Hyaluronic Acid-Based Nanoparticles Loaded with Rutin as Vasculo-Protective Tools against Anthracycline-Induced Endothelial Damages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hybrid Nanoparticles
2.3. TEM Analyses
2.4. Particle Size and Zeta Potential Measurements
2.5. Stability Study
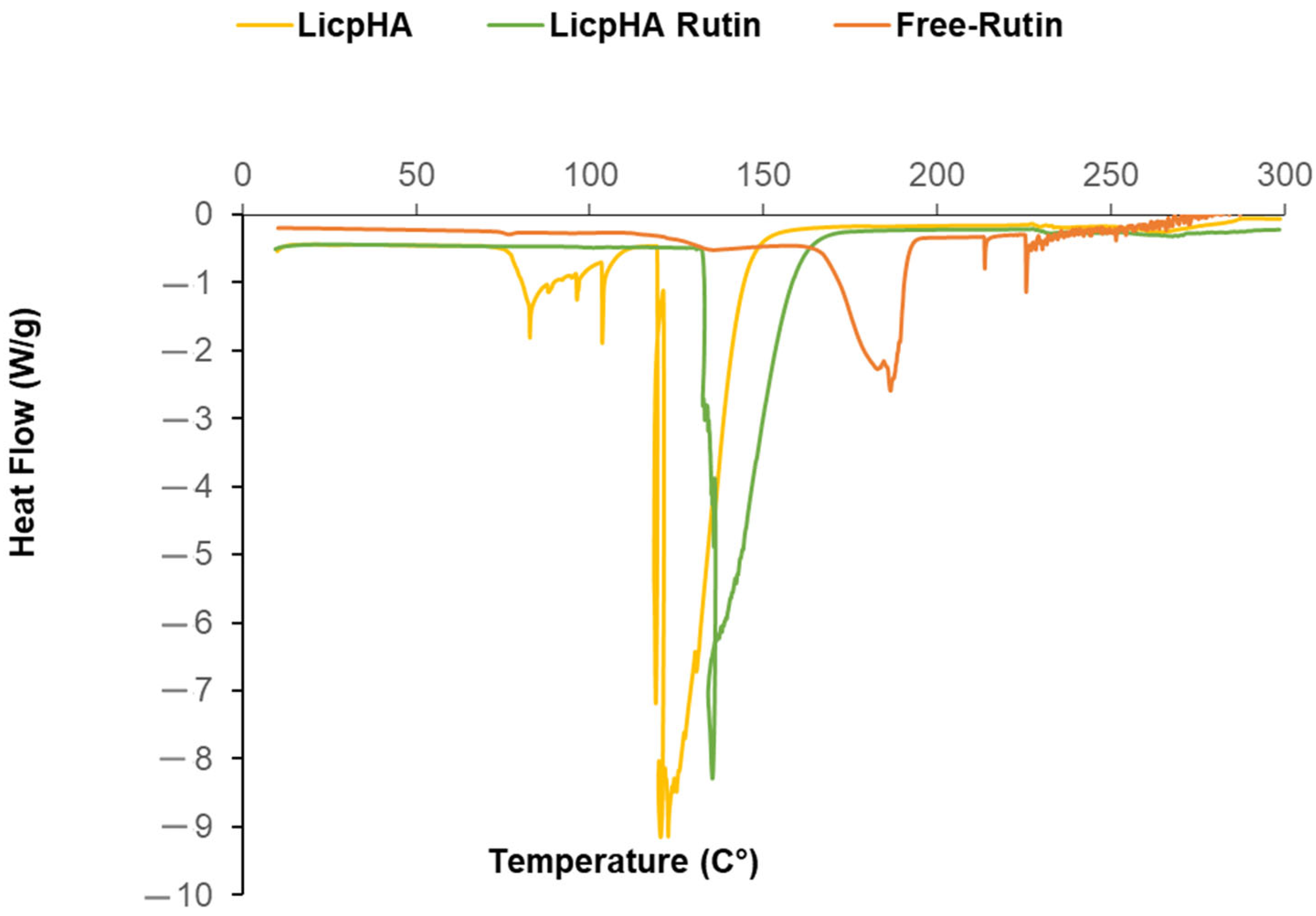
2.6. Differential Scanning Calorimetry (DSC) Analysis
2.7. Determination of the Total Amount of Rutin Content and the Encapsulation Efficiency
2.8. Release Kinetics of Rutin
2.9. Cell Culture and Treatments
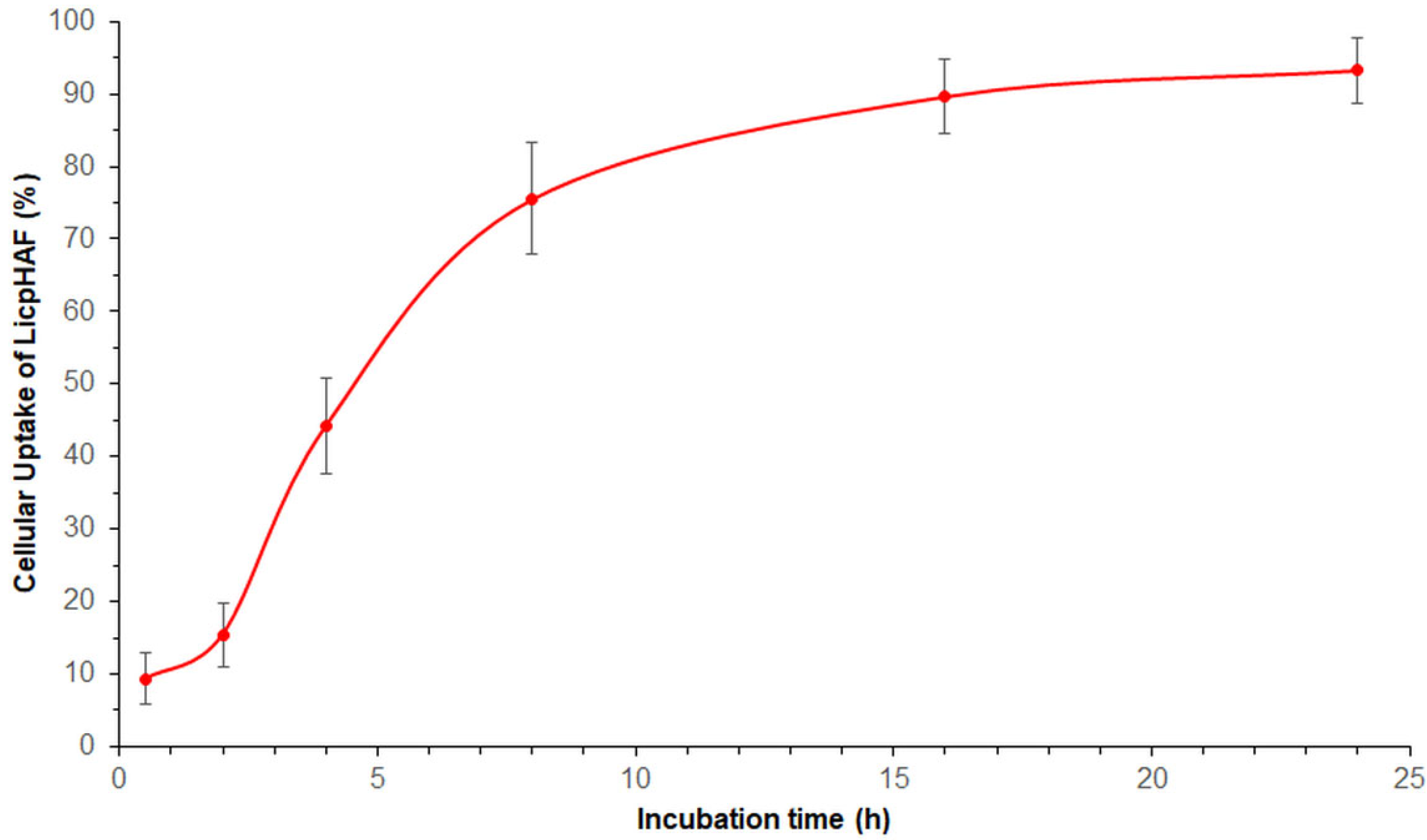
2.10. Cellular Uptake Studies
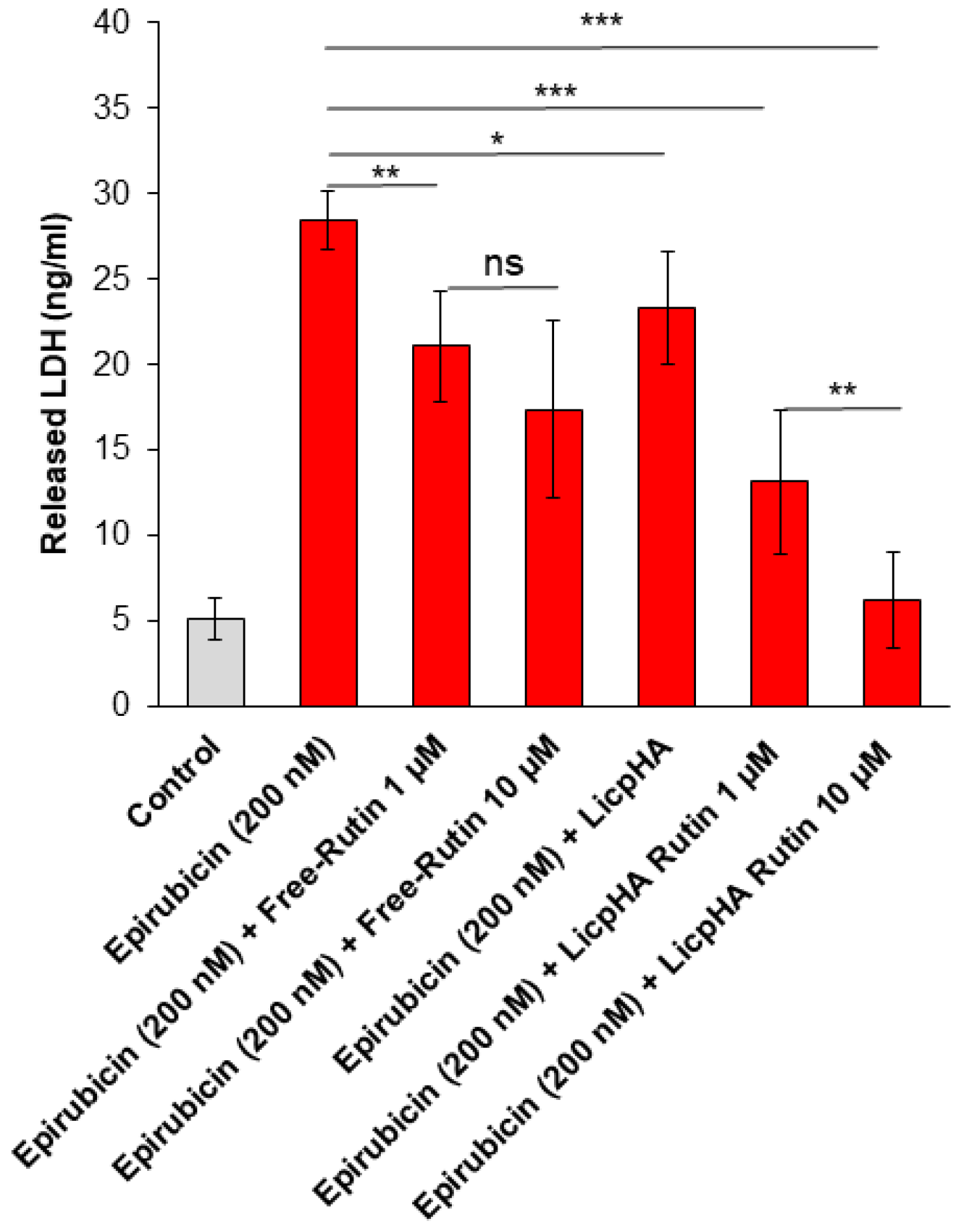
2.11. Assessment of Cell Survival and LDH Release
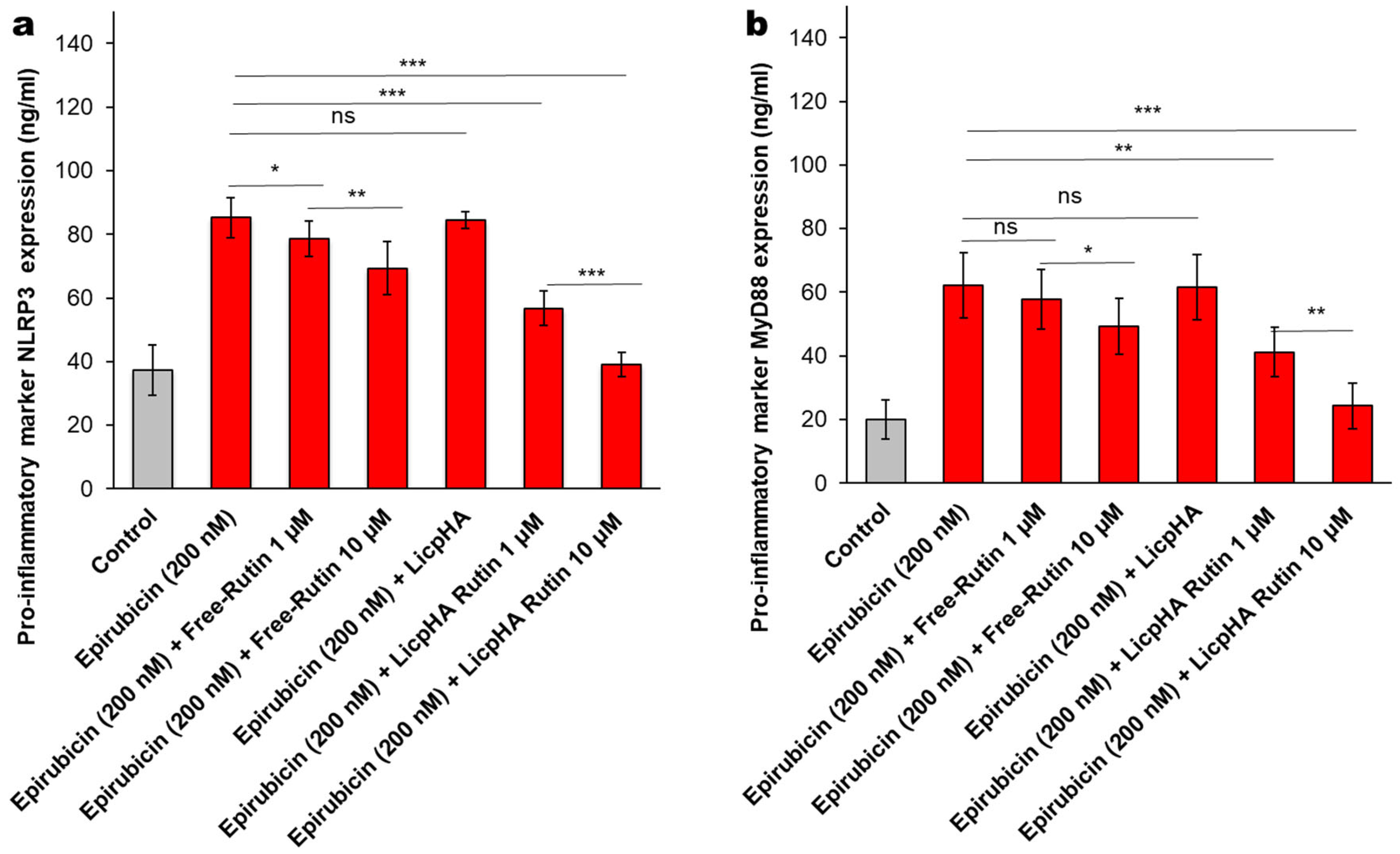
2.12. NLRP-3 and MyD-88 Expression Studies
2.13. Cytokines, Chemokines, and Growth Factors Assay
2.14. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Hybrid Nanoparticles
3.2. Physicochemical Characterisation of the Hybrid Nanoparticles
3.3. Release Kinects
3.4. Cellular Uptake Study
3.5. Cell Viability and LDH Release of Endothelial Cells
3.5.1. NLRP-3 and Myd-88 Expression in Endothelial Cells
3.5.2. Cytokines and Chemokines Levels in Endothelial Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients 2018, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Grakova, E.V.; Shilov, S.N.; Kopeva, K.V.; Berezikova, E.N.; Popova, A.A.; Neupokoeva, M.N.; Ratushnyak, E.T.; Teplyakov, A.T. Anthracycline-Induced Cardiotoxicity: The Role of Endothelial Dysfunction. Cardiology 2021, 146, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Luu, A.Z.; Chowdhury, B.; Al-Omran, M.; Teoh, H.; Hess, D.A.; Verma, S. Role of Endothelium in Doxorubicin-Induced Cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Cerulla, N.; Arcusa, À.; Navarro, J.-B.; De La Osa, N.; Garolera, M.; Enero, C.; Chico, G.; Fernández-Morales, L. Cognitive Impairment Following Chemotherapy for Breast Cancer: The Impact of Practice Effect on Results. J. Clin. Exp. Neuropsychol. 2019, 41, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Adebisi, G. Epirubicin Treatment Induces Neurobehavioral, Oxido-Inflammatory and Neurohistology Alterations in Rats: Protective Effect of the Endogenous Metabolite of Tryptophan− 3-Indolepropionic Acid. Neurochem. Res. 2023, 48, 2767–2783. