Synthesis, Characterization, and Cytotoxicity Evaluation of Chlorambucil-Functionalized Mesoporous Silica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Mesoporous Silica Nanoparticles (MSNs)
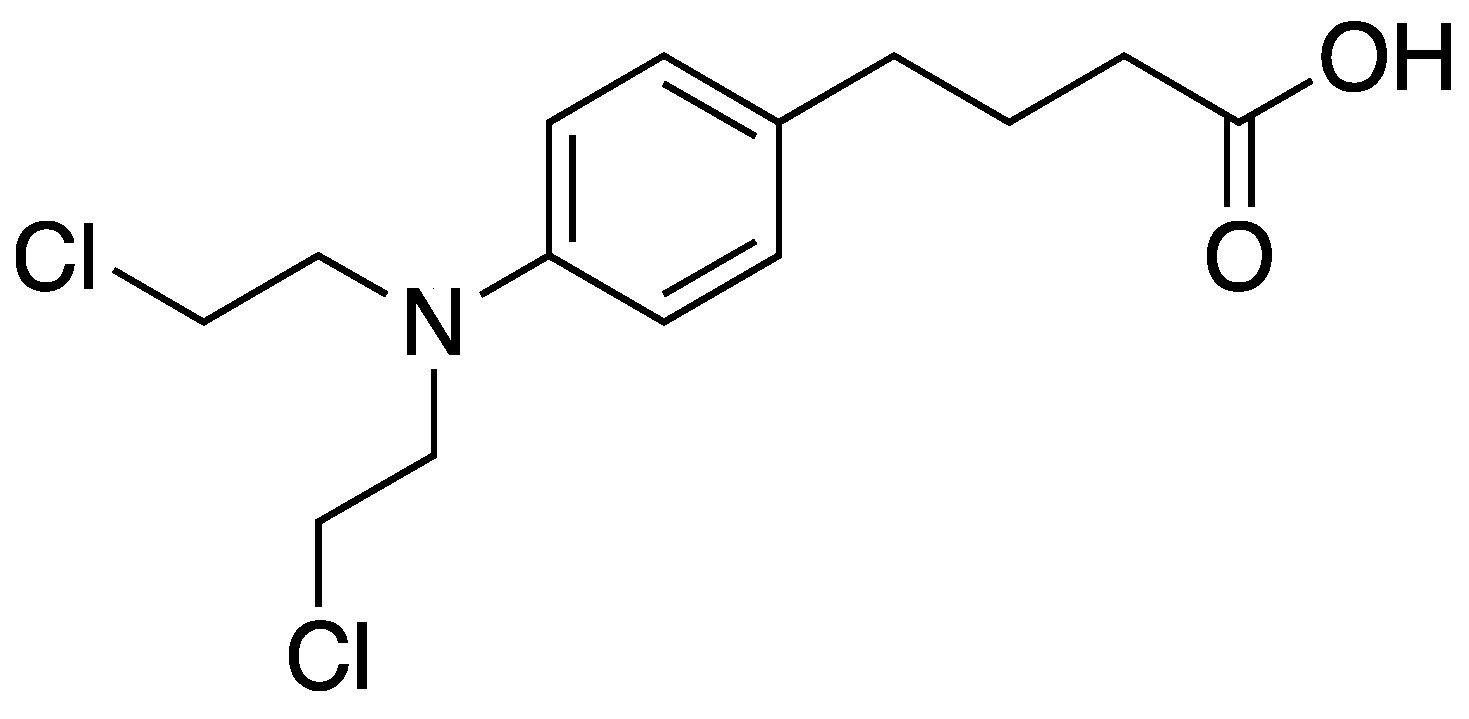
2.3. Synthesis of Chlorambucil Grafted MSNs
2.4. Ninhydrin Assay (2,2-Dihydroxyindane-1,3-dione)
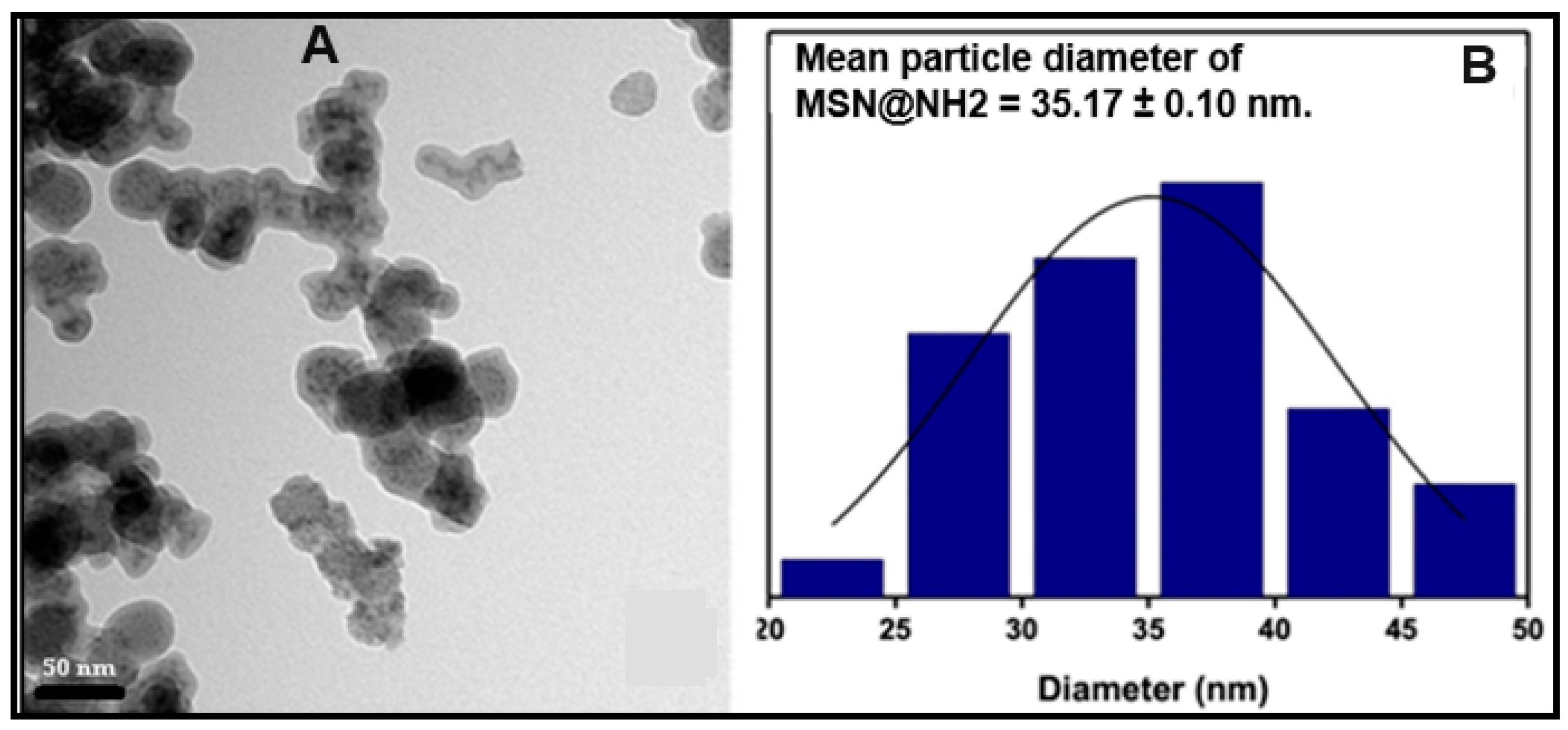
2.5. Particle Size, Size Distribution
2.6. Scanning Electron Microscopy—Field Emission Gun (SEM-FEG)
2.7. Transmission Electron Microscopy (TEM)
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Detecting Free Amino Groups on MSN@NH2-CLB Nanoparticle Surface
2.10. CHN and Cl Elemental Analyses
2.11. Thermogravimetric Analysis (TGA)
2.12. Cytotoxicity Assay
3. Results and Discussion
3.1. Nanoparticle Synthesis and Characterization
3.2. Preparing MSN Surfaces for Functionalization
3.3. MSNs@NH2-CLB Characterization
3.4. Elemental Analysis for Chlorine to Quantify CLB Loaded in Nanoparticles
3.5. Thermogravimetric Analysis
3.6. Cytotoxic Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunt, N. The global challenge of cancer governance. World Med. Health Policy 2023, 15, 672–681. [Google Scholar] [CrossRef]
- Rahikkala, A.; Pereira, S.A.P.; Figueiredo, P.; Passos, M.L.C.; Araújo, A.R.T.S.; Saraiva, M.L.M.F.S.; Santos, H.A. Mesoporous Silica Nanoparticles for Targeted and Stimuli Responsive Delivery of Chemotherapeutics: A Review. Adv. Biosyst. 2018, 2, 1800020. [Google Scholar] [CrossRef]
- Santos, M.O. Estimativa 2018: Incidência de Câncer no Brasil. Rev. Bras. Cancerol. 2018, 64, 119–120. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jia, Y.; Song, W.; Zhang, L. Therapeutic Potential of Nitrogen Mustard Based Hybrid Molecules. Front. Pharmacol. 2018, 9, 1453. [Google Scholar] [CrossRef]
- Leung, A.W.Y.; Kalra, J.; Santos, N.; Bally, M.B.; Anglesio, M.S. Harnessing the Potential of Lipid-Based Nanomedicines for Type-Specific Ovarian Cancer Treatments. Nanomedicine 2014, 9, 501–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Lin, H.Y.; Hung, S.K.; Chiou, W.Y.; Lee, M.S. Role of modern radiotherapy in managing patients with hepatocellular carcinoma. World J. Gastroenterol. 2021, 27, 2434–2457. [Google Scholar] [CrossRef] [PubMed]
