Antibiofilm and Antihemolytic Activities of Actinostemma lobatum Extract Rich in Quercetin against Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Materials
2.2. Bacterial Strains and Cell Growth Measurements
2.3. Analyses of Quercetin Using HPLC and NMR
2.4. Crystal Violet Biofilm Assay
2.5. Microscopic Analysis of Biofilms
2.6. Hemolytic Activity Assay
2.7. Statistical Analysis
3. Results
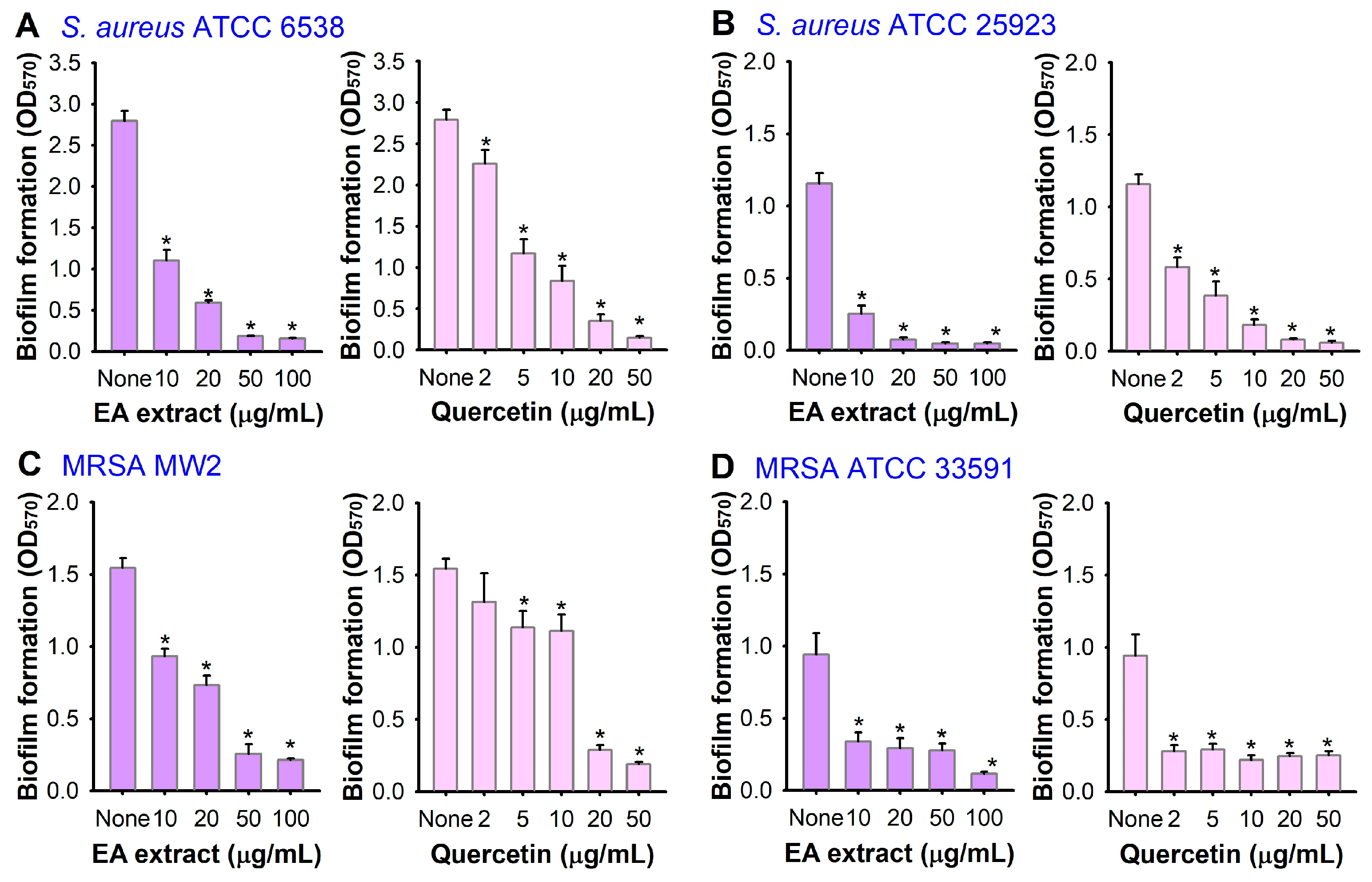
3.1. Antibiofilm Activity of A. lobatum Extract
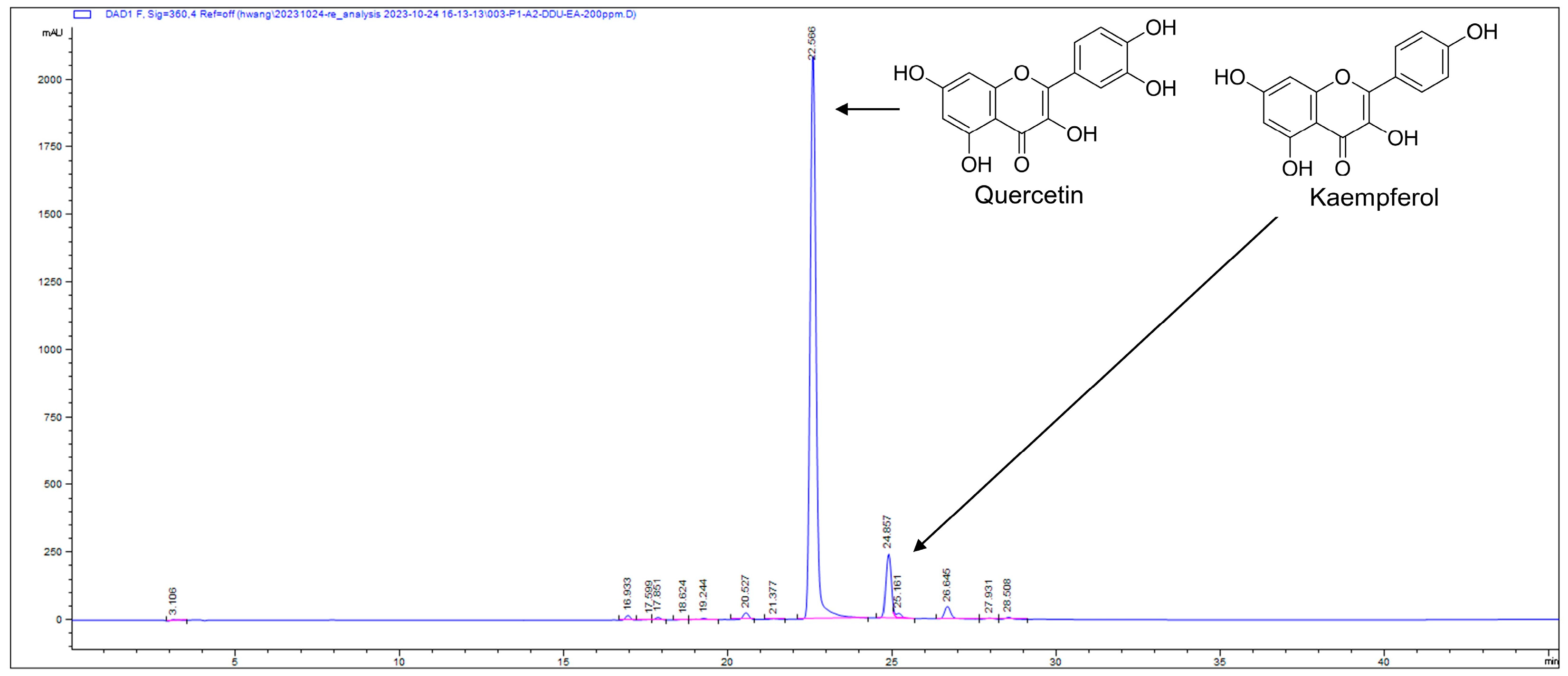
3.2. Bioassay-Guided Fractionation and Isolation
3.3. Identification and Quantification of Active Compound Quercetin
3.4. Microscopic Examination of the Antibiofilm Activity of the A. lobatum Extract against S. aureus
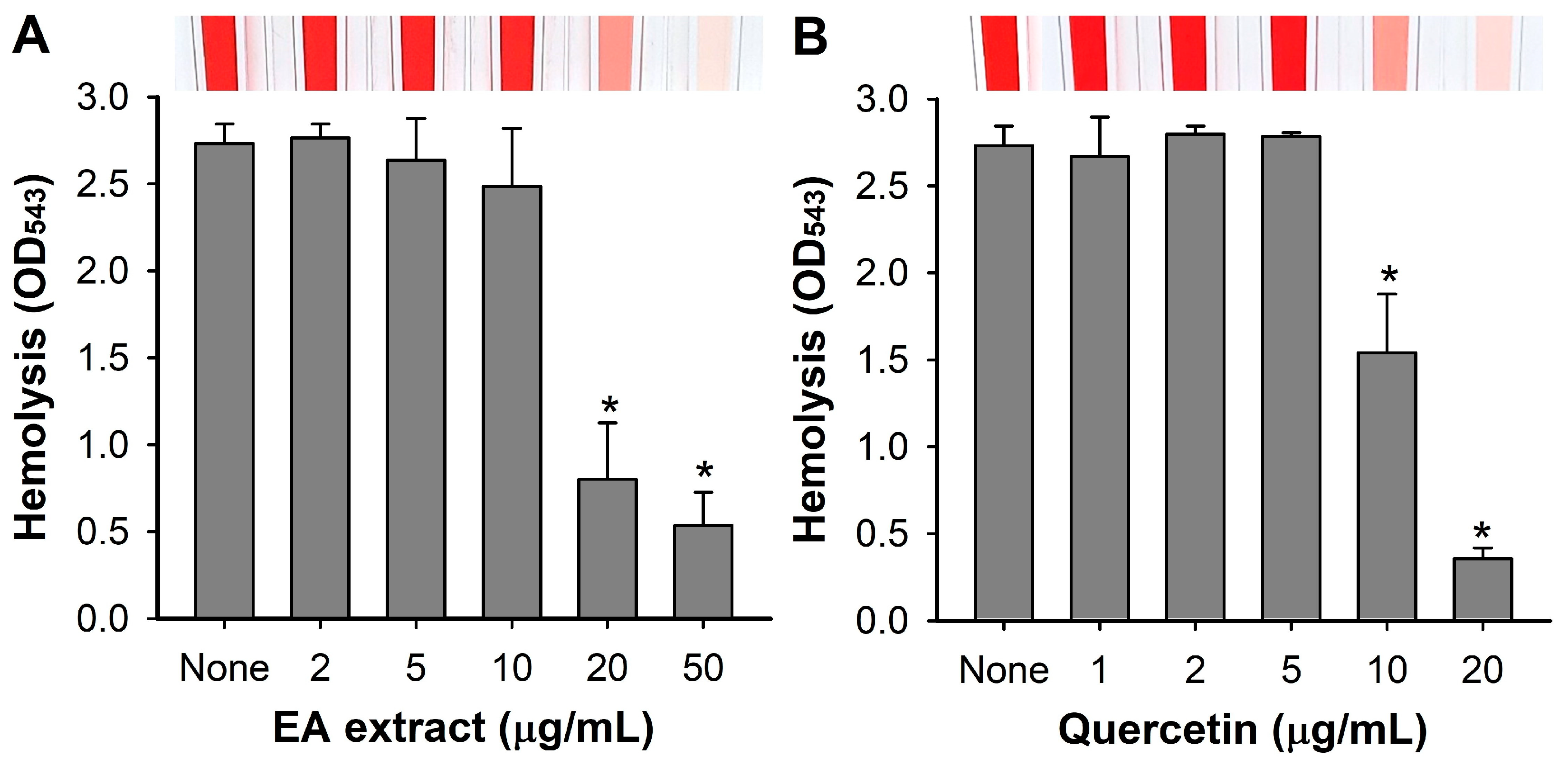
3.5. Inhibition of Hemolytic Activity by A. lobatum Extract
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus aureus toxins: An update on their pathogenic properties and potential treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmolle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, L.; Lasalvia, A.; Fabiano, M.G.; D’Intino, E.; Cioppo, F.D.; Fraschetti, C.; Filippi, A.; Ammendolia, M.G.; Conte, A.L.; Forte, J.; et al. Lentisk (Pistacia lentiscus) oil nanoemulsions loaded with levofloxacin: Phytochemical profiles and antibiofilm activity against Staphylococcus spp. Pharmaceutics 2024, 16, 927. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Park, J.-H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.-H.; Kim, S.I.; Cho, M.H.; Lee, J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Park, S.; Hu, L.; Lee, J. Phytopigment alizarin inhibits multispecies biofilm development by Cutibacterium acnes, Staphylococcus aureus, and Candida albicans. Pharmaceutics 2022, 14, 1047. [Google Scholar] [CrossRef]
