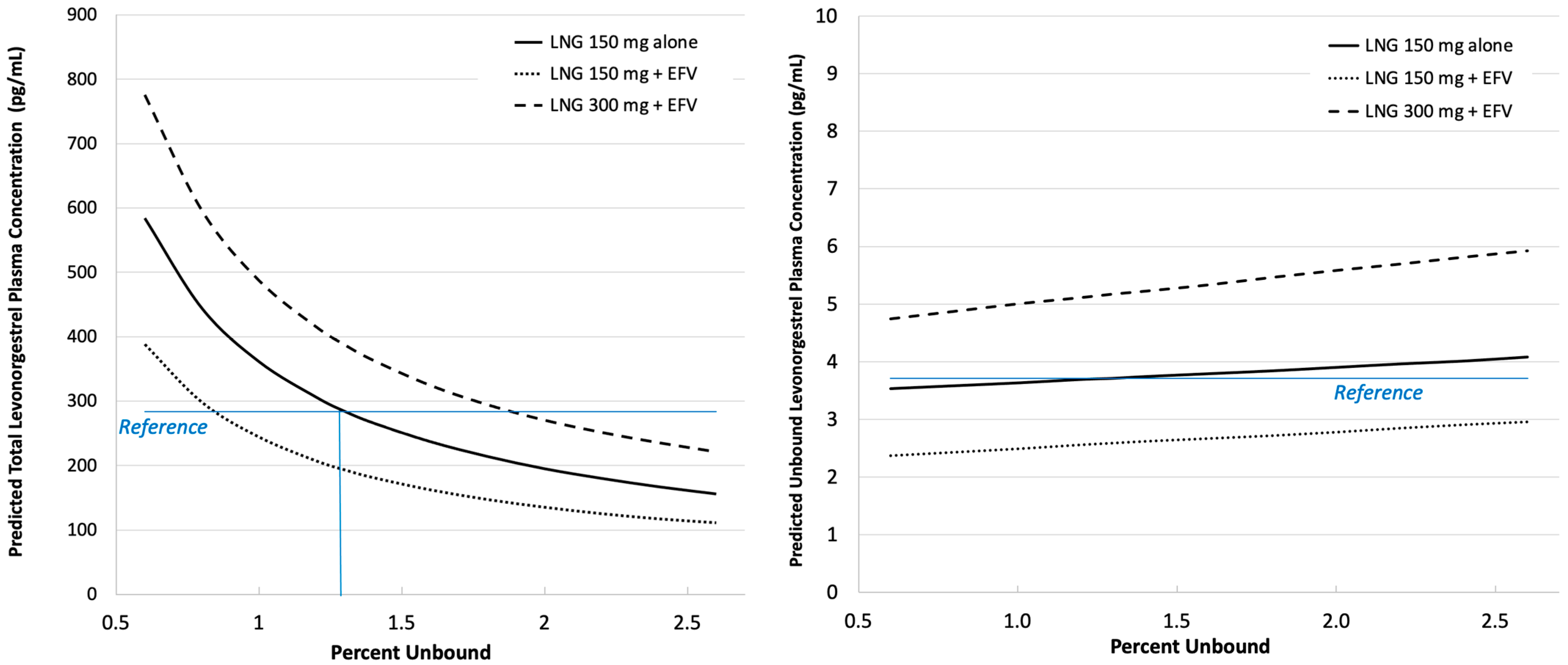
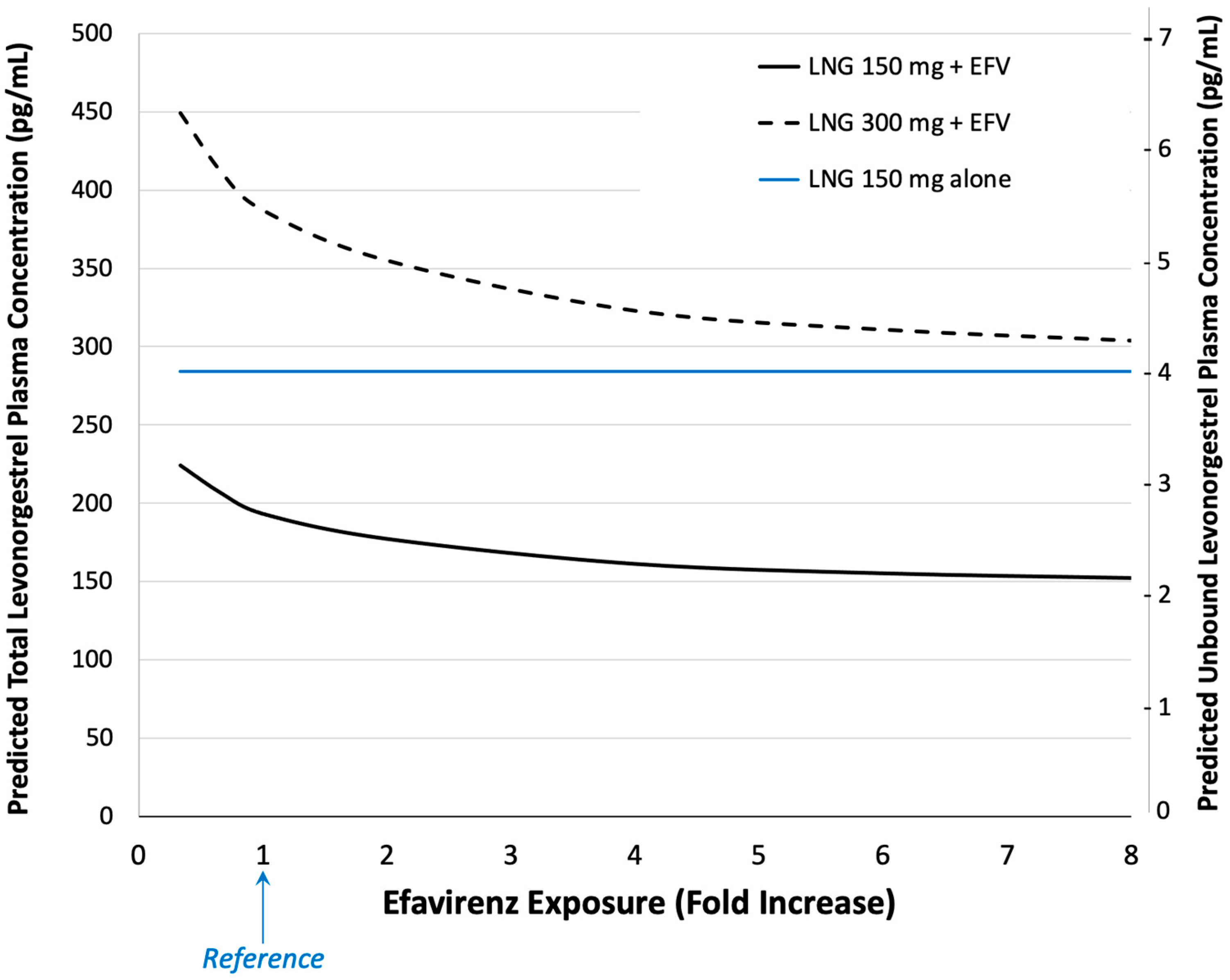
2.3.2. Effects of Protein Binding and Efavirenz Exposure
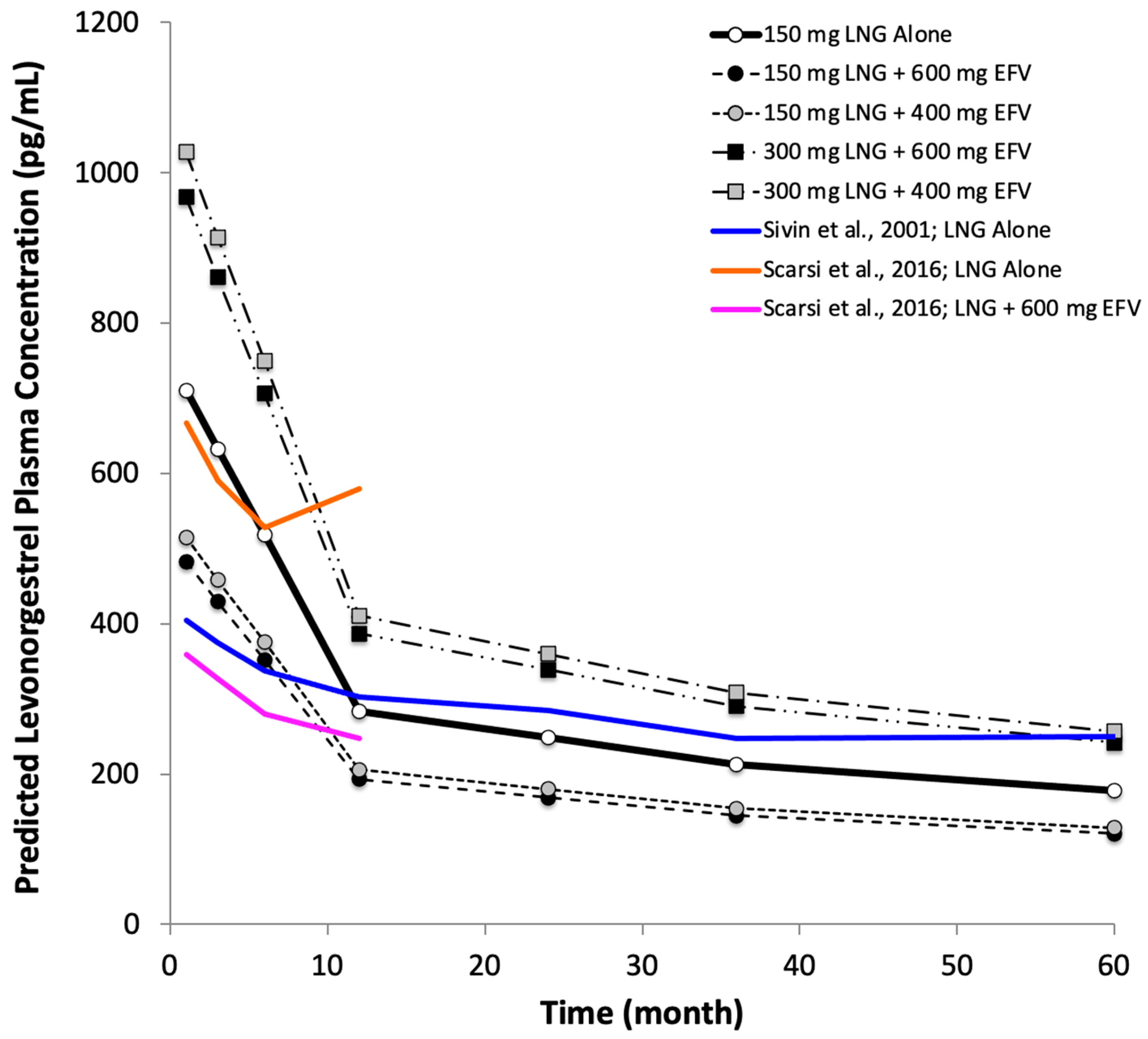
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global HIV & AIDS Statistics—Fact Sheet. UNAIDS. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 3 May 2024).
- Reynolds, H.W.; Janowitz, B.; Homan, R.; Johnson, L. The value of contraception to prevent perinatal HIV transmission. Sex. Transm. Dis. 2006, 33, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.W.; Janowitz, B.; Wilcher, R.; Cates, W. Contraception to prevent HIV-positive births: Current contribution and potential cost savings in PEPFAR countries. Sex. Transm. Infect. 