Revitalizing Bacillus Calmette–Guérin Immunotherapy for Bladder Cancer: Nanotechnology and Bioengineering Approaches
Abstract
:1. Introduction
2. The History and Current State of BCG Immunotherapy
3. The Immune Mechanisms of BCG Bladder Cancer Treatment
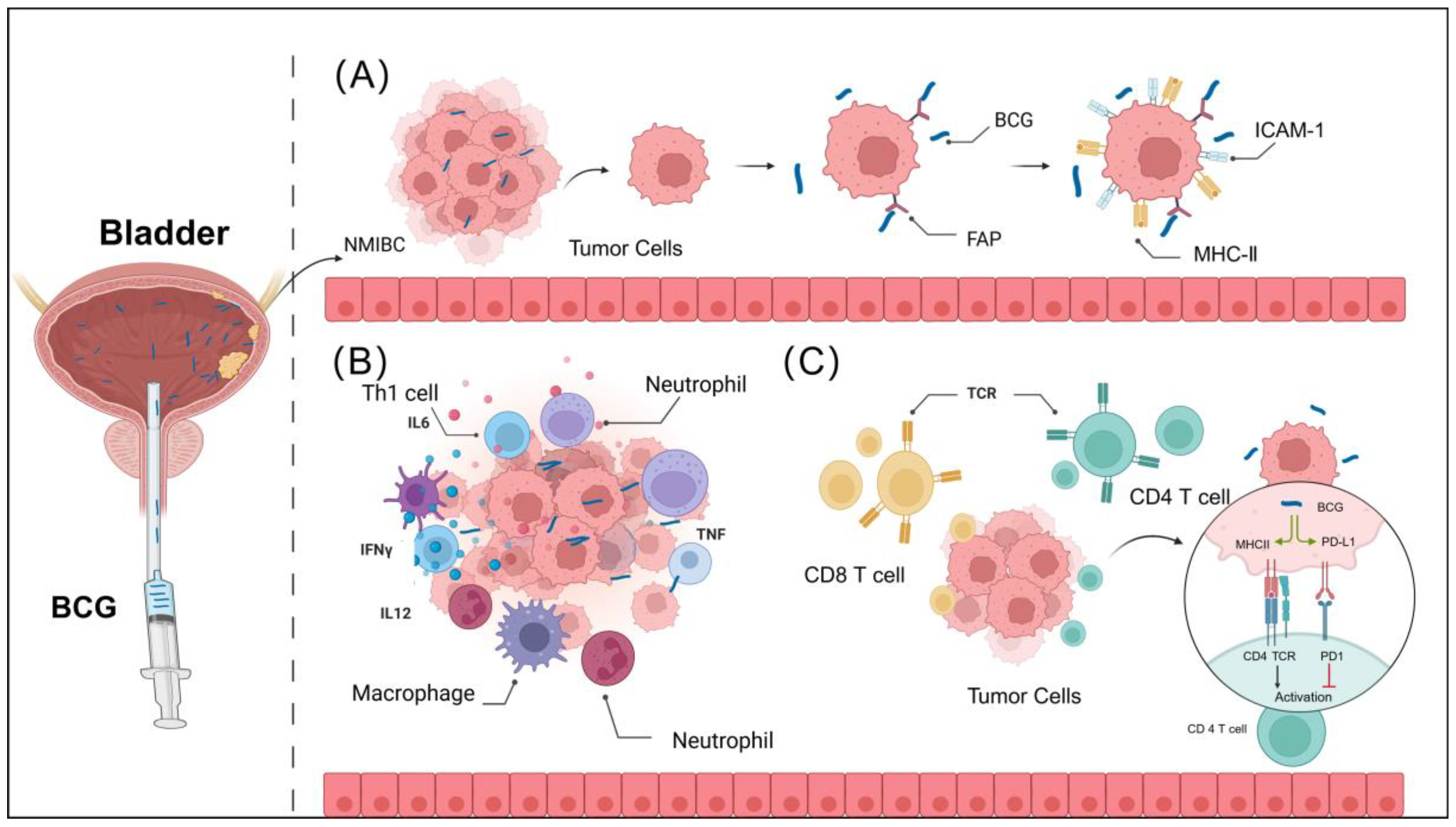
3.1. BCG Attachment and Internalization in Bladder Urothelium
3.2. BCG Enhances Innate Immune Activation
3.3. BCG Enhances Adaptive Immune Activation
4. Where Is BCG’S Future?
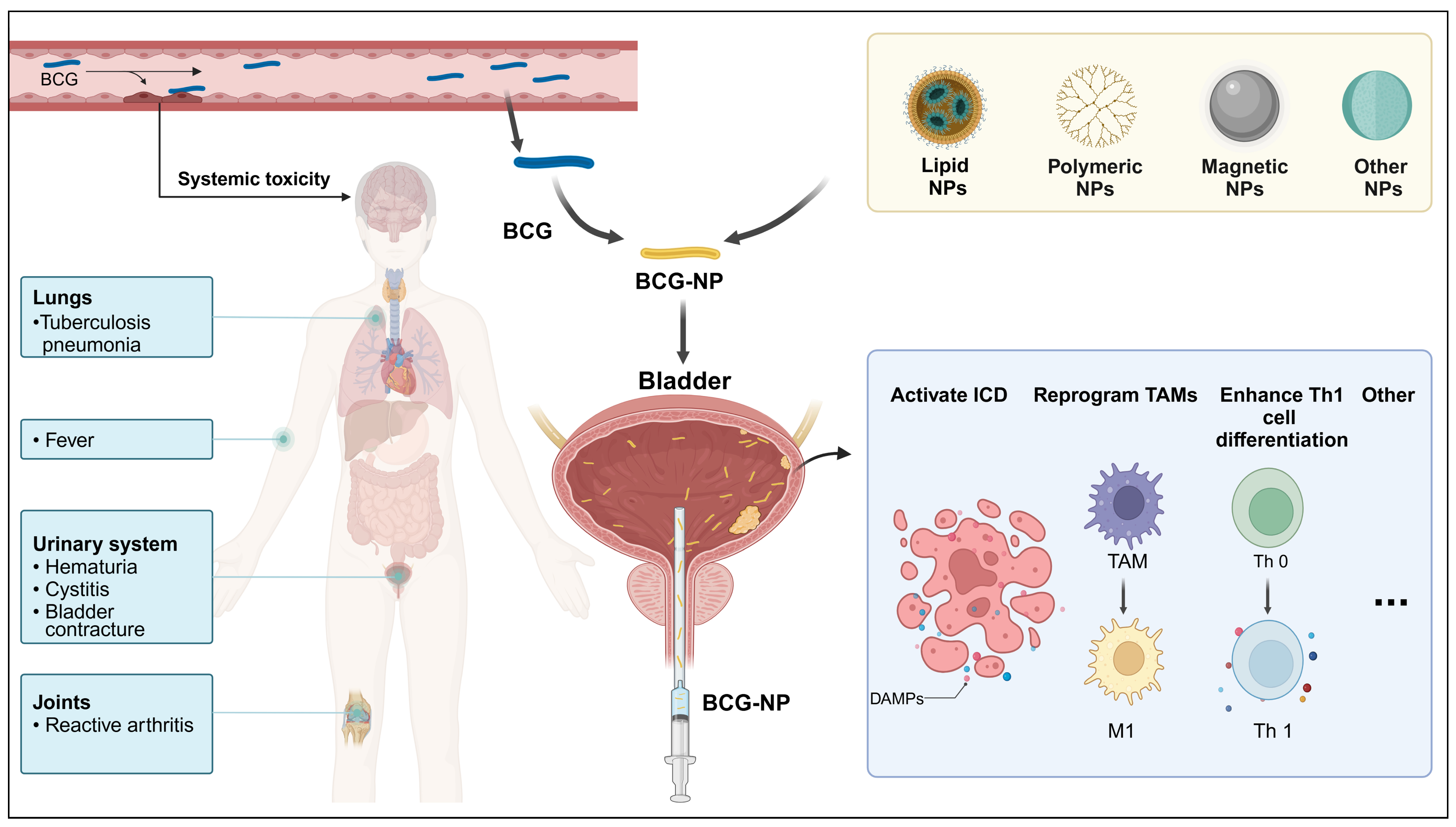
4.1. Challenges of BCG Use in Bladder Cancer Treatment
4.2. Nanotechnology and Engineered BCG
4.3. Application of BCG in Novel Immune Delivery Systems
4.3.1. BCG Bacterial Cell Walls: Advancing Drug Delivery
4.3.2. Lipid Nanoparticles for Delivering Bacterial Cell Walls
4.3.3. Polymeric Nanoparticles
4.3.4. Hybrid Formulations
5. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA. Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder Cancer. Nat. Rev. Dis. Primers 2023, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Seisen, T.; Peyronnet, B.; Dominguez-Escrig, J.L.; Bruins, H.M.; Yuan, C.Y.; Babjuk, M.; Böhle, A.; Burger, M.; Compérat, E.M.; Cowan, N.C.; et al. Oncologic Outcomes of Kidney-Sparing Surgery versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-Muscle Invasive Bladder Cancer Guidelines Panel. Eur. Urol. 2016, 70, 1052–1068. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current Best Practice for Bladder Cancer: A Narrative Review of Diagnostics and Treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M. Bladder Cancer: A Review. JAMA 2020, 324, 1980. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (Ta, T1, and Carcinoma In Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Holzbeierlein, J.M.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Holmes, R.; James, A.C.; Kirkby, E.; McKiernan, J.M.; Schuckman, A.K. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. J. Urol. 2024, 211, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the Treatment of Superficial Bladder Tumors. J. Urol. 1976, 116, 180–183. [Google Scholar] [CrossRef]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG Immunotherapy and Its Outlook for Bladder Cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef]
- Lamm, D.L.; Blumenstein, B.A.; Crissman, J.D.; Montie, J.E.; Gottesman, J.E.; Lowe, B.A.; Sarosdy, M.F.; Bohl, R.D.; Grossman, H.B.; Beck, T.M.; et al. Maintenance Bacillus Calmette-Guerin Immunotherapy for Recurrent TA, T1 and Carcinoma In Situ Transitional Cell Carcinoma of the Bladder: A Randomized Southwest Oncology Group Study. J. Urol. 2000, 163, 1124–1129. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.M.; Witjes, J.A.; Kurth, K. Bacillus Calmette-Guerin versus Chemotherapy for the Intravesical Treatment of Patients with Carcinoma In Situ of the Bladder: A Meta-Analysis of the Published Results of Randomized Clinical Trials. J. Urol. 2005, 174, 86–91; discussion 91–92. [Google Scholar] [CrossRef] [PubMed]
- Ritz, N.; Hanekom, W.A.; Robins-Browne, R.; Britton, W.J.; Curtis, N. Influence of BCG Vaccine Strain on the Immune Response and Protection against Tuberculosis. FEMS Microbiol. Rev. 2008, 32, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Hoption Cann, S.A.; van Netten, J.P.; van Netten, C. Dr William Coley and Tumour Regression: A Place in History or in the Future. Postgrad. Med. J. 2003, 79, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Hawgood, B.J. Doctor Albert Calmette 1863-1933: Founder of Antivenomous Serotherapy and of Antituberculous BCG Vaccination. Toxicon 1999, 37, 1241–1258. [Google Scholar] [CrossRef] [PubMed]
- Pearl, R. On the Pathological Relations between Cancer and Tuberculosis. Proc. Soc. Exp. Biol. Med. 1928, 26, 73–75. [Google Scholar] [CrossRef]