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-Induced Cognitive Impairment: The Mechanistic Insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Naqvi, S.; Shams, S.; Sheikh, R.A.; Al-Abbasi, F.A.; Asseri, A.H.; Baig, M.R.; Kumar, V. Nanomedicines: Intervention in Inflammatory Pathways of Cancer. Inflammopharmacology 2023, 31, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic Benefits of Rutin and Its Nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef]
- Ramalingayya, G.V.; Cheruku, S.P.; Nayak, P.; Kishore, A.; Shenoy, R.; Rao, C.M.; Krishnadas, N. Rutin Protects against Neuronal Damage in Vitro and Ameliorates Doxorubicin-Induced Memory Deficits in Vivo in Wistar Rats. Drug Des. Devel. Ther. 2017, 11, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. The Interactions of Flavonoids within Neuronal Signalling Pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The Impact of Flavonoids on Memory: Physiological and Molecular Considerations. Chem. Soc. Rev. 2009, 38, 1152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Wu, J.; Cai, J. The In Vivo Synaptic Plasticity Mechanism of EGb 761-Induced Enhancement of Spatial Learning and Memory in Aged Rats: EGb 761 Improves in Vivo LTP and Memory. Br. J. Pharmacol. 2006, 148, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Dwarampudi, L.P.; Kadiyala, M.; Kuppuswamy, G.; Veera Venkata Satyanarayana Reddy, K.; Kumar, C.K.A.; Paranjothy, M. Formulation and Characterization of Chitosan Encapsulated Phytoconstituents of Curcumin and Rutin Nanoparticles. Int. J. Biol. Macromol. 2017, 104, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, X.; Zhang, Q.; Wang, L.; Li, Y.; Li, Y. Characterization and Evaluation of the Solubility and Oral Bioavailability of Rutin–Ethanolate Solvate. AAPS PharmSciTech 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Ashafaq, M.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Rutin-Encapsulated Chitosan Nanoparticles Targeted to the Brain in the Treatment of Cerebral Ischemia. Int. J. Biol. Macromol. 