Synthesis and Antimicrobial Activity of Newly Synthesized Nicotinamides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Synthesized Compounds
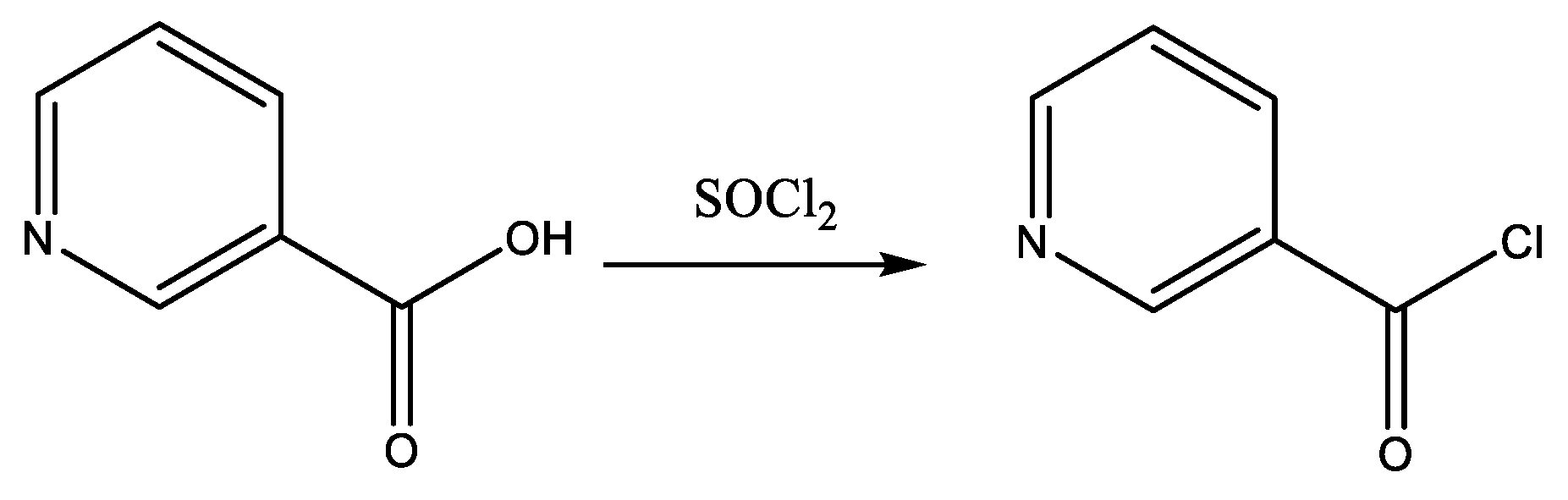
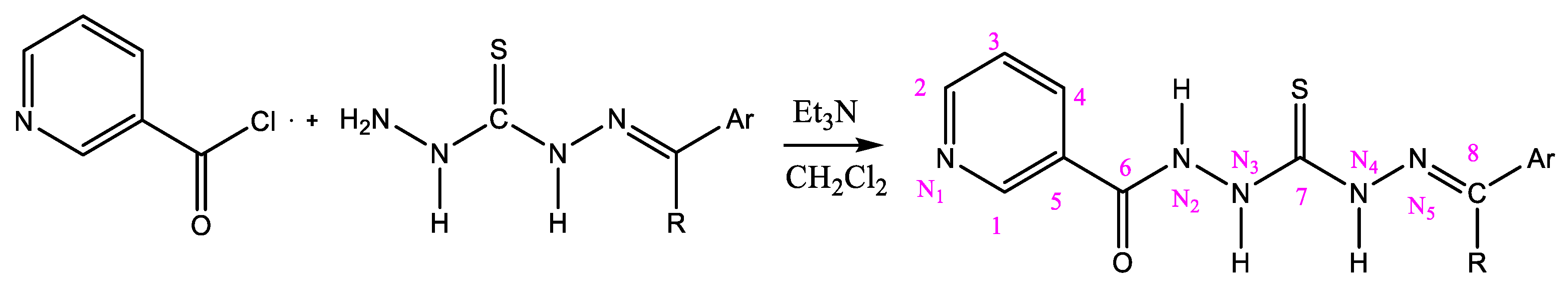
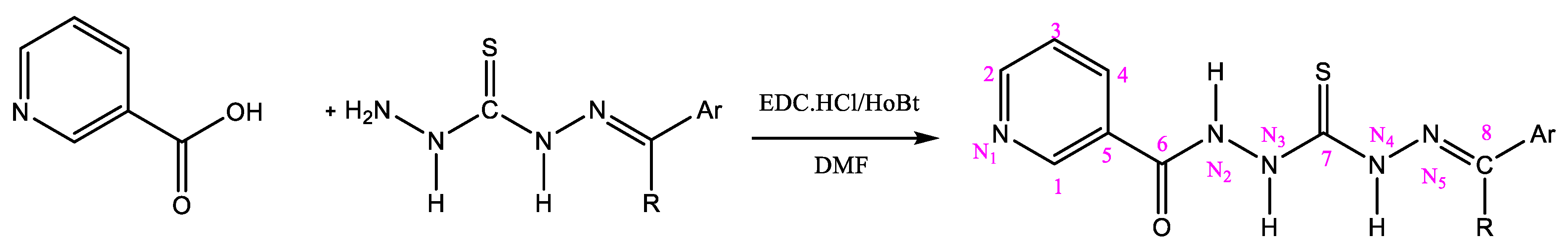
2.3. General Procedure for the Preparation of Nicotinamides (NC 1–7)
2.4. Antimicrobial Susceptibility Testing by Resazurin Assay
2.5. Microbe Growth Curve Analysis
2.6. Target Fishing
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemistry
3.1.1. (NC 1) 1-[(2-Hydroxyphenyl) methyl]-5-(pyridine-3-carbonyl) Dihydrazide Thiocarbonyl Acid
3.1.2. (NC 2) 1-(2-Pyridinylmethylene)-5-(pyridine-3-carbonyl) Dihydrazide Thiocarbonyl Acid
3.1.3. (NC 3) 1-[1-(2-Pyridinyl)ethylidene]-5-(pyridine-3-carbonyl) Dihydrazide Thiocarbonyl Acid
3.1.4. (NC 4) 1-(8-Quinolylmethylene)-5-(pyridine-3-carbonyl) Dyhidrazide Thiocarbonyl Acid
3.1.5. (NC 5) 1-(2-Quinolylmethylene)-5-(pyridine-3-carbonyl) Dihydrazide Thiocarbonyl Acid
3.1.6. (NC 6) 1-[(8-Hydroxy)-2-quinolylmethylene]-5-(pyridine-3-carbonyl) Dihydrazide Thiocarbonyl Acid
3.1.7. (NC 7) 1-(Phenyl-methylen)-5-(pyridine-3-carbonyl) Dihydrazide Thiocarbonyl Acid
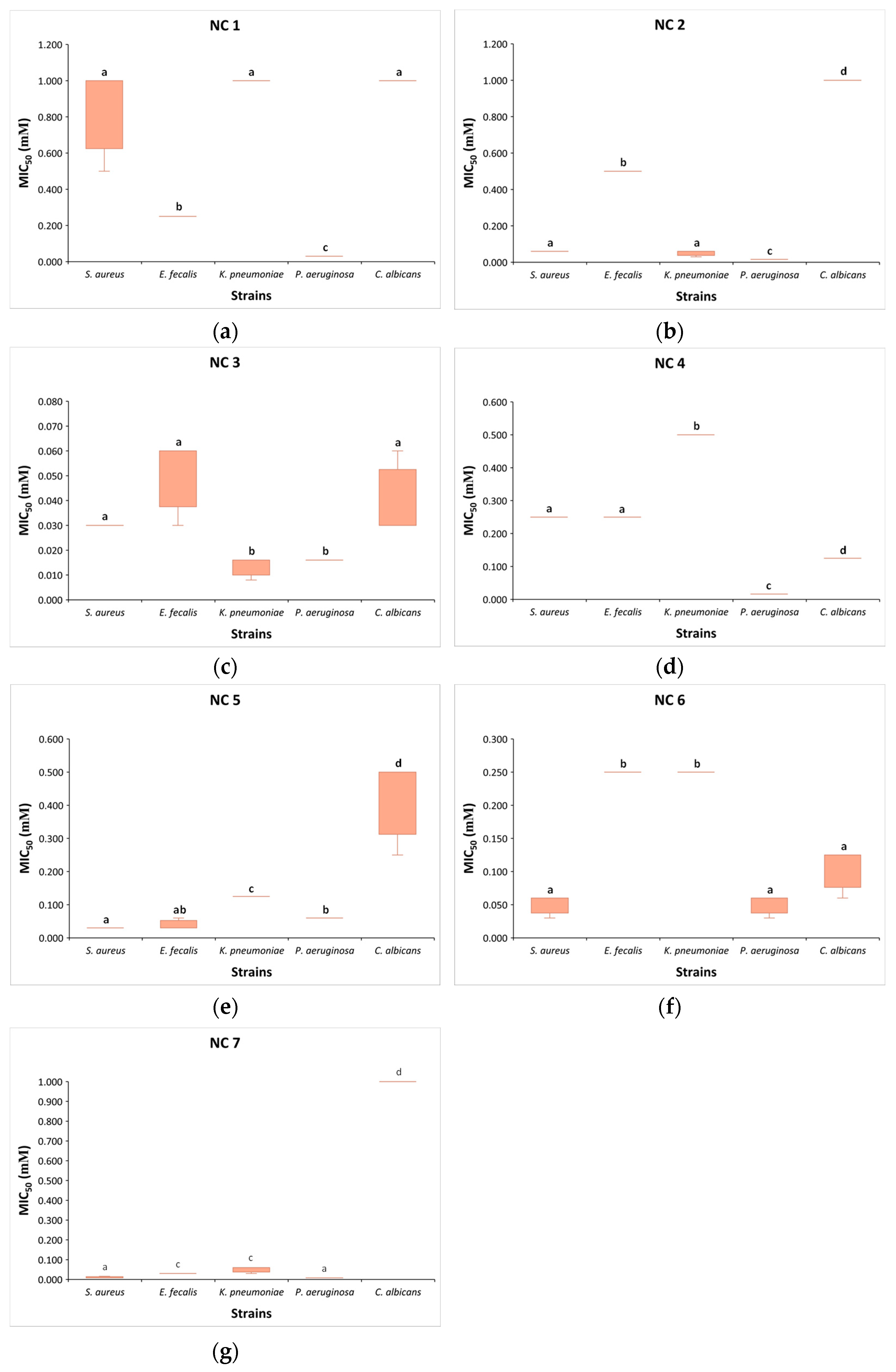
3.2. Minimum Inhibitory Concentrations of NC 1–7
3.3. Growth Curve Analysis
3.4. Target Fishing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, P629–P655. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Surveillance in Europe 2023—2021 Data; European Centre for Disease Prevention and Control and World Health Organization: Stockholm, Sweden, 2023; ISBN 9789289058537. Available online: www.who.int/europe/publications/i/item/9789289058537 (accessed on 2nd June 2024).
- Denning, D.W. Antifungal drug resistance: An update. Eur. J. Hosp. Pharm. 2022, 29, 109–112. [Google Scholar] [CrossRef]
- Raut, A.; Huy, N.T. Rising incidence of mucormycosis in patients with COVID-19: Another challenge for India amidst the second wave? Lancet Respir. Med. 2021, 9, e77. [Google Scholar] [CrossRef]
- Nnadi, N.E.; Carter, D.A. Climate change and the emergence of fungal pathogens. PLoS Pathog. 2021, 17, e1009503. [Google Scholar] [CrossRef]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006024-1. Available online: www.who.int/publications/i/item/9789240060241 (accessed on 4 February 2024).
