The Antinociceptive Role of Nrf2 in Neuropathic Pain: From Mechanisms to Clinical Perspectives
Abstract
1. Introduction
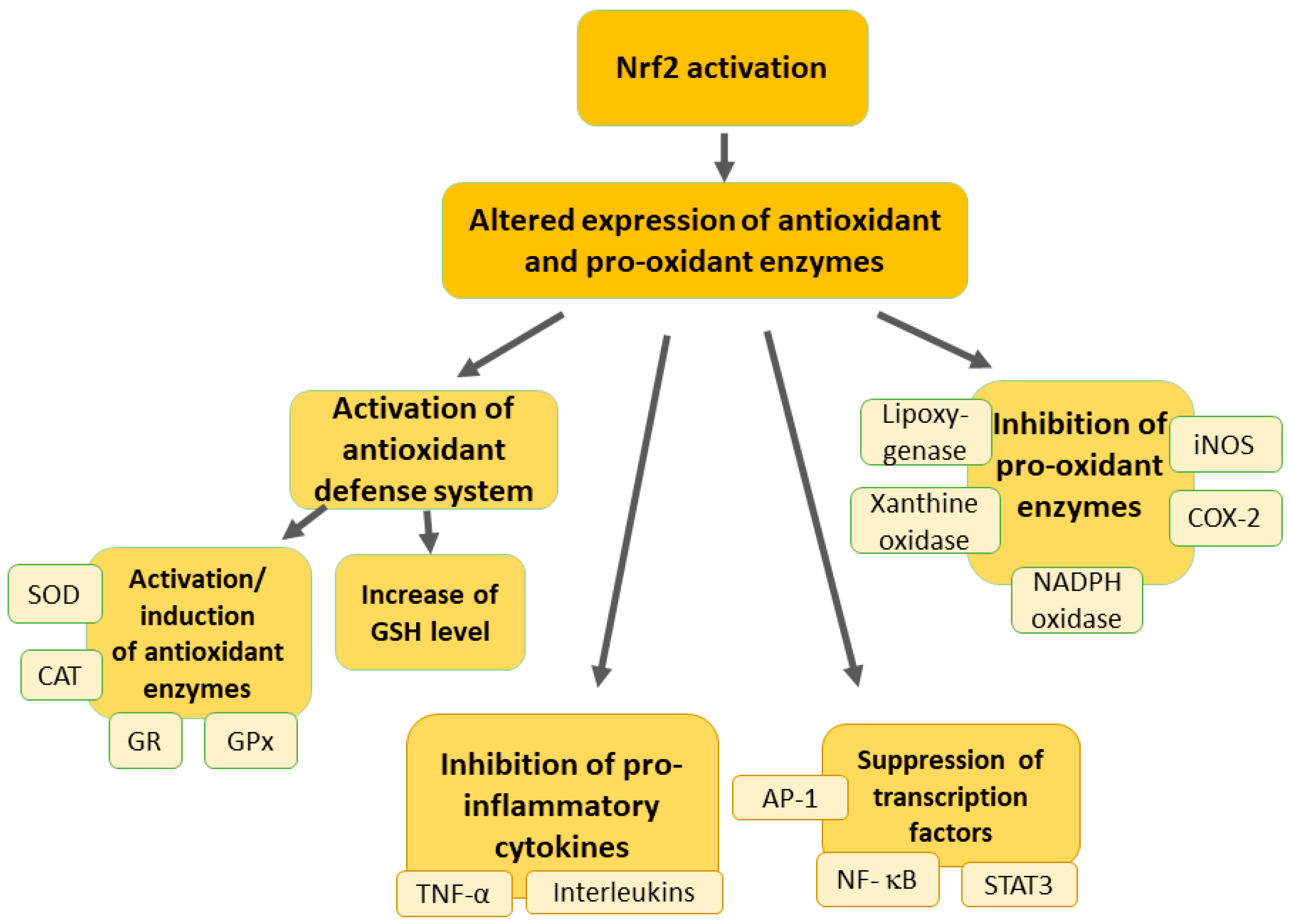
2. Overview of the Nrf2 Pathway
3. Modulation of Oxidative Stress in Neuropathic Pain by Nrf2 Activation
4. Protective Effects of Nrf2 against Inflammation in Neuropathic Pain
5. Mitochondrial Function and Neuropathic Pain: The Role of Nrf2
6. Nrf2 Modulation in Neuropathic Pain: Pharmacological and Alternative Strategies
7. Challenges and Limitations of Antinociceptive Effects of Nrf2 Modulators in Neuropathic Pain
8. Clinical Perspectives of Nrf2 Modulators in Neuropathic Pain
9. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Jayakar, S.; Shim, J.; Jo, S.; Bean, B.P.; Singeç, I.; Woolf, C.J. Developing nociceptor-selective treatments for acute and chronic pain. Sci. Transl. Med. 2021, 13, eabj9837. [Google Scholar] [CrossRef] [PubMed]
- International Association for the Study of Pain (IASP). IASP Terminology. 2024. Available online: https://www.iasp-pain.org/terminology (accessed on 27 June 2024).
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.M.; Kelleher, E.; Irani, A.; Schrepf, A.; Clauw, D.J.; Harte, S.E. Deciphering nociplastic pain: Clinical features, risk factors and potential mechanisms. Nat. Rev. Neurol. 2024, 20, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical Assessment of Inflammatory Pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Migliorati, G.; Delfino, D.V. Association of inflammatory mediators with pain perception. Biomed. Pharmacother. 2017, 96, 1445–1452. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, J.; Han, X.; Yu, W.; Gu, X. Potential novel therapeutic strategies for neuropathic pain. Front. Mol. Neurosci. 2023, 16, 1138798. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.; Kersebaum, D.; Lawn, T.; Sachau, J.; Sendel, M.; Vollert, J. Improving neuropathic pain treatment—By rigorous stratification from bench to bedside. J. Neurochem. 2023; early view. [Google Scholar] [CrossRef]
- Ma, Y.C.; Kang, Z.B.; Shi, Y.Q.; Ji, W.Y.; Zhou, W.M.; Nan, W. The Complexity of Neuropathic Pain and Central Sensitization: Exploring Mechanisms and Therapeutic Prospects. J. Integr. Neurosci. 2024, 23, 89. [Google Scholar] [CrossRef]
- Sacerdote, P.; Franchi, S.; Moretti, S.; Castelli, M.; Procacci, P.; Magnaghi, V.; Panerai, A.E. Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain. J. Neuroimmune Pharmacol. 2013, 8, 202–211. [Google Scholar] [CrossRef]
- Gao, Y.J.; Ji, R.R. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010, 7, 482–493. [Google Scholar] [CrossRef]
- Yang, D.; Xu, K.; Xu, X.; Xu, P. Revisiting prostaglandin E2: A promising therapeutic target for osteoarthritis. Clin. Immunol. 2024, 260, 109904. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.X.; Zhai, M.N.; Zhu, M.; He, C.; Wang, H.; Wang, J.; Zhang, Z.J. Inflammation in pathogenesis of chronic pain: Foe and friend. Mol. Pain 2023, 19, 17448069231178176. [Google Scholar] [CrossRef]
- Seifert, O.; Baerwald, C. Interaction of pain and chronic inflammation. Z. Rheumatol. 2021, 80, 205–213. [Google Scholar] [CrossRef]
- Sommer, C.; Leinders, M.; Üçeyler, N. Inflammation in the pathophysiology of neuropathic pain. Pain 2018, 159, 595–602. [Google Scholar] [CrossRef]
- Silva Santos Ribeiro, P.; Willemen, H.; Eijkelkamp, N. Mitochondria and sensory processing in inflammatory and neuropathic pain. Front. Pain Res. 2022, 3, 1013577. [Google Scholar] [CrossRef]
- Basu, P.; Averitt, D.L.; Maier, C.; Basu, A. The Effects of Nuclear Factor Erythroid 2 (NFE2)-Related Factor 2 (Nrf2) Activation in Preclinical Models of Peripheral Neuropathic Pain. Antioxidants 2022, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Mei, W.; Tian, X.B.; Tian, Y.K.; Liu, D.Q.; Ye, D.W. The therapeutic potential of Nrf2 inducers in chronic pain: Evidence from preclinical studies. Pharmacol. Ther. 2021, 225, 107846. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Luo, Y.; Deng, M. New advances in Nrf2-mediated analgesic drugs. Phytomedicine 2023, 110, 154598. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Antonucci, L.; Karin, M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis 2020, 41, 405–416. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef]
- Ulasov, A.V.; Rosenkranz, A.A.; Georgiev, G.P.; Sobolev, A.S. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 2022, 291, 120111. [Google Scholar] [CrossRef]
- Rushmore, T.H.; Morton, M.R.; Pickett, C.B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991, 266, 11632–11639. [Google Scholar] [CrossRef]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T. NRF2 and cancer: The good, the bad and the importance of context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- Shah, A.; Varma, M.; Bhandari, R. Exploring sulforaphane as neurotherapeutic: Targeting Nrf2-Keap & Nf-Kb pathway crosstalk in ASD. Metab. Brain Dis. 2024, 39, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Mamun, A.A.; Jakaria, M.; Thangapandiyan, S.; Ahmad, J.; Rahman, M.A.; Mathew, B.; Abdel-Daim, M.M.; Aleya, L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ. 2020, 707, 135624. [Google Scholar] [CrossRef]
- Yagishita, Y.; Gatbonton-Schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current Landscape of NRF2 Biomarkers in Clinical Trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef]
- Glorieux, C.; Enríquez, C.; González, C.; Aguirre-Martínez, G.; Buc Calderon, P. The Multifaceted Roles of NRF2 in Cancer: Friend or Foe? Antioxidants 2024, 13, 70. [Google Scholar] [CrossRef]
- Occhiuto, C.J.; Moerland, J.A.; Leal, A.S.; Gallo, K.A.; Liby, K.T. The Multi-Faceted Consequences of NRF2 Activation throughout Carcinogenesis. Mol. Cells 2023, 46, 176–186. [Google Scholar] [CrossRef]
- Smith, R.E. The Effects of Dietary Supplements that Overactivate the Nrf2/ARE System. Curr. Med. Chem. 2020, 27, 2077–2094. [Google Scholar] [CrossRef]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The Role of Nrf2 in Cardiovascular Function and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef]