- Gerson, S.L.; Caimi, P.F.; William, B.M.; Creger, R.J. Pharmacology and molecular mechanisms of antineoplastic agents for hematologic malignancies. In Hematology: Basic Principles and Practice, 7th ed.; Hoffman, R., Silberstein, L.E., Weitz, J.I., Salama, M.E., Benz, E.J., Jr., Heslop, H.E., Anastasi, J., Abutalib, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 849–912. [Google Scholar]
- Zhang, W.; Zhu, W.; He, R.; Fang, S.; Zhang, Y.; Yao, C.; Ismail, M.; Li, X. Improvement of Stability and Anticancer Activity of Chlorambucil—Tetrapeptide Conjugate Vesicles. Chin. J. Chem. 2016, 34, 609–616. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Guterres, S.S.; Freitas, L.L.; Pohlmann, A.R. Caracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Química Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Ventola, C. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Shen, S.C.; Ng, W.K.; Chia, L.S.O.; Dong, Y.C.; Tan, R.B.H. Applications of mesoporous materials as excipients for innovative drug delivery and formulation. Curr. Pharm. Des. 2013, 19, 6270–6289. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, A.S.; Nakhjiri, A.T.; Khakzad, M.J.; Rezayat, S.M.; Ebrahimnejad, P.; Heydarinasab, A.; Akbarzadeh, A.; Marjani, A. Mesoporous silica nanoparticles as a versatile nanocarrier for cancer treatment: A review. J. Mol. Liq. 2021, 328, 115417. [Google Scholar] [CrossRef]
- Ahmadi, F.; Taleghani, A.S.; Ebrahimnejad, P.; Moghaddam, S.P.H.; Ebrahimnejad, F.; Asare-Addo, K.; Nokhodchi, A. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. Int. J. Pharm. 2022, 625, 122099. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Ghoran, S.H.; Niakan, M.H.; Jamali, K.; Moeini, Z.; Jangjou, A.; Izadpanah, P.; Amani, A.M. Mesoporous silica nanoparticle: Heralding a brighter future in cancer nanomedicine. Microporous Mesoporous Mater. 2021, 319, 110967. [Google Scholar] [CrossRef]
- Chircov, C.; Spoială, A.; Păun, C.; Crăciun, L.; Ficai, D.; Ficai, A.; Andronescu, E.; Turculeƫ, S.C. Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents. Molecules 2020, 25, 3814. [Google Scholar] [CrossRef]
- Li, T.; Shi, S.; Goel, S.; Shen, X.; Xie, X.; Chen, Z.; Zhang, H.; Li, S.; Qin, X.; Yang, H.; et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater. 2019, 89, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, X.; Zhang, J.; Jin, G.; Luan, Y. Chlorambucil prodrug-participating catanionic aggregates for sustained drug release and improved antitumour activity. J. Mol. Liq. 2019, 274, 556–561. [Google Scholar] [CrossRef]