- Mijatovic, S.; Stankovic, J.A.; Calovski, I.C.; Dubljanin, E.; Pljevljakusic, D.; Bigovic, D.; Dzamic, A. Antifungal Activity of Lavandula angustifolia Essential Oil against Candida albicans: Time-Kill Study on Pediatric Sputum Isolates. Molecules 2022, 27, 6300. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.A.; Chistyakov, E.M. Progress in dental materials: Application of natural ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Yudaev, P.; Butorova, I.; Chuev, V.; Posokhova, V.; Klyukin, B.; Chistyakov, E. Wound Gel with Antimicrobial Effects Based on Polyvinyl Alcohol and Functional Aryloxycyclotriphosphazene. Polymers 2023, 15, 2831. [Google Scholar] [CrossRef]
- Yılmaz, G.E.; Göktürk, I.; Ovezova, M.; Yılmaz, F.; Kılıç, S.; Denizli, A. Antimicrobial Nanomaterials: A Review. Hygiene 2023, 3, 269–290. [Google Scholar] [CrossRef]
- Jiang, H.; Li, L.; Li, Z.; Chu, X. Metal-based nanoparticles in antibacterial application in biomedical field: Current development and potential mechanisms. Biomed. Microdevices. 2024, 26, 12. [Google Scholar] [CrossRef]
- AlSaleh, A.; Shahid, M.; Farid, E.; Kamal, N.; Bindayna, K. Synergistic antimicrobial effect of ascorbic acid and nicotinamide with rifampicin and vancomycin against SCCmec type IV methicillin-resistant Staphylococcus aureus (MRSA). Access. Microbiol. 2023, 5, 000475.v4. [Google Scholar] [CrossRef]
- Mourenza, Á.; Gil, J.A.; Mateos, L.M.; Letek, M. Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance. Antioxidants 2020, 9, 361. [Google Scholar] [CrossRef]
- Thomas, A.B.; Nanda, R.K.; Kothapalli, L.P.; Deshpande, A.D. Synthesis and antimicrobial activity of N-[2-(aryl/substituted aryl)-4-oxo-1,3-thiazolidin-3-yl]pyridine-4-carboxamide. J. Kor. Chem. Soc. 2011, 55, 960–968. [Google Scholar] [CrossRef]
- da Silva, I.M.; da Silva Filho, J.; Santiago, P.B.; do Egito, M.S.; de Souza, C.A.; Gouveia, F.L.; Ximenes, R.M.; de Sena, K.X.; de Faria, A.R.; Brondani, D.J.; et al. Synthesis and antimicrobial activities of 5-Arylidene-thiazolidine-2,4-dione derivatives. Biomed. Res. Int. 2014, 2014, 316082. [Google Scholar] [CrossRef]