- Hoang, L.; Benes, F.; Fenclova, M.; Kronusova, O.; Svarcova, V.; Rehorova, K.; Svecova, E.B.; Vosatka, M.; Hajslova, J.; Kastanek, P.; et al. Phytochemical composition and in vitro biological activity of Iris spp. (Iridaceae): A new source of bioactive constituents for the inhibition of oral bacterial biofilms. Antibiotics 2020, 9, 403. [Google Scholar] [CrossRef]
- Xu, Z.; Chang, L. Identification and Control of Common Weeds; Springer: Singapore, 2017; Volume 3, p. 417. [Google Scholar]
- Cao, J.Q.; Li, W.; Tang, Y.; Zhang, X.R.; Li, W.; Zhao, Y.Q. Three new triterpene saponins from Actinostemma lobatum MAXIM (Cucurbitaceae) and their in vitro cytotoxicity. Phytochem. Lett. 2015, 11, 301–305. [Google Scholar] [CrossRef]
- Kim, D.K. Antioxidative constituents from the whole plant of Actinostemma lobatum maxim. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 746–751. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Okabe, H.; Mihashi, K.; Lee, K.-H. Antitumor agents 171. Cytotoxicities of lobatosides B, C, D, and E, cyclic bisdesmosides isolated from Actinostemma lobatum maxim. Bioorg. Med. Chem. Lett. 1996, 6, 2807–2810. [Google Scholar] [CrossRef]
- Li, W.; Cao, J.; Tang, Y.; Zhang, L.; Xie, Q.; Shen, H.; Zhao, Y. Cyclic bisdesmosides from Actinostemma lobatum MAXIM (Cucurbitaceae) and their in vitro cytotoxicity. Fitoterapia 2012, 83, 147–152. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, H.J.; Lee, J.H.; Jang, Y.S.; Kim, D.K.; Shim, B.S.; Cho, K.H.; Ko, S.G.; Ahn, K.S.; Kim, S.H. Blockade of glycoprotein IIb/IIIa mediates the antithrombotic activity of butanol fraction of Actinostemma lobatum Maxim. J. Ethnopharmacol. 2008, 116, 431–438. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, Y.T.; Lee, D.Y.; Cho, E.; Hwang, B.S.; Jeon, J. Large-scale screening of the plant extracts for antifungal activity against the plant pathogenic fungi. Plant Pathol. J. 2022, 38, 685–691. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhang, T.; Xie, L.L.; Karrar, E.; Shi, L.K.; Jin, J.; Wang, X.G.; Jin, Q.Z. Physicochemical characteristics of Actinostemma lobatum Maxim. kernel oil by supercritical fluid extraction and conventional methods. Ind. Crops Prod. 2020, 152, 112516. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Lee, J. Antibiofilm activity of lawsone against polymicrobial Enterohemorrhagic coli O157:H7 and Candida albicans by suppression of curli production and hyphal growth. Phytomedicine 2024, 124, 155306. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.F.; Chen, S.N.; Simmler, C.; Lankin, D.C.; Gödecke, T.; Jaki, B.U.; Friesen, J.B.; McAlpine, J.B.; Napoitano, J.G. Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay. J. Med. Chem. 2014, 57, 9220–9231. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Lee, J. Inhibition of Staphylococcus aureus biofilm formation and virulence factor production by petroselinic acid and other unsaturated C18 fatty acids. Microbiol. Spectr. 2022, 10, e0133022. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Kang, X.; Chen, X.; Luo, X.; Li, C.; Wang, G. Quercetin targets SarA of methicillin-resistant Staphylococcus aureus to mitigate biofilm formation. Microbiol. Spectr. 