Green Solid Lipid Nanoparticles by Fatty Acid Coacervation: An Innovative Nasal Delivery Tool for Drugs Targeting Cerebrovascular and Neurological Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cells
2.3. Animals
2.4. Synthesis and Characterization of Conjugated Linoleic and α-Linolenic Acids
2.5. Rhod B-AOT Ion Pair Formation and Characterization
2.6. Saponification of Vegetal Butters and Fats
2.7. SLN Formulation
2.8. SLN Characterization
2.8.1. Size, Morphology, Differential Scanning Calorimetry (DSC), and Nuclear Magnetic Resonance (NMR)
2.8.2. Drug and Fluorescent Probe % Recovery and Entrapment Efficiency
2.8.3. PDA-HPLC
2.9. Lipid Matrix and Unsaponifiable Fraction Characterization
2.9.1. Gas Chromatography Coupled to Mass Spectrometry (GC-MS)
2.9.2. Ultra-High-Performance Liquid Chromatography Coupled to Photodiode Array Detector and Mass Spectrometry (UHPLC-PDA-MS)
2.10. Release Studies
2.10.1. Release Studies of Fluorescent Probes
2.10.2. Release Studies of Drugs
2.11. Cell Studies
2.11.1. Cell Viability Assay
2.11.2. CSF Secretion
2.12. Vasoprotection in Rat Aorta with Endothelium Impairment Induced by Pyrogallol
2.13. Animal Studies
2.13.1. Pharmacokinetics after Intranasal Administration
2.13.2. Biodistribution after Intranasal Administration
2.13.3. HPLC–Fluorimetry
2.14. Statistical Analysis
3. Results
3.1. SLN Characterization
3.1.1. Size, Morphology, and DSC
3.1.2. Drug and Fluorescent Probe Loading
3.2. Lipid Matrix and Unsaponifiable Fraction Characterization
3.2.1. Gas Chromatography Coupled to Mass Spectrometry (GC-MS)
3.2.2. NMR
- 1H-NMR Shea fatty acids ((CD3)2SO), 0.85 (t, CH2-CH3), 1.23 (m, CH2), 1.47 (q, CH2-CH2-COOH), 1.95–2.05 (m, CH2-CH=CH), 2.14–2.20 (t, CH2-CH2-COOH), 2.73 (t, CH=CH-CH2-CH=CH), 3.38 (br. s, COOH/OH), 3.83–4.05 (CH2-OH), and 5.28–5.35 (m, CH=CH/-CH-OCO).
- 1H-NMR Mango fatty acids ((CD3)2SO), 0.85 (t, CH2-CH3), 1.23 (m, CH2), 1.47 (q, CH2-CH2-COOH), 1.95–2.04 (m, CH2-CH=CH), 2.14–2.20 (t, CH2-CH2-COOH), 2.73 (t, CH=CH-CH2-CH=CH), 4.06 (br. s, COOH/OH), 3.83–4.05 (m, CH2-OH), and 5.28–5.35 (m, CH=CH/-CH-OCO).
3.2.3. Ultra-High-Performance Liquid Chromatography Coupled to Photodiode Array Detector and Mass Spectrometry (UHPLC-PDA-MS)
3.3. Release Studies
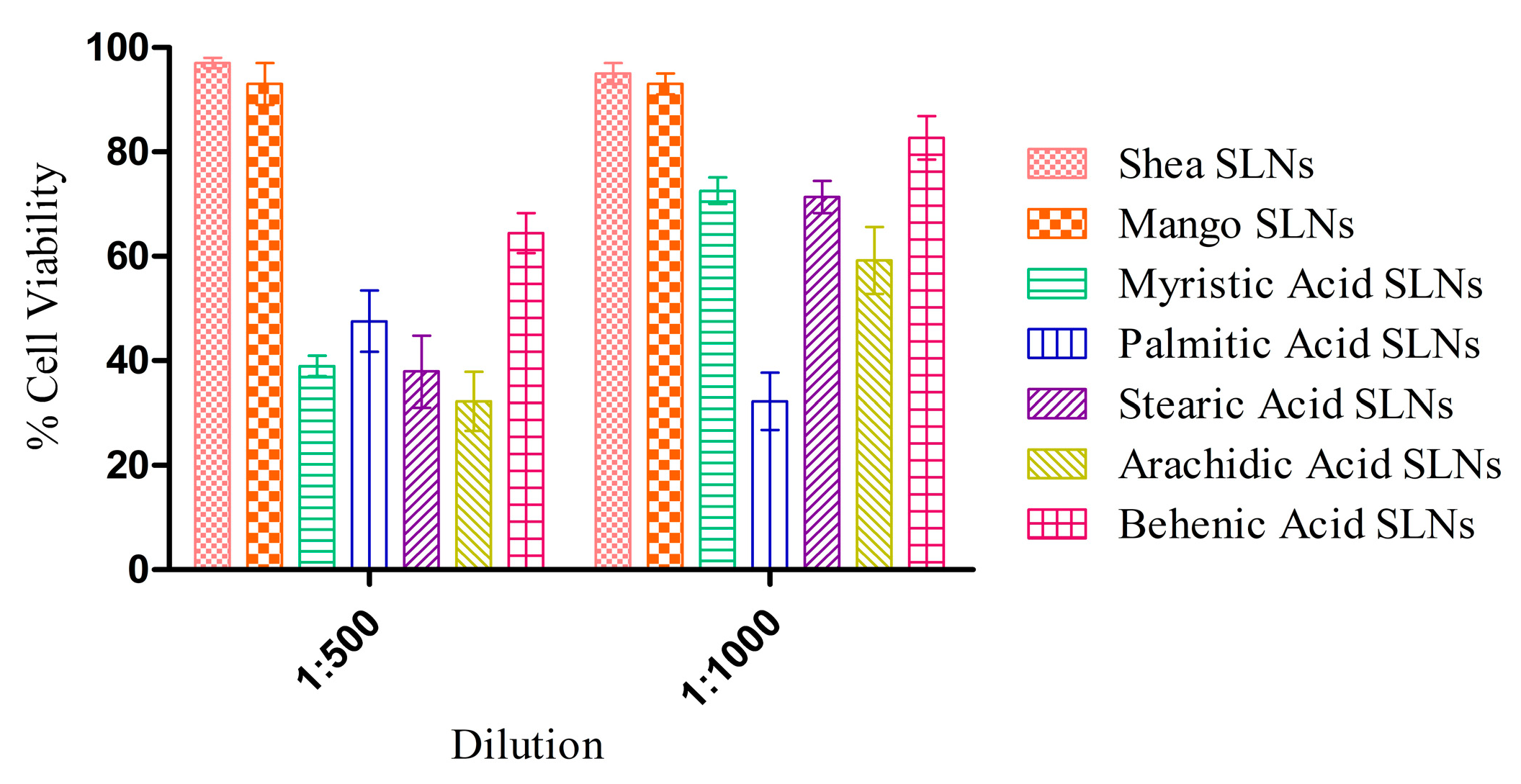
3.4. Cell Studies
3.4.1. Cell Viability Assay
3.4.2. CSF Secretion
3.5. Ex Vivo Studies on Isolated Vessels
3.6. Animal Studies
4. Discussion
4.1. Physico-Chemical Standpoint
4.2. Composition–Activity Relationship
4.3. Pharmacological Standpoint
4.4. In Vivo Fate
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andjelkovic, A.V.; Keep, R.F.; Wang, M.M. Molecular Mechanisms of Cerebrovascular Diseases. Int. J. Mol. Sci. 2022, 23, 7161. [Google Scholar] [CrossRef] [PubMed]