- Lamm, D.L.; Thor, D.E.; Harris, S.C.; Reyna, J.A.; Stogdill, V.D.; Radwin, H.M. Bacillus Calmette-Guerin Immunotherapy of Superficial Bladder Cancer. J. Urol. 1980, 124, 38–40. [Google Scholar] [CrossRef]
- Lamm, D.L.; Blumenstein, B.A.; Crawford, E.D.; Montie, J.E.; Scardino, P.; Grossman, H.B.; Stanisic, T.H.; Smith, J.A.; Sullivan, J.; Sarosdy, M.F. A Randomized Trial of Intravesical Doxorubicin and Immunotherapy with Bacille Calmette-Guérin for Transitional-Cell Carcinoma of the Bladder. N. Engl. J. Med. 1991, 325, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Wu, P.-C.; Chancellor, M.; Yoshimura, N.; Huang, L. Recent Advances in Intravesical Drug/Gene Delivery. Mol. Pharm. 2006, 3, 369–379. [Google Scholar] [CrossRef] [PubMed]
- GuhaSarkar, S.; Banerjee, R. Intravesical Drug Delivery: Challenges, Current Status, Opportunities and Novel Strategies. J. Control. Release 2010, 148, 147–159. [Google Scholar] [CrossRef]
- Wiker, H.G.; Harboe, M. The Antigen 85 Complex: A Major Secretion Product of Mycobacterium Tuberculosis. Microbiol. Rev. 1992, 56, 648–661. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Kavoussi, L.R.; Catalona, W.J. Role of Fibronectin in Intravesical BCG Therapy for Superficial Bladder Cancer. J. Urol. 1988, 139, 410–414. [Google Scholar] [CrossRef]
- Zhao, W.; Schorey, J.S.; Bong-Mastek, M.; Ritchey, J.; Brown, E.J.; Ratliff, T.L. Role of a Bacillus Calmette-Guérin Fibronectin Attachment Protein in BCG-Induced Antitumor Activity. Int. J. Cancer 2000, 86, 83–88. [Google Scholar] [CrossRef]
- Durek, C.; Richter, E.; Basteck, A.; Rüsch-Gerdes, S.; Gerdes, J.; Jocham, D.; Böhle, A. The Fate of Bacillus Calmette-Guerin after Intravesical Instillation. J. Urol. 2001, 165, 1765–1768. [Google Scholar] [CrossRef]
- Bevers, R.F.; Kurth, K.H.; Schamhart, D.H. Role of urothelial cells in BCG immunotherapy for superficial bladder cancer. Br. J. Cancer 2004, 91, 607–612. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Berglund, R.K.; Maschino, A.C.; Savage, C.J.; Herr, H.W. Fibrin Clot Inhibitor Medication and Efficacy of Bacillus Calmette-Guerin for Bladder Urothelial Cancer. J. Urol. 2009, 182, 1306–1312. [Google Scholar] [CrossRef]
- Witjes, J.A.; vd Meijden, A.P.; Doesburg, W.; Debruyne, F.M. Influence of Fibrin Clot Inhibitors on the Efficacy of Intravesical Bacillus Calmette-Guérin in the Treatment of Superficial Bladder Cancer. The Dutch Southeast Cooperative Urological Group. Eur. Urol. 1993, 23, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, M.J.; Badalato, G.M.; Motamedinia, P.; Hruby, G.W.; McKiernan, J.M. The Effect of Fibrin Clot Inhibitors on the Immunomodulatory Efficacy of Bacillus Calmette-Guérin Therapy for Non-Muscle-Invasive Bladder Cancer. Urology 2013, 81, 1273–1278. [Google Scholar] [CrossRef]
- Prescott, S.; James, K.; Hargreave, T.B.; Chisholm, G.D.; Smyth, J.F. Intravesical Evans Strain BCG Therapy: Quantitative Immunohistochemical Analysis of the Immune Response within the Bladder Wall. J. Urol. 1992, 147, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Biot, C.; Rentsch, C.A.; Gsponer, J.R.; Birkhäuser, F.D.; Jusforgues-Saklani, H.; Lemaître, F.; Auriau, C.; Bachmann, A.; Bousso, P.; Demangel, C.; et al. Preexisting BCG-Specific T Cells Improve Intravesical Immunotherapy for Bladder Cancer. Sci. Transl. Med. 2012, 4, 137ra72. [Google Scholar] [CrossRef]
- Durek, C.; Brandau, S.; Ulmer, A.J.; Flad, H.D.; Jocham, D.; Böhle, A. Bacillus-Calmette-Guérin (BCG) and 3D tumors: An in vitro model for the study of adhesion and invasion. J. Urol. 1999, 162, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Bevers, R.F.; de Boer, E.C.; Kurth, K.H.; Schamhart, D.H. BCG-induced interleukin-6 upregulation and BCG internalization in well and poorly differentiated human bladder cancer cell lines. Eur. Cytokine Netw. 1998, 9, 181–186. [Google Scholar] [PubMed]
