An Investigation on the Relationship between Dust Emission and Air Flow as Well as Particle Size with a Novel Containment Two-Chamber Setup
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
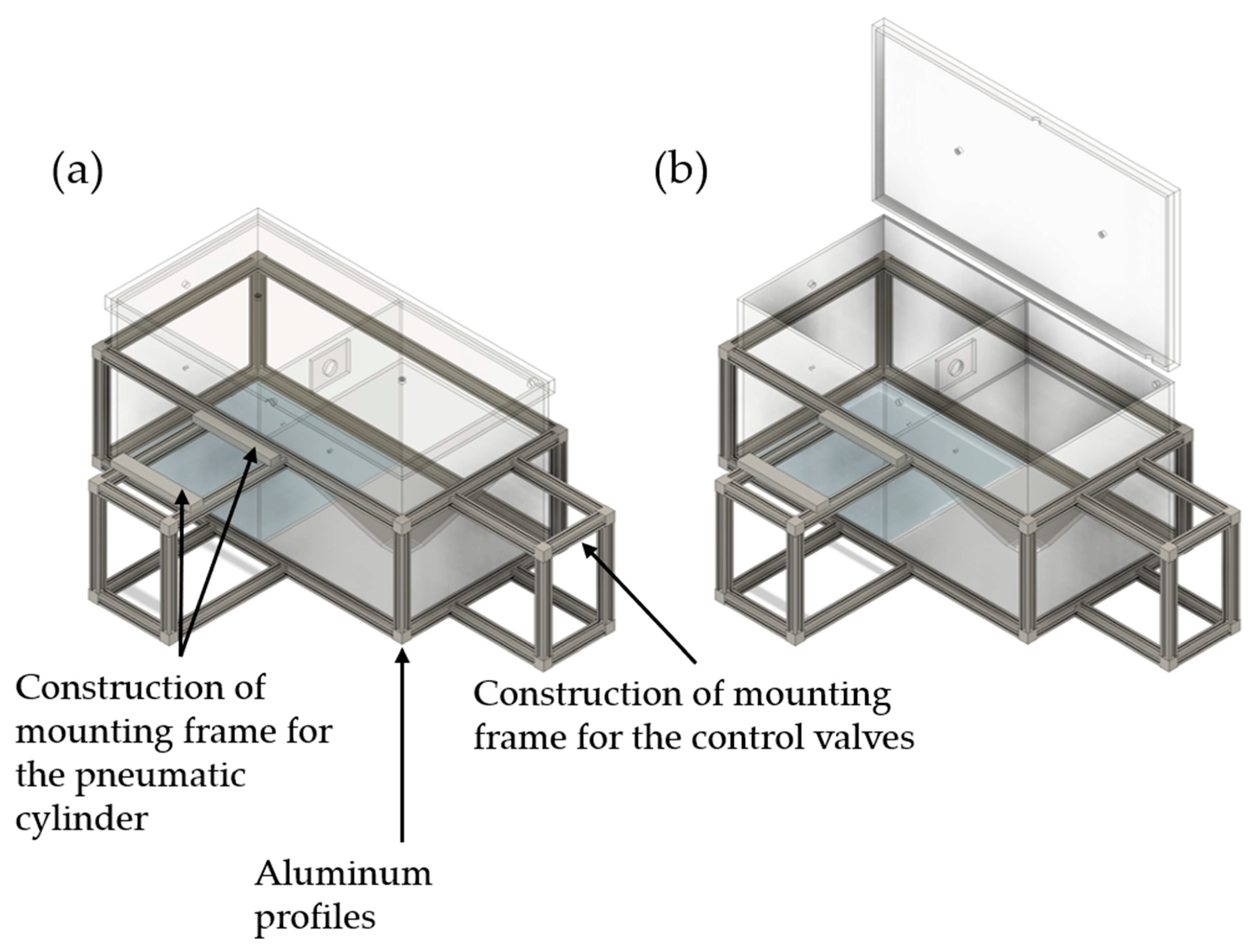
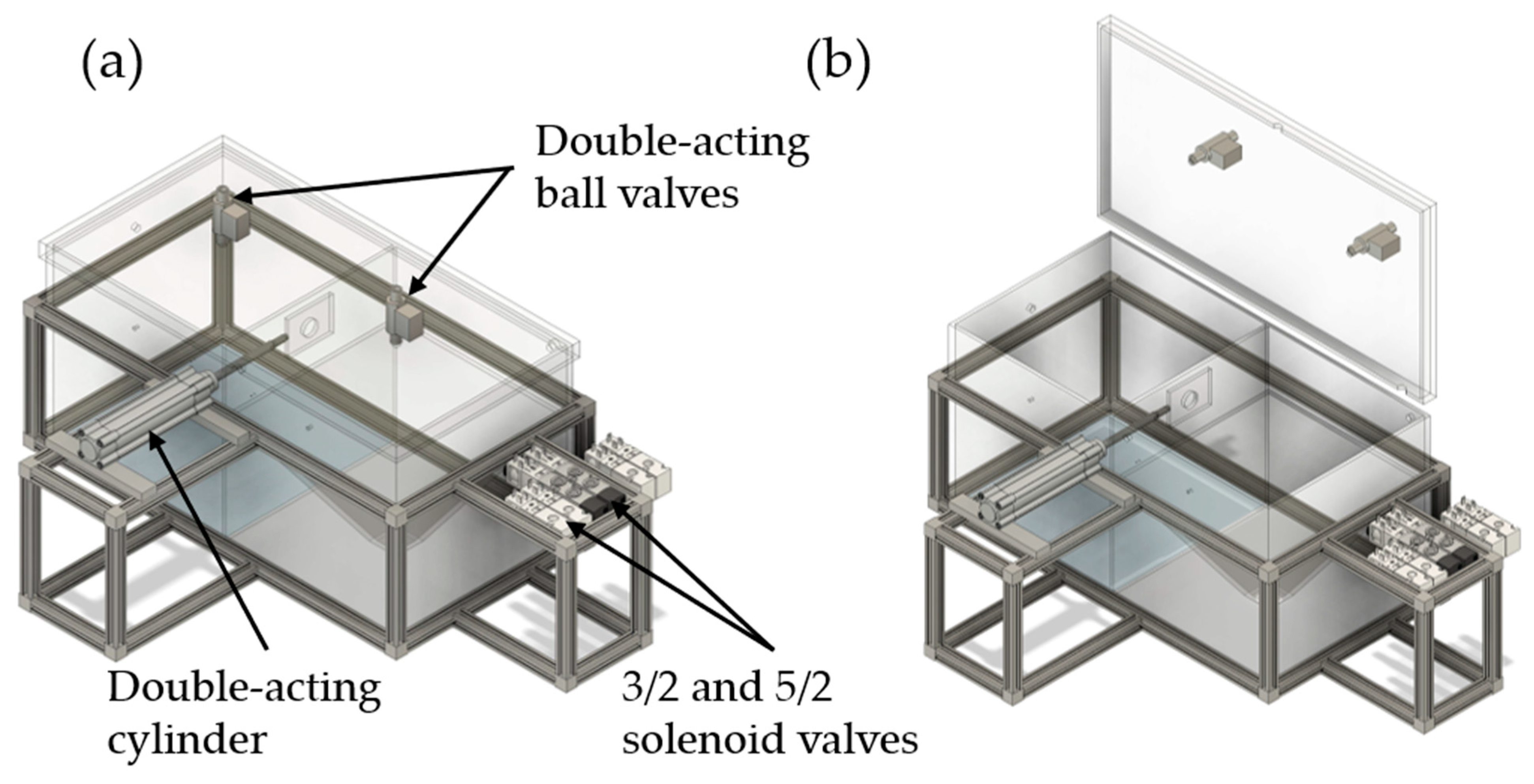
2.2. Two-Chamber Setup (TSC)
