Advances in Cyclodextrins and Their Derivatives in Nano-Delivery Systems
Abstract
:1. Introduction
2. Cyclodextrins (CDs)
2.1. The Types and Characteristics of Natural CDs
2.2. Formation of the CD Inclusion Complex
2.3. Types and Characteristics of CD Polymers
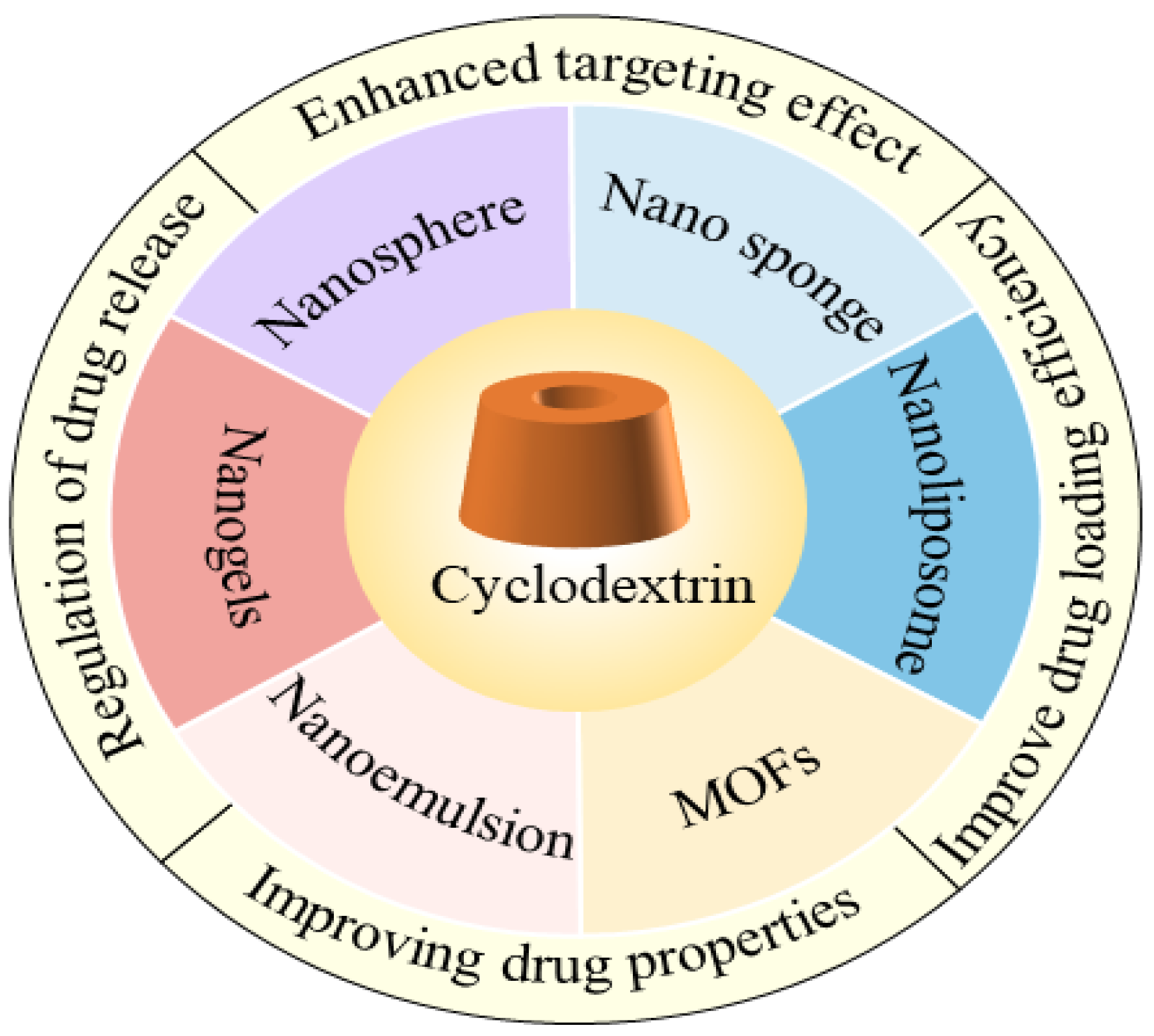
3. CD-Based Nano-Delivery System
3.1. Nanospheres
3.2. Nano-Sponges
3.3. Liposomes
3.4. Metal–Organic Frameworks
3.5. Nanogels
3.6. Emulsions
4. The Application of CDs and Their Derivatives in Nano-Delivery Systems
4.1. Enhanced Targeting Effect
4.2. Regulation of Drug Release
4.3. Improving Drug Properties
4.4. Improved Drug-Loading Efficiency
5. Conclusions and Prospects
Funding
Conflicts of Interest
References
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Bai, H.; Wang, J.; Phan, C.U.; Chen, Q.; Hu, X.; Shao, G.; Zhou, J.; Lai, L.; Tang, G. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat. Commun. 2021, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Lukova, P.; Katsarov, P.; Pilicheva, B. Application of starch, cellulose, and their derivatives in the development of microparticle drug-delivery systems. Polymers 2023, 15, 3615. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Yang, X.; Chen, S.; Wang, Q.; Zhang, A.; Tang, B. Cyclodextrin-based host–guest supramolecular hydrogels for local drug delivery. Coord. Chem. Rev. 2022, 454, 214352. [Google Scholar] [CrossRef]
- Taharabaru, T.; Kihara, T.; Obata, A.; Onodera, R.; Wen, Y.; Li, J.; Motoyama, K.; Higashi, T. Cyclodextrin-based tailored polyrotaxanes for highly efficient delivery of the genome-editing molecule. Carbohydr. Polym. 2024, 323, 121443. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Motoyama, K.; Arima, H. Cyclodextrin-based polyrotaxanes and polypseudorotaxanes as drug delivery carriers. J. Drug Deliv. Sci. Technol. 2013, 23, 523–529. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.-G. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Yakupova, L.R.; Skuredina, A.A.; Kopnova, T.Y.; Kudryashova, E.V. In Vitro Biological Properties of Cyclodextrin-Based Polymers: Interaction with Human Serum Albumin, Red Blood Cells and Bacteria. Polysaccharides 2023, 4, 343–357. [Google Scholar] [CrossRef]
- Ma, P.; Huang, J. Nanoformulation of Paclitaxel: Exploring the Cyclodextrin/PLGA Nano Delivery Carrier to Slow Down Paclitaxel Release, Enhance Accumulation in Vivo. J. Cancer 2023, 14, 759–769. [Google Scholar] [CrossRef]
- Arya, P.; Raghav, N. In-vitro studies of Curcumin-β-cyclodextrin inclusion complex as sustained release system. J. Mol. Struct. 2021, 1228, 129774. [Google Scholar] [CrossRef]
- Kim, J.S. Study of Flavonoid/Hydroxypropyl-beta-Cyclodextrin Inclusion Complexes by UV-Vis, FT-IR, DSC, and X-Ray Diffraction Analysis. Prev. Nutr. Food Sci. 2020, 25, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Shelley, H.; Babu, R.J. Role of Cyclodextrins in Nanoparticle-Based Drug Delivery Systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef]
- Narayanan, G.; Shen, J.; Matai, I.; Sachdev, A.; Boy, R.; Tonelli, A.E. Cyclodextrin-based nanostructures. Prog. Mater. Sci. 2022, 124, 100869. [Google Scholar] [CrossRef]
- Villiers, A. Sur la fermentation de la fécule par l’action du ferment butyrique. Compt. Rend. Acad. Sci. 1891, 112, 536–538. [Google Scholar]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent advances in host–guest self-assembled cyclodextrin carriers: Implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, R.; Banerjee, U. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Shieh, W.J.; Hedges, A.R. Properties and Applications of Cyclodextrins. J. Macromol. Sci. Part A 1996, 33, 673–683. [Google Scholar] [CrossRef]
- da Silva Júnior, W.F.; de Oliveira Pinheiro, J.G.; Moreira, C.D.; de Souza, F.J.; de Lima, Á.A. Alternative technologies to improve solubility and stability of poorly water-soluble drugs. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 281–305. [Google Scholar]