- Keep, R.F.; Jones, H.C.; Hamilton, M.G.; Drewes, L.R. A year in review: Brain barriers and brain fluids research in 2022. Fluids Barriers CNS 2023, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Perrelli, A.; Fatehbasharzad, P.; Benedetti, V.; Ferraris, C.; Fontanella, M.; Luca, E.D.; Moglianetti, M.; Battaglia, L.; Retta, S.F. Towards precision nanomedicine for cerebrovascular diseases with emphasis on Cerebral Cavernous Malformation (CCM). Expert Opin. Drug Deliv. 2021, 18, 849–876. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.J.; Iliff, J.J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2016, 1862, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal Fluid Secretion by the Choroid Plexus. Physiol. Rev. 2013, 93, 1847–1892. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Z.; Chen, Y.; Liao, J.; Wang, Y.; Liu, J.; Lin, Z.; Xiao, G. Choroid plexus epithelium and its role in neurological diseases. Front. Mol. Neurosci. 2022, 15, 949231. [Google Scholar] [CrossRef] [PubMed]
- Andren, K.; Wikkelso, C.; Tisell, M.; Hellstrom, P. Natural course of idiopathic normal pressure hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2014, 85, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Orešković, D.; Klarica, M. Development of hydrocephalus and classical hypothesis of cerebrospinal fluid hydrodynamics: Facts and illusions. Prog. Neurobiol. 2011, 94, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Panciani, P.P.; Migliorati, K.; Muratori, A.; Gelmini, M.; Padovani, A.; Fontanella, M. Computerized gait analysis with inertial sensor in the management of idiopathic normal pressure hydrocephalus. Eur. J. Phys. Rehabil. Med. 2018, 54, 724–729. [Google Scholar] [CrossRef]
- Williams, H. The venous hypothesis of hydrocephalus. Med. Hypotheses 2008, 70, 743–747. [Google Scholar] [CrossRef]
- Browd, S.R.; Gottfried, O.N.; Ragel, B.T.; Kestle, J.R.W. Failure of Cerebrospinal Fluid Shunts: Part II: Overdrainage, Loculation, and Abdominal Complications. Pediatr. Neurol. 2006, 34, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Moreira, J.N.; Lobo, J.M.S.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal Drug Delivery: How, Why and What for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. Eur. J. Pharm. Biopharm. 2020, 149, 192–217. [Google Scholar] [CrossRef]
- Battaglia, L.; Panciani, P.P.; Muntoni, E.; Capucchio, M.T.; Biasibetti, E.; Bonis, P.D.; Mioletti, S.; Fontanella, M.; Swaminathan, S. Lipid nanoparticles for intranasal administration: Application to nose-to-brain delivery. Expert Opin. Drug Deliv. 2018, 15, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M.; Cavalli, R.; Trotta, M. Solid lipid nanoparticles produced through a coacervation method. J. Microencapsul. 2010, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.N.; Jansen, H.; Kennedy, R.L.; Malabu, U.H. Hydroxyoctadecadienoic acids: Oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 2016, 785, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lowry, J.R.; Marshall, N.; Wenzel, T.J.; Murray, T.E.; Klegeris, A. The dietary fatty acids α-linolenic acid (ALA) and linoleic acid (LA) selectively inhibit microglial nitric oxide production. Mol. Cell Neurosci. 2020, 109, 103569. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, E.; Marini, E.; Ahmadi, N.; Milla, P.; Ghè, C.; Bargoni, A.; Capucchio, M.T.; Biasibetti, E.; Battaglia, L. Lipid nanoparticles as vehicles for oral delivery of insulin and insulin analogs: Preliminary ex vivo and in vivo studies. Acta Diabetol. 2019, 56, 1283–1292. [Google Scholar] [CrossRef]