2016, 91, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric Lipid Hybrid Nanoparticles (PLNs) as Emerging Drug Delivery Platform—A Comprehensive Review of Their Properties, Preparation Methods, and Therapeutic Applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid–Polymer Hybrid Nanoparticles as a next-Generation Drug Delivery Platform: State of the Art, Emerging Technologies, and Perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Ishak, R.A.H.; Mostafa, N.M.; Kamel, A.O. Stealth Lipid Polymer Hybrid Nanoparticles Loaded with Rutin for Effective Brain Delivery—Comparative Study with the Gold Standard (Tween 80): Optimization, Characterization and Biodistribution. Drug Deliv. 2017, 24, 1874–1890. [Google Scholar] [CrossRef]
- Júlio, A.; Caparica, R.; Costa Lima, S.A.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; Santos De Almeida, T.; Fonte, P. Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. Nanomaterials 2019, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Giarra, S.; Serri, C.; Russo, L.; Zeppetelli, S.; De Rosa, G.; Borzacchiello, A.; Biondi, M.; Ambrosio, L.; Mayol, L. Spontaneous Arrangement of a Tumor Targeting Hyaluronic Acid Shell on Irinotecan Loaded PLGA Nanoparticles. Carbohydr. Polym. 2016, 140, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lian, Y.; Fang, Q.; Liu, L.; Zhang, J.; Li, J. Hyaluronic-Acid-Modified Lipid-Polymer Hybrid Nanoparticles as an Efficient Ocular Delivery Platform for Moxifloxacin Hydrochloride. Int. J. Biol. Macromol. 2018, 116, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Hörmann, N.; Gust, R.; Ganner, A. Synthesis, Characterization and Evaluation of Hyaluronic Acid-Based Polymers for Nasal Delivery. Int. J. Pharm. 2023, 631, 122496. [Google Scholar] [CrossRef] [PubMed]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic Acid: A Review on Its Biology, Aspects of Drug Delivery, Route of Administrations and a Special Emphasis on Its Approved Marketed Products and Recent Clinical Studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.R.; More, M.P.; Patil, P.B.; Mujumdar, A.; Naik, J.B. Statistical Optimization of Voriconazole Nanoparticles Loaded Carboxymethyl Chitosan-Poloxamer Based in Situ Gel for Ocular Delivery: In Vitro, Ex Vivo, and Toxicity Assessment. Drug Deliv. Transl. Res. 2022, 12, 3063–3082. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of Thymoquinone through Hyaluronic Acid-Decorated Mixed Pluronic® Nanoparticles to Attenuate Angiogenesis and Metastasis of Triple-Negative Breast Cancer. J. Control. Release 2020, 322, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Abdel Hady, M.; Awad, G.A.S. Phospholipid Based Colloidal Poloxamer–Nanocubic Vesicles for Brain Targeting via the Nasal Route. Colloids Surf. B Biointerfaces 2012, 100, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-Based Intranasal Thermoreversible Gel of Zolmitriptan-Loaded Nanoethosomes: Formulation, Optimization, Evaluation and Permeation Studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef]
- Serri, C.; Quagliariello, V.; Iaffaioli, R.V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination Therapy for the Treatment of Pancreatic Cancer through Hyaluronic Acid-decorated Nanoparticles Loaded with Quercetin and Gemcitabine: A Preliminary In Vitro Study. J. Cell. Physiol. 2019, 234, 4959–4969. [Google Scholar] [CrossRef]
- De La Hoz-Camacho, R.; Rivera-Lazarín, A.L.; Vázquez-Guillen, J.M.; Caballero-Hernández, D.; Mendoza-Gamboa; Martínez-Torres, A.C.; Rodríguez-Padilla, C. Cyclophosphamide and Epirubicin Induce High Apoptosis in Microglia Cells While Epirubicin Provokes DNA Damage and Microglial Activation at Sub-Lethal Concentrations. EXCLI J. 2022, 21, 197. [Google Scholar] [CrossRef]
- Sauter, K.A.D.; Wood, L.J.; Wong, J.; Iordanov, M.; Magun, B.E. Doxorubicin and Daunorubicin Induce Processing and Release of Interleukin-1β through Activation of the NLRP3 Inflammasome: Progress at a Snail’s Pace. Cancer Biol. Ther. 2011, 11, 1008–1016. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Rolim, A.; Maciel, C.P.M.; Kaneko, T.M.; Consiglieri, V.O.; Salgado-Santos, I.M.N.; Velasco, M.V.R. Validation Assay for Total Flavonoids, as Rutin Equivalents, from Trichilia Catigua Adr. Juss (Meliaceae) and Ptychopetalum Olacoides Bentham (Olacaceae) Commercial Extract. J. AOAC Int. 2005, 88, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Rolim, A.; Oishi, T.; Maciel, C.P.M.; Zague, V.; Pinto, C.A.S.O.; Kaneko, T.M.; Consiglieri, V.O.; Velasco, M.V.R. Total Flavonoids Quantification from O/W Emulsion with Extract of Brazilian Plants. Int. J. Pharm. 2006, 308, 107–114. [Google Scholar] [CrossRef]