- Liu, J.Y.; Zhang, X.; Tian, B. Selective modifications at the different positions of cyclodextrins: A review of strategies. Turk. J. Chem. 2020, 44, 261–278. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. gamma-Cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Sabadini, E.; Cosgrove, T.; Egidio Fdo, C. Solubility of cyclomaltooligosaccharides (cyclodextrins) in H2O and D2O: A comparative study. Carbohydr. Res. 2006, 341, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y. Multicharged cyclodextrin supramolecular assemblies. Chem. Soc. Rev. 2022, 51, 4786–4827. [Google Scholar] [CrossRef] [PubMed]
- Najm, A.; Niculescu, A.-G.; Bolocan, A.; Rădulescu, M.; Grumezescu, A.M.; Beuran, M.; Gaspar, B.S. Chitosan and Cyclodextrins—Versatile Materials Used to Create Drug Delivery Systems for Gastrointestinal Cancers. Pharmaceutics 2023, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, chemical, and physical properties, and applications. Polysaccharides 2021, 3, 1–31. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Eid, E.E.; Almaiman, A.A.; Alshehade, S.A.; Alsalemi, W.; Kamran, S.; Suliman, F.O.; Alshawsh, M.A. Characterization of thymoquinone-sulfobutylether-β-cyclodextrin inclusion complex for anticancer applications. Molecules 2023, 28, 4096. [Google Scholar] [CrossRef] [PubMed]
- Arisaka, Y.; Yui, N. Polyrotaxane-based biointerfaces with dynamic biomaterial functions. J. Mater. Chem. B 2019, 7, 2123–2129. [Google Scholar] [CrossRef]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef]
- Crivello, G.; Orlandini, G.; Morena, A.G.; Torchio, A.; Mattu, C.; Boffito, M.; Tzanov, T.; Ciardelli, G. Lignin–cobalt nano-enabled poly (pseudo) rotaxane supramolecular hydrogel for treating chronic wounds. Pharmaceutics 2023, 15, 1717. [Google Scholar] [CrossRef]
- Evangelista, T.F.; Andrade, G.R.; Nascimento, K.N.; Dos Santos, S.B.; Santos, M.D.F.C.; D’Oca, C.D.R.M.; Estevam, C.D.S.; Gimenez, I.F.; Almeida, L.E. Supramolecular polyelectrolyte complexes based on cyclodextrin-grafted chitosan and carrageenan for controlled drug release. Carbohydr. Polym. 2020, 245, 116592. [Google Scholar] [CrossRef]
- Omtvedt, L.A.; Dalheim, M.Ø.; Nielsen, T.T.; Larsen, K.L.; Strand, B.L.; Aachmann, F.L. Efficient grafting of cyclodextrin to alginate and performance of the hydrogel for release of model drug. Sci. Rep. 2019, 9, 9325. [Google Scholar] [CrossRef]
- Osman, S.K.; Brandl, F.P.; Zayed, G.M.; Teßmar, J.K.; Göpferich, A.M. Cyclodextrin based hydrogels: Inclusion complex formation and micellization of adamantane and cholesterol grafted polymers. Polymer 2011, 52, 4806–4812. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Xiong, J.; Wu, D.; Li, S.; Tao, Y.; Qin, Y.; Kong, Y. Facile synthesis of chitosan-grafted beta-cyclodextrin for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2019, 125, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Khodayari, H.; Heydarinasab, A.; Moniri, E.; Miralinaghi, M. Synthesis and characterization of magnetic nanoparticles-grafted-hyaluronic acid/β-cyclodextrin as a novel pH-sensetive nanocarrier for targeted delivery of doxorubicin. Inorg. Chem. Commun. 2023, 148, 110366. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Decher, G. Self-assembled smart nanocarriers for targeted drug delivery. Adv. Mater. 2016, 28, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier drug delivery systems: Characterization, limitations, future perspectives and implementation of artificial intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A versatile nano-delivery system for biomedical applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv. Pharm. Bull. 2020, 10, 166. [Google Scholar] [CrossRef]
- Pandey, D.; Panwar, V.S.; Mishra, H.; Adhikari, L.; Pandey, M.; Semalty, M. Cyclodextrin Based Nanoparticles For Improved Solubility and Drug Delivery. J. Mt. Res. 2021, 16, 187–199. [Google Scholar] [CrossRef]