2024, 12, e0272223. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Lee, J.-H.; Cho, M.H.; Lee, J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2015, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Ma, Q.; Wang, G.; Li, N.; Mao, Y.; Wang, X.; Wang, Y.; Wang, G. Potential mechanisms of quercetin influence the ClfB protein during biofilm formation of Staphylococcus aureus. Front. Pharmacol. 2022, 13, 825489. [Google Scholar] [CrossRef]
- Jing, S.; Kong, X.; Wang, L.; Wang, H.; Feng, J.; Wei, L.; Meng, Y.; Liu, C.; Chang, X.; Qu, Y.; et al. Quercetin reduces the virulence of S. aureus by targeting ClpP to protect mice from MRSA-induced lethal pneumonia. Microbiol. Spectr. 2022, 10, e0234021. [Google Scholar] [CrossRef] [PubMed]
- Memariani, H.; Memariani, M.; Ghasemian, A. An overview on anti-biofilm properties of quercetin against bacterial pathogens. World J. Microbiol. Biotechnol. 2019, 35, 143. [Google Scholar] [CrossRef]
- Tan, Y.; Lin, Q.; Yao, J.; Zhang, G.; Peng, X.; Tian, J. In vitro outcomes of quercetin on Candida albicans planktonic and biofilm cells and in vivo effects on vulvovaginal candidiasis. Evidences of its mechanisms of action. Phytomedicine 2023, 114, 154800. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Zavodnik, I.B. Antimicrobial activity of quercetin, naringenin and catechin: Flavonoids inhibit Staphylococcus aureus-induced hemolysis and modify membranes of bacteria and erythrocytes. Molecules 2023, 28, 1252. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Willard, J.; Yeaman, M.R.; Cheung, A.L.; Bayer, A.S. Regulation of Staphylococcus aureus alpha-toxin gene (hla) expression by agr, sarA, and sae in vitro and in experimental infective endocarditis. J. Infect. Dis. 2006, 194, 1267–1275. [Google Scholar] [CrossRef]