- Shahzad, S.; Ashraf, M.A.; Sajid, M.; Shahzad, A.; Rafique, A.; Mahmood, M.S. Evaluation of synergistic antimicrobial effect of vitamins (A, B1, B2, B6, B12, C, D, E and K) with antibiotics against resistant bacterial strains. J. Glob. Antimicrob. Resist. 2018, 13, 231. [Google Scholar] [CrossRef]
- Wu, J.; Kang, S.; Luo, L.; Shi, Q.; Ma, J.; Yin, J.; Song, B.; Hu, D.; Yang, S. Synthesis and antifungal activities of novel nicotinamide derivatives containing 1,3,4-oxadiazole. Chem. Central J. 2013, 7, 64. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, D.; Kuang, J.; Cai, H.; Wu, S.; Xue, W. Synthesis and antifungal activity of N-(substituted pyridinyl)-1-methyl(phenyl)-3-(trifluoromethyl)-1H-pyrazole-4-carboxa-mide derivatives. Molecules 2012, 17, 14205–14218. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Liao, Z.; Tan, F.; Zhu, Z.; Jiang, Y.; Cao, Y. Effect of Nicotinamide against Candida albicans. Front. Microbiol. 2019, 10, 595. [Google Scholar] [CrossRef]
- Tcherniuk, S.O.; Chesnokova, O.; Oleinikov, I.V.; Oleinikov, A.V. Nicotinamide inhibits the growth of P. falciparum and enhances the antimalarial effect of artemisinin, chloroquine and pyrimethamine. Mol. Biochem. Parasitol. 2017, 216, 14–20. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, Z.; Yin, S.; Duan, D.; Liao, X.; Xiao, Y.; He, J.; Zhong, J.; Zeng, Z.; Su, L.; et al. Nicotinamide mitigates visceral leishmaniasis by regulating inflammatory response and enhancing lipid metabolism. Parasit Vectors. 2024, 1, 288. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ Metabolism and Its Roles in Cellular Processes during Ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Bratic, A.; Larsson, N.-G. The Role of Mitochondria in Aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Zeidler, J.D.; Kashyap, S.; Warner, G.; Chini, E.N. Evolving Concepts in NAD+ Metabolism. Cell Metab. 2021, 33, 1076–1087. [Google Scholar] [CrossRef]
- Soma, M.; Lalam, S.K. The Role of Nicotinamide Mononucleotide (NMN) in Anti-Aging, Longevity, and Its Potential for Treating Chronic Conditions. Mol. Biol. Rep. 2022, 49, 9737–9748. [Google Scholar] [CrossRef]