- Mirankó, M.; Tóth, J.; Bartos, C.; Ambrus, R.; Feczkó, T. Nano-spray-dried levocetirizine dihydrochloride with mucoadhesive carriers and cyclodextrins for nasal administration. Pharmaceutics 2023, 15, 317. [Google Scholar] [CrossRef]
- Ooi, Y.J.; Wen, Y.; Zhu, J.; Song, X.; Li, J. Codelivery of Doxorubicin and p53 Gene by β-Cyclodextrin-Based Supramolecular Nanoparticles Formed via Host–Guest Complexation and Electrostatic Interaction. Biomacromolecules 2024, 25, 2980–2989. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.; Zhang, L.; Wang, Z.; Zhou, Q.; Huang, T.; Ge, C.; Xu, H.; Zhu, M.; Zhao, F.; et al. Cyclodextrin-mediated formation of porous RNA nanospheres and their application in synergistic targeted therapeutics of hepatocellular carcinoma. Biomaterials 2020, 261, 120304. [Google Scholar] [CrossRef]
- Khairnar, P.; Kolipaka, T.; Pandey, G.; Phatale, V.; Shah, S.; Srinivasarao, D.A.; Saraf, S.; Srivastava, S. Nanosponge-mediated oligonucleotide delivery: A cutting-edge technology towards cancer management. J. Drug Deliv. Sci. Technol. 2023, 91, 105226. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Nanosponges for drug delivery and cancer therapy: Recent advances. Nanomaterials 2022, 12, 2440. [Google Scholar] [CrossRef]
- Utzeri, G.; Matias, P.M.; Murtinho, D.; Valente, A.J. Cyclodextrin-based nanosponges: Overview and opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Q.J.; Li, W.; Zuberi, Z.; Feng, J.X.; Lin, Q.L.; Ren, J.L.; Luo, F.J.; Ding, Q.M.; Zeng, X.X.; et al. Toward improvements for carrying capacity of the cyclodextrin-based nanosponges: Recent progress from a material and drug delivery. J. Mater. Sci. 2021, 56, 5995–6015. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2012, 75, 315–322. [Google Scholar] [CrossRef]
- Duchene, D.; Bochot, A. Thirty years with cyclodextrins. Int. J. Pharm. 2016, 514, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery—Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-beta-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef]
- Kumar, S.; Dalal, P.; Rao, R. Cyclodextrin nanosponges: A promising approach for modulating drug delivery. Colloid Sci. Pharm. Nanotechnol. 2020, 79. [Google Scholar] [CrossRef]
- Dai, Y.; Li, Q.; Zhang, S.; Shi, S.; Li, Y.; Zhao, X.; Zhou, L.; Wang, X.; Zhu, Y.; Li, W. Smart GSH/pH dual-bioresponsive degradable nanosponges based on β-CD-appended hyper-cross-linked polymer for triggered intracellular anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2021, 64, 102650. [Google Scholar] [CrossRef]
- Aguilar-Perez, K.M.; Aviles-Castrillo, J.I.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M.N. Insight Into Nanoliposomes as Smart Nanocarriers for Greening the Twenty-First Century Biomedical Settings. Front. Bioeng. Biotechnol. 2020, 8, 579536. [Google Scholar] [CrossRef]
- Hashemi, M.; Ghadyani, F.; Hasani, S.; Olyaee, Y.; Raei, B.; Khodadadi, M.; Ziyarani, M.F.; Basti, F.A.; Tavakolpournegari, A.; Matinahmadi, A.; et al. Nanoliposomes for doxorubicin delivery: Reversing drug resistance, stimuli-responsive carriers and clinical translation. J. Drug Deliv. Sci. Technol. 2023, 80, 104112. [Google Scholar] [CrossRef]
- Yang, J.; Wen, C.; Pan, C.; Guo, H.; Zhao, W.; Zhang, J.; Zhu, Y.; Zhang, Y.; Zhang, L. Nanoliposomal multi-drug delivery system with reduced toxicity and multi-drug resistance. J. Mater. Sci. 2019, 54, 9718–9728. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Chen, H.; Cai, Y.; Sheng, T.; Wang, P.; Li, Z.; Yang, F.; Gu, N. Magnet-activatable nanoliposomes as intracellular bubble microreactors to enhance drug delivery efficacy and burst cancer cells. Nanoscale 2019, 11, 18854–18865. [Google Scholar] [CrossRef]
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Betamethasone-in-cyclodextrin-in-liposome: The effect of cyclodextrins on encapsulation efficiency and release kinetics. Int. J. Pharm. 2006, 312, 75–82. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Li, C.; Otkur, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Onodera, S.; Ikejima, T. Silibinin treatment protects human skin cells from UVB injury through upregulation of estrogen receptors. J. Photochem. Photobiol. B Biol. 2021, 216, 112147. [Google Scholar] [CrossRef] [PubMed]