- Muntoni, E.; Anfossi, L.; Milla, P.; Marini, E.; Ferraris, C.; Capucchio, M.T.; Colombino, E.; Segale, L.; Porta, M.; Battaglia, L. Glargine insulin loaded lipid nanoparticles: Oral delivery of liquid and solid oral dosage forms. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 691–698. [Google Scholar] [CrossRef]
- Metayer, T.; Orset, C.; Ali, C.; Furon, J.; Szabla, N.; Emery, E.; Vivien, D.; Gaberel, T. Bumetanide lowers acute hydrocephalus in a rat model of subarachnoid hemorrhage. Acta Neurochir. 2022, 164, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Karimy, J.K.; Zhang, J.; Kurland, D.B.; Theriault, B.C.; Duran, D.; Stokum, J.A.; Furey, C.G.; Zhou, X.; Mansuri, M.S.; Montejo, J.; et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 2017, 23, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Wagner, K.R. Bumetanide decreases canine cerebrospinal fluid production. In vivo evidence for NaCl cotransport in the central nervous system. J. Clin. Investig. 1993, 92, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Kaila, K. CNS pharmacology of NKCC1 inhibitors. Neuropharmacology 2022, 205, 108910. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, E.; Marini, E.; Ferraris, C.; Garelli, S.; Capucchio, M.T.; Colombino, E.; Panciani, P.P.; Battaglia, L. Intranasal lipid nanocarriers: Uptake studies with fluorescently labeled formulations. Colloids Surf. B Biointerfaces 2022, 214, 112470. [Google Scholar] [CrossRef] [PubMed]
- Sirignano, C.; Nadembega, P.; Poli, F.; Romano, B.; Lucariello, G.; Rigano, D.; Taglialatela-Scafati, O. Triterpenoids from Vitellaria paradoxa Stem Barks Reduce Nitrite Levels in LPS-Stimulated Macrophages. Plants 2021, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, C.; Brucato, L.; Gallicchio, M.; Rosa, A.C.; Collino, M.; Fantozzi, R. Celecoxib modulates adhesion of HT29 colon cancer cells to vascular endothelial cells by inhibiting ICAM-1 and VCAM-1 expression. Br. J. Pharmacol. 2008, 153, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Miyazawa, T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett. 2000, 148, 173–179. [Google Scholar] [CrossRef]
- Shi, J.; Chen, L. Determination of rhodamine B in lipsticks by high performance liquid chromatography after extraction with AOT reversed micelles. Anal. Methods 2014, 6, 8627–8632. [Google Scholar] [CrossRef]
- Marengo, A.; Maxia, A.; Sanna, C.; Bertea, C.M.; Bicchi, C.; Ballero, M.; Cagliero, C.; Rubiolo, P. Characterization of four wild edible Carduus species from the Mediterranean region via phytochemical and biomolecular analyses. Food Res. Int. 2017, 100, 822–831. [Google Scholar] [CrossRef]
- Dianzani, C.; Monge, C.; Miglio, G.; Serpe, L.; Martina, K.; Cangemi, L.; Ferraris, C.; Mioletti, S.; Osella, S.; Gigliotti, C.L.; et al. Nanoemulsions as Delivery Systems for Poly-Chemotherapy Aiming at Melanoma Treatment. Cancers 2020, 12, 1198. [Google Scholar] [CrossRef] [PubMed]