2.2.1. Design of the TCS
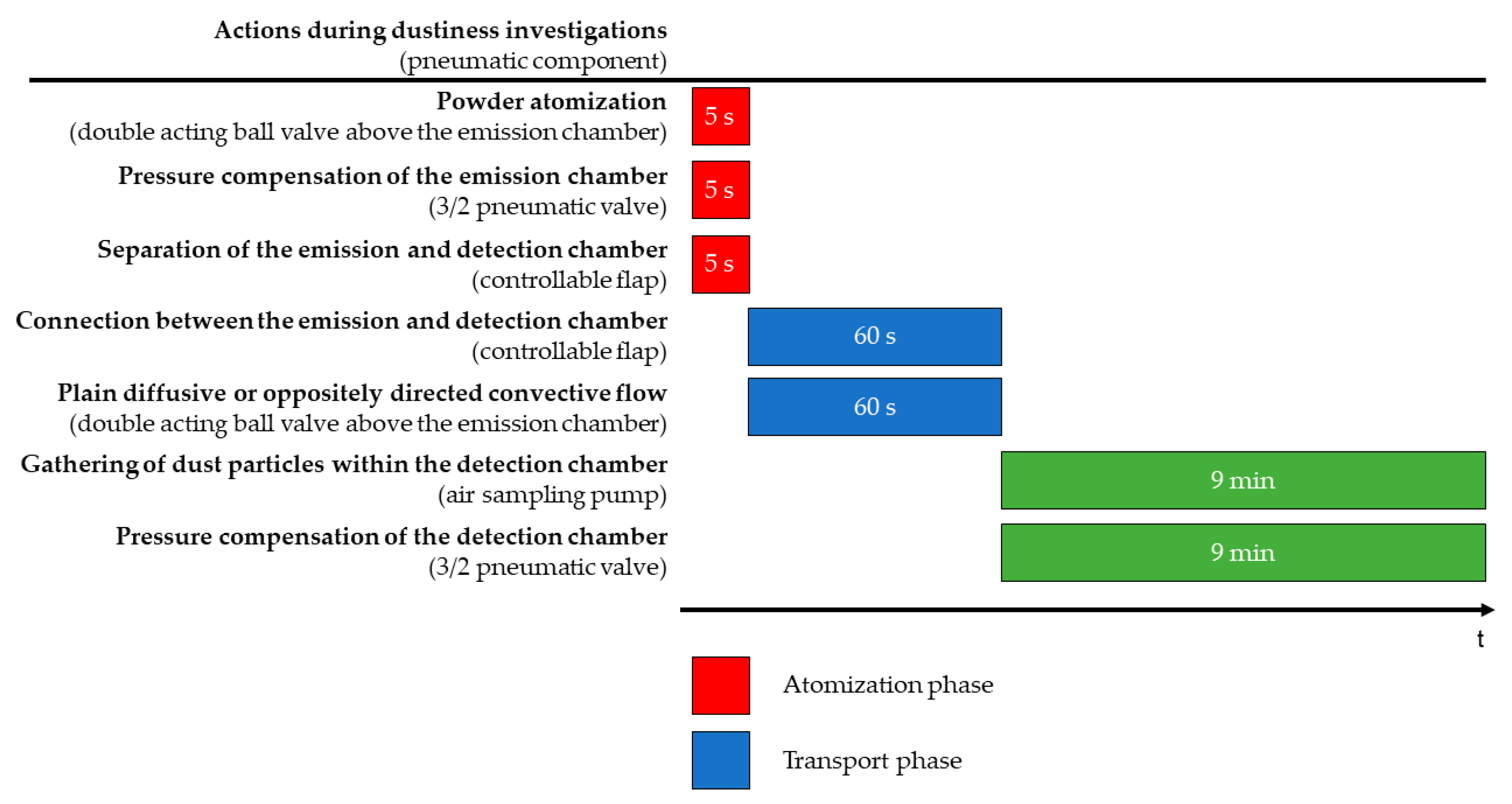
2.2.2. TCS Measurement Phases
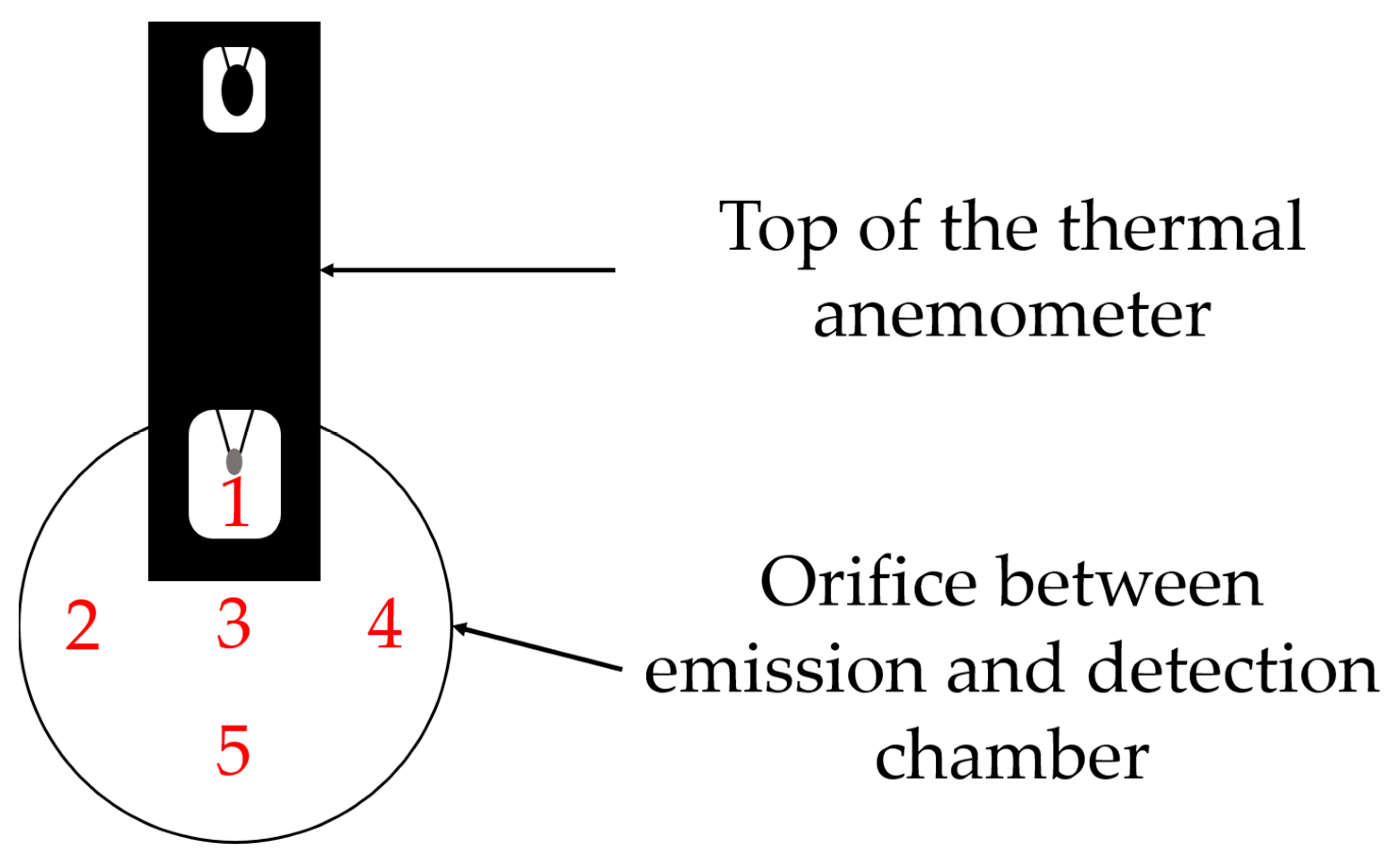
2.2.3. Flow Velocities within the TCS