- Crino, A.; Schia ni, R.; Manfrini, S.; Mesturino, C.; Visalli, N.; Beretta Anguissola, G.; Suraci, C.; Pitocco, D.; Spera, S.; Corbi, S.; et al. A randomized trial of nicotinamide and vitamin e in children with recent onset type 1 diabetes (imdiab ix). Eur. J. Endocrinol. 2004, 150, 719–724. [Google Scholar] [CrossRef]
- Gale, E.A.; Bingley, P.J.; Emmett, C.L.; Collier, T.; European Nicotinamide Diabetes Intervention Trial Group. European nicotinamide diabetes intervention trial (endit): A randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004, 363, 925–931. [Google Scholar] [CrossRef]
- Yang, J.; Klaidman, L.K.; Nalbandian, A.; Oliver, J.; Chang, M.L.; Chan, P.H.; Adams, J.D., Jr. The efects of nicotinamide on energy metabolism following transient focal cerebral ischemia in wistar rats. Neurosci. Lett. 2002, 333, 91–94. [Google Scholar] [CrossRef]
- Anderson, D.W.; Bradbury, K.A.; Schneider, J.S. Broad neuroprotective profile of nicotinamide in different mouse models of mptp-induced parkinsonism. Eur. J. Neurosci. 2008, 28, 610–617. [Google Scholar] [CrossRef]
- Wang, P.; Miao, C.Y. Nampt as a therapeutic target against stroke. Trends Pharmacol. Sci. 2015, 36, 891–905. [Google Scholar] [CrossRef]
- Hathorn, T.; Snyder-Keller, A.; Messer, A. Nicotinamide improves motor deficits and upregulates pgc-1alpha and bdnf gene expression in a mouse model of huntington’s disease. Neurobiol. Dis. 2011, 41, 43–50. [Google Scholar] [CrossRef]
- Naia, L.; Rosenstock, T.R.; Oliveira, A.M.; Oliveira-Sousa, S.I.; Caldeira, G.L.; Carmo, C.; Laco, M.N.; Hayden, M.R.; Oliveira, C.R.; Rego, A.C. Comparative mitochondrial-based protective effects of resveratrol and nicotinamide in huntington’s disease models. Mol. Neurobiol. 2017, 54, 5385–5399. [Google Scholar] [CrossRef]
- Green, K.N.; Steffan, J.S.; Martinez-Coria, H.; Sun, X.; Schreiber, S.S.; Thompson, L.M.; LaFerla, F.M. Nicotinamide restores cognition in alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of thr231-phosphotau. J. Neurosci. 2008, 28, 11500–11510. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.G.; Matsui, T.C.; Godin, A.M.; Gomides, L.F.; Pereira-Silva, P.E.; Duarte, I.D.; Menezes, G.B.; Coelho, M.M.; Klein, A. Neutrophil recruitment is inhibited by nicotinamide in experimental pleurisy in mice. Eur. J. Pharmacol. 2012, 685, 198–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snaidr, V.A.; Damian, D.L.; Halliday, G.M. Nicotinamide for photoprotection and skin cancer chemoprevention: A review of efficacy and safety. Exp. Dermatol. 2019, 28 (Suppl. 1), 15–22. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.F. Nicotinamide: An oral antimicrobial agent with activity against both Mycobacterium tuberculosis and human immunodeficiency virus. Clin. Infect. Dis. 2003, 36, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.F.; Srinivasan, A. Nicotinamide inhibits hiv-1 in both acute and chronic in vitro infection. Biochem. Biophys. Res. Commun. 1995, 210, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Sarkany, R.P.; Breathnach, S.M.; Morris, A.A.; Weismann, K.; Flynn, P.D. Metabolic and nutritional disorders. In Rook’s Textbook of Dermatology, 8th ed.; Burns, T., Breathnach, S., Cox, N., Griffiths, C., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 59–63. Available online: https://insight.cumbria.ac.uk/id/eprint/2737/1/Cox_RooksTextbookOfDermatology.pdf (accessed on 3 July 2024).
- Dedee, F.; Murrell, M.R.-Q. Management and Prognosis of Bullous Pemphigoid. 2019. Available online: https://www.uptodate.com/contents/management-and-prognosis-of-bullous-pemphigoid (accessed on 5 August 2024).
- Damian, D.L.; Patterson, C.R.; Stapelberg, M.; Park, J.; Barnetson, R.S.; Halliday, G.M. Uv radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J. Investig. Dermatol. 2008, 128, 447–454. [Google Scholar] [CrossRef]
- Song, S.B.; Park, J.S.; Chung, G.J.; Lee, I.H.; Hwang, E.S. Diverse therapeutic eficacies and more diverse mechanisms of nicotinamide. Metabolomics 2019, 15, 137. [Google Scholar] [CrossRef]
- Assaleh, M. Imino Derivatives of Carbonothionic Dihydrazides and Cinnamic Acids Amides: Structure-Activity Relationship Studies. Doctoral Dissertation, NaRDuS, Provence, France, 2022; p. 17. Available online: https://nardus.mpn.gov.rs/handle/123456789/21484 (accessed on 10 May 2024).