- Baehr, C.; Reichel, V.; Fricker, G. Choroid plexus epithelial monolayers—A cell culture model from porcine brain. Cerebrospinal Fluid Res. 2006, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Ameli, P.A.; Madan, M.; Chigurupati, S.; Yu, A.; Chan, S.L.; Pattisapu, J.V. Effect of Acetazolamide on Aquaporin-1 and Fluid Flow in Cultured Choroid Plexus. In Acta Neurochirurgica Supplementum; Springer: Vienna, Austria, 2012; pp. 59–64. [Google Scholar] [CrossRef]
- Hakvoort, A.; Haselbach, M.; Galla, H.-J. Active transport properties of porcine choroid plexus cells in culture. Brain Res. 1998, 795, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Brusotti, G.; Papetti, A.; Serra, M.; Temporini, C.; Marini, E.; Orlandini, S.; Sanda, A.K.; Watcho, P.; Kamtchouing, P. Allanblackia floribunda Oliv.: An aphrodisiac plant with vasorelaxant properties. J. Ethnopharmacol. 2016, 192, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.-H.; Qian, L.-B.; Chen, S.; Li, J.; Wang, H.P.; Bruce, I.C.; Lin, J.; Xia, Q. Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur. J. Pharmacol. 2009, 616, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, B.; Westesen, K. Thermoanalysis of the recrystallization process of melt-homogenized glyceride nanoparticles. Colloids Surf. B Biointerfaces 1994, 3, 159–175. [Google Scholar] [CrossRef]
- Bunjes, H.; Siekmann, B.; Westesen, K. Emulsions of Supercooled Melts—A Novel Drug Delivery System. In Submicron Emulsions in Drug Targeting and Delivery; CRC Press: Boca Raton, FL, USA, 2019; pp. 175–204. [Google Scholar]
- Isidorov, V.A.; Rusak, M.; Szczepaniak, L.; Witkowski, S. Gas chromatographic retention indices of trimethylsilyl derivatives of mono- and diglycerides on capillary columns with non-polar stationary phases. J. Chromatogr. A 2007, 1166, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Thi, H.T.; Le, B.A.; Le, H.N.T.; Okitsu, K.; Imamura, K.; Takenaka, N.; Luu, B.V.; Maeda, Y. Screening of fatty acids, saccharides, and phytochemicals in Jatropha curcas seed kernel as their trimethylsilyl derivatives using gas chromatography/mass spectrometry. J. Chromatogr. B 2018, 1102, 66–73. [Google Scholar] [CrossRef]
- Murru, E.; Carta, G.; Manca, C.; Sogos, V.; Pistis, M.; Melis, M.; Banni, S. Conjugated Linoleic Acid and Brain Metabolism: A Possible Anti-Neuroinflammatory Role Mediated by PPARα Activation. Front. Pharmacol. 2021, 11, 587140. [Google Scholar] [CrossRef]
- Gong, M.; Hu, Y.; Wei, W.; Jin, Q.; Wang, X. Production of conjugated fatty acids: A review of recent advances. Biotechnol. Adv. 2019, 37, 107454. [Google Scholar] [CrossRef]