- Tao, S.; Rojo de la Vega, M.; Chapman, E.; Ooi, A.; Zhang, D.D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol. Carcinog. 2018, 57, 182–192. [Google Scholar] [CrossRef]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Hao, W.; Li, M.; Cai, Q.; Wu, S.; Li, X.; He, Q.; Hu, Y. Roles of NRF2 in Fibrotic Diseases: From Mechanisms to Therapeutic Approaches. Front. Physiol. 2022, 13, 889792. [Google Scholar] [CrossRef]
- Dodson, M.; Shakya, A.; Anandhan, A.; Chen, J.; Garcia, J.G.N.; Zhang, D.D. NRF2 and Diabetes: The Good, the Bad, and the Complex. Diabetes 2022, 71, 2463–2476. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Carrasco, C.; Naziroǧlu, M.; Rodríguez, A.B.; Pariente, J.A. Neuropathic Pain: Delving into the Oxidative Origin and the Possible Implication of Transient Receptor Potential Channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, L.; Albino-Teixeira, A.; Pinho, D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: Focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol. Res. 2020, 162, 105280. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Mika, J. Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of pain and its implications for therapeutic interventions. Signal Transduct. Target. Ther. 2024, 9, 155. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Li, N.; Loh, P.; Guo, Y.; Hu, X.; Zhang, J.; Dou, B.; Wang, L.; Yang, C.; et al. Role of nerve signal transduction and neuroimmune crosstalk in mediating the analgesic effects of acupuncture for neuropathic pain. Front. Neurol. 2023, 14, 1093849. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Profumo, E.; Tucci, P.; Saso, L. A Perspective on Nrf2 Signaling Pathway for Neuroinflammation: A Potential Therapeutic Target in Alzheimer’s and Parkinson’s Diseases. Front. Cell. Neurosci. 2021, 15, 787258. [Google Scholar] [CrossRef]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front. Immunol. 2019, 10, 3009. [Google Scholar] [CrossRef]
- Orfali, R.; Alwatban, A.Z.; Orfali, R.S.; Lau, L.; Chea, N.; Alotaibi, A.M.; Nam, Y.W.; Zhang, M. Oxidative stress and ion channels in neurodegenerative diseases. Front. Physiol. 2024, 15, 1320086. [Google Scholar] [CrossRef]
- Ślęczkowska, M.; Misra, K.; Santoro, S.; Gerrits, M.M.; Hoeijmakers, J.G.J. Ion Channel Genes in Painful Neuropathies. Biomedicines 2023, 11, 2680. [Google Scholar] [CrossRef]
- Özgül, C.; Nazıroğlu, M. TRPM2 channel protective properties of N-acetylcysteine on cytosolic glutathione depletion dependent oxidative stress and Ca2+ influx in rat dorsal root ganglion. Physiol. Behav. 2012, 106, 122–128. [Google Scholar] [CrossRef]
- Zeng, T.; Li, J.; Xie, L.; Dong, Z.; Chen, Q.; Huang, S.; Xie, S.; Lai, Y.; Li, J.; Yan, W.; et al. Nrf2 regulates iron-dependent hippocampal synapses and functional connectivity damage in depression. J. Neuroinflamm. 2023, 20, 212. [Google Scholar] [CrossRef]
- Vasavda, C.; Xu, R.; Liew, J.; Kothari, R.; Dhindsa, R.S.; Semenza, E.R.; Paul, B.D.; Green, D.P.; Sabbagh, M.F.; Shin, J.Y.; et al. Identification of the NRF2 transcriptional network as a therapeutic target for trigeminal neuropathic pain. Sci. Adv. 2022, 8, eabo5633. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Santos, C.L.; de Souza Almeida, R.R.; da Silva, A.; Thomaz, N.K.; Costa, N.L.F.; Weber, F.B.; Schmitz, I.; Medeiros, L.S.; Medeiros, L.; et al. Gliotoxicity and Glioprotection: The Dual Role of Glial Cells. Mol. Neurobiol. 2021, 58, 6577–6592. [Google Scholar] [CrossRef]
- Rosa, A.O.; Egea, J.; Lorrio, S.; Rojo, A.I.; Cuadrado, A.; López, M.G. Nrf2-mediated haeme oxygenase-1 up-regulation induced by cobalt protoporphyrin has antinociceptive effects against inflammatory pain in the formalin test in mice. Pain 2008, 137, 332–339. [Google Scholar] [CrossRef]
- Li, W.; Jiang, D.; Li, Q.; Yao, S.; Sun, X.; Yang, Y.; Meng, Z.; Liu, W. Lipopolysaccharide-induced preconditioning protects against traumatic spinal cord injury by upregulating Nrf2 expression in rats. Life Sci. 2016, 162, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tao, X.; Wang, Q.; Yu, X.; Dong, D. Diosmetin alleviates neuropathic pain by regulating the Keap1/Nrf2/NF-κB signaling pathway. Biomed. Pharmacother. 2024, 170, 116067. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Wang, J.Q.; Wu, Y.H. MG53 ameliorates nerve injury induced neuropathic pain through the regulation of Nrf2/HO-1 signaling in rats. Behav. Brain Res. 2023, 449, 114489. [Google Scholar] [CrossRef]
- Elsayed, H.R.H.; Ali, E.M.T.; Rabei, M.R.; El Nashar, E.M.; Alghamdi, M.A.; Al-Zahrani, N.S.; Alshehri, S.H.; Aldahhan, R.A.; Morsy, A.I. Angiotensin II Type 1 receptor blockade attenuates the neuropathological changes in the spinal cords of diabetic rats with modulation of nuclear factor erythroid 2-related factor 2/heme oxygenase 1 system. Tissue Cell 2024, 88, 102420. [Google Scholar] [CrossRef]
- Xue, C.; Kui, W.; Huang, A.; Li, Y.; Li, L.; Gu, Z.; Xie, L.; Kong, S.; Yu, J.; Ruan, H.; et al. Electroacupuncture suppresses neuronal ferroptosis to relieve chronic neuropathic pain. J. Cell. Mol. Med. 2024, 28, e18240. [Google Scholar] [CrossRef]
- Zhang, M.W.; Sun, X.; Xu, Y.W.; Meng, W.; Tang, Q.; Gao, H.; Liu, L.; Chen, S.H. Curcumin relieves oxaliplatin-induced neuropathic pain via reducing inflammation and activating antioxidant response. Cell Biol. Int. 2024, 48, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Xu, J.; Xu, D.; Ma, X.; Zhao, X.; Liu, L. Nociceptive behavior induced by chemotherapeutic paclitaxel and beneficial role of antioxidative pathways. Physiol. Res. 2019, 68, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, L.; Wang, Y.; Wang, G.; Zhao, Y.; Zhang, Y. Electroacupuncture enhances antioxidative signal pathway and attenuates neuropathic pain induced by chemotherapeutic paclitaxel. Physiol. Res. 2019, 68, 501–510. [Google Scholar] [CrossRef]
- Sun, H.; Guo, X.; Wang, Z.; Wang, P.; Zhang, Z.; Dong, J.; Zhuang, R.; Zhou, Y.; Ma, G.; Cai, W. Alphalipoic Acid Prevents Oxidative Stress and Peripheral Neuropathy in Nab-Paclitaxel-Treated Rats through the Nrf2 Signalling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 3142732. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, K.; Chen, Y.; Wang, Y.; Wang, Y.; Lian, N.; Zhang, K.; Yu, Y. Nrf2/HO-1 signaling pathway participated in the protection of hydrogen sulfide on neuropathic pain in rats. Int. Immunopharmacol. 2019, 75, 105746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Chen, N.; Sun, J.; Wang, X.M.; Cao, F.; Tian, Y.K.; Ye, D.W. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol. Sin. 2020, 41, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P. Mechanisms for Reducing Neuropathic Pain. Mol. Neurobiol. 2020, 57, 67–87. [Google Scholar] [CrossRef]