- Takke, A.; Shende, P. Potential of cyclodextrin in hybrid liposomes for improving the solubility, bioavailability and stability of silibinin. Chem. Pap. 2022, 76, 6579–6589. [Google Scholar] [CrossRef]
- Aloisio, C.; Antimisiaris, S.G.; Longhi, M.R. Liposomes containing cyclodextrins or meglumine to solubilize and improve the bioavailability of poorly soluble drugs. J. Mol. Liq. 2017, 229, 106–113. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Azzi, J.; Jraij, A.; Auezova, L.; Fourmentin, S.; Greige-Gerges, H. Novel findings for quercetin encapsulation and preservation with cyclodextrins, liposomes, and drug-in-cyclodextrin-in-liposomes. Food Hydrocoll. 2018, 81, 328–340. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Vadivel, V. Development and characterization of catechin-in-cyclodextrin-in-phospholipid liposome to eradicate MRSA-mediated surgical site infection: Investigation of their anti-infective efficacy through in vitro and in vivo studies. Int. J. Pharm. 2021, 609, 121130. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–organic framework nanocarriers for drug delivery in biomedical applications. Nano-Micro Lett. 2020, 12, 1–29. [Google Scholar]
- Fatima, S.F.; Sabouni, R.; Garg, R.; Gomaa, H. Recent advances in Metal-Organic Frameworks as nanocarriers for triggered release of anticancer drugs: Brief history, biomedical applications, challenges and future perspective. Colloids Surf. B Biointerfaces 2023, 225, 113266. [Google Scholar] [CrossRef]
- Dummert, S.V.; Saini, H.; Hussain, M.Z.; Yadava, K.; Jayaramulu, K.; Casini, A.; Fischer, R.A. Cyclodextrin metal–organic frameworks and derivatives: Recent developments and applications. Chem. Soc. Rev. 2022, 51, 5175–5213. [Google Scholar] [CrossRef]
- Forgan, R.S.; Smaldone, R.A.; Gassensmith, J.J.; Furukawa, H.; Cordes, D.B.; Li, Q.; Wilmer, C.E.; Botros, Y.Y.; Snurr, R.Q.; Slawin, A.M.; et al. Nanoporous carbohydrate metal-organic frameworks. J. Am. Chem. Soc. 2012, 134, 406–417. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Sun, H.; Wu, D.; Wang, C.; Ren, X.; Shao, Q.; York, P.; Tong, J.; Zhu, J.; et al. Antioxidant Biodegradable Covalent Cyclodextrin Frameworks as Particulate Carriers for Inhalation Therapy against Acute Lung Injury. ACS Appl. Mater. Interfaces 2022, 14, 38421–38435. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; Wang, C.; Qin, W.; Hu, X.; Tong, J.; Yu, L.; Zhang, G.; Ren, X.; Li, Z.; et al. Paeonol loaded cyclodextrin metal-organic framework particles for treatment of acute lung injury via inhalation. Int. J. Pharm. 2020, 587, 119649. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-loaded composite nanogels for cancer treatment. J. Control. Release 2020, 328, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Rangaraj, N.; Laxmikeshav, K.; Sampathi, S. Nanogels as drug carriers–Introduction, chemical aspects, release mechanisms and potential applications. Int. J. Pharm. 2020, 581, 119268. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: Drug delivery and beyond. Carbohydr. Polym. 2022, 297, 120033. [Google Scholar] [CrossRef]
- Kubeil, M.; Suzuki, Y.; Casulli, M.A.; Kamal, R.; Hashimoto, T.; Bachmann, M.; Hayashita, T.; Stephan, H. Exploring the Potential of Nanogels: From Drug Carriers to Radiopharmaceutical Agents. Adv. Healthc. Mater. 2024, 13, 2301404. [Google Scholar] [CrossRef]
- Si, X.; Ma, S.; Xu, Y.; Zhang, D.; Shen, N.; Yu, H.; Zhang, Y.; Song, W.; Tang, Z.; Chen, X. Hypoxia-sensitive supramolecular nanogels for the cytosolic delivery of ribonuclease A as a breast cancer therapeutic. J. Control. Release 2020, 320, 83–95. [Google Scholar] [CrossRef]