- Tvrzicka, E.; Kremmyda, L.-S.; Stankova, B.; Zak, A. Fatty acids as Biocompounds: Their role in human metabolism, health and disease—A review part 1: Classification, dietary sources and biological functions. Biomed. Pap. 2011, 155, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, L.; Wang, D.; Li, P.; Shahidi, F. Conjugated Fatty Acids in Muscle Food Products and Their Potential Health Benefits: A Review. J. Agric. Food Chem. 2020, 68, 13530–13540. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, Z.; Boeglin, W.E.; Brash, A.R. Robust inhibitory effects of conjugated linolenic acids on a cyclooxygenase-related linoleate 10S-dioxygenase: Comparison with COX-1 and COX-2. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.; Cheng, X.; Luo, L.; Yang, L.; Zhao, Y.; Zhou, Y.; Peng, L.; Jin, X.; Cui, L.; Liu, F.; et al. Cinnamic Acid Improved Lipopolysaccharide-Induced Depressive-Like Behaviors by Inhibiting Neuroinflammation and Oxidative Stress in Mice. Pharmacology 2022, 107, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Kojima, N.; Kikuchi, T.; Yasukawa, K.; Tokuda, H.; Masters, E.T.; Manosroi, A.; Manosroi, J. Anti-Infl ammatory and Chemopreventive Effects of Triterpene Cinnamates and Acetates from Shea Fat. J. Oleo Sci. 2010, 59, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Abe, M.; Akihisa, T. Anti-inflammatory and Other Bioactivities of Triterpene Esters in Shea Butter. Acc. Mater. Surf. Res 2017, 2, 127–136. [Google Scholar]
- Maranz, S.; Wiesman, Z.; Garti, N. Phenolic Constituents of Shea (Vitellaria paradoxa) Kernels. J. Agric. Food Chem. 2003, 51, 6268–6273. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, A.M.; Salama, W.H.; Hamed, M.B.; Fahmy, A.S.; Mohamed, S.A. Phenolic-antioxidant capacity of mango seed kernels: Therapeutic effect against viper venoms. Rev. Bras. Farmacogn. 2018, 28, 594–601. [Google Scholar] [CrossRef]
- Melis, M.P.; Angioni, E.; Carta, G.; Murru, E.; Scanu, P.; Spada, S.; Banni, S. Characterization of conjugated linoleic acid and its metabolites by RP-HPLC with diode array detector. Eur. J. Lipid Sci. Technol. 2001, 103, 617–621. [Google Scholar] [CrossRef]
- Taherali, F.; Varum, F.; Basit, A.W. A slippery slope: On the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 2018, 124, 16–33. [Google Scholar] [CrossRef]
- Gallarate, M.; Serpe, L.; Foglietta, F.; Zara, G.; Giordano, S.; Peira, E.; Chirio, D.; Battaglia, L. Solid Lipid Nanoparticles Loaded with Fluorescent-labelled Cyclosporine A: Anti-Inflammatory Activity In Vitro. Protein Pept. Lett. 2014, 21, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xing, J.; Zheng, Z.; Song, F.; Liu, Z.; Liu, S. Ultrahigh performance liquid chromatography–triple quadrupole mass spectrometry inhibitors fishing assay: A novel method for simultaneously screening of xanthine oxidase inhibitor and superoxide anion scavenger in a single analysis. Anal. Chim. Acta 2012, 715, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Pascual, M.; Gómez-Aguado, I.; Rodríguez-Castejón, J.; Rodríguez-Gascón, A.; Muntoni, E.; Battaglia, L.; del Pozo-Rodríguez, A.; Aspiazu, M.Á.S. Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production. Pharmaceutics 2020, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Stella, B.; Peira, E.; Dianzani, C.; Gallarate, M.; Battaglia, L.; Gigliotti, C.L.; Boggio, E.; Dianzani, U.; Dosio, F. Development and Characterization of Solid Lipid Nanoparticles Loaded with a Highly Active Doxorubicin Derivative. Nanomaterials 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Clemente, N.; Ferrara, B.; Gigliotti, C.; Boggio, E.; Capucchio, M.; Biasibetti, E.; Schiffer, D.; Mellai, M.; Annovazzi, L.; Cangemi, L.; et al. Solid Lipid Nanoparticles Carrying Temozolomide for Melanoma Treatment. Preliminary In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Pascual, M.; Albano, A.; Solinís, M.Á.; Serpe, L.; Rodríguez-Gascón, A.; Foglietta, F.; Muntoni, E.; Torrecilla, J.; del Pozo-Rodríguez, A.; Battaglia, L. Gene Delivery in the Cornea: In Vitro & Ex Vivo Evaluation of Solid Lipid Nanoparticle-Based Vectors. Nanomedicine 2018, 13, 1847–1854. [Google Scholar] [CrossRef]
- Muntoni, E.; Martina, K.; Marini, E.; Giorgis, M.; Lazzarato, L.; Salaroglio, I.C.; Riganti, C.; Lanotte, M.; Battaglia, L. Methotrexate-Loaded Solid Lipid Nanoparticles: Protein Functionalization to Improve Brain Biodistribution. Pharmaceutics 2019, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wen, Z.; He, X.; He, S. Effects of cinnamic acid on expression of tissue factor induced by TNFα in endothelial cells and its mechanisms. J. Chin. Med. Assoc. 2006, 69, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kose, S.B.E.; Kocasari, F.S. Protective effect of cinnamic acid on orthophenylphenol-induced oxidative stress in rats. Vet. Res. Forum 2022, 13, 187–192. [Google Scholar] [CrossRef]
- Kaduce, T.L.; Figard, P.H.; Leifur, R.; Spector, A.A. Formation of 9-Hydroxyoctadecadienoic Acid from Linoleic Acid in Endothelial Cells. J. Biol. Chem. 1989, 264, 6823–6830. [Google Scholar] [CrossRef]
- Haas, T.A.; Bertomeu, M.-C.; Bastida, E.; Buchanan, M.R. Cyclic AMP regulation of endothelial cell triacylglycerol turnover, 13-hydroxyoctadecadienoic acid (13-HODE) synthesis and endothelial cell thrombogenecity. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 1990, 1051, 174–178. [Google Scholar] [CrossRef]
- Setty, B.N.Y.; Berger, M.; Stuart, M.J. 13-Hydroxyoctadecadienoic acid (13-HODE) stimulates prostacyclin production by endothelial cells. Biochem. Biophys. Res. Commun. 1987, 146, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kim, Y.-I.; Hirai, S.; Goto, T.; Ohyane, C.; Tsugane, T.; Konishi, C.; Fujii, T.; Inai, S.; Iijima, Y.; et al. Comparative and Stability Analyses of 9- and 13-Oxo-octadecadienoic Acids in Various Species of Tomato. Biosci. Biotechnol. Biochem. 2012, 76, 2012E2. [Google Scholar] [CrossRef]
- Cullingford, T. Peroxisome proliferator-activated receptor alpha and the ketogenic diet. Epilepsia 2008, 49, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, A.B.; Oernbo, E.K.; Stoica, A.; Gerkau, N.J.; Barbuskaite, D.; Tritsaris, K.; Rose, C.R.; MacAulay, N. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat. Commun. 2018, 9, 2167. [Google Scholar] [CrossRef]
- Southwell, B.R.; Duan, W.; Alcorn, D.; Brack, C.; Richardson, S.J.; Köhrle, J.; Schreiber, G. Thyroxine transport to the brain: Role of protein synthesis by the choroid plexus. Endocrinology 1993, 133, 2116–2126. [Google Scholar] [CrossRef] [PubMed]