- Kuroda, K.; Brown, E.J.; Telle, W.B.; Russell, D.G.; Ratliff, T.L. Characterization of the Internalization of Bacillus Calmette-Guerin by Human Bladder Tumor Cells. J. Clin. Investig. 1993, 91, 69–76. [Google Scholar] [CrossRef]
- Cosma, C.L.; Sherman, D.R.; Ramakrishnan, L. The Secret Lives of the Pathogenic Mycobacteria. Annu. Rev. Microbiol. 2003, 57, 641–676. [Google Scholar] [CrossRef]
- Mitropoulos, D.N. Novel Insights into the Mechanism of Action of Intravesical Immunomodulators. Vivo 2005, 19, 611–621. [Google Scholar]
- Flynn, J.L.; Chan, J.; Lin, P.L. Macrophages and Control of Granulomatous Inflammation in Tuberculosis. Mucosal Immunol. 2011, 4, 271–278. [Google Scholar] [CrossRef]
- Darieva, Z.; Lasunskaia, E.B.; Campos, M.N.N.; Kipnis, T.L.; Da Silva, W.D. Activation of Phosphatidylinositol 3-Kinase and c-Jun-N-Terminal Kinase Cascades Enhances NF-kappaB-Dependent Gene Transcription in BCG-Stimulated Macrophages through Promotion of P65/P300 Binding. J. Leukoc. Biol. 2004, 75, 689–697. [Google Scholar] [CrossRef]
- Bisiaux, A.; Boussier, J.; Duffy, D.; Quintana-Murci, L.; Fontes, M.; Albert, M.L. Deconvolution of the Response to Bacillus Calmette–Guérin Reveals NF-κB-Induced Cytokines as Autocrine Mediators of Innate Immunity. Front. Immunol. 2017, 8, 796. [Google Scholar] [CrossRef] [PubMed]
- Bisiaux, A.; Thiounn, N.; Timsit, M.-O.; Eladaoui, A.; Chang, H.-H.; Mapes, J.; Mogenet, A.; Bresson, J.-L.; Prié, D.; Béchet, S.; et al. Molecular Analyte Profiling of the Early Events and Tissue Conditioning Following Intravesical Bacillus Calmette-Guerin Therapy in Patients with Superficial Bladder Cancer. J. Urol. 2009, 181, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, X.; O’Donnell, M.A. Role of Th1 and TH2 Cytokines in BCG-Induced IFN-Gamma Production: Cytokine Promotion and Simulation of BCG Effect. Cytokine 2003, 21, 17–26. [Google Scholar] [CrossRef]
- De Boer, E.C.; De Jong, W.H.; Steerenberg, P.A.; Aarden, L.A.; Tetteroo, E.; De Groot, E.R.; Van der Meijden, A.P.; Vegt, P.D.; Debruyne, F.M.; Ruitenberg, E.J. Induction of Urinary Interleukin-1 (IL-1), IL-2, IL-6, and Tumour Necrosis Factor during Intravesical Immunotherapy with Bacillus Calmette-Guérin in Superficial Bladder Cancer. Cancer Immunol. Immunother. CII 1992, 34, 306–312. [Google Scholar] [CrossRef]
- Stefanini, G.F.; Bercovich, E.; Mazzeo, V.; Grigioni, W.F.; Emili, E.; D’Errico, A.; Lo Cigno, M.; Tamagnini, N.; Mazzetti, M. Class I and Class II HLA Antigen Expression by Transitional Cell Carcinoma of the Bladder: Correlation with T-Cell Infiltration and BCG Treatment. J. Urol. 1989, 141, 1449–1453. [Google Scholar] [CrossRef]
- Prescott, S.; James, K.; Busuttil, A.; Hargreave, T.B.; Chisholm, G.D.; Smyth, J.F. HLA-DR Expression by High Grade Superficial Bladder Cancer Treated with BCG. Br. J. Urol. 1989, 63, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Lattime, E.C.; Gomella, L.G.; McCue, P.A. Murine Bladder Carcinoma Cells Present Antigen to BCG-Specific CD4+ T-Cells. Cancer Res. 1992, 52, 4286–4290. [Google Scholar]
- Suttmann, H.; Riemensberger, J.; Bentien, G.; Schmaltz, D.; Stöckle, M.; Jocham, D.; Böhle, A.; Brandau, S. Neutrophil Granulocytes Are Required for Effective Bacillus Calmette-Guérin Immunotherapy of Bladder Cancer and Orchestrate Local Immune Responses. Cancer Res. 2006, 66, 8250–8257. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Knudson, M.J. Mycobacterium Bovis Bacillus Calmette-Guérin-Induced Macrophage Cytotoxicity against Bladder Cancer Cells. Clin. Dev. Immunol. 2010, 2010, 357591. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, T.L.; Ritchey, J.K.; Yuan, J.J.; Andriole, G.L.; Catalona, W.J. T-Cell Subsets Required for Intravesical BCG Immunotherapy for Bladder Cancer. J. Urol. 1993, 150, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- de Boer, E.C.; de Jong, W.H.; van der Meijden, A.P.; Steerenberg, P.A.; Witjes, F.; Vegt, P.D.; Debruyne, F.M.; Ruitenberg, E.J. Leukocytes in the Urine after Intravesical BCG Treatment for Superficial Bladder Cancer. A Flow Cytofluorometric Analysis. Urol. Res. 1991, 19, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Riemensberger, J.; Jacobsen, M.; Kemp, D.; Zhao, W.; Zhao, X.; Jocham, D.; Ratliff, T.L.; Böhle, A. NK Cells Are Essential for Effective BCG Immunotherapy. Int. J. Cancer 2001, 92, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Flad, H.D.; Böhle, A.; Chen, Y.Q.; Ulmer, A.J. Cellular Cytotoxicity of Human Natural Killer Cells and Lymphokine-Activated Killer Cells against Bladder Carcinoma Cell Lines. Immunol. Lett. 1991, 27, 191–197. [Google Scholar] [CrossRef]
- Ludwig, A.T.; Moore, J.M.; Luo, Y.; Chen, X.; Saltsgaver, N.A.; O’Donnell, M.A.; Griffith, T.S. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand: A Novel Mechanism for Bacillus Calmette-Guérin-Induced Antitumor Activity. Cancer Res. 2004, 64, 3386–3390. [Google Scholar] [CrossRef]
- Simons, M.P.; O’Donnell, M.A.; Griffith, T.S. Role of Neutrophils in BCG Immunotherapy for Bladder Cancer. Urol. Oncol. 2008, 26, 341–345. [Google Scholar] [CrossRef]
- Leitch, A.E.; Lucas, C.D.; Rossi, A.G. Editorial: Neutrophil Apoptosis: Hot on the TRAIL of Inflammatory Resolution. J. Leukoc. Biol. 2011, 90, 841–843. [Google Scholar] [CrossRef]
- Pryor, K.; Goddard, J.; Goldstein, D.; Stricker, P.; Russell, P.; Golovsky, D.; Penny, R. Bacillus Calmette-Guerin (BCG) Enhances Monocyte- and Lymphocyte-Mediated Bladder Tumour Cell Killing. Br. J. Cancer 1995, 71, 801–807. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Shapiro, A.; Catalona, W.J. Inhibition of Murine Bladder Tumor Growth by Bacille Calmette-Guerin: Lack of a Role of Natural Killer Cells. Clin. Immunol. Immunopathol. 1986, 41, 108–115. [Google Scholar] [CrossRef]
- Suttmann, H.; Jacobsen, M.; Reiss, K.; Jocham, D.; Böhle, A.; Brandau, S. Mechanisms of Bacillus Calmette-Guerin Mediated Natural Killer Cell Activation. J. Urol. 2004, 172, 1490–1495. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Kalinski, P. Breaking Barriers: Modulation of Tumor Microenvironment to Enhance Bacillus Calmette–Guérin Immunotherapy of Bladder Cancer. Cells 2024, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.M.; Basse, P.H.; Jiang, W.; Guru, K.; Chatta, G.; Kalinski, P. NFκB-Activated COX2/PGE2/EP4 Axis Controls the Magnitude and Selectivity of BCG-Induced Inflammation in Human Bladder Cancer Tissues. Cancers 2021, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Zuiverloon, T.C.M.; Nieuweboer, A.J.M.; Vékony, H.; Kirkels, W.J.; Bangma, C.H.; Zwarthoff, E.C. Markers Predicting Response to Bacillus Calmette-Guérin Immunotherapy in High-Risk Bladder Cancer Patients: A Systematic Review. Eur. Urol. 2012, 61, 128–145. [Google Scholar] [CrossRef]
- Riemensberger, J.; Böhle, A.; Brandau, S. IFN-Gamma and IL-12 but Not IL-10 Are Required for Local Tumour Surveillance in a Syngeneic Model of Orthotopic Bladder Cancer. Clin. Exp. Immunol. 2002, 127, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Saint, F.; Patard, J.J.; Maille, P.; Soyeux, P.; Hoznek, A.; Salomon, L.; Abbou, C.C.; Chopin, D.K. Prognostic Value of a T Helper 1 Urinary Cytokine Response after Intravesical Bacillus Calmette-Guerin Treatment for Superficial Bladder Cancer. J. Urol. 2002, 167, 364–367. [Google Scholar] [CrossRef]