- Duan, Q.Y.; Zhu, Y.X.; Jia, H.R.; Guo, Y.; Zhang, X.; Gu, R.; Li, C.; Wu, F.G. Platinum-Coordinated Dual-Responsive Nanogels for Universal Drug Delivery and Combination Cancer Therapy. Small 2022, 18, 2203260. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H.; Salehi, R. Dual anticancer drug delivery of D-galactose-functionalized stimuli-responsive nanogels for targeted therapy of the liver hepatocellular carcinoma. Eur. Polym. J. 2022, 167, 111061. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein nanoparticles for Pickering emulsions: A comprehensive review on their shapes, preparation methods, and modification methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef]
- Cai, L.; Cao, M.; Regenstein, J. Slow-release and nontoxic Pickering emulsion platform for antimicrobial peptide. J. Agric. Food Chem. 2020, 68, 7453–7466. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cheng, J.; Huang, Q. Food-grade Pickering emulsions stabilized by ovotransferrin fibrils. Food Hydrocoll. 2019, 94, 592–602. [Google Scholar] [CrossRef]
- Ming, L.; Wu, H.; Liu, A.; Naeem, A.; Dong, Z.; Fan, Q.; Zhang, G.; Liu, H.; Li, Z. Evolution and critical roles of particle properties in Pickering emulsion: A review. J. Mol. Liq. 2023, 388, 122775. [Google Scholar] [CrossRef]
- Wu, L.; Liao, Z.; Liu, M.; Yin, X.; Li, X.; Wang, M.; Lu, X.; Lv, N.; Singh, V.; He, Z.; et al. Fabrication of non-spherical Pickering emulsion droplets by cyclodextrins mediated molecular self-assembly. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 163–172. [Google Scholar] [CrossRef]
- Pandey, P.; Gulati, N.; Makhija, M.; Purohit, D.; Dureja, H. Nanoemulsion: A Novel Drug Delivery Approach for Enhancement of Bioavailability. Recent. Pat. Nanotechnol. 2020, 14, 276–293. [Google Scholar] [CrossRef]
- Yuan, C.; Cheng, C.; Cui, B. Pickering Emulsions Stabilized by Cyclodextrin Nanoparticles: A Review. Starch-Stärke 2021, 73, 2100077. [Google Scholar] [CrossRef]
- Leclercq, L.; Dechézelles, J.-F.; Rauwel, G.; Nardello-Rataj, V. In vitro study of versatile drug formulations based on α-cyclodextrin and polyethylene glycol using colloidal tectonics. J. Drug Deliv. Sci. Technol. 2020, 59, 101913. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Im, D.; Ud Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. New potential application of hydroxypropyl-beta-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr. Polym. 2021, 271, 118433. [Google Scholar] [CrossRef] [PubMed]
- Velhal, K.; Barage, S.; Roy, A.; Lakkakula, J.; Yamgar, R.; Alqahtani, M.S.; Yadav, K.K.; Ahn, Y.; Jeon, B.-H. A promising review on cyclodextrin conjugated paclitaxel nanoparticles for cancer treatment. Polymers 2022, 14, 3162. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Kara, G.; Calin, G.A.; Ozpolat, B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv. Drug Deliv. Rev. 2022, 182, 114113. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.J.; Nguyen, D.T.; Kim, D.; Yoo, S.Y.; Lee, S.M.; Lee, J.Y.; Kim, D.D. Tailoring renal-clearable zwitterionic cyclodextrin for colorectal cancer-selective drug delivery. Nat. Nanotechnol. 2023, 18, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Varan, C.; Ozturk, S.C.; Benito, J.M.; Esendagli, G.; Bilensoy, E. Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo. Nanomaterials 2021, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, J.; Zou, L.; Tang, J.; Zheng, J.; Luo, M.; Wang, G.; Liang, D.; Li, Y.; Chen, B.; et al. Preparation, Characterization, and Staphylococcus aureus Biofilm Elimination Effect of Baicalein-Loaded beta-Cyclodextrin-Grafted Chitosan Nanoparticles. Int. J. Nanomed. 2022, 17, 5287–5302. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; d’Avanzo, N.; Mancuso, A.; De Gaetano, A.; Paladini, G.; Caridi, F.; Venuti, V.; Paolino, D.; Ventura, C.A. Chitosan/Cyclodextrin Nanospheres for Potential Nose-to-Brain Targeting of Idebenone. Pharmaceuticals 2022, 15, 1206. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef]