- Islam, B.; Islam, I.; Nath, N.; Emran, B.T.; Rahman, R.M.; Sharma, R.; Matin, M.M. Recent Advances in Pyridine Scaffold: Focus on Chemistry, Synthesis, and Antibacterial Activities. Biomed. Res. Int. 2023, 2023, 9967591. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0, 2024. Available online: http://www.eucast.org (accessed on 22 April 2024).
- Stevenson, K.; McVey, A.F.; Clark, I.B.N.; Swain, P.S.; Pilizota, T. General calibration of microbial growth in microplate readers. Sci. Rep. 2016, 6, 38828. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic. Acids. Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, C.; Gong, J.; Liu, X.; Li, H. Enhancing the Enrichment of Pharmacophore-Based Target Prediction for the Polypharmacological Profiles of Drugs. J. Chem. Inf. Model. 2016, 56, 1175–1183. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, F.; Ouyang, S.; Wang, X.; Li, H.; Jiang, H. Cyndi: A multi-objective evolution algorithm based method for bioactive molecular conformational generation. BMC Bioinform. 2009, 10, 101. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.H.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA suite of programs: An versatile platform for cheminformatics and drug design projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lavogina, D.; Lust, H.; Tahk, M.J.; Laasfeld, T.; Vellama, H.; Nasirova, N.; Vardja, M.; Eskla, K.L.; Salumets, A.; Rinken, A.; et al. Revisiting the Resazurin-Based Sensing of Cellular Viability: Widening the Application Horizon. Biosensors 2022, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Kafa, A.H.T.; Tüzün, G.; Güney, E.; Aslan, R.; Sayın, K.; Tüzün, B.; Ataseven, H. Synthesis, computational analyses, antibacterial and antibiofilm properties of nicotinamide derivatives. Struct. Chem. 2022, 33, 1189–1197. [Google Scholar] [CrossRef]
- Dang, T.; Nizamov, I.S.; Salikhov, R.Z.; Sabirzyanova, L.R.; Vorobev, V.V.; Burganova, T.I.; Shaidoullina, M.M.; Batyeva, E.S.; Cherkasov, R.A.; Abdullin, T.I. Synthesis and characterization of pyridoxine, nicotine and nicotinamide salts of dithiophosphoric acids as antibacterial agents against resistant wound infection. Bioorg. Med. Chem. 2019, 27, 100–109. [Google Scholar] [CrossRef]
- Adamiec, M.; Adamus, J.; Ciebiada, I.; Denys, A.; Gebicki, J. Search for drugs of the combined anti-inflammatory and anti-bacterial properties: 1-methyl-N’-(hydroxymethyl)nicotinamide. Pharmacol. Rep. 2006, 58, 246–249. Available online: http://if-pan.krakow.pl/pjp/pdf/2006/2_246.pdf (accessed on 15 June 2024).
- Wang, W.; Liu, X.J.; Lin, G.T.; Wu, J.P.; Xu, G.; Xu, D. Novel N-(1H-Pyrazol-5-yl)nicotinamide Derivatives: Design, Synthesis and Antifungal Activity. Chem. Biodivers. 2022, 19, e202101032. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, G.; Khalaf, H.; Naglah, A.; Al-Wasidi, A.; Al-Jafshar, N.; Awad, H. The Synthesis of Molecular Docking Studies, In Vitro Antimicrobial and Antifungal Activities of Novel Dipeptide Derivatives Based on N-(2-(2-Hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide. Molecules 2018, 23, 761. [Google Scholar] [CrossRef]