- Luo, Y. Blocking IL-10 Enhances Bacillus Calmette-Guérin Induced T Helper Type 1 Immune Responses and Anti-Bladder Cancer Immunity. Oncoimmunology 2012, 1, 1183–1185. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Gillen, D.; Catalona, W.J. Requirement of a Thymus Dependent Immune Response for BCG-Mediated Antitumor Activity. J. Urol. 1987, 137, 155–158. [Google Scholar] [CrossRef]
- Pichler, R.; Fritz, J.; Zavadil, C.; Schäfer, G.; Culig, Z.; Brunner, A. Tumor-Infiltrating Immune Cell Subpopulations Influence the Oncologic Outcome after Intravesical Bacillus Calmette-Guérin Therapy in Bladder Cancer. Oncotarget 2016, 7, 39916–39930. [Google Scholar] [CrossRef]
- Messing, E.M. Words of Wisdom. Re: Preexisting BCG-Specific T Cells Improve Intravesical Immunotherapy for Bladder Cancer. Eur. Urol. 2012, 62, 935–936. [Google Scholar] [CrossRef]
- Zhou, T.C.; Sankin, A.I.; Porcelli, S.A.; Perlin, D.S.; Schoenberg, M.P.; Zang, X. A Review of the PD-1/PD-L1 Checkpoint in Bladder Cancer: From Mediator of Immune Escape to Target for Treatment. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 14–20. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Cao, Y.-W.; Yang, X.-C.; Niu, H.-T.; Sun, L.-J.; Wang, X.-S.; Liu, J. Effect of TLR4 and B7-H1 on Immune Escape of Urothelial Bladder Cancer and Its Clinical Significance. Asian Pac. J. Cancer Prev. 2014, 15, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Kates, M.; Matoso, A.; Choi, W.; Baras, A.S.; Daniels, M.J.; Lombardo, K.; Brant, A.; Mikkilineni, N.; McConkey, D.J.; Kamat, A.M.; et al. Adaptive Immune Resistance to Intravesical BCG in Non-Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin. Cancer Res. 2020, 26, 882–891. [Google Scholar] [CrossRef]
- Jallad, S.; Goubet, S.; Symes, A.; Larner, T.; Thomas, P. Prognostic Value of Inflammation or Granuloma after Intravesival BCG in Non-Muscle-Invasive Bladder Cancer. BJU Int. 2014, 113, E22–E27. [Google Scholar] [CrossRef]
- Pérez-Jacoiste Asín, M.A.; Fernández-Ruiz, M.; López-Medrano, F.; Lumbreras, C.; Tejido, Á.; San Juan, R.; Arrebola-Pajares, A.; Lizasoain, M.; Prieto, S.; Aguado, J.M. Bacillus Calmette-Guérin (BCG) Infection Following Intravesical BCG Administration as Adjunctive Therapy for Bladder Cancer: Incidence, Risk Factors, and Outcome in a Single-Institution Series and Review of the Literature. Medicine 2014, 93, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Brausi, M.; Oddens, J.; Sylvester, R.; Bono, A.; van de Beek, C.; van Andel, G.; Gontero, P.; Turkeri, L.; Marreaud, S.; Collette, S.; et al. Side Effects of Bacillus Calmette-Guérin (BCG) in the Treatment of Intermediate—And High-Risk Ta, T1 Papillary Carcinoma of the Bladder: Results of the EORTC Genito-Urinary Cancers Group Randomised Phase 3 Study Comparing One-Third Dose with Full Dose and 1 Year with 3 Years of Maintenance BCG. Eur. Urol. 2014, 65, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Messing, E.M. The BCG Shortage. Bladder Cancer Amst. Neth. 2017, 3, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.J.; Hwang, T.J.; Kesselheim, A.S. Ensuring Access to Injectable Generic Drugs—The Case of Intravesical BCG for Bladder Cancer. N. Engl. J. Med. 2017, 376, 1401–1403. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R. There’s Plenty of Room at the Bottom. In Feynman and Computation; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-429-50045-9. [Google Scholar]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, J.; Wang, W.; Geng, H.; Wang, Y.; Gao, B. Current Advances in the Application of Nanomedicine in Bladder Cancer. Biomed. Pharmacother. 2023, 157, 114062. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Karimi-Maleh, H.; Taheriazam, A.; Mirzaei, S.; Hashemi, M.; Hushmandi, K.; Makvandi, P.; Nazarzadeh Zare, E.; Sharifi, E.; et al. (Nano)Platforms in Bladder Cancer Therapy: Challenges and Opportunities. Bioeng. Transl. Med. 2023, 8, e10353. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.-Q.; Qin, Y.; You, S.-H.; Min, J.-J. Bacteria-Cancer Interactions: Bacteria-Based Cancer Therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a Butyrate-Producing Probiotic, Inhibits Intestinal Tumor Development through Modulating Wnt Signaling and Gut Microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-X.; Niu, M.-T.; Qin, Y.-T.; Sun, Y.-X.; Zhang, X.-Z. Progress of Engineered Bacteria for Tumor Therapy. Adv. Drug Deliv. Rev. 2022, 185, 114296. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Wu, X.; Zhang, Y.; Mannion, C.; Brouchkov, A.; Man, Y.-G.; Chen, T. Oncolytic Bacteria and Their Potential Role in Bacterium-Mediated Tumour Therapy: A Conceptual Analysis. J. Cancer 2019, 10, 4442–4454. [Google Scholar] [CrossRef]
- Huang, X.; Pan, J.; Xu, F.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Bacteria-Based Cancer Immunotherapy. Adv. Sci. 2021, 8, 2003572. [Google Scholar] [CrossRef]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-Targeting Bacteria Engineered to Fight Cancer. Nat. Rev. Cancer 2018, 18, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, F.; Chen, W.; Liu, S.; Qiu, L.; Chen, J. Bacteria-Mediated Cancer Therapies: Opportunities and Challenges. Biomater. Sci. 2021, 9, 5732–5744. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Q.; Liu, W.; Wen, Z.; Li, Y. Precision Strategies for Cancer Treatment by Modifying the Tumor-Related Bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 6183–6197. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, X. Advances in Bacteria-Based Therapy for Drug Delivery. Adv. Drug Deliv. Rev. 2022, 190, 114565. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yue, H.; Wang, S.; Li, X.; Wang, X.; Guo, P.; Ma, G.; Wei, W. Advances of Bacteria-Based Delivery Systems for Modulating Tumor Microenvironment. Adv. Drug Deliv. Rev. 2022, 188, 114444. [Google Scholar] [CrossRef]
- Joraku, A.; Homhuan, A.; Kawai, K.; Yamamoto, T.; Miyazaki, J.; Kogure, K.; Yano, I.; Harashima, H.; Akaza, H. Immunoprotection against Murine Bladder Carcinoma by Octaarginine-Modified Liposomes Incorporating Cell Wall of Mycobacterium Bovis Bacillus Calmette-Guérin. BJU Int. 2009, 103, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Kawai, K.; Kojima, T.; Oikawa, T.; Joraku, A.; Shimazui, T.; Nakaya, A.; Yano, I.; Nakamura, T.; Harashima, H.; et al. The Liposome-Incorporating Cell Wall Skeleton of Mycobacterium Bovis Bacillus Calmette-Guéin Can Directly Enhance the Susceptibility of Cancer Cells to Lymphokine-Activated Killer Cells through up-Regulation of Natural-Killer Group 2, Member D Ligands. BJU Int. 2011, 108, 1520–1526. [Google Scholar] [CrossRef]
- Miyazaki, J.; Nishiyama, H.; Yano, I.; Nakaya, A.; Kohama, H.; Kawai, K.; Joraku, A.; Nakamura, T.; Harashima, H.; Akaza, H. The Therapeutic Effects of R8-Liposome-BCG-CWS on BBN-Induced Rat Urinary Bladder Carcinoma. Anticancer Res. 2011, 31, 2065–2071. [Google Scholar]
- Kato, T.; Bilim, V.; Yuuki, K.; Naito, S.; Yamanobe, T.; Nagaoka, A.; Yano, I.; Akaza, H.; Tomita, Y. Bacillus Calmette-Guerin and BCG Cell Wall Skeleton Suppressed Viability of Bladder Cancer Cells in Vitro. Anticancer Res. 2010, 30, 4089–4096. [Google Scholar]
- Samaddar, S.; Mazur, J.; Sargent, J.; Thompson, D.H. Immunostimulatory Response of RWFV Peptide-Targeted Lipid Nanoparticles on Bladder Tumor Associated Cells. ACS Appl. Bio Mater. 2021, 4, 3178–3188. [Google Scholar] [CrossRef]