- He, Y.; Xiong, T.; He, S.; Sun, H.; Huang, C.; Ren, X.; Wu, L.; Patterson, L.H.; Zhang, J. Pulmonary targeting crosslinked cyclodextrin metal–organic frameworks for lung cancer therapy. Adv. Funct. Mater. 2021, 31, 2004550. [Google Scholar] [CrossRef]
- Matshetshe, K.I.; Parani, S.; Manki, S.M.; Oluwafemi, O.S. Preparation, characterization and in vitro release study of beta-cyclodextrin/chitosan nanoparticles loaded Cinnamomum zeylanicum essential oil. Int. J. Biol. Macromol. 2018, 118, 676–682. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-L.; Zhang, J.; Jin, W.; Chen, X.-Y.; Yang, J.-M.; Chi, S.-M.; Ruan, Q.; Zhao, Y. Oral administration of pH-responsive polyamine modified cyclodextrin nanoparticles for controlled release of anti-tumor drugs. React. Funct. Polym. 2022, 172, 105175. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Q.; Mei, L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy. Chin. Chem. Lett. 2020, 31, 1345–1356. [Google Scholar] [CrossRef]
- Mrowczynski, R.; Jedrzak, A.; Szutkowski, K.; Grzeskowiak, B.F.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Enoch, I.V.M.V.; Rex Jeya Rajkumar, S. Polymeric cyclodextrin-dextran spooled nickel ferrite nanoparticles: Expanded anticancer efficacy of loaded camptothecin. Mater. Lett. 2020, 261, 127114. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, X.; Wu, C.; Wang, X.; Wang, L.J.; Loh, X.J.; Li, Z.; Wu, Y.L. Cyclodextrin-Based Star-Like Amphiphilic Cationic Polymer as a Potential Pharmaceutical Carrier in Macrophages. Macromol. Rapid Commun. 2019, 40, e1800207. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Pharmacokinetic study of solid-lipid-nanoparticles of altretamine complexed epichlorohydrin-beta-cyclodextrin for enhanced solubility and oral bioavailability. Int. J. Biol. Macromol. 2017, 101, 24–31. [Google Scholar] [CrossRef]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kanabar, D.D.; Muth, A.; Gupta, V. Cyclodextrin Complexation for Enhanced Stability and Non-invasive Pulmonary Delivery of Resveratrol-Applications in Non-small Cell Lung Cancer Treatment. AAPS PharmSciTech 2020, 21, 183. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Chaudhari, J.; Trotta, F.; Caldera, F. Investigation of Cyclodextrin-Based Nanosponges for Solubility and Bioavailability Enhancement of Rilpivirine. AAPS PharmSciTech 2018, 19, 2358–2369. [Google Scholar] [CrossRef]
- Vij, M.; Dand, N.; Kumar, L.; Wadhwa, P.; Wani, S.U.D.; Mahdi, W.A.; Alshehri, S.; Alam, P.; Shakeel, F. Optimisation of a Greener-Approach for the Synthesis of Cyclodextrin-Based Nanosponges for the Solubility Enhancement of Domperidone, a BCS Class II Drug. Pharmaceuticals 2023, 16, 567. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Sharma, K.; Kadian, V.; Kumar, A.; Mahant, S.; Rao, R. Evaluation of solubility, photostability and antioxidant activity of ellagic acid cyclodextrin nanosponges fabricated by melt method and microwave-assisted synthesis. J. Food Sci. Technol. 2022, 59, 898–908. [Google Scholar] [CrossRef]
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal-Organic Frameworks and Their Applications. Acc. Chem. Res. 2021, 54, 1440–1453. [Google Scholar] [CrossRef]
- Ding, H.; Wu, L.; Guo, T.; Zhang, Z.; Garba, B.M.; Gao, G.; He, S.; Zhang, W.; Chen, Y.; Lin, Y.; et al. CD-MOFs Crystal Transformation from Dense to Highly Porous Form for Efficient Drug Loading. Cryst. Growth Des. 2019, 19, 3888–3894. [Google Scholar] [CrossRef]
- Lin, E.Y.; Chen, Y.S.; Li, Y.S.; Chen, S.R.; Lee, C.H.; Huang, M.H.; Chuang, H.M.; Harn, H.J.; Yang, H.H.; Lin, S.Z.; et al. Liposome Consolidated with Cyclodextrin Provides Prolonged Drug Retention Resulting in Increased Drug Bioavailability in Brain. Int. J. Mol. Sci. 2020, 21, 4408. [Google Scholar] [CrossRef]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Drug-in-cyclodextrin-in-liposomes as a carrier system for volatile essential oil components: Application to anethole. Food Chem. 2017, 218, 365–371. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of high-drug-loading nanoparticles. ChemPlusChem 2020, 85, 2143–2157. [Google Scholar] [CrossRef]