- Hakvoort, A.; Haselbach, M.; Wegener, J.; Hoheisel, D.; Galla, H.-J. The Polarity of Choroid Plexus Epithelial Cells In Vitro Is Improved in Serum-Free Medium. J. Neurochem. 1998, 71, 1141–1150. [Google Scholar] [CrossRef]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef]
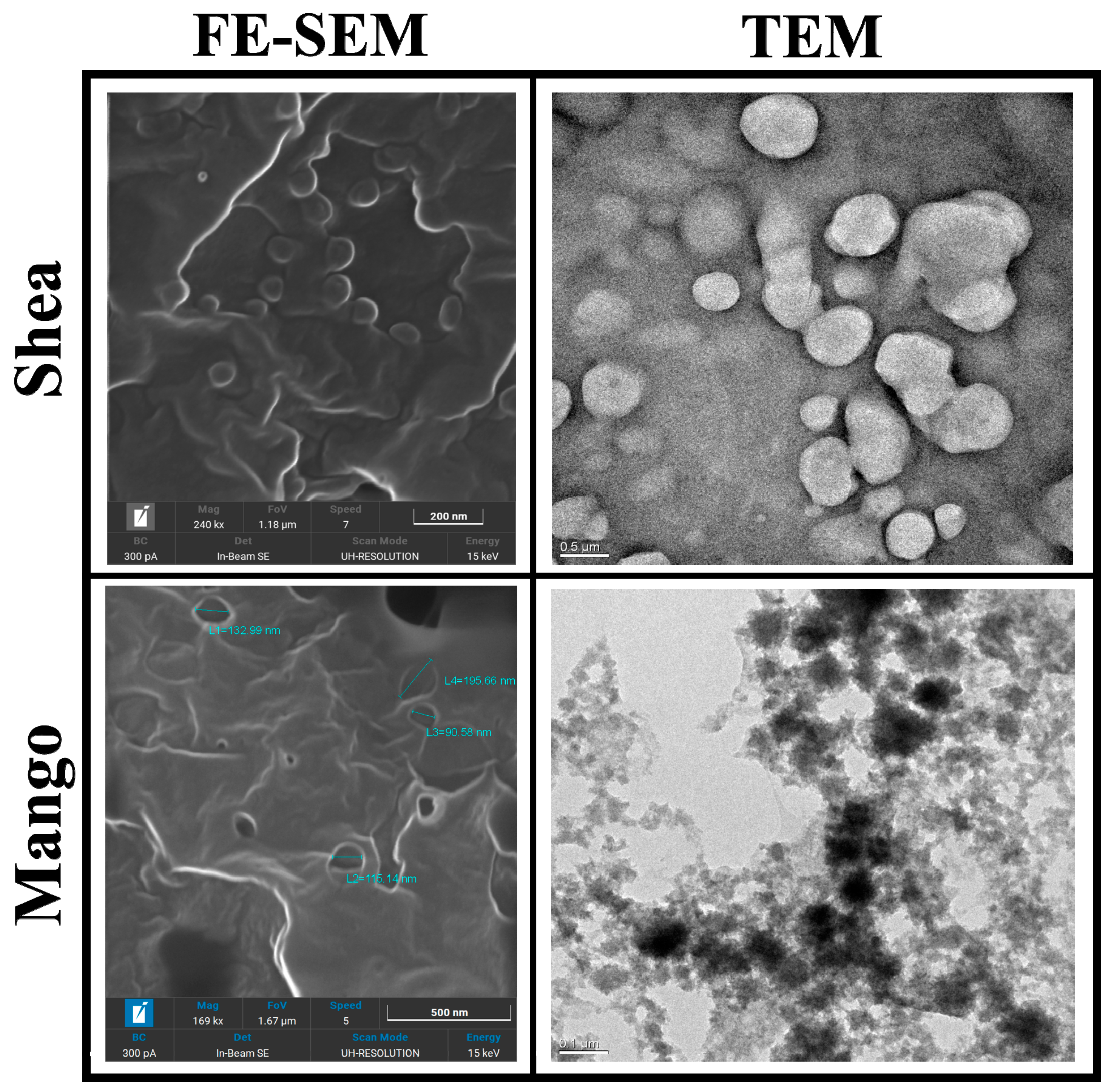




| Saponification | Precipitation Acid | Soap | Mean Particle Size (nm) | Polydispersion | % Recovery | EE% | ||
|---|---|---|---|---|---|---|---|---|
| Cold | Hot | |||||||
| Mango SLNs | x | H3PO4 | 1% | 259.1 ± 2.5 | 0.179 | - | - | |
| Shea SLNs | 299.5 ± 1.7 | 0.173 | - | - | ||||
| Shea SLNs | x | 4% | 120.4 ± 1.0 | 0.138 | - | - | ||
| Mango SLNs | x | 1% | 178.9 ± 0.8 | 0.202 | - | - | ||
| Shea SLNs | 131.1 ± 1.2 | 0.197 | - | - | ||||
| Mango SLNs | x | 1% | 191.8 ± 1.9 | 0.081 | 6-cou 79 | 6-cou 93 | ||
| Shea SLNs | 143.1 ± 3.4 | 0.056 | 6-cou 95 | 6-cou 92 | ||||
| Mango SLNs | x | 1% | 360.5 ± 2.6 | 0.110 | rhod B-AOT 77 | rhod B-AOT 86 | ||
| Shea SLNs | 298.7 ± 1.6 | 0.173 | rhod B-AOT 89 | rhod B-AOT 85 | ||||
| Mango SLNs | x | 1% | 323.6 ± 5.0 | 0.106 | rhod B 64 | rhod B 38 | ||
| Shea SLNs | 443.9 ± 4.0 | 0.084 | rhod B 72 | rhod B 55 | ||||
| Mango SLNs | x | C3H6O3 | 1% | 228.5 ± 0.7 | 0.226 | Bume 72 ± 6.13 | Bume 74 ± 3.3 | |
| Dexa73 ± 11.98 | Dexa 25 ± 8.33 | |||||||
| Shea SLNs | 201.1 ± 1.4 | 0.108 | Bume 70 ± 6.05 | Bume 83 ± 1.63 | ||||
| Dexa 80 ± 4.33 | Dexa 53 ± 12.93 | |||||||
| Tonset | Tpeak | Enthalpy (J/g) | |
|---|---|---|---|
| Mango SLNs | 54.06 | 59.61 | 21.29 |
| Mango fatty acids | 56.66 | 59.61 | 11.38 |
| Shea SLNs | 44.72 | 53.81 | 17.28 |
| Shea fatty acids | 45.69 | 52.7 | 22.98 |
| Palmitic acid | 59.64 | 63.59 | 195.23 |
| Stearic acid | 68.06 | 69.17 | 188.61 |
| Stearic acid by coacervation | 51.8 | 54.71 | 165.43 |
| Arachidic acid | 70.71 | 71.83 | 202.94 |
| It a | Cold Saponification | Hot Saponification | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak N° | Retention Time | Experimental | Reference b | Compound Name c | MW (TMS) | Mango Pellet | Shea Pellet | Mango Pellet | Shea Pellet |
| 1 | 23.864 | 1288 | 1290 | Glycerol 3TMS | 308 | 0.73% (rsd%:12.7%) | 0.77% (rsd%:3.7%) | 0.83% (rsd%:17.4%) | 1.11% (rsd%:19.9%) |
| 2 | 52.905 | 2054 | 2053 | Palmitic acid TMS | 328 | 6.84% (rsd%:10.8%) | 3.31% (rsd%:10.5%) | 2.83% (rsd%:33.2%) | 3.84% (rsd%:11.7%) |
| 3 | 57.928 | 2217 | 2217 | Linoleic acid TMS | 352 | 3.49% (rsd%:18.3%) | 3.94% (rsd%:1.2%) | 0.37% (rsd%:39%) | 2.54% (rsd%:25.6%) |
| 4 | 58.076 | 2222 | 2224 | Oleic acid TMS | 354 | 35.99% (rsd%:12.2%) | 36.81% (rsd%:8.3%) | 30.84% (rsd%:21%) | 47.55% (rsd%:14.1%) |
| 5 | 58.880 | 2249 | 2250 | Stearic acid TMS | 356 | 24.87% (rsd%:11.4%) | 25.68% (rsd%:10.7%) | 24.69% (rsd%:27.3%) | 26.98% (rsd%:3.6%) |
| 6 | 64.511 | 2449 | 2451 | Arachidic acid TMS | 384 | 0.97% (rsd%:8.1%) | 0.91% (rsd%:18.7%) | 2.65% (rsd%:9.4%) | 0.84% (rsd%:6.6%) |
| 7 | 68.780 | 2611 | 2611 | Glyceryl palmitate 2TMS | 474 | 0.32% (rsd%:26.9%) | 0.28% (rsd%:9.6%) | <0.25% (~0.12%) (rsd%:34.2%) | <0.25% (~0.24%) (rsd%:30.4%) |