2008, 84 (Suppl. S2), ii49–ii53. [Google Scholar] [CrossRef] [PubMed]
- Tsui, A.O.; Brown, W.; Li, Q. Contraceptive Practice in Sub-Saharan Africa. Popul. Dev. Rev. 2017, 43 (Suppl. 1), 166–191. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Choi, Y.; Rimon, J.G.; Alzouma, S.; Gichangi, P.; Guiella, G.; Kayembe, P.; Kibira, S.P.; Makumbi, F.; OlaOlorun, F.; et al. Trends in contraceptive prevalence rates in sub-Saharan Africa since the 2012 London Summit on Family Planning: Results from repeated cross-sectional surveys. Lancet Glob. Health 2019, 7, e904–e911. [Google Scholar] [CrossRef] [PubMed]
- Kanyangarara, M.; Sakyi, K.; Laar, A. Availability of integrated family planning services in HIV care and support sites in sub-Saharan Africa: A secondary analysis of national health facility surveys. Reprod. Health 2019, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Jacobstein, R.; Stanley, H. Contraceptive implants: Providing better choice to meet growing family planning demand. Glob. Health Sci. Pract. 2013, 1, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bayer New Zealand. Jadelle Data Sheet. Published Online 9 September 2015. Available online: http://www.bayerresources.com.au/resources/uploads/DataSheet/file9537.pdf (accessed on 28 July 2024).