- De Gaetano, F.; Marino, A.; Marchetta, A.; Bongiorno, C.; Zagami, R.; Cristiano, M.C.; Paolino, D.; Pistara, V.; Ventura, C.A. Development of Chitosan/Cyclodextrin Nanospheres for Levofloxacin Ocular Delivery. Pharmaceutics 2021, 13, 1293. [Google Scholar] [CrossRef] [PubMed]
- Varan, G.; Akkin, S.; Demirturk, N.; Benito, J.M.; Bilensoy, E. Erlotinib entrapped in cholesterol-depleting cyclodextrin nanoparticles shows improved antitumoral efficacy in 3D spheroid tumors of the lung and the liver. J. Drug Target. 2021, 29, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.L.; Gu, W.; Lu, S.S.; Chen, Z.P.; Cai, B.C.; Yang, X.X. Drug-in-cyclodextrin-in-liposomes: A promising delivery system for hydrophobic drugs. Expert Opin. Drug Deliv. 2014, 11, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bao, X.; Fang, A.; Li, H.; Zhou, Y.; Liu, Y.; Jiang, C.; Wu, J.; Song, X. Nanoliposome-Encapsulated Brinzolamide-hydropropyl-beta-cyclodextrin Inclusion Complex: A Potential Therapeutic Ocular Drug-Delivery System. Front. Pharmacol. 2018, 9, 91. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr. Polym. 2019, 224, 115168. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Meirelles, G.C.; Barp, C.G.; Assreuy, J.; Silva, M.A.S.; Ponchel, G. Cyclodextrin based nanosponge of norfloxacin: Intestinal permeation enhancement and improved antibacterial activity. Carbohydr. Polym. 2018, 195, 586–592. [Google Scholar] [CrossRef]
- Yakupova, L.R.; Skuredina, A.A.; Markov, P.O.; Le-Deygen, I.M.; Kudryashova, E.V. Cyclodextrin Polymers as a Promising Drug Carriers for Stabilization of Meropenem Solutions. Appl. Sci. 2023, 13, 3608. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Usman Minhas, M. Hydroxypropyl-β-cyclodextrin hybrid nanogels as nano-drug delivery carriers to enhance the solubility of dexibuprofen: Characterization, in vitro release, and acute oral toxicity studies. Adv. Polym. Technol. 2018, 37, 2171–2185. [Google Scholar] [CrossRef]
- Rizvi, S.S.B.; Akhtar, N.; Minhas, M.U.; Mahmood, A.; Khan, K.U. Synthesis and Characterization of Carboxymethyl Chitosan Nanosponges with Cyclodextrin Blends for Drug Solubility Improvement. Gels 2022, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Duggi Vamshidhar Reddy, A.S.R. Development And Evaluation Of Nanosponges Based Controlled Release Tapentadol Tablets By Box-Behnken Design. Nveo-Nat. Volatiles Essent. Oils J. 2021, 8, 5000–5016. [Google Scholar]
- He, Y.; Hou, X.; Guo, J.; He, Z.; Guo, T.; Liu, Y.; Zhang, Y.; Zhang, J.; Feng, N. Activation of a gamma-cyclodextrin-based metal-organic framework using supercritical carbon dioxide for high-efficient delivery of honokiol. Carbohydr. Polym. 2020, 235, 115935. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Alsotari, S.; Buqaien, R.; Ismail, S.; Awidi, A.; Al Bawab, A. Remote loading of curcumin-in-modified beta-cyclodextrins into liposomes using a transmembrane pH gradient. RSC Adv. 2019, 9, 37148–37161. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Sang, S.; Julian McClements, D.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. Preparation, characterization and in vitro digestive behaviors of emulsions synergistically stabilized by gamma-cyclodextrin/sodium caseinate/alginate. Food Res. Int. 2022, 160, 111634. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Zhou, Y.; Xu, Y.; Chen, Y.; Yudintceva, N.; Shevtsov, M.; Gao, H. Supramolecular nanomedicines based on host–guest interactions of cyclodextrins. Exploration 2023, 3, 20210111. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, L.; Li, S.; Li, S.; Wu, Y.; Li, Z.; Qiu, H. Green materials with promising applications: Cyclodextrin-based deep eutectic supramolecular polymers. Green Chem. 2023, 25, 4180–4195. [Google Scholar] [CrossRef]