- Singh, G.; Nenavathu, B.P.; Imtiyaz, K.; Rizvi, M.M.A. Fabrication of chlorambucil loaded graphene- oxide nanocarrier and its application for improved antitumor activity. Biomed. Pharmacother. 2020, 129, 110443. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, Y.; Mu, G.; Yang, L.; Wang, W.; Liu, J.; Liu, J. A peptide–drug hydrogel to enhance the anti-cancer activity of chlorambucil. Biomater. Sci. 2020, 8, 5638–5646. [Google Scholar] [CrossRef] [PubMed]
- Hussein-Al-Ali, S.H.; Hussein, M.Z.; Bullo, S.; Arulselvan, P. Chlorambucil-Iron Oxide Nanoparticles as a Drug Delivery System for Leukemia Cancer Cells. Int. J. Nanomed. 2021, 16, 6205–6216. [Google Scholar] [CrossRef] [PubMed]
- Kulig, K.; Ziabka, M.; Pilarczyk, K.; Owczarzy, A.; Rogóz, W.; Maciazek-Jurczyk, M. Physicochemical Study of Albumin Nanoparticles with Chlorambucil. Processes 2022, 10, 1170. [Google Scholar] [CrossRef]
- Juárez, J.M.; Cussa, J.; Anunziata, O.A.; Costa, M.B.G. Mesoporous Cellular Foam (MCF): An efficient and biocompatible nanomaterial for the controlled release of Chlorambucil. J. Porous Mater. 2022, 29, 1507–1517. [Google Scholar] [CrossRef]
- Ovejero, P.K.; Díaz-Garcia, D.; Mena-Palomo, I.; Marciello, M.; Lozano-Chamizo, L.; Morato, Y.L.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Synthesis of a theragnostic platform based on fibrous silica nanoparticles for the enhanced treatment of triple-negative breast cancer promoted by a combination of chemotherapeutic agents. Biomater. Adv. 2022, 137, 212823. [Google Scholar]
- Nandiyanto, A.B.D.; Kim, S.G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Osseni, S.A. New nanoplatform based on Gd2O2S:Eu3+ core: Synthesis, characterization and use for in vitro bio-labelling. J. Mater. 2011, 21, 18365–18372. [Google Scholar] [CrossRef]
- Vijayashree, I.S.; Niranjana, P.; Prabhu, G.; Sureshbabu, V.V.; Manjanna, J. Conjugation of Au Nanoparticles with Chlorambucil for Improved Anticancer Activity. J. Clust. Sci. 2017, 28, 133–148. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Soliman, H.M.; El-Shweihy, N.M. Extracellular cholesterol oxidase production by Streptomyces aegypti, in vitro anticancer activities against rhabdomyosarcoma, breast cancer cell-lines and in vivo apoptosis. Sci. Rep. 2018, 8, 2706. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-R.; Lin, R.; Li, H.J.; He, W.L.; Du, J.Z.; Wang, J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 11, e1519. [Google Scholar] [CrossRef] [PubMed]