- Ni, T.; Xie, F.; Li, L.; Hao, Y.; Chi, X.; Yan, L.; Zhang, D.; Jiang, Y.; Lv, Q. Design, Synthesis and Structure-Activity Relationship Studies of Nicotinamide Derivatives as Potent Antifungal Agents by Disrupting Cell Wall. Molecules 2023, 28, 1135. [Google Scholar] [CrossRef]
- Assaleh, M.H.; Bjelogrlic, S.; Prlainovic, N.; Cvijetic, I.; Bozic, A.; Arandjelovic, I.; Vukovic, D.; Marinkovic, A. Antimycobacterial and anticancer activity of newly designed cinnamic acid hydrazides with favorable toxicity profile. Arab. J. Chem. 2022, 15, 103532. [Google Scholar] [CrossRef]
- Maruyama, M.; Kumagai, T.; Matoba, Y.; Hayashida, M.; Fujii, T.; Hata, Y.; Sugiyama, M. Crystal structures of the transposon Tn5-carried bleomycin resistance determinant uncomplexed and complexed with bleomycin. J. Biol. Chem. 2001, 276, 9992–9999. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Miller, M.C.; Grkovic, S.; Brown, M.H.; Skurray, R.A.; Brennan, R.G. Structural mechanisms of QacR induction and multidrug recognition. Science 2001, 294, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Sugantino, M.; Roderick, S.L. Crystal structure of Vat(D): An acetyltransferase that inactivates streptogramin group A antibiotics. Biochemistry 2002, 41, 2209–2216. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]



| Compounds | Ar | R | |
|---|---|---|---|
| NC 1 | 1-[(2-hydroxyphenyl)methyl]-5-(pyridine-3-carbonyl)dihydrazide thiocarbonyl acid |  | H |
| NC 2 | 1-(2-pyridinylmethylene)-5-(pyridine-3-carbonyl)dyhidrazide thiocarbonyl acid | 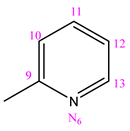 | H |
| NC 3 | 1-[1-(2-pyridinyl)ethylidene]-5-(pyridine-3-carbonyl)dyhidrazide thiocarbonyl acid | 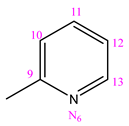 | CH3 |
| NC 4 | 1-(8-hinolylmethylene)-5-(pyridine-3-carbonyl)dyhidrazide thiocarbonyl acid | 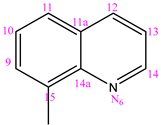 | H |
| NC 5 | 1-(2-hynolilmethylene)-5-(pyridine-3-carbonyl)dyhidrazide thiocarbonyl acid | 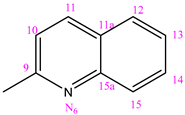 | H |
| NC 6 | 1-[(8-hydroxy)-2-hynolilmethilen]-5-(pyridine-3-carbonyl)dyhidrazide thiocarbonyl acid | 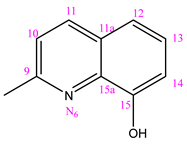 | H |
| NC 7 | 1-(phenyl-methylen)-5-(pyridine-3-carbonyl)dyhidrazide thiocarbonyl acid | 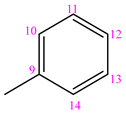 | H |
| Compound | MIC | Microorganism | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus (ATCC 6538) | E. faecalis (ATCC 29212) | K. pneumoniae (ATCC 27853) | P. aeruginosa (NCIMB 9111) | C. albicans (ATCC 24433) | |||||||
| mg/mL | mM | mg/mL | mM | mg/mL | mM | mg/mL | mM | mg/mL | mM | ||
| NC 1 | MICR | >0.3154 | >1 | >0.3154 | >1 | >0.3154 | >1 | 0.3154 | 1 | >0.3154 | >1 |
| MIC50 | 0.3154 | 1 | 0.0788 | 0.25 | 0.3154 | 1 | 0.0101 | 0.032 | 0.3154 | 1 | |
| MIC90 | >0.3154 | >1 | >0.3154 | >1 | >0.3154 | >1 | 0.3154 | 1 | >0.3154 | >1 | |
| NC 2 | MICR | >0.3033 | >1 | >0.3033 | >1 | 0.1517 | 0.5 | 0.3033 | 1 | >0.3033 | >1 |
| MIC50 | 0.0194 | 0.064 | 0.1517 | 0.5 | 0.0194 | 0.064 | 0.0194 | 0.016 | 0.3033 | 1 | |
| MIC90 | >0.3033 | >1 | >0.3033 | >1 | 0.1517 | 0.5 | 0.1517 | 0.5 | >0.3033 | >1 | |
| NC 3 | MICR | >0.3144 | >1 | >0.3144 | >1 | 0.0101 | 0.032 | 0.0101 | 0.032 | 0.3144 | 1 |