- Baby, A.R.; Maciel, C.P.M.; Kaneko, T.M.; Velasco, M.V.R. UV Spectrophotometric Determination of Bioflavonoids from a Semisolid Pharmaceutical Dosage Form Containing Trichilia Catigua Adr. Juss and Ptychopetalum Olacoides Bentham Standardized Extract: Analytical Method Validation and Statistical Procedures. J. AOAC Int. 2006, 89, 1532–1537. [Google Scholar] [CrossRef]
- Quagliariello, V.; Masarone, M.; Armenia, E.; Giudice, A.; Barbarisi, M.; Caraglia, M.; Barbarisi, A.; Persico, M. Chitosan-Coated Liposomes Loaded with Butyric Acid Demonstrate Anticancer and Anti-Inflammatory Activity in Human Hepatoma HepG2 Cells. Oncol. Rep. 2018, 41, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Hooftman, A.; Angiari, S.; Hester, S.; Corcoran, S.E.; Runtsch, M.C.; Ling, C.; Ruzek, M.C.; Slivka, P.F.; McGettrick, A.F.; Banahan, K.; et al. The Immunomodulatory Metabolite Itaconate Modifies NLRP3 and Inhibits Inflammasome Activation. Cell Metab. 2020, 32, 468–478.e7. [Google Scholar] [CrossRef]
- Malich, G.; Markovic, B.; Winder, C. The Sensitivity and Specificity of the MTS Tetrazolium Assay for Detecting the In Vitro Cytotoxicity of 20 Chemicals Using Human Cell Lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Wu, T.; Zhu, C.; Wang, X.; Kong, Q.; Guo, T.; He, Z.; He, Y.; Ruan, S.; Ruan, H.; Pei, L.; et al. Cholesterol and Phospholipid-Free Multilamellar Niosomes Regulate Transdermal Permeation of a Hydrophobic Agent Potentially Administrated for Treating Diseases in Deep Hair Follicles. J. Pharm. Sci. 2022, 111, 1785–1797. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Delfino, I.; Crucianelli, M.; Conte, N.; Avitabile, D.; Saladino, R. Green and Scalable Preparation of Colloidal Suspension of Lignin Nanoparticles and Its Application in Eco-Friendly Sunscreen Formulations. ACS Omega 2021, 6, 21444–21456. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Barone, A.; Mancuso, A.; Torella, D.; Paolino, D. Rutin-Loaded Nanovesicles for Improved Stability and Enhanced Topical Efficacy of Natural Compound. J. Funct. Biomater. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Leonyza, A.; Surini, S. Optimization of sodium deoxycholate-based transfersomes for percutaneous delivery of peptides and proteins. Int. J. Appl. Pharm. 2019, 11, 329–332. [Google Scholar] [CrossRef]
- Chiesa, E.; Greco, A.; Riva, F.; Dorati, R.; Conti, B.; Modena, T.; Genta, I. Hyaluronic Acid-Based Nanoparticles for Protein Delivery: Systematic Examination of Microfluidic Production Conditions. Pharmaceutics 2021, 13, 1565. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Phosphatidylcholine-Rutin Complex as a Potential Nanocarrier for Food Applications. J. Funct. Foods 2017, 33, 134–141. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Fagnola, M.; Pagani, M.P.; Maffioletti, S.; Tavazzi, S.; Papagni, A. Hyaluronic Acid in Hydrophilic Contact Lenses: Spectroscopic Investigation of the Content and Release in Solution. Contact Lens Anterior Eye 2009, 32, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Serri, C.; Argirò, M.; Piras, L.; Mita, D.G.; Saija, A.; Mita, L.; Forte, M.; Giarra, S.; Biondi, M.; Crispi, S.; et al. Nano-Precipitated Curcumin Loaded Particles: Effect of Carrier Size and Drug Complexation with (2-Hydroxypropyl)-β-Cyclodextrin on Their Biological Performances. Int. J. Pharm. 2017, 520, 21–28. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Cristiano, M.C.; Venuti, V.; Crupi, V.; Majolino, D.; Paladini, G.; Acri, G.; Testagrossa, B.; Irrera, A.; Paolino, D.; et al. Rutin-Loaded Solid Lipid Nanoparticles: Characterization and In Vitro Evaluation. Molecules 2021, 26, 1039. [Google Scholar] [CrossRef]
- Zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery—Drug Release and Release Mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Quagliariello, V.; Gennari, A.; Jain, S.A.; Rosso, F.; Iaffaioli, R.V.; Barbarisi, A.; Barbarisi, M.; Tirelli, N. Double-Responsive Hyaluronic Acid-Based Prodrugs for Efficient Tumour Targeting. Mater. Sci. Eng. C 2021, 131, 112475. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Vasco, C.; Girgenti, V.; Fugnanesi, V.; Calatozzolo, C.; Canazza, A.; Salmaggi, A.; Rivoltini, L.; Morbin, M.; Ciusani, E. Melanoma Cells Homing to the Brain: An In Vitro Model. BioMed Res. Int. 2015, 2015, 476069. [Google Scholar] [CrossRef]