- Fanizza, E.; Urso, C.; Iacobazzi, R.M.; Depalo, N.; Corricelli, M.; Panniello, A.; Agostiano, A.; Denora, N.; Laquintana, V.; Striccoli, M.; et al. Fabrication of photoactive heterostructures based on quantum dots decorated with Au nanoparticles. Sci. Technol. Adv. Mater. 2016, 17, 98–108. [Google Scholar] [CrossRef]
- Ibrahim, A.S.S.; Al-Salamah, A.A.; El-Toni, A.M.; El-Tayeb, M.A.; Elbadawi, Y.B. Cyclodextrin glucan transferase immobilization onto functionalized magnetic double mesoporous core–shell silica nanospheres. Electron. J. Biotechnol. 2014, 17, 55–64. [Google Scholar] [CrossRef]
- Santos, I.M.D.; Pereira, S.P.; Mezacasa, A.; Cáceres, O.I.A.; Timóteo, F.; Lopes, A.S.; Del Pino, K.F.; Duarte, A.P.; Cardoso, T.F.M.; Castro, G.R.; et al. Emodin-containing MCM-41 Type Mesoporous Silica Nanoparticle Drug Delivery System. Theor. Exper. Chem. 2020, 56, 174–182. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A.; Nowak, I. APTES-functionalized mesoporous silica as a vehicle for antipyrine—Adsorption and release studies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 187–196. [Google Scholar] [CrossRef]
- Bruice, P.Y. Química Orgânica, 4th ed.; Pearson Education do Brasil: São Paulo, Brazil, 2006; 547p. [Google Scholar]
- Luo, C.-Q.; Zhou, Y.X.; Zhou, T.J.; Xing, L.; Cui, P.F.; Sun, M.; Jin, L.; Lu, N.; Jiang, H.L. Reactive oxygen species-responsive nanoprodrug with quinone methides-mediated GSH depletion for improved chlorambucil breast cancers therapy. J. Control. Release 2018, 274, 56–68. [Google Scholar] [CrossRef]
- Martines, M.A.U.; Davolos, M.R.; Jafelicci, M., Jr.; Souza, D.F.; Nunes, L.A.O. Cr3+ and Cr4+ luminescence in glass ceramic silica. J. Lumin. 2008, 128, 1787–1790. [Google Scholar] [CrossRef]
- Freitas, L.B.D.O.; Bravo, I.J.G.; Macedo, W.A.A.; Sousa, E.M.B. Mesoporous silica materials functionalized with folic acid: Preparation, characterization, and release profile study with methotrexate. J. Sol.-Gel. Sci. Technol. 2016, 77, 186–204. [Google Scholar] [CrossRef]
- Freitas, L.B.D.O.; Corgosinho, L.M.; Faria, J.A.Q.A.; Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; Sousa, E.M.B. Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Microporous Mesoporous Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]