- Hoven, G.v.; Qin, Q.; Neukirch, C.; Husmann, M.; Hellmann, N. Staphylococcus aureus α-toxin: Small pore, large consequences. Biol. Chem. 2019, 400, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Int. J. Med. Update 2007, 2, 20–35. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kim, Y.-G.; Choi, J.-S.; Jeong, Y.T.; Hwang, B.S.; Lee, J. Antibiofilm and Antihemolytic Activities of Actinostemma lobatum Extract Rich in Quercetin against Staphylococcus aureus. Pharmaceutics 2024, 16, 1075. https://doi.org/10.3390/pharmaceutics16081075
Lee J-H, Kim Y-G, Choi J-S, Jeong YT, Hwang BS, Lee J. Antibiofilm and Antihemolytic Activities of Actinostemma lobatum Extract Rich in Quercetin against Staphylococcus aureus. Pharmaceutics. 2024; 16(8):1075. https://doi.org/10.3390/pharmaceutics16081075
Chicago/Turabian StyleLee, Jin-Hyung, Yong-Guy Kim, Ji-Su Choi, Yong Tae Jeong, Buyng Su Hwang, and Jintae Lee. 2024. "Antibiofilm and Antihemolytic Activities of Actinostemma lobatum Extract Rich in Quercetin against Staphylococcus aureus" Pharmaceutics 16, no. 8: 1075. https://doi.org/10.3390/pharmaceutics16081075
APA StyleLee, J.-H., Kim, Y.-G., Choi, J.-S., Jeong, Y. T., Hwang, B. S., & Lee, J. (2024). Antibiofilm and Antihemolytic Activities of Actinostemma lobatum Extract Rich in Quercetin against Staphylococcus aureus. Pharmaceutics, 16(8), 1075. https://doi.org/10.3390/pharmaceutics16081075