- Hung, A.L.; Lim, M.; Doshi, T.L. Targeting cytokines for treatment of neuropathic pain. Scand. J. Pain 2017, 17, 287–293. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Kishioka, S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr. Opin. Pharmacol. 2012, 12, 55–61. [Google Scholar] [CrossRef]
- Smith, P.A. Neuropathic pain; what we know and what we should do about it. Front. Pain Res. 2023, 4, 1220034. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio 2022, 14, 100223. [Google Scholar] [CrossRef]
- An, J.; Chen, B.; Kang, X.; Zhang, R.; Guo, Y.; Zhao, J.; Yang, H. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am. J. Transl. Res. 2020, 12, 2353–2378. [Google Scholar]
- Xu, Y.; Jia, B.; Li, J.; Li, Q.; Luo, C. The Interplay between Ferroptosis and Neuroinflammation in Central Neurological Disorders. Antioxidants 2024, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- Gayatri, V.; Krishna Prasad, M.; Mohandas, S.; Nagarajan, S.; Kumaran, K.; Ramkumar, K.M. Crosstalk between inflammasomes, inflammation, and Nrf2: Implications for gestational diabetes mellitus pathogenesis and therapeutics. Eur. J. Pharmacol. 2024, 963, 176241. [Google Scholar] [CrossRef]
- Khassafi, N.; Azami Tameh, A.; Mirzaei, H.; Rafat, A.; Barati, S.; Khassafi, N.; Vahidinia, Z. Crosstalk between Nrf2 signaling pathway and inflammation in ischemic stroke: Mechanisms of action and therapeutic implications. Exp. Neurol. 2024, 373, 114655. [Google Scholar] [CrossRef]
- Oronsky, B.; Takahashi, L.; Gordon, R.; Cabrales, P.; Caroen, S.; Reid, T. RRx-001: A chimeric triple action NLRP3 inhibitor, Nrf2 inducer, and nitric oxide superagonist. Front. Oncol. 2023, 13, 1204143. [Google Scholar] [CrossRef]
- Kim, H.K.; Wang, Q.; Hwang, S.H.; Dougherty, P.M.; Wang, J.; Abdi, S. Bardoxolone Methyl Ameliorates Chemotherapy-Induced Neuropathic Pain by Activation of Phosphorylated Nuclear Factor Erythroid 2-Related Factor 2 in the Dorsal Root Ganglia. Anesth. Analg. 2024, 138, 664–675. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Tsai, H.P.; Tsai, T.H.; Miao, H.C.; Zhang, Z.H.; Wu, C.H. RTA-408 Regulates p-NF-κB/TSLP/STAT5 Signaling to Ameliorate Nociceptive Hypersensitivity in Chronic Constriction Injury Rats. Mol. Neurobiol. 2024, 61, 1714–1725. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, F.; Cao, W.; Zhang, W.; Liu, W.; Dai, F. Betulinic acid alleviates neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Neurosci. Lett. 2023, 813, 137429. [Google Scholar] [CrossRef]
- Mokhtari, T.; Lu, M.; El-Kenawy, A.E. Potential anxiolytic and antidepressant-like effects of luteolin in a chronic constriction injury rat model of neuropathic pain: Role of oxidative stress, neurotrophins, and inflammatory factors. Int. Immunopharmacol. 2023, 122, 110520. [Google Scholar] [CrossRef]
- Su, C.J.; Zhang, J.T.; Zhao, F.L.; Xu, D.L.; Pan, J.; Liu, T. Resolvin D1/N-formyl peptide receptor 2 ameliorates paclitaxel-induced neuropathic pain through the activation of IL-10/Nrf2/HO-1 pathway in mice. Front. Immunol. 2023, 14, 1091753. [Google Scholar] [CrossRef]
- Khan, A.; Shal, B.; Ullah Khan, A.; Ullah Shah, K.; Saniya Zahra, S.; Ul Haq, I.; Ud Din, F.; Ali, H.; Khan, S. Neuroprotective mechanism of Ajugarin-I against Vincristine-Induced neuropathic pain via regulation of Nrf2/NF-κB and Bcl2 signalling. Int. Immunopharmacol. 2023, 118, 110046. [Google Scholar] [CrossRef] [PubMed]
- Yardim, A.; Gur, C.; Comakli, S.; Ozdemir, S.; Kucukler, S.; Celik, H.; Kandemir, F.M. Investigation of the effects of berberine on bortezomib-induced sciatic nerve and spinal cord damage in rats through pathways involved in oxidative stress and neuro-inflammation. Neurotoxicology 2022, 89, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Makinde, E.A.; Eze, F.N.; Olatunji, O.J. Securidaca inappendiculata Polyphenol Rich Extract Counteracts Cognitive Deficits, Neuropathy, Neuroinflammation and Oxidative Stress in Diabetic Encephalopathic Rats via p38 MAPK/Nrf2/HO-1 Pathways. Front. Pharmacol. 2021, 12, 737764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Wang, X.Q.; Wan, Z.H.; Liu, Y.Q.; Zhang, M.J. Dexmedetomidine Relieves Neuropathic Pain in Rats With Chronic Constriction Injury via the Keap1-Nrf2 Pathway. Front. Cell Dev. Biol. 2021, 9, 714996. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Liao, X.; Tang, Y.; Liu, J. Dexmedetomidine alleviates inflammation in neuropathic pain by suppressing NLRP3 via Nrf2 activation. Exp. Ther. Med. 2021, 22, 1046. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Adamson, D.C. Neuronal-astrocyte metabolic interactions: Understanding the transition into abnormal astrocytoma metabolism. J. Neuropathol. Exp. Neurol. 2011, 70, 167–176. [Google Scholar] [CrossRef]
- Rumpf, S.; Sanal, N.; Marzano, M. Energy metabolic pathways in neuronal development and function. Oxf. Open Neurosci. 2023, 2, kvad004. [Google Scholar] [CrossRef] [PubMed]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Walters, G.C.; Usachev, Y.M. Mitochondrial calcium cycling in neuronal function and neurodegeneration. Front. Cell Dev. Biol. 2023, 11, 1094356. [Google Scholar] [CrossRef]
- Doyle, T.M.; Salvemini, D. Mini-Review: Mitochondrial dysfunction and chemotherapy-induced neuropathic pain. Neurosci. Lett. 2021, 760, 136087. [Google Scholar] [CrossRef] [PubMed]
- Baev, A.Y.; Vinokurov, A.Y.; Novikova, I.N.; Dremin, V.V.; Potapova, E.V.; Abramov, A.Y. Interaction of Mitochondrial Calcium and ROS in Neurodegeneration. Cells 2022, 11, 706. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Falabella, M.; Vernon, H.J.; Hanna, M.G.; Claypool, S.M.; Pitceathly, R.D.S. Cardiolipin, Mitochondria, and Neurological Disease. Trends Endocrinol. Metab. 2021, 32, 224–237. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Shilovsky, G.A.; Ashapkin, V.V. Transcription Factor Nrf2 and Mitochondria—Friends or Foes in the Regulation of Aging Rate. Biochemistry 2022, 87, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.J. The contribution of mitochondria to sensory processing and pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 119–146. [Google Scholar] [CrossRef]