- Erdoğar, N.; Iskit, A.B.; Eroğlu, H.; Sargon, M.F.; Mungan, N.A.; Bilensoy, E. Antitumor Efficacy of Bacillus Calmette-Guerin Loaded Cationic Nanoparticles for Intravesical Immunotherapy of Bladder Tumor Induced Rat Model. J. Nanosci. Nanotechnol. 2015, 15, 10156–10164. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhong, X.; Liu, R.; Yang, X.; Xie, Z.; Zhang, Y.; Xu, Y.; Wang, H.; He, C.; Du, G.; et al. Co-Delivery of Oxaliplatin Prodrug Liposomes with Bacillus Calmette-Guérin for Chemo-Immunotherapy of Orthotopic Bladder Cancer. J. Control. Release 2024, 365, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, P.; Li, P.; Xue, A.; Zhang, X.; Zhang, H.; Jin, X. A Magnetic Chitosan Hydrogel for Sustained and Prolonged Delivery of Bacillus Calmette–Guérin in the Treatment of Bladder Cancer. Biomaterials 2013, 34, 10258–10266. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, L.; Peng, J.; Lyu, Y.; Li, Y.; Duan, D.; Zhang, W.; Wei, G.; Li, T.; Niu, Y.; et al. Drug-Loaded Bacillus Calmette–Guérin Bacteria for Immuno-Chemo Combo Therapy in Bladder Cancer. Adv. Mater. 2024, 36, 2310735. [Google Scholar] [CrossRef] [PubMed]
- Freund, J. The Mode of Action of Immunologic Adjuvants. Bibl. Tuberc. 1956, 10, 130–148. [Google Scholar]
- Larson, C.L.; Bell, J.F.; List, R.H.; Ribi, E.; Wicht, W.C. II. Host-Reactive Properties of Cell Walls and Protoplasm from Mycobacteria. Bacteriol. Rev. 1963, 27, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Azuma, I.; Kishimoto, S.; Yamamura, Y.; Petit, J.F. Adjuvanticity of Mycobacterial Cell Walls. Jpn. J. Microbiol. 1971, 15, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Azuma, I.; Ribi, E.E.; Meyer, T.J.; Zbar, B. Biologically Active Components from Mycobacterial Cell Walls. I. Isolation and Composition of Cell Wall Skeleton and Component P3. J. Natl. Cancer Inst. 1974, 52, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.J.; Ribi, E.E.; Azuma, I.; Zbar, B. Biologically Active Components from Mycobacterial Cell Walls. II. Suppression and Regression of Strain-2 Guinea Pig Hepatoma. J. Natl. Cancer Inst. 1974, 52, 103–111. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Azuma, I.; Sakatani, M.; Nishikawa, H.; Ogura, T. Effect of Oil-Attached BCG Cell-Wall Skeleton on the Induction of Pleural Fibrosarcomas in Mice. GANN Jpn. J. Cancer Res. 1976, 67, 441–445. [Google Scholar] [CrossRef]
- Yamamura, Y.; Sakatani, M.; Ogura, T.; Azuma, I. Adjuvant Immunotherapy of Lung Cancer with BCG Cell Wall Skeleton (BCG-CWS). Cancer 1979, 43, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.C.; Hata, K.; Lee, K.B.; Azuma, I. Inhibitory Effect of BCG Cell-Wall Skeletons (BCG-CWS) Emulsified in Squalane on Tumor Growth and Metastasis in Mice. Arch. Pharm. Res. 2002, 25, 522–527. [Google Scholar] [CrossRef]
- Nakajima, H.; Kawasaki, K.; Oka, Y.; Tsuboi, A.; Kawakami, M.; Ikegame, K.; Hoshida, Y.; Fujiki, F.; Nakano, A.; Masuda, T.; et al. WT1 Peptide Vaccination Combined with BCG-CWS Is More Efficient for Tumor Eradication than WT1 Peptide Vaccination Alone. Cancer Immunol. Immunother. 2004, 53, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Nishida, Y.; Yoshii, S.; Kim, S.Y.; Uda, H.; Hamasaki, T. Immunotherapy of Ovarian Cancer with Cell Wall Skeleton of Mycobacterium Bovis Bacillus Calmette-Guérin: Effect of Lymphadenectomy. Cancer Sci. 2009, 100, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, H.; Takeda, J.; Onoé, K.; Morikawa, K. Histological Studies on Adjuvanticity of BCG-Cell Walls: Comparison of Adjuvanticity between Oil-in-Water and Water-in-Oil Forms. Bull. Inst. Immunol. Sci. 1978, 38, 13–23. [Google Scholar]
- Nakamura, T.; Fukiage, M.; Suzuki, Y.; Yano, I.; Miyazaki, J.; Nishiyama, H.; Akaza, H.; Harashima, H. Mechanism Responsible for the Antitumor Effect of BCG-CWS Using the LEEL Method in a Mouse Bladder Cancer Model. J. Control. Release 2014, 196, 161–167. [Google Scholar] [CrossRef]