- World Health Organization. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV. World Health Organization. December 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/277395/WHO-CDS-HIV-18.51-eng.pdf?ua=1 (accessed on 3 September 2019).
- Zash, R.; Makhema, J.; Shapiro, R.L. Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. N. Engl. J. Med. 2018, 379, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Dugdale, C.M.; Ciaranello, A.L.; Bekker, L.-G.; Stern, M.E.; Myer, L.; Wood, R.; Sax, P.E.; Abrams, E.J.; Freedberg, K.A.; Walensky, R.P. Risks and Benefits of Dolutegravir- and Efavirenz-Based Strategies for South African Women with HIV of Child-Bearing Potential: A Modeling Study. Ann. Intern. Med. 2019, 170, 614. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.H.; Swamy, P.; Preidis, G.A.; Mwanyumba, A.; Motsa, N.; Sarero, H.N. Implementing the Jadelle implant for women living with HIV in a resource-limited setting: Concerns for drug interactions leading to unintended pregnancies. AIDS 2014, 28, 791–793. [Google Scholar] [CrossRef]
- Patel, R.C.; Onono, M.; Gandhi, M.; Blat, C.; Hagey, J.; Shade, S.B.; Vittinghoff, E.; Bukusi, E.A.; Newmann, S.J.; Cohen, C.R. Pregnancy rates in HIV-positive women using contraceptives and efavirenz-based or nevirapine-based antiretroviral therapy in Kenya: A retrospective cohort study. Lancet HIV 2015, 2, e474–e482. [Google Scholar] [CrossRef] [PubMed]
- Scarsi, K.K.; Darin, K.M.; Nakalema, S.; Back, D.J.; Byakika-Kibwika, P.; Else, L.J.; Dilly Penchala, S.; Buzibye, A.; Cohn, S.E.; Merry, C.; et al. Unintended Pregnancies Observed with Combined Use of the Levonorgestrel Contraceptive Implant and Efavirenz-based Antiretroviral Therapy: A Three-Arm Pharmacokinetic Evaluation Over 48 Weeks. Clin. Infect. Dis. 2016, 62, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Chappell, C.A.; Lamorde, M.; Nakalema, S.; Chen, B.A.; Mackline, H.; Riddler, S.A.; Cohn, S.E.; Darin, K.M.; Achilles, S.L.; Scarsi, K.K. Efavirenz decreases etonogestrel exposure: A pharmacokinetic evaluation of implantable contraception with antiretroviral therapy. AIDS 2017, 31, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- HHS Panel on Antiretroviral Guidelines for Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://www.ncbi.nlm.nih.gov/books/NBK586306/ (accessed on 28 July 2024).
- Dickinson, L.; Amin, J.; Else, L.; Boffito, M.; Egan, D.; Owen, A.; Khoo, S.; Back, D.; Orrell, C.; Clarke, A.; et al. Pharmacokinetic and Pharmacodynamic Comparison of Once-Daily Efavirenz (400 mg vs. 600 mg) in Treatment-Naïve HIV-Infected Patients: Results of the ENCORE1 Study. Clin. Pharmacol. Ther. 2015, 98, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.; Amin, J.; Else, L.; Boffito, M.; Egan, D.; Owen, A.; Khoo, S.; Back, D.; Orrell, C.; Clarke, A.; et al. Comprehensive Pharmacokinetic, Pharmacodynamic and Pharmacogenetic Evaluation of Once-Daily Efavirenz 400 and 600 mg in Treatment-Naïve HIV-Infected Patients at 96 Weeks: Results of the ENCORE1 Study. Clin. Pharmacokinet. 2016, 55, 861–873. [Google Scholar] [CrossRef] [PubMed]
- ENCORE1 Study Group; Carey, D.; Puls, R.; Amin, J.; Losso, M.; Phanupak, P.; Foulkes, S.; Mohapi, L.; Crabtree-Ramirez, B.; Jessen, H.; et al. Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study. Lancet Infect. Dis. 2015, 15, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Dravid, A.; Pilawan, A.S.; S., A.; Morkar, D.N.; Ramapuram, J.T.; Madhukarrao, K.M.; Naik, K.S.; Bhrusundi, M.; R., R.K.; Nageswaramma, S.; et al. Efficacy and safety of 400 mg efavirenz versus standard 600 mg dose when taken with tenofovir and lamivudine combination in Indian adult patients with HIV-1 infection: An open-label, interventional, randomized, non-inferiority trial. Medicine 2022, 101, e31982. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Peng, W.; Song, X.; Li, Y.; Han, Y.; Zhu, T.; Fu, Q.; Du, X.; Cao, W.; Li, T. Pharmacodynamics of efavirenz 400 mg in treatment-naïve Chinese HIV-infected patients in a prospective cohort study. BMC Infect. Dis. 2021, 21, 112. [Google Scholar] [CrossRef]
- Kuhnz, W.; Al-Yacoub, G.; Fuhrmeister, A. Pharmacokinetics of levonorgestrel and ethinylestradiol in 9 women who received a low-dose oral contraceptive over a treatment period of 3 months and, after a wash-out phase, a single oral administration of the same contraceptive formulation. Contraception 1992, 46, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Bristol Myer Squibb. Sustiva Drug Label. 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020972s038lbl.pdf (accessed on 28 July 2024).