- Shin, H.J.; Park, H.; Shin, N.; Kwon, H.H.; Yin, Y.; Hwang, J.A.; Song, H.J.; Kim, J.; Kim, D.W.; Beom, J. Pink1-Mediated Chondrocytic Mitophagy Contributes to Cartilage Degeneration in Osteoarthritis. J. Clin. Med. 2019, 8, 1849. [Google Scholar] [CrossRef]
- Toyama, S.; Shimoyama, N.; Ishida, Y.; Koyasu, T.; Szeto, H.H.; Shimoyama, M. Characterization of acute and chronic neuropathies induced by oxaliplatin in mice and differential effects of a novel mitochondria-targeted antioxidant on the neuropathies. Anesthesiology 2014, 120, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Willemen, H.; Kavelaars, A.; Prado, J.; Maas, M.; Versteeg, S.; Nellissen, L.J.J.; Tromp, J.; Gonzalez Cano, R.; Zhou, W.; Jakobsson, M.E.; et al. Identification of FAM173B as a protein methyltransferase promoting chronic pain. PLoS Biol. 2018, 16, e2003452. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, Y.; Lu, X.; Weng, Q.; Dai, G.; Yu, Y.; Yu, K.; Gao, W. Inhibition of Histone Deacetylase 6 by Tubastatin A Attenuates the Progress of Osteoarthritis via Improving Mitochondrial Function. Am. J. Pathol. 2020, 190, 2376–2386. [Google Scholar] [CrossRef]
- Willemen, H.; Santos Ribeiro, P.S.; Broeks, M.; Meijer, N.; Versteeg, S.; Tiggeler, A.; de Boer, T.P.; Małecki, J.M.; Falnes, P.; Jans, J.; et al. Inflammation-induced mitochondrial and metabolic disturbances in sensory neurons control the switch from acute to chronic pain. Cell Rep. Med. 2023, 4, 101265. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Arese, M.; Oberley-Deegan, R.E.; Saso, L.; Chatterjee, A. NRF2: A crucial regulator for mitochondrial metabolic shift and prostate cancer progression. Front. Physiol. 2022, 13, 989793. [Google Scholar] [CrossRef] [PubMed]
- Kerins, M.J.; Ooi, A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Signal. 2018, 29, 1756–1773. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Nucleus-encoded regulators of mitochondrial function: Integration of respiratory chain expression, nutrient sensing and metabolic stress. Biochim. Biophys. Acta 2012, 1819, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.R.; Moraes, C.T. Nuclear-Mitochondrial Interactions. Biomolecules 2022, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Chan, A.; Woodhead, J.S.T.; Reynolds, J.C.; Kumagai, H.; Kim, S.J.; Lee, C. Mitochondrial-derived peptides in energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E659–E666. [Google Scholar] [CrossRef]
- Miller, B.; Kim, S.J.; Kumagai, H.; Mehta, H.H.; Xiang, W.; Liu, J.; Yen, K.; Cohen, P. Peptides derived from small mitochondrial open reading frames: Genomic, biological, and therapeutic implications. Exp. Cell Res. 2020, 393, 112056. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef]
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial biogenesis and clearance: A balancing act. FEBS J. 2017, 284, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016, 1, 80–91. [Google Scholar] [CrossRef]
- Arnould, T.; Michel, S.; Renard, P. Mitochondria Retrograde Signaling and the UPR mt: Where Are We in Mammals? Int. J. Mol. Sci. 2015, 16, 18224–18251. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, J.; Feng, M.; Peng, J.; Du, X.; Chu, H.; Chen, X. The Mitochondrial-Derived Peptide MOTS-c Attenuates Oxidative Stress Injury and the Inflammatory Response of H9c2 Cells through the Nrf2/ARE and NF-κB Pathways. Cardiovasc. Eng. Technol. 2022, 13, 651–661. [Google Scholar] [CrossRef]
- Wan, W.; Zhang, L.; Lin, Y.; Rao, X.; Wang, X.; Hua, F.; Ying, J. Mitochondria-derived peptide MOTS-c: Effects and mechanisms related to stress, metabolism and aging. J. Transl. Med. 2023, 21, 36. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, Z.; Wang, T. MOTS-c: A promising mitochondrial-derived peptide for therapeutic exploitation. Front. Endocrinol. 2023, 14, 1120533. [Google Scholar] [CrossRef]
- Abdelnaser, M.; Alaaeldin, R.; Attya, M.E.; Fathy, M. Modulating Nrf-2/HO-1, apoptosis and oxidative stress signaling pathways by gabapentin ameliorates sepsis-induced acute kidney injury. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 947–958. [Google Scholar] [CrossRef]
- Sałat, K.; Gdula-Argasińska, J.; Malikowska, N.; Podkowa, A.; Lipkowska, A.; Librowski, T. Effect of pregabalin on contextual memory deficits and inflammatory state-related protein expression in streptozotocin-induced diabetic mice. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Chen, J.; Pang, L.; Chen, C.; Ye, J.; Liu, H.; Chen, H.; Zhang, S.; Liu, S.; Liu, B.; et al. The Acid Sphingomyelinase Inhibitor Amitriptyline Ameliorates TNF-α-Induced Endothelial Dysfunction. Cardiovasc. Drugs Ther. 2024, 38, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, D.; Bris, Á.G.; MacDowell, K.S.; García-Bueno, B.; Madrigal, J.L.; Leza, J.C.; Caso, J.R. Modulation of the antioxidant nuclear factor (erythroid 2-derived)-like 2 pathway by antidepressants in rats. Neuropharmacology 2016, 103, 79–91. [Google Scholar] [CrossRef]
- Peng, C.; Xue, L.; Yue, Y.; Chen, W.; Wang, W.; Shen, J. Duloxetine HCl Alleviates Asthma Symptoms by Regulating PI3K/AKT/mTOR and Nrf2/HO-1 Signaling Pathways. Inflammation 2023, 46, 2449–2469. [Google Scholar] [CrossRef]
- Engel, D.F.; de Oliveira, J.; Lieberknecht, V.; Rodrigues, A.L.S.; de Bem, A.F.; Gabilan, N.H. Duloxetine Protects Human Neuroblastoma Cells from Oxidative Stress-Induced Cell Death through Akt/Nrf-2/HO-1 Pathway. Neurochem. Res. 2018, 43, 387–396. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; He, N. Lidocaine attenuates hypoxia/reoxygenation-induced inflammation, apoptosis and ferroptosis in lung epithelial cells by regulating the p38 MAPK pathway. Mol. Med. Rep. 2022, 25, 150. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, Z.; Zhou, Q. Lidocaine relieves murine allergic rhinitis by regulating the NF-κB and p38 MAPK pathways. Exp. Ther. Med. 2022, 23, 193. [Google Scholar] [CrossRef]
- Xiao, S.; Zhou, Y.; Wang, Q.; Yang, D. Ketamine Attenuates Airway Inflammation via Inducing Inflammatory Cells Apoptosis and Activating Nrf2 Pathway in a Mixed-Granulocytic Murine Asthma Model. Drug Des. Dev. Ther. 2022, 16, 4411–4428. [Google Scholar] [CrossRef]