| MIC50 | 0.0101 | 0.032 | 0.0201 | 0.064 | 0.0050 | 0.016 | 0.0050 | 0.016 | 0.0101 | 0.032 | |
| MIC90 | >0.3144 | >1 | >0.3144 | >1 | 0.0201 | 0.064 | 0.0101 | 0.032 | 0.0201 | 0.064 | |
| NC 4 | MICR | >0.3504 | >1 | >0.3504 | >1 | >0.3504 | >1 | 0.3504 | 1 | 0.1752 | 0.5 |
| MIC50 | 0.0876 | 0.25 | 0.0876 | 0.25 | 0,1752 | 0.5 | 0.0056 | 0.016 | 0.0438 | 0.125 | |
| MIC90 | >0.3504 | >1 | >0.3504 | >1 | >0.3504 | >1 | 0.0438 | 0.125 | 0.0876 | 0.25 | |
| NC 5 | MICR | >0.3504 | >1 | >0.3504 | >1 | >0.3504 | >1 | 0.3504 | 1 | >0.3504 | >1 |
| MIC50 | 0,0112 | 0.032 | 0.0112 | 0.032 | 0.0438 | 0.125 | 0.0224 | 0.064 | 0.1752 | 0.5 | |
| MIC90 | >0.3504 | >1 | >0.3504 | >1 | 0.1752 | 0.5 | 0.3504 | 1 | >0.3504 | >1 | |
| NC 6 | MICR | >0.3064 | >1 | >0.3064 | >1 | 0.3064 | 1 | >0.3064 | >1 | >0.3064 | >1 |
| MIC50 | 0.0196 | 0.064 | 0.0766 | 0.25 | 0.0766 | 0.25 | 0.0196 | 0.064 | 0.0383 | 0.125 | |
| MIC90 | >0.3064 | >1 | >0.3064 | >1 | >0.3064 | >1 | 0.3064 | 1 | >0.3064 | >1 | |
| NC 7 | MICR | >0.2994 | >1 | >0.2994 | >1 | 0.1497 | 0.5 | >0.2994 | >1 | >0.2994 | >1 |
| MIC50 | 0.0024 | 0.008 | 0.0096 | 0.032 | 0.0147 | 0.06 | 0.0024 | 0.008 | 0.2994 | 1 | |
| MIC90 | >0.2994 | >1 | >0.2994 | >1 | >0.2994 | >1 | >0.2994 | >1 | >0.2994 | >1 | |
| Levofloxacin | MICR | μg/mL | μg/mL | μg/mL | μg/mL | N/A | |||||
| 0.001 | 4 | 0.125 | 0.5 | ||||||||
| Amikacin | MICR | μg/mL | N/A | μg/mL | μg/mL | N/A | |||||
| 16 | 8 | 16 | |||||||||
| Fluconazole | MICR | N/A | N/A | N/A | N/A | μg/mL | |||||
| 2 | |||||||||||
| Target | PDB Code | Biological Role | z-Score | Reference |
|---|---|---|---|---|
| Bleomycin resistance protein | 1EWJ | Confers defense against bleomycin-induced DNA cleavage in cells | 4.10 | [62] |
| HTH-type transcriptional regulator QacR | 1JT6 | Regulates the expression of the QacA multidrug efflux pump in S. aureus | 2.81 | [63] |
| Streptogramin A acetyltransferase | 1KHR | Xenobiotic acetyltransferase responsible for streptogramin resistance phenotype | 2.12 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, B.A.; Marinković, A.; Stanković, J.A.; Mijatović, S.; Cvijetić, I.; Simić, M.; Arandjelović, I. Synthesis and Antimicrobial Activity of Newly Synthesized Nicotinamides. Pharmaceutics 2024, 16, 1084. https://doi.org/10.3390/pharmaceutics16081084
Marković BA, Marinković A, Stanković JA, Mijatović S, Cvijetić I, Simić M, Arandjelović I. Synthesis and Antimicrobial Activity of Newly Synthesized Nicotinamides. Pharmaceutics. 2024; 16(8):1084. https://doi.org/10.3390/pharmaceutics16081084
Chicago/Turabian StyleMarković, Bojana Anić, Aleksandar Marinković, Jelena Antić Stanković, Stefan Mijatović, Ilija Cvijetić, Milena Simić, and Irena Arandjelović. 2024. "Synthesis and Antimicrobial Activity of Newly Synthesized Nicotinamides" Pharmaceutics 16, no. 8: 1084. https://doi.org/10.3390/pharmaceutics16081084
APA StyleMarković, B. A., Marinković, A., Stanković, J. A., Mijatović, S., Cvijetić, I., Simić, M., & Arandjelović, I. (2024). Synthesis and Antimicrobial Activity of Newly Synthesized Nicotinamides. Pharmaceutics, 16(8), 1084. https://doi.org/10.3390/pharmaceutics16081084