| Property | α-CD | β-CD | γ-CD |
|---|---|---|---|
| Molecular formula | C36H60O30 | C42H70O35 | C48H80O40 |
| Number of glucose units | 6 | 7 | 8 |
| Molar mass (g/mol) | 972.85 | 1134.99 | 1297.13 |
| Solubility in water at room temperature (mg/mL) | 129.5 ± 0.7 | 18.4 ± 0.2 | 249.2 ± 0.2 |
| Moisture content (%w/w) | 10.2 | 13.0–15.0 | 8–18 |
| Nano-Delivery System | CD Type | Size/nm | Active Ingredients | Effect | References |
|---|---|---|---|---|---|
| Nanoparticles | HPCD-HMD | 120–200 | Meropenem | Improved the solubility of drugs in aqueous solutions. | [127] |
| Mannose-modified γ-CD | 100–300 | Regorafenib (RG) | Improved the biodistribution and pharmacokinetic and pharmaceutical properties of RG. | [2] | |
| 6OcaproβCD and PC βCDC6 | 113 ± 4 and 82 ± 2 | Paclitaxel | Improved the antitumor effect. | [97] | |
| Nanogels | HP-β-CD | 310.65 ± 18.75 | Dexibuprofen | This nanogel, which has porous and amorphous shapes, can significantly enhance drug release, and the formulation demonstrated good biocompatibility. | [128] |
| β-CD-conjugated hyaluronic acid (HA-βCD) | 36.0 ± 4.5 | Small molecules and proteins | The HPC nanogels were a robust and universal drug delivery nanoplatform. | [81] | |
| β-CD | 657 | Methotrexate (MTX) and doxorubicin (DOX) | These nanogels were double-responsive (pH and temperature) and photoluminescent. | [82] | |
| Nanospheres | Am-CD/RNA | 390 | SiRNA/sorafenib | The nanogel achieved synergistic therapy for hepatocellular carcinoma. | [44] |
| HP-β-CD | 140 | Idebenone (IDE) | There was a higher permeation/interaction of IDE-loaded CS NPs with respect to free IDE. | [99] | |
| α- and β-CD | 88–270 | Erlotinib (ERL) | The nanospheres could increase ERL’s anticancer efficacy with conventional and 3D tumor models made in lung and hepatocellular carcinoma cells. | [122] | |
| Nano-sponge | β-CD-CMC-g-poly | 195–250 | Docetaxel | The water solubility of docetaxel significantly improved (by up to 14 times). | [129] |
| β-CD | 51.38–154.56 | Tapentadol | The drug release rate in 6 h was 51.62–82.34%, which significantly improved the controlled-release ability. | [130] | |
| MOFs | γ-CD | The mean pore size of CD-MOFs is 1.4 nm. | Paeonol (PAE) | The permeability of PAE-CD-MOF was 5 times higher than that of free PAE. | [74] |
| γ-CD | 200–500 | Honokiol (HNK) | The MOF improved the solubility and dissolution rate of HNK. | [131] | |
| Liposome | E-βCD/D-βCD/βCD | 146–163 | Curcumin | The encapsulation efficiency of liposome was more than 5 times higher than that of normal liposome. | [132] |
| HP-β-CD | 82.29 ± 6.20 | Brinzolamide (BRZ) | The liposome had an entrapment efficiency (EE) of 92.50 ± 2.10%. | [124] | |
| Emulsion | γ-CD/sodium caseinate/alginate (Alg) | 138 ± 6 and 206 ± 12 | Curcumin | The liposomes were stable under the conditions of high acidity (pH 3.0), high alkalinity (pH 11.0), and high temperature (90 °C). | [133] |
| α-CDs were modified with octenylsuccinic anhydride (OSA) | 10–100 μm | Curcumin | It possessed good storage stability after 30 days of storage. In addition, emulsion with a smaller particle size had a higher free fatty acid release and increased bioavailability by 10.3%. | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.-Y.; Zou, Y.-X.; Lei, H.-F.; Bi, Y.; Yang, R.; Tang, J.-H.; Jin, Q.-R. Advances in Cyclodextrins and Their Derivatives in Nano-Delivery Systems. Pharmaceutics 2024, 16, 1054. https://doi.org/10.3390/pharmaceutics16081054
Ji X-Y, Zou Y-X, Lei H-F, Bi Y, Yang R, Tang J-H, Jin Q-R. Advances in Cyclodextrins and Their Derivatives in Nano-Delivery Systems. Pharmaceutics. 2024; 16(8):1054. https://doi.org/10.3390/pharmaceutics16081054
Chicago/Turabian StyleJi, Xin-Yu, Yi-Xuan Zou, Han-Fang Lei, Yong Bi, Rui Yang, Ji-Hui Tang, and Qing-Ri Jin. 2024. "Advances in Cyclodextrins and Their Derivatives in Nano-Delivery Systems" Pharmaceutics 16, no. 8: 1054. https://doi.org/10.3390/pharmaceutics16081054