- Liang, J.; Wu, S.; Xie, W.; He, H. Ketamine ameliorates oxidative stress-induced apoptosis in experimental traumatic brain injury via the Nrf2 pathway. Drug Des. Dev. Ther. 2018, 12, 845–853. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Q.; She, Y.; Bi, X.; Zhao, B. Ketamine reduces LPS-induced HMGB1 via activation of the Nrf2/HO-1 pathway and NF-κB suppression. J. Trauma. Acute Care Surg. 2015, 78, 784–792. [Google Scholar] [CrossRef]
- McAnally, H.; Bonnet, U.; Kaye, A.D. Gabapentinoid Benefit and Risk Stratification: Mechanisms Over Myth. Pain Ther. 2020, 9, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Meaadi, J.; Obara, I.; Eldabe, S.; Nazar, H. The safety and efficacy of gabapentinoids in the management of neuropathic pain: A systematic review with meta-analysis of randomised controlled trials. Int. J. Clin. Pharm. 2023, 45, 556–565. [Google Scholar] [CrossRef]
- Kremer, M.; Salvat, E.; Muller, A.; Yalcin, I.; Barrot, M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience 2016, 338, 183–206. [Google Scholar] [CrossRef]
- Sindrup, S.H.; Otto, M.; Finnerup, N.B.; Jensen, T.S. Antidepressants in the treatment of neuropathic pain. Basic. Clin. Pharmacol. Toxicol. 2005, 96, 399–409. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chen, P.P. A review of SSRIs and SNRIs in neuropathic pain. Expert. Opin. Pharmacother. 2010, 11, 2813–2825. [Google Scholar] [CrossRef]
- Fornasari, D.; Magni, A.; Pais, P.; Palao, T.; Polati, E.; Sansone, P. Changing the paradigm in postherpetic neuralgia treatment: Lidocaine 700 mg medicated plaster. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3664–3676. [Google Scholar] [CrossRef]
- Varshney, V.; Osborn, J.; Chaturvedi, R.; Shah, V.; Chakravarthy, K. Advances in the interventional management of neuropathic pain. Ann. Transl. Med. 2021, 9, 187. [Google Scholar] [CrossRef]
- Voute, M.; Morel, V.; Pickering, G. Topical Lidocaine for Chronic Pain Treatment. Drug Des. Dev. Ther. 2021, 15, 4091–4103. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Cerqueira, A.R.; Soares, A.G.; Costa, S.K. Capsaicin and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 91–125. [Google Scholar] [CrossRef]
- Varshney, V.; Kumar, A.; Parashar, V.; Kumar, A.; Goyal, A.; Garabadu, D. Therapeutic Potential of Capsaicin in various Neurodegenerative Diseases with Special Focus on Nrf2 Signaling. Curr. Pharm. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain. Med. 2019, 20, S2–S12. [Google Scholar] [CrossRef]
- Muthuraman, A.; Singh, N.; Jaggi, A.S.; Ramesh, M. Drug therapy of neuropathic pain: Current developments and future perspectives. Curr. Drug Targets 2014, 15, 210–253. [Google Scholar] [CrossRef]
- Yang, Y.; Maher, D.P.; Cohen, S.P. Emerging concepts on the use of ketamine for chronic pain. Expert. Rev. Clin. Pharmacol. 2020, 13, 135–146. [Google Scholar] [CrossRef]
- Dosenovic, S.; Jelicic Kadic, A.; Miljanovic, M.; Biocic, M.; Boric, K.; Cavar, M.; Markovina, N.; Vucic, K.; Puljak, L. Interventions for Neuropathic Pain: An Overview of Systematic Reviews. Anesth. Analg. 2017, 125, 643–652. [Google Scholar] [CrossRef]
- Sisignano, M.; Gribbon, P.; Geisslinger, G. Drug Repurposing to Target Neuroinflammation and Sensory Neuron-Dependent Pain. Drugs 2022, 82, 357–373. [Google Scholar] [CrossRef]
- Cavalu, S.; Sharaf, H.; Saber, S.; Youssef, M.E.; Abdelhamid, A.M.; Mourad, A.A.E.; Ibrahim, S.; Allam, S.; Elgharabawy, R.M.; El-Ahwany, E.; et al. Ambroxol, a mucolytic agent, boosts HO-1, suppresses NF-κB, and decreases the susceptibility of the inflamed rat colon to apoptosis: A new treatment option for treating ulcerative colitis. FASEB J. 2022, 36, e22496. [Google Scholar] [CrossRef] [PubMed]
- Sunkari, S.; Thatikonda, S.; Pooladanda, V.; Challa, V.S.; Godugu, C. Protective effects of ambroxol in psoriasis like skin inflammation: Exploration of possible mechanisms. Int. Immunopharmacol. 2019, 71, 301–312. [Google Scholar] [CrossRef]
- Doğan Ünlü, M.; Aşçı, S.; İmeci, O.; Milletsever, A.; Özmen, Ö.; Sezer, S.; Demirci, S. A novel insight into the neuroprotective effects of cannabidiol: Maintained apelin/dopamine synthesis, NRF2 signaling, and AKT/CREB/BDNF gene expressions. Acta Neurobiol. Exp. 2024, 84, 98–110. [Google Scholar] [CrossRef]
- Hokmabadi, V.; Khalili, A.; Hashemi, S.A.; Hedayatyanfard, K.; Parvari, S.; Changizi-Ashtiyani, S.; Bayat, G. Cannabidiol interacts with the FXR/Nrf2 pathway and changes the CB1/CB2 receptors ratio in gentamicin-induced kidney injury in rats. Iran. J. Basic. Med. Sci. 2023, 26, 343–350. [Google Scholar] [CrossRef]
- Kumar Kalvala, A.; Bagde, A.; Arthur, P.; Kumar Surapaneni, S.; Ramesh, N.; Nathani, A.; Singh, M. Role of Cannabidiol and Tetrahydrocannabivarin on Paclitaxel-induced neuropathic pain in rodents. Int. Immunopharmacol. 2022, 107, 108693. [Google Scholar] [CrossRef] [PubMed]
- Bakare, A.O.; Owoyele, B.V. Antinociceptive and neuroprotective effects of bromelain in chronic constriction injury-induced neuropathic pain in Wistar rats. Korean J. Pain 2020, 33, 13–22. [Google Scholar] [CrossRef]
- Shang, B.; Shi, H.; Wang, X.; Guo, X.; Wang, N.; Wang, Y.; Dong, L. Protective effect of melatonin on myenteric neuron damage in experimental colitis in rats. Fundam. Clin. Pharmacol. 2016, 30, 117–127. [Google Scholar] [CrossRef]
- Tao, Y.; Zhao, Q.; Lu, C.; Yong, W.; Xu, M.; Wang, Z.; Leng, X. Melatonin suppresses atherosclerosis by ferroptosis inhibition via activating NRF2 pathway. FASEB J. 2024, 38, e23678. [Google Scholar] [CrossRef]
- Zhao, T.; Fang, R.; Ding, J.; Liu, Y.; Cheng, M.; Zhou, F.; Liu, F.; Li, W.; Li, S.; Jiang, K.; et al. Melatonin ameliorates multiorgan injuries induced by severe acute pancreatitis in mice by regulating the Nrf2 signaling pathway. Eur. J. Pharmacol. 2024, 975, 176646. [Google Scholar] [CrossRef]
- Wang, L.L.; Huang, Y.H.; Yan, C.Y.; Wei, X.D.; Hou, J.Q.; Pu, J.X.; Lv, J.X. N-acetylcysteine Ameliorates Prostatitis via miR-141 Regulating Keap1/Nrf2 Signaling. Inflammation 2016, 39, 938–947. [Google Scholar] [CrossRef]
- Hota, K.B.; Hota, S.K.; Chaurasia, O.P.; Singh, S.B. Acetyl-L-carnitine-mediated neuroprotection during hypoxia is attributed to ERK1/2-Nrf2-regulated mitochondrial biosynthesis. Hippocampus 2012, 22, 723–736. [Google Scholar] [CrossRef]