- Drolez, A.; Vandenhaute, E.; Julien, S.; Gosselet, F.; Burchell, J.; Cecchelli, R.; Delannoy, P.; Dehouck, M.-P.; Mysiorek, C. Selection of a Relevant In Vitro Blood-Brain Barrier Model to Investigate Pro-Metastatic Features of Human Breast Cancer Cell Lines. PLoS ONE 2016, 11, e0151155. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Ubogu, E.E.; Williams, K.A.; Tucky, B.; Callahan, M.K.; Ransohoff, R.M. Human Brain Microvascular Endothelial Cells and Umbilical Vein Endothelial Cells Differentially Facilitate Leukocyte Recruitment and Utilize Chemokines for T Cell Migration. Clin. Dev. Immunol. 2008, 2008, 384982. [Google Scholar] [CrossRef]
- Boyle, S.; Dobson, V.; Duthie, S.; Hinselwood, D.; Kyle, J.; Collins, A. Bioavailability and Efficiency of Rutin as an Antioxidant: A Human Supplementation Study. Eur. J. Clin. Nutr. 2000, 54, 774–782. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic Potential and Recent Advances in Drug Delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Quagliariello, V.; Paccone, A.; Iovine, M.; Cavalcanti, E.; Berretta, M.; Maurea, C.; Canale, M.L.; Maurea, N. Interleukin-1 Blocking Agents as Promising Strategy for Prevention of Anticancer Drug-Induced Cardiotoxicities: Possible Implications in Cancer Patients with COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6797–6812. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Sadeer, N.; Edoo, M.; Venugopala, K.N. The Potential Application of Novel Drug Delivery Systems for Phytopharmaceuticals and Natural Extracts—Current Status and Future Perspectives. Mini-Rev. Med. Chem. 2021, 21, 2731–2746. [Google Scholar] [CrossRef]
- Carneiro, J.; Döll-Boscardin, P.M.; Fiorin, B.C.; Nadal, J.M.; Farago, P.V.; Paula, J.P.D. Development and Characterization of Hyaluronic Acid-Lysine Nanoparticles with Potential as Innovative Dermal Filling. Braz. J. Pharm. Sci. 2016, 52, 645–651. [Google Scholar] [CrossRef]