| 8 | 72.949 | 2779 | 2788 | Glyceryl oleate 2TMS | 500 | 5.24% (rsd%:18.7%) | 7.30% (rsd%:12.8%) | 1.59% (rsd%:36.3%) | 3.52% (rsd%:22.8%) |
| 9 | 73.540 | 2803 | 2806 | Glyceryl stearate TMS | 502 | 4.61% (rsd%:17%) | 6.48% (rsd%:18.6%) | 1.61% (rsd%:37.3%) | 3.48% (rsd%:19.8%) |
| Quantification as µg/mg Soap (RSD%) | PAp/(PAp + PAs) b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak N° | Retention Time | UV | ESI+ | ESI− | MW | Compound | Reference | Mango | Shea | Mango | Shea |
| 1 | 28.043 | 277 | 190 [M+H+CH3CN]+ | 147 [M-H-]−, 193 [M-H+HCOOH]− | 148 | Cinnamic acid a* | [49,50] | 0.28 (8.4%) | 0.42 (5.7%) | 0.25 | 0.20 |
| 2 | 45.825 | 224/270/ 261/281 | 279 [M+H]+ | / | 278 | Conjugated trienoic fatty acid derivatives ** | [51] | 0.09% (17.4%) | 0.15% (9.7%) | 1 | 1 |
| 3 | 73.614 | 223/273 | 409 [M+H-C9H8O2]+/ 557 [M+H]+ | / | 556 | Lupeol Cinnamate a*** | [26,47] | 2.21 (17%) | 0.72 (14.6%) | 0.70 | 0.62 |
| 4 | 75.955 | 223/273 | 409 [M+H-C9H8O2]+ | / | / | Triterpene cinnamyl ester derivative *** | [26,47] | 2.98 (17%) | 1.01 (14.6%) | 1 | 1 |
| 5 | 80.862 | 224/272 | 409 [M+H-C9H8O2]+ | / | / | Triterpene cinnamyl ester derivative *** | [26,47] | 1.24 (16.6%) | 0.41 (17.8%) | 1 | 1 |
| 6 | 83.964 | 224/273 | 409 [M+H-C9H8O2-H2O]+ | / | / | Triterpene cinnamyl ester derivative *** | [26,47] | 1.64 (12.8%) | 0.52 (11.3%) | 1 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozza, A.; Bordano, V.; Marengo, A.; Muntoni, E.; Marini, E.; Lazzarato, L.; Dianzani, C.; Monge, C.; Rosa, A.C.; Cangemi, L.; et al. Green Solid Lipid Nanoparticles by Fatty Acid Coacervation: An Innovative Nasal Delivery Tool for Drugs Targeting Cerebrovascular and Neurological Diseases. Pharmaceutics 2024, 16, 1051. https://doi.org/10.3390/pharmaceutics16081051
Bozza A, Bordano V, Marengo A, Muntoni E, Marini E, Lazzarato L, Dianzani C, Monge C, Rosa AC, Cangemi L, et al. Green Solid Lipid Nanoparticles by Fatty Acid Coacervation: An Innovative Nasal Delivery Tool for Drugs Targeting Cerebrovascular and Neurological Diseases. Pharmaceutics. 2024; 16(8):1051. https://doi.org/10.3390/pharmaceutics16081051
Chicago/Turabian StyleBozza, Annalisa, Valentina Bordano, Arianna Marengo, Elisabetta Muntoni, Elisabetta Marini, Loretta Lazzarato, Chiara Dianzani, Chiara Monge, Arianna Carolina Rosa, Luigi Cangemi, and et al. 2024. "Green Solid Lipid Nanoparticles by Fatty Acid Coacervation: An Innovative Nasal Delivery Tool for Drugs Targeting Cerebrovascular and Neurological Diseases" Pharmaceutics 16, no. 8: 1051. https://doi.org/10.3390/pharmaceutics16081051
APA StyleBozza, A., Bordano, V., Marengo, A., Muntoni, E., Marini, E., Lazzarato, L., Dianzani, C., Monge, C., Rosa, A. C., Cangemi, L., Valsania, M. C., Colitti, B., Camisassa, E., & Battaglia, L. (2024). Green Solid Lipid Nanoparticles by Fatty Acid Coacervation: An Innovative Nasal Delivery Tool for Drugs Targeting Cerebrovascular and Neurological Diseases. Pharmaceutics, 16(8), 1051. https://doi.org/10.3390/pharmaceutics16081051