- Barhwal, K.; Hota, S.K.; Jain, V.; Prasad, D.; Singh, S.B.; Ilavazhagan, G. Acetyl-l-carnitine (ALCAR) prevents hypobaric hypoxia-induced spatial memory impairment through extracellular related kinase-mediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience 2009, 161, 501–514. [Google Scholar] [CrossRef]
- Zhang, H.; Xiang, D.; Liu, X.; Xiang, L. PPARα agonist relieves spinal cord injury in rats by activating Nrf2/HO-1 via the Raf-1/MEK/ERK pathway. Aging 2021, 13, 24640–24654. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, H.M.; Qi, L.M.; Li, X.; Huang, Q.; Xie, X.; Xia, Q. Deciphering resveratrol’s role in modulating pathological pain: From molecular mechanisms to clinical relevance. Phytother. Res. 2024, 38, 59–73. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, H.; Lin, Q.; Liu, X.; Cheng, Y.; Deng, B. Anti-inflammatory effect of resveratrol attenuates the severity of diabetic neuropathy by activating the Nrf2 pathway. Aging 2021, 13, 10659–10671. [Google Scholar] [CrossRef]
- Recalde, M.D.; Miguel, C.A.; Noya-Riobó, M.V.; González, S.L.; Villar, M.J.; Coronel, M.F. Resveratrol exerts anti-oxidant and anti-inflammatory actions and prevents oxaliplatin-induced mechanical and thermal allodynia. Brain Res. 2020, 1748, 147079. [Google Scholar] [CrossRef]
- Chen, B.; He, Q.; Chen, C.; Lin, Y.; Xiao, J.; Pan, Z.; Li, M.; Li, S.; Yang, J.; Wang, F.; et al. Combination of curcumin and catalase protects against chondrocyte injury and knee osteoarthritis progression by suppressing oxidative stress. Biomed. Pharmacother. 2023, 168, 115751. [Google Scholar] [CrossRef]
- Lu, X.; Xu, G.; Lin, Z.; Song, J.; Zhang, Y.; Wang, H.; Lu, F.; Xia, X.; Ma, X.; Zou, F.; et al. Sulforaphane Delays Intervertebral Disc Degeneration by Alleviating Endoplasmic Reticulum Stress in Nucleus Pulposus Cells via Activating Nrf-2/HO-1. Oxidative Med. Cell. Longev. 2023, 2023, 3626091. [Google Scholar] [CrossRef]
- Komine, S.; Miura, I.; Miyashita, N.; Oh, S.; Tokinoya, K.; Shoda, J.; Ohmori, H. Effect of a sulforaphane supplement on muscle soreness and damage induced by eccentric exercise in young adults: A pilot study. Physiol. Rep. 2021, 9, e15130. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, M.; Xu, L.; Ni, H.; Zhao, B.; Ni, C.; Huang, M.; Zhu, J.; Luo, G.; Yao, M. Sulforaphane alleviates hyperalgesia and enhances analgesic potency of morphine in rats with cancer-induced bone pain. Eur. J. Pharmacol. 2021, 909, 174412. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Lu, X.; Meng, J.; Qin, X.; Jiang, J. Anti-nociceptive and anti-inflammatory effects of sulforaphane on sciatic endometriosis in a rat model. Neurosci. Lett. 2020, 723, 134858. [Google Scholar] [CrossRef]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Leánez, S.; Pol, O. Sulforaphane Inhibited the Nociceptive Responses, Anxiety- and Depressive-Like Behaviors Associated With Neuropathic Pain and Improved the Anti-allodynic Effects of Morphine in Mice. Front. Pharmacol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Russo, M.A.; Baron, R.; Dickenson, A.H.; Kern, K.U.; Santarelli, D.M. Ambroxol for neuropathic pain: Hiding in plain sight? Pain 2023, 164, 3–13. [Google Scholar] [CrossRef]
- Salat, K.; Gryzlo, B.; Kulig, K. Experimental Drugs for Neuropathic Pain. Curr. Neuropharmacol. 2018, 16, 1193–1209. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. The molecular activity of cannabidiol in the regulation of Nrf2 system interacting with NF-κB pathway under oxidative stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef]
- Ambriz-Tututi, M.; Rocha-González, H.I.; Cruz, S.L.; Granados-Soto, V. Melatonin: A hormone that modulates pain. Life Sci. 2009, 84, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Zakaria, R.; Jeet Singh, H.; Acuna-Castroviejo, D. Melatonin and its agonists in pain modulation and its clinical application. Arch. Ital. Biol. 2012, 150, 274–289. [Google Scholar] [CrossRef]
- Posa, L.; De Gregorio, D.; Gobbi, G.; Comai, S. Targeting Melatonin MT2 Receptors: A Novel Pharmacological Avenue for Inflammatory and Neuropathic Pain. Curr. Med. Chem. 2018, 25, 3866–3882. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, N.; Govoni, S.; Allegri, M. Non-drug pain relievers active on non-opioid pain mechanisms. Pain Pract. 2022, 22, 255–275. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef]
- Boyd, A.; Bleakley, C.; Hurley, D.A.; Gill, C.; Hannon-Fletcher, M.; Bell, P.; McDonough, S. Herbal medicinal products or preparations for neuropathic pain. Cochrane Database Syst. Rev. 2019, 4, CD010528. [Google Scholar] [CrossRef]
- Jahromi, B.; Pirvulescu, I.; Candido, K.D.; Knezevic, N.N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 251. [Google Scholar] [CrossRef]
- Santos, W.; Guimarães, J.O.; Pina, L.T.S.; Serafini, M.R.; Guimarães, A.G. Antinociceptive effect of plant-based natural products in chemotherapy-induced peripheral neuropathies: A systematic review. Front. Pharmacol. 2022, 13, 1001276. [Google Scholar] [CrossRef] [PubMed]
- Rolim, L.C.; da Silva, E.M.; Flumignan, R.L.; Abreu, M.M.; Dib, S.A. Acetyl-L-carnitine for the treatment of diabetic peripheral neuropathy. Cochrane Database Syst. Rev. 2019, 6, Cd011265. [Google Scholar] [CrossRef]
- Rowin, J. Integrative neuromuscular medicine: Neuropathy and neuropathic pain: Consider the alternatives. Muscle Nerve 2019, 60, 124–136. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Di Lascio, S.; Fornasari, D. Acetyl-L-carnitine in chronic pain: A narrative review. Pharmacol. Res. 2021, 173, 105874. [Google Scholar] [CrossRef]
- Lang-Illievich, K.; Klivinyi, C.; Lasser, C.; Brenna, C.T.A.; Szilagyi, I.S.; Bornemann-Cimenti, H. Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients 2023, 15, 1350. [Google Scholar] [CrossRef]
- Miguel, C.A.; Noya-Riobó, M.V.; Mazzone, G.L.; Villar, M.J.; Coronel, M.F. Antioxidant, anti-inflammatory and neuroprotective actions of resveratrol after experimental nervous system insults. Special focus on the molecular mechanisms involved. Neurochem. Int. 2021, 150, 105188. [Google Scholar] [CrossRef]
- Shen, C.L.; Castro, L.; Fang, C.Y.; Castro, M.; Sherali, S.; White, S.; Wang, R.; Neugebauer, V. Bioactive compounds for neuropathic pain: An update on preclinical studies and future perspectives. J. Nutr. Biochem. 2022, 104, 108979. [Google Scholar] [CrossRef]