- Affandi, B.; Cekan, S.Z.; Boonkasemsanti, W.; Samil, R.S.; Diczfalusy, E. The interaction between sex hormone binding globulin and levonorgestrel released from Norplant®, an implantable contraceptive. Contraception 1987, 35, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Cirrincione, L.R.; Nakalema, S.; Chappell, C.A.; Byakika-Kibwika, P.; Kyohairwe, I.; Winchester, L.; Mackline, H.; Pham, M.M.; Cohn, S.E.; Siccardi, M.; et al. Effect of double-dose levonorgestrel subdermal implant in women taking efavirenz-based antiretroviral therapy: The DoubLNG pharmacokinetic study. Contraception 2023, 122, 109975. [Google Scholar] [CrossRef] [PubMed]
- Kuhnz, W.; Staks, T.; Jütting, G. Pharmacokinetics of levonorgestrel and ethinylestradiol in 14 women during three months of treatment with a tri-step combination oral contraceptive: Serum protein binding of levonorgestrel and influence of treatment on free and total testosterone levels in the serum. Contraception 1994, 50, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Wanke, R.; Harjivan, S.G.; Pereira, S.A.; Marques, M.M.; Antunes, A.M.M. The role of competitive binding to human serum albumin on efavirenz–warfarin interaction: A nuclear magnetic resonance study. Int. J. Antimicrob. Agents 2013, 42, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Hoener, B. Changes in plasma protein binding have little clinical relevance. Clin. Pharmacol. Ther. 2002, 71, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Desta, Z.; Saussele, T.; Ward, B.; Blievernicht, J.; Li, L.; Klein, K.; Flockhart, D.A.; Zanger, U.M. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 2007, 8, 547–558. [Google Scholar] [CrossRef] [PubMed]
- di lulio, J.; Fayet, A.; Arab-Alameddine, M.; Rotger, M.; Lubomirov, R.; Cavassini, M.; Furrer, H.; Günthard, H.F.; Colombo, S.; Csajka, C.; et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet. Genom. 2009, 19, 300–309. [Google Scholar] [CrossRef]
- Klein, K.; Lang, T.; Saussele, T.; Barbosa-Sicard, E.; Schunck, W.-H.; Eichelbaum, M.; Schwab, M.; Zanger, U.M. Genetic variability of CYP2B6 in populations of African and Asian origin: Allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet. Genom. 2005, 15, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Gatanaga, H.; Tachikawa, N.; Teruya, K.; Kikuchi, Y.; Yoshino, M.; Kuwahara, T.; Shirasaka, T.; Kimura, S.; Oka, S. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem. Biophys. Res. Commun. 2004, 319, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Arab-Alameddine, M.; Di Iulio, J.; Buclin, T.; Rotger, M.; Lubomirov, R.; Cavassini, M.; Fayet, A.; Décosterd, L.; Eap, C.; Biollaz, J.; et al. Pharmacogenetics-Based Population Pharmacokinetic Analysis of Efavirenz in HIV-1-Infected Individuals. Clin. Pharmacol. Ther. 2009, 85, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.A.; Gorski, J.C.; Jones, D.R.; Hall, S.D.; Flockhart, D.A.; Desta, Z. The Cytochrome P450 2B6 (CYP2B6) Is the Main Catalyst of Efavirenz Primary and Secondary Metabolism: Implication for HIV/AIDS Therapy and Utility of Efavirenz as a Substrate Marker of CYP2B6 Catalytic Activity. J. Pharmacol. Exp. Ther. 2003, 306, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Almond, L.M.; Mukadam, S.; Gardner, I.; Okialda, K.; Wong, S.; Hatley, O.; Tay, S.; Rowland-Yeo, K.; Jamei, M.; Rostami-Hodjegan, A.; et al. Prediction of Drug-Drug Interactions Arising from CYP3A induction Using a Physiologically Based Dynamic Model. Drug Metab. Dispos. 