- Karthik, S.; Jana, A.; Saha, B.; Kalyani, B.K.; Ghosh, S.K.; Zhao, Y.; Singh, N.D.P. Synthesis, and in vitro evaluation of charge reversal photo responsive quinoline tethered mesoporous silica for targeted drug delivery. J. Mater. Chem. B 2014, 2, 7971–7977. [Google Scholar] [CrossRef] [PubMed]
- Bézivin, C.; Tomasi, F.; Lohézie-Le, D.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef]


| Nanoparticles | Assignments (cm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| υ(H-O-H) | υ(C-H) | υ(C=O) | υ(O-H) | υ(N-H) | υ(Si-O) | υ(Si-OH) | υ(Si-O-Si) | υ(Si-O) | |
| MSN | ~3400 | - | - | 1640 | - | 1100 | 970 | 814 | 465 |
| MSN@NH2 | ~3400 | 2949 | - | 1635 | 1523 | 1112 | 960 | 802 | 467 |
| MSN@NH2-CLB | ~3400 | 2943 | 1620 | - | 1515 | 1110 | 962 | 804 | 469 |
| Mass | Nanoparticle | ||
|---|---|---|---|
| CLB | MSN@NH2 | MSN@NH2-CLB | |
| Chlorine (%) | 23.25 ± 0.05 | 0 | 1.63 ± 0.01 |
| CLB (mg) | 1000 ± 1.23 | 0 | 70,00 ± 0.05 |
| Substances | Cell Line | Selectivity Index | |||
|---|---|---|---|---|---|
| NIH-3T3 | A549 | CT26WT | A549 | CT26WT | |
| Chlorambucil | 85.50 ± 1.18 | 115.2 ± 1.21 | 62.39 ± 1.52 | 0.74 | 1.37 |
| MSN@NH2-CLB | 115.9 ± 1.13 | 17.71 ± 1.26 | 27.67 ± 1.17 | 6.54 | 4.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karnopp, J.C.F.; Jorge, J.; da Silva, J.R.; Boldo, D.; Del Pino Santos, K.F.; Duarte, A.P.; de Castro, G.R.; de Azevedo, R.B.; Prada, A.L.; Amado, J.R.R.; et al. Synthesis, Characterization, and Cytotoxicity Evaluation of Chlorambucil-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics 2024, 16, 1086. https://doi.org/10.3390/pharmaceutics16081086
Karnopp JCF, Jorge J, da Silva JR, Boldo D, Del Pino Santos KF, Duarte AP, de Castro GR, de Azevedo RB, Prada AL, Amado JRR, et al. Synthesis, Characterization, and Cytotoxicity Evaluation of Chlorambucil-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics. 2024; 16(8):1086. https://doi.org/10.3390/pharmaceutics16081086
Chicago/Turabian StyleKarnopp, Juliana Camila Fischer, Juliana Jorge, Jaqueline Rodrigues da Silva, Diego Boldo, Kristiane Fanti Del Pino Santos, Adriana Pereira Duarte, Gustavo Rocha de Castro, Ricardo Bentes de Azevedo, Ariadna Lafourcade Prada, Jesús Rafael Rodríguez Amado, and et al. 2024. "Synthesis, Characterization, and Cytotoxicity Evaluation of Chlorambucil-Functionalized Mesoporous Silica Nanoparticles" Pharmaceutics 16, no. 8: 1086. https://doi.org/10.3390/pharmaceutics16081086