- Portaccio, M.; Faramarzi, B.; Lepore, M. Probing Biochemical Differences in Lipid Components of Human Cells by Means of ATR-FTIR Spectroscopy. Biophysica 2023, 3, 524–538. [Google Scholar] [CrossRef]




| Formulation | Mean Diameter (nm) | PDI | Zeta Potential (mV) | Total Amount of Rutin (%) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| LicpHA | 179 ± 4 * | 1.0 ± 0.0 | −35 ± 1 * | - | - |
| LicpHA Rutin | 209 ± 5 * | 1.1 ± 0.0 | −30 ± 1 * | 69 ± 2 | 45 ± 1 |
| Formulation | 4 °C | RPMI 37 °C | |||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 15 | Day 30 | Day 0 | Day 1 | Day 2 | Day 3 | |
| LicpHA | 179 ± 4 | 199 ± 3 | 190 ± 3 | 186 ± 3 | 182 ± 3 | 194 ± 2 | 189 ± 2 |
| LicpHA Rutin | 209 ± 5 | 209 ± 2 | 206 ±2 | 202 ± 1 | 202 ± 1 | 208 ± 3 | 210 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serri, C.; Quagliariello, V.; Cruz-Maya, I.; Guarino, V.; Maurea, N.; Giunchedi, P.; Rassu, G.; Gavini, E. Hyaluronic Acid-Based Nanoparticles Loaded with Rutin as Vasculo-Protective Tools against Anthracycline-Induced Endothelial Damages. Pharmaceutics 2024, 16, 985. https://doi.org/10.3390/pharmaceutics16080985
Serri C, Quagliariello V, Cruz-Maya I, Guarino V, Maurea N, Giunchedi P, Rassu G, Gavini E. Hyaluronic Acid-Based Nanoparticles Loaded with Rutin as Vasculo-Protective Tools against Anthracycline-Induced Endothelial Damages. Pharmaceutics. 2024; 16(8):985. https://doi.org/10.3390/pharmaceutics16080985
Chicago/Turabian StyleSerri, Carla, Vincenzo Quagliariello, Iriczalli Cruz-Maya, Vincenzo Guarino, Nicola Maurea, Paolo Giunchedi, Giovanna Rassu, and Elisabetta Gavini. 2024. "Hyaluronic Acid-Based Nanoparticles Loaded with Rutin as Vasculo-Protective Tools against Anthracycline-Induced Endothelial Damages" Pharmaceutics 16, no. 8: 985. https://doi.org/10.3390/pharmaceutics16080985
APA StyleSerri, C., Quagliariello, V., Cruz-Maya, I., Guarino, V., Maurea, N., Giunchedi, P., Rassu, G., & Gavini, E. (2024). Hyaluronic Acid-Based Nanoparticles Loaded with Rutin as Vasculo-Protective Tools against Anthracycline-Induced Endothelial Damages. Pharmaceutics, 16(8), 985. https://doi.org/10.3390/pharmaceutics16080985