2016, 44, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Ke, A.; Barter, Z.; Rowland-Yeo, K.; Almond, L. Towards a Best Practice Approach in PBPK Modeling: Case Example of Developing a Unified Efavirenz Model Accounting for Induction of CYPs 3A4 and 2B6: Best Practice Approach in Efavirenz PBPK Modeling. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Beal, S.; Sheiner, L.B.; Boeckmann, A.; Bauer, R.J. NONMEM User’s Guide; Icon Development Solutions: Ellicott City, MD, USA, 2009. [Google Scholar]
- Back, D.J.; Grimmer, S.F.; Rogers, S.; Stevenson, P.J.; Orme, M.L. Comparative pharmacokinetics of levonorgestrel and ethinyloestradiol following intravenous, oral and vaginal administration. Contraception 1987, 36, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kook, K.; Gabelnick, H.; Duncan, G. Pharmacokinetics of levonorgestrel 0.75 mg tablets. Contraception 2002, 66, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Carten, M.L.; Kiser, J.J.; Kwara, A.; Mawhinney, S.; Cu-Uvin, S. Pharmacokinetic Interactions between the Hormonal Emergency Contraception, Levonorgestrel (Plan B), and Efavirenz. Infect. Dis. Obstet. Gynecol. 2012, 2012, 137192. [Google Scholar] [CrossRef]
- Arizona Software. GraphClick for Mac. Available online: https://graphclick.en.softonic.com/mac (accessed on 28 July 2024).
- Sivin, I.; Wan, L.; Ranta, S.; Alvarez, F.; Brache, V.; Mishell, D.R.; Darney, P.; Biswas, A.; Diaz, S.; Kiriwat, O.; et al. Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception 2001, 64, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.W.; Ribaudo, H.J.; Kim, R.B.; Tierney, C.; Wilkinson, G.R.; Gulick, R.M.; Clifford, D.B.; Hulgan, T.; Marzolini, C.; Acosta, E.P. Pharmacogenetics of efavirenz and central nervous system side effects: An Adult AIDS Clinical Trials Group study. AIDS 2004, 18, 2391–2400. [Google Scholar] [PubMed]
- Roberts, O.; Rajoli, R.K.R.; Back, D.J.; Owen, A.; Darin, K.M.; Fletcher, C.V.; Lamorde, M.; Scarsi, K.K.; Siccardi, M. Physiologically based pharmacokinetic modelling prediction of the effects of dose adjustment in drug–drug interactions between levonorgestrel contraceptive implants and efavirenz-based ART. J. Antimicrob. Chemother. 2018, 73, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Chappell, C.A.; Lamorde, M.; Nakalema, S.; Kyohairwe, I.; Byakika-Kibwika, P.; Meyn, L.A.; Pham, M.M.; Scarsi, K.K. A randomized trial of double vs single-dose etonogestrel implant to overcome the interaction with efavirenz-based antiretroviral therapy. Am. J. Obstet. Gynecol. 2024, 231, 242.e1–242.e9. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, F.Z.; Roy, S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception 1990, 42, 67–96. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.; Chadwick, D.J.; Martin, C.; Tjia, J.; Back, D.J.; Orme, M. The interaction of phenytoin and carbamazepine with combined oral contraceptive steroids. Br. J. Clin. Pharmacol. 1990, 30, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Sanofi-Aventis U.S. LLC. Ketek (Telithromycin): Drug Label. 2010. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021144s014lbl.pdf (accessed on 28 July 2024).