- Sun, J.; Chen, F.; Braun, C.; Zhou, Y.Q.; Rittner, H.; Tian, Y.K.; Cai, X.Y.; Ye, D.W. Role of curcumin in the management of pathological pain. Phytomedicine 2018, 48, 129–140. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef]
- Roganović, J.; Petrović, N. Clinical Perspectives of Non-Coding RNA in Oral Inflammatory Diseases and Neuropathic Pain: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 8278. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, L. Alleviative effect of microRNA-497 on diabetic neuropathic pain in rats in relation to decreased USP15. Cell Biol. Toxicol. 2023, 39, 1–16. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Liu, W.; Yan, B.; Hu, X.; Yang, F. miRNA-155 silencing reduces sciatic nerve injury in diabetic peripheral neuropathy. J. Mol. Endocrinol. 2019, 63, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Franzini, M.; Valdenassi, L.; Pandolfi, S.; Tirelli, U.; Ricevuti, G.; Chirumbolo, S. The Role of Ozone as an Nrf2-Keap1-ARE Activator in the Anti-Microbial Activity and Immunity Modulation of Infected Wounds. Antioxidants 2023, 12, 1985. [Google Scholar] [CrossRef]
- Clavo, B.; Martínez-Sánchez, G.; Rodríguez-Esparragón, F.; Rodríguez-Abreu, D.; Galván, S.; Aguiar-Bujanda, D.; Díaz-Garrido, J.A.; Cañas, S.; Torres-Mata, L.B.; Fabelo, H.; et al. Modulation by Ozone Therapy of Oxidative Stress in Chemotherapy-Induced Peripheral Neuropathy: The Background for a Randomized Clinical Trial. Int. J. Mol. Sci. 2021, 22, 2802. [Google Scholar] [CrossRef]
- Kalkman, H.O. Inhibition of Microglial GSK3β Activity Is Common to Different Kinds of Antidepressants: A Proposal for an In Vitro Screen to Detect Novel Antidepressant Principles. Biomedicines 2023, 11, 806. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Rockwell, C.E.; Jin, Y.; Boss, A.P.; Kaiser, L.M.; Awali, S. The Complicated Role of Nuclear Factor Erythroid-Derived 2-Like 2 in Allergy and Asthma. Drug Metab. Dispos. 2022, 50, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jeon, J.H. Recent Advances in Understanding Nrf2 Agonism and Its Potential Clinical Application to Metabolic and Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 2846. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yue, Z.; Liu, Z.; Liu, H.; Zhang, J.; Zhang, F.; Hu, T.; Fu, J. The impact of Nrf2 knockout on the neuroprotective effects of dexmedetomidine in a mice model of cognitive impairment. Behav. Brain Res. 2024, 469, 115006. [Google Scholar] [CrossRef]
- Antochi, F. Dimethyl fumarate—A new player in oral treatment options for relapsing forms of multiple sclerosis. Maedica 2014, 9, 408–409. [Google Scholar]
- Kanda, H.; Yamawaki, K. Bardoxolone methyl: Drug development for diabetic kidney disease. Clin. Exp. Nephrol. 2020, 24, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Pant, T.; Uche, N.; Juric, M.; Zielonka, J.; Bai, X. Regulation of immunomodulatory networks by Nrf2-activation in immune cells: Redox control and therapeutic potential in inflammatory diseases. Redox Biol. 2024, 70, 103077. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxidative Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Copple, I.M. Advances and challenges in therapeutic targeting of NRF2. Trends Pharmacol. Sci. 2023, 44, 137–149. [Google Scholar] [CrossRef]
- Cho, H.Y.; Marzec, J.; Kleeberger, S.R. Functional polymorphisms in Nrf2: Implications for human disease. Free Radic. Biol. Med. 2015, 88, 362–372. [Google Scholar] [CrossRef]
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Helgueta Romero, S.; Frauenknecht, K.B.M.; Mittelbronn, M.; Sinkkonen, L. Normal and Pathological NRF2 Signalling in the Central Nervous System. Antioxidants 2022, 11, 1426. [Google Scholar] [CrossRef]
- Morgenstern, C.; Lastres-Becker, I.; Demirdöğen, B.C.; Costa, V.M.; Daiber, A.; Foresti, R.; Motterlini, R.; Kalyoncu, S.; Arioz, B.I.; Genc, S.; et al. Biomarkers of NRF2 signalling: Current status and future challenges. Redox Biol. 2024, 72, 103134. [Google Scholar] [CrossRef]


| Compound | Experimental Model | Dose/Concentration | Possible Mechanism | Ref. |
|---|---|---|---|---|
| Gabapentin | Sepsis-induced acute kidney injury in rats | 50 and 100 mg/kg i.p. for 4 days | Upregulation of Nrf2/HO-1 pathway, suppressed apoptosis, attenuated oxidative stress | [129] |
| Pregabalin | Streptozotocin-induced diabetic mice | 10 mg/kg i.p. | Higher expression of Nrf2 in brain, immunomodulation, suppression of inflammation | [130] |
| Amitriptyline | HUVEC cells | 2.5 µM for 24 h | Alleviated inflammation due to suppressed NF-κB activity | [131] |
| Duloxetine | Rat mild chronic stress model | 15 mg/kg i.p. for 7 days | Increased expression of Nrf2 and antioxidant enzymes in prefrontal cortex. | [132] |
| Asthmatic BALB/c mice model | 30 mg/kg i.g. for 10 days | Decreased inflammation due to Nrf2/HO-1 pathway activation in lungs | [133] | |
| Human neuroblastoma SH-SY5Y cells | 1–5 µM for 24 h | Neuroprotective activity against oxidative stress and cell death via Akt/Nfr2/HO-1 pathway, nuclear translocation of Nrf2 | [134] | |
| Lidocaine | Lung A549 cells | 0.5–10 mM | Attenuation of inflammation, apoptosis and ferroptosis due to regulation of p38 MAPK signaling pathway | [135] |
| Murine allergic rhinitis model | 1, 3, 5 mg/kg intranasally for 7 days | Reduced inflammation, suppressed NF-κB and p38 MAPK signaling pathways | [136] | |
| Ketamine | Mixed-granulocyte murine asthma model | 75 mg/kg i.p. for 4 days | Reduced inflammation due to activation of Nrf2/Keap-1 signaling pathway | [137] |
| Experimental traumatic brain injury in mice | 30, 60, 100 mg/kg i.p. | Increased Nrf2 level and induced expression of Nrf2 signaling pathway-related proteins | [138] | |
| Lipopolysaccharide-stimulated macrophages | 100–1000 µM for 12 h | Activated Nrf2/HO-1 signaling pathway, suppressed NF-κB. | [139] |
| Compound | Experimental Model | Dose/Concentration | Possible Mechanism | Ref. |
|---|---|---|---|---|
| Ambroxol | Acetic acid rat model of ulcerative colitis | 100, 200 mg/kg per os for 8 days | Enhanced Nrf2 activity, upregulated HO-1 and catalase, inactivated NF-κB signaling, suppressed IL-6 and TNF-α. | [155] |