- Nakamura, T.; Fukiage, M.; Higuchi, M.; Nakaya, A.; Yano, I.; Miyazaki, J.; Nishiyama, H.; Akaza, H.; Ito, T.; Hosokawa, H.; et al. Nanoparticulation of BCG-CWS for Application to Bladder Cancer Therapy. J. Control. Release 2014, 176, 44–53. [Google Scholar] [CrossRef]
- Li, S.; Meng, C.; Hao, Q.; Zhou, R.; Dai, L.; Guo, Y.; Zhao, S.; Zhou, X.; Lou, C.; Xu, J.; et al. “On/off”-Switchable Crosslinked PTX-Nanoformulation with Improved Precise Delivery for NSCLC Brain Metastases and Restrained Adverse Reaction over Nab-PTX. Biomaterials 2024, 307, 122537. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef]
- Li, S.; Meng, C.; Hao, Q.; Dai, L.; Shi, J.; Xu, J.; Zhou, X.; Zhao, S.; Yang, J.; Kang, H.; et al. A Multistage-responsive Antibody-delivery Strategy to Improve Immunotherapy for NSCLC Brain Metastasis by Ultrasensitive Releasing and Tumor-anchoring. Adv. Funct. Mater. 2024, 34, 2312595. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ramanathan, R.K.; Borad, M.J.; Laheru, D.A.; Smith, L.S.; Wood, T.E.; Korn, R.L.; Desai, N.; Trieu, V.; Iglesias, J.L.; et al. Gemcitabine plus Nab-Paclitaxel Is an Active Regimen in Patients with Advanced Pancreatic Cancer: A Phase I/II Trial. J. Clin. Oncol. 2011, 29, 4548–4554. [Google Scholar] [CrossRef] [PubMed]
- Forssen, E.A. The Design and Development of DaunoXome(R) for Solid Tumor Targeting In Vivo. Adv. Drug Deliv. Rev. 1997, 24, 133–150. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chatterjee, A.; Qian, C.; Lagree, K.; Wang, Y.; Becker, C.A.; Freeman, M.R.; Murali, R.; Yang, W.; Underhill, D.M. Profiling Phagosome Proteins Identifies PD-L1 as a Fungal-Binding Receptor. Nature 2024, 630, 736–743. [Google Scholar] [CrossRef] [PubMed]

| Base-Nanocarriers | Material | Therapeutic Agent | Advantages in Immunotherapy | Ref |
|---|---|---|---|---|
| Lipid nanoparticles | Liposomes | BCG-CW | Enhanced the internalization of BCG-CW into bladder cancer cells and induced an anti-tumor immune response | [87] |
| Liposomes | BCG-CWS | Enhanced the expression of NKG2D ligands and targeting integrin α5β1 promoted the tumor uptake of CWS, activated APCs, and enhanced Th1 cell differentiation | [88,89,90] | |
| DOTAP+ DOPE+CHEMS | RWFV, CpG | Initiated a potent immune-stimulatory response and target macrophage, and activated APCs | [91] | |
| Polymeric nanoparticles | Chitosan | BCG | - | [92] |
| Chitosan | Oxaliplatin, BCG | Activated ICD, activated APCs, enhanced both cell-mediated and humoral immune response, and reprogrammed TAMs towards the M1 | [93] | |
| Magnetic nanoparticles | Fe3O4-MNP+ chitosan+ β-glycerophosphate | BCG | Enhanced the retention of BCG in the bladder and induced Th1 immune response | [94] |
| Live BCG | PLGA | Live BCG, DOX | Synergistic effect tumor ICD on BCG immunity. Enhanced DC activation and antigen presentation, activated APCs, and reprogrammed TAMs towards the M1 | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, M.; Shang, S.; Liu, K.; Wang, Y.; Xu, P.; Song, H.; Zhang, J.; Sun, Z.; Yan, Y.; Zhu, Z.; et al. Revitalizing Bacillus Calmette–Guérin Immunotherapy for Bladder Cancer: Nanotechnology and Bioengineering Approaches. Pharmaceutics 2024, 16, 1067. https://doi.org/10.3390/pharmaceutics16081067
Lv M, Shang S, Liu K, Wang Y, Xu P, Song H, Zhang J, Sun Z, Yan Y, Zhu Z, et al. Revitalizing Bacillus Calmette–Guérin Immunotherapy for Bladder Cancer: Nanotechnology and Bioengineering Approaches. Pharmaceutics. 2024; 16(8):1067. https://doi.org/10.3390/pharmaceutics16081067
Chicago/Turabian StyleLv, Maoxin, Shihao Shang, Kepu Liu, Yuliang Wang, Peng Xu, Hao Song, Jie Zhang, Zelong Sun, Yuhao Yan, Zheng Zhu, and et al. 2024. "Revitalizing Bacillus Calmette–Guérin Immunotherapy for Bladder Cancer: Nanotechnology and Bioengineering Approaches" Pharmaceutics 16, no. 8: 1067. https://doi.org/10.3390/pharmaceutics16081067