- Davis, A.R.; Westhoff, C.L.; Stanczyk, F.Z. Carbamazepine coadministration with an oral contraceptive: Effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia 2011, 52, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Sabo, J.P.; Lang, B.; Elgadi, M.; Huang, F. Effect of the hepatitis C virus protease inhibitor faldaprevir on the pharmacokinetics of an oral contraceptive containing ethinylestradiol and levonorgestrel in healthy female volunteers. Antimicrob. Agents Chemother. 2015, 59, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Fattore, C.; Cipolla, G.; Gatti, G.; Limido, G.L.; Sturm, Y.; Bernasconi, C.; Perucca, E. Induction of ethinylestradiol and levonorgestrel metabolism by oxcarbazepine in healthy women. Epilepsia 1999, 40, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Aouri, M.; Barcelo, C.; Ternon, B.; Cavassini, M.; Anagnostopoulos, A.; Yerly, S.; Hugues, H.; Vernazza, P.; Günthard, H.F.; Buclin, T.; et al. In Vivo Profiling and Distribution of Known and Novel Phase I and Phase II Metabolites of Efavirenz in Plasma, Urine, and Cerebrospinal Fluid. Drug Metab. Dispo.s 2016, 44, 151–161. [Google Scholar] [CrossRef]
- Shou, M.; Hayashi, M.; Pan, Y.; Xu, Y.; Morrissey, K.; Xu, L.; Skiles, G.L. Modeling, Prediction, and in Vitro in Vivo Correlation of CYP3A4 Induction. Drug Metab. Dispos. 2008, 36, 2355–2370. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, E.D.; Whittington, D.; Ensign, D.; Hoffer, C.; Bedynek, P.S.; Campbell, S.; Stubbert, K.; Crafford, A.; London, A.; Kim, T. Mechanism of Efavirenz Influence on Methadone Pharmacokinetics and Pharmacodynamics. Clin. Pharmacol. Ther. 2012, 91, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Maldarelli, F.; Natarajan, V.; Formentini, E.; Alfaro, R.; Penzak, S. Efavirenz Induces CYP2B6-Mediated Hydroxylation of Bupropion in Healthy Subjects. JAIDS J. Acquir. Immune Defic. Syndr. 2008, 49, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Desta, Z. In vitro Analysis and Quantitative Prediction of Efavirenz Inhibition of Eight Cytochrome P450 (CYP) Enzymes: Major Effects on CYPs 2B6, 2C8, 2C9 and 2C19. Drug Metab. Pharmacokinet. 2013, 28, 362. [Google Scholar] [CrossRef] [PubMed]
- Burhenne, J.; Matthée, A.-K.; Pasáková, I.; Röder, C.; Heinrich, T.; Haefeli, W.E.; Mikus, G.; Weiss, J. No evidence for induction of ABC transporters in peripheral blood mononuclear cells in humans after 14 days of efavirenz treatment. Antimicrob. Agents Chemother. 2010, 54, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Berruet, N.; Sentenac, S.; Auchere, D.; Gimenez, F.; Farinotti, R.; Fernandez, C. Effect of efavirenz on intestinal p-glycoprotein and hepatic p450 function in rats. J. Pharm. Pharm. Sci. 2005, 8, 226–234. [Google Scholar] [PubMed]
- Rotger, M.; Colombo, S.; Furrer, H.; Bleiber, G.; Buclin, T.; Lee, B.L.; Keiser, O.; Biollaz, J.; Décosterd, L.; Telenti, A.; et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet. Genom. 2005, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, S.E.; Santos, D.; Valverde, M.P.; Domínguez-Gil, A.; González, F.; Luna, G.; García, M.J. Influence of the Cytochrome P450 2B6 Genotype on Population Pharmacokinetics of Efavirenz in Human Immunodeficiency Virus Patients. Antimicrob. Agents Chemother. 2009, 53, 2791–2798. [Google Scholar] [CrossRef]