| Mouse macrophages (RAW 264.7) Human keratinocytes (HaCaT) cell line | 7.8 to 500 μM | Decreased the protein expression of nitrotyrosine, iNOS, NF-κB and MAPKs signaling cascade, increases expression of Nrf-2 and SOD-1 in RAW 264.7 cells and skin tissue | [156] | |
| Psoriasis-like BALB/c mice skin inflammation model | 10, 30 mg/kg/day topically for 6 days | |||
| Cannabidiol | Lipopolysaccharide-induced rat neuroinflammation model | 5 mg/kg i.p. | Suppressed neuroinflammation via regulating AKT, CREB, BDNF expressions, Nrf2 signaling, apelin and tyrosine hydroxylase synthesis. | [157] |
| Gentamicin-induced rat nephrotoxicity | 2.5, 5, and 10 mg/kg/day for 10 days | Activation of the FXR/Nrf2 pathway | [158] | |
| Paclitaxel-induced murine peripheral neuropathy | 10 mg/kg i.p. twice a week for six weeks | Restored p-AMPK, SIRT1, Nrf2, HO-1, SOD2 levels | [159] | |
| Bromelain | Rat chronic constriction injury | 30, 50 mg/kg per os | Nrf2 activation and translocation to nucleus | [160] |
| Melatonin | Rat myenteric neuron damage during colitis model | 2.5 mg/kg i.p. | Alleviated inflammation and oxidative stress via increased the Nrf2 and HO-1 levels. | [161] |
| ApoE−/− mice | 20 mg/kg/day i.p. | Inhibited macrophage ferroptosis due to activation of Nrf2 pathway | [162] | |
| Severe acute pancreatitis murine model | 5, 20 50 mg/kg i.p. | Protected from multiorgan damage via increased Nrf2 expression | [163] | |
| N-acetyl-L-cysteine | Rat chronic prostatitis model | 300 mg/kg i.p. | Activated the Keap1/Nrf2 signaling | [164] |
| Acetyl-L-carnitine | Rat hypoxia model | 75 mg/kg per os | Mitochondrial biogenesis via ERK-Nrf2 mediated pathway | [165] |
| Rat hypobaric hypoxia model | 50 mg/kg i.p. | Reduced oxidative damage via ERK-Nrf2 mediated pathway | [166] | |
| Palmitoylethanol-amide | Rat spinal cord injury model | 2 mg/kg i.p. | Inhibited inflammatory responses and oxidative stress, due to activation of Nrf2/HO-1 pathway | [167] |
| Resveratrol | Rat chronic constriction injury | 20 mg/kg, per os | Activation of Nrf2 and antioxidant enzymes | [168] |
| Mice diabetic peripheral neuropathy | 10% 10 mL/kg, i.g. | Suppression of the expression of NF-κB by upregulating the activation of Nrf2 and the expression of antioxidant enzymes | [169] | |
| Rat chemotherapy-induced peripheral neuropathic pain | 6.28 and 7.81 mg/kg/day p.o. | NF-κB inhibition, Nrf2 activation | [170] | |
| Curcumin | Oxaliplatin-induced neuropathic pain mouse model | 10 mg/kg i.p. for 7 days | Inhibited the NLRP3 inflammasome-mediated inflammatory response, increased Nrf2/GPx4-mediated antioxidant responses, and decreased mitochondrial oxidative generation | [71] |
| Knee osteoarthritis rat model | 100 μg/mL 100 μL injection into a knee cavity 2× week for 8 weeks | Antioxidant activity via Nrf2/HO-1 signaling pathway | [171] | |
| Sulforaphane | Human nucleus pulposus tissue Rat intervertebral disc degeneration model | 10 μM for 24 h 10 μmol/L intradiscal injection every second week for 8 weeks | Delayed intervertebral disc degeneration by alleviating endoplasmic reticulum stress in nucleus pulposus cells via activating Nrf2/HO-1 | [172] |
| Human eccentric exercise model | 30 mg/day for 7 days | Increased NQO1 mRNA expression in peripheral blood mononuclear cells | [173] | |
| Rat cancer-induced bone pain model | 0.1, 0.5, 1, 5 or 10 mg/kg injected intrathecally for 7 days | Alleviated cancer pain via Nrf 2, HO-1 activation, NF-κB inhibition, inflammatory marker suppression. Also enhanced pain relief by morphine. | [174] | |
| Rat sciatic endo metriosis | 5, 15, 30 and 60 mg/kg, i.p. for 28 days | Keap1/Nrf 2 pathway upregulation, suppression of inflammation and oxidative stress | [175] | |
| CCI-induced murine neuropathic pain | 10 mg/kg, i.p. for 7 days | Nrf 2/HO-1/NQO-pathway activation, microglial activation, MAPK phosphorylation | [176] |
| Compound | Developer/Trial | Original Use | Effects | Ref. |
|---|---|---|---|---|
| Dimethyl fumarate | Fumaderm® (Biogen, Cambridge, MA, USA) | In combination with mono ethylfumarate—for moderate to severe plaque psoriasis | Prevents degradation of Nrf2 activity, enhances expression of genes protecting from inflammation and oxidative damage. | [206] |
| Skilarence® (Almirall Limited, Uxbridge, Middlesex, UK) | For moderate to severe plaque psoriasis | |||
| BG-12, Tecfidera® (Biogen, Cambridge, MA, USA) | Approved for relapsing-remitting multiple sclerosis | |||
| Bardoxolone methyl | BEACON trial | For treatment of chronic kidney disease and other inflammatory conditions | Nrf2 inducer with broad-spectrum anti-inflammatory and antioxidant effects | [207] |
| CARDINAL trial | For use in Alport syndrome | |||
| Oltipraz | Developed by Rhône-Poulenc (Vitry-sur-Seine, France) | For schistosomiasis | Could induce enzymes that maintain reduced GSH pools and detoxify electrophiles | [41] |
| Sulforaphane | Few clinical studies on autism, schizophrenia, cardiovascular disease, and diabetes | n.d. | Can improve markers of oxidative stress and inflammation in patients with chronic diseases | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrikonis, K.; Bernatoniene, J.; Kopustinskiene, D.M.; Casale, R.; Davinelli, S.; Saso, L. The Antinociceptive Role of Nrf2 in Neuropathic Pain: From Mechanisms to Clinical Perspectives. Pharmaceutics 2024, 16, 1068. https://doi.org/10.3390/pharmaceutics16081068
Petrikonis K, Bernatoniene J, Kopustinskiene DM, Casale R, Davinelli S, Saso L. The Antinociceptive Role of Nrf2 in Neuropathic Pain: From Mechanisms to Clinical Perspectives. Pharmaceutics. 2024; 16(8):1068. https://doi.org/10.3390/pharmaceutics16081068
Chicago/Turabian StylePetrikonis, Kestutis, Jurga Bernatoniene, Dalia M. Kopustinskiene, Roberto Casale, Sergio Davinelli, and Luciano Saso. 2024. "The Antinociceptive Role of Nrf2 in Neuropathic Pain: From Mechanisms to Clinical Perspectives" Pharmaceutics 16, no. 8: 1068. https://doi.org/10.3390/pharmaceutics16081068
APA StylePetrikonis, K., Bernatoniene, J., Kopustinskiene, D. M., Casale, R., Davinelli, S., & Saso, L. (2024). The Antinociceptive Role of Nrf2 in Neuropathic Pain: From Mechanisms to Clinical Perspectives. Pharmaceutics, 16(8), 1068. https://doi.org/10.3390/pharmaceutics16081068