- Patel, R.C.; Stalter, R.M.; Thomas, K.K.; Tamraz, B.; Blue, S.W.; Erikson, D.W.; Kim, C.J.; Kelley, E.J.; Nanda, K.; Kourtis, A.P.; et al. A pharmacokinetic and pharmacogenetic evaluation of contraceptive implants and antiretroviral therapy among women in Kenya and Uganda. AIDS 2019, 33, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Neary, M.; Lamorde, M.; Olagunju, A.; Darin, K.M.; Merry, C.; Byakika-Kibwika, P.; Back, D.J.; Siccardi, M.; Owen, A.; Scarsi, K.K. The Effect of Gene Variants on Levonorgestrel Pharmacokinetics When Combined with Antiretroviral Therapy Containing Efavirenz or Nevirapine. Clin. Pharmacol. Ther. 2017, 102, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.; Aplenc, R.; TenHave, T.; Foulkes, A.S.; Thakur, R.; Mosepele, M.; Barrett, J.S.; Flexner, C.; Strom, B.L.; Bisson, G. Slow Efavirenz Metabolism Genotype Is Common in Botswana. JAIDS J. Acquir. Immune Defic. Syndr. 2008, 49, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Nyakutira, C.; Röshammar, D.; Chigutsa, E.; Chonzi, P.; Ashton, M.; Nhachi, C.; Masimirembwa, C. High prevalence of the CYP2B6 516G-->T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur. J. Clin. Pharmacol. 2008, 64, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.W.; Smeaton, L.M.; Shafer, R.W.; Robbins, G.K.; Morse, G.D.; Labbe, L.; Wilkinson, G.R.; Clifford, D.B.; D’Aquila, R.T.; De Gruttola, V.; et al. Pharmacogenetics of long-term responses to antiretroviral regimens containing Efavirenz and/or Nelfinavir: An Adult Aids Clinical Trials Group Study. J. Infect. Dis. 2005, 192, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Wyen, C.; Hendra, H.; Vogel, M.; Hoffmann, C.; Knechten, H.; Brockmeyer, N.H.; Bogner, J.R.; Rockstroh, J.; Esser, S.; Jaeger, H.; et al. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J. Antimicrob. Chemother. 2008, 61, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, H.J.; Haas, D.W.; Tierney, C.; Kim, R.B.; Wilkinson, G.R.; Gulick, R.M.; Clifford, D.B.; Marzolini, C.; Fletcher, C.V.; Tashima, K.T.; et al. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: An Adult AIDS Clinical Trials Group Study. Clin. Infect. Dis. 2006, 42, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Mouly, S.; Lown, K.S.; Kornhauser, D.; Joseph, J.L.; Fiske, W.D.; Benedek, I.H.; Watkins, P.B. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin. Pharmacol. Ther. 2002, 72, 1–9. [Google Scholar] [CrossRef]

| Parameter | Value | Source |
|---|---|---|
| B/P | 0.671 | calculated |
| fu | 0.013 | [22,26] |
| CLintrinsic,CYP3A4 (μL/min/pmol) | 0.0843 | RD |
| fu,mic | 0.449 | calculated |
| HLM (μL/min/mg protein) | 128 | RD |
| Q (L/h) | 10.2 | MEM |
| Vss (L/kg) | 2.27 | MEM |
| Release rate a (μg/day) | ||
| month 1 | 100 | [8] |
| month 3 | 89 b | |
| month 6 | 73 b | |
| month 12 | 40 | |
| month 24 | 35 b | |
| month 36 | 30 | |
| month 60 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeojo, L.W.; Patel, R.C.; Sambol, N.C. A Physiologically-Based Pharmacokinetic Simulation to Evaluate Approaches to Mitigate Efavirenz-Induced Decrease in Levonorgestrel Exposure with a Contraceptive Implant. Pharmaceutics 2024, 16, 1050. https://doi.org/10.3390/pharmaceutics16081050
Adeojo LW, Patel RC, Sambol NC. A Physiologically-Based Pharmacokinetic Simulation to Evaluate Approaches to Mitigate Efavirenz-Induced Decrease in Levonorgestrel Exposure with a Contraceptive Implant. Pharmaceutics. 2024; 16(8):1050. https://doi.org/10.3390/pharmaceutics16081050
Chicago/Turabian StyleAdeojo, Lilian W., Rena C. Patel, and Nancy C. Sambol. 2024. "A Physiologically-Based Pharmacokinetic Simulation to Evaluate Approaches to Mitigate Efavirenz-Induced Decrease in Levonorgestrel Exposure with a Contraceptive Implant" Pharmaceutics 16, no. 8: 1050. https://doi.org/10.3390/pharmaceutics16081050