2.3. Investigated Powder Fractions
2.4. Powder Characterization
2.4.1. True Density
2.4.2. Bulk and Tapped Density
2.4.3. Laser Diffractometry
2.4.4. Residual Moisture Content
2.5. HPLC Analysis
3. Results and Discussion
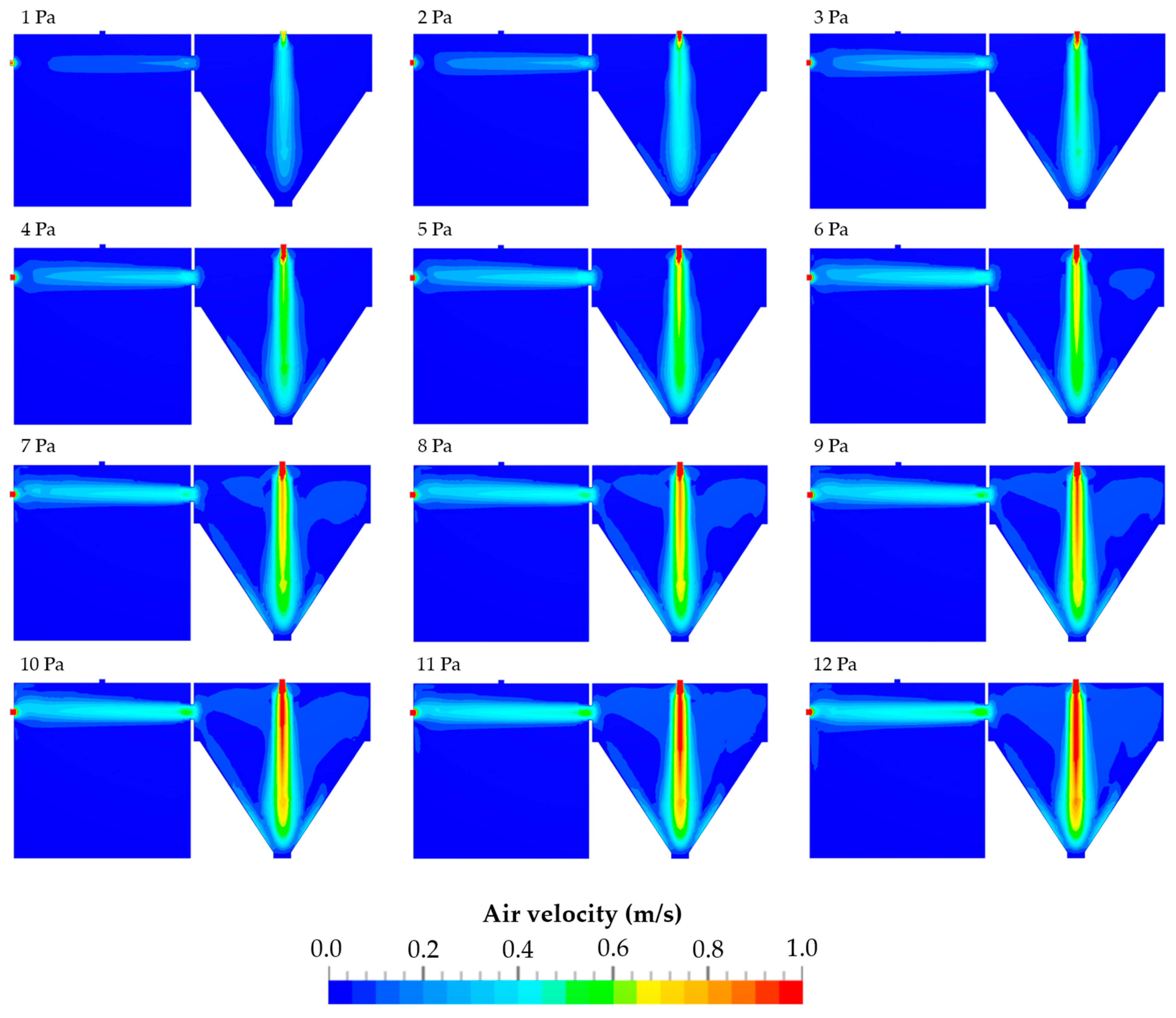
3.1. Air Flow Conditions within the TCS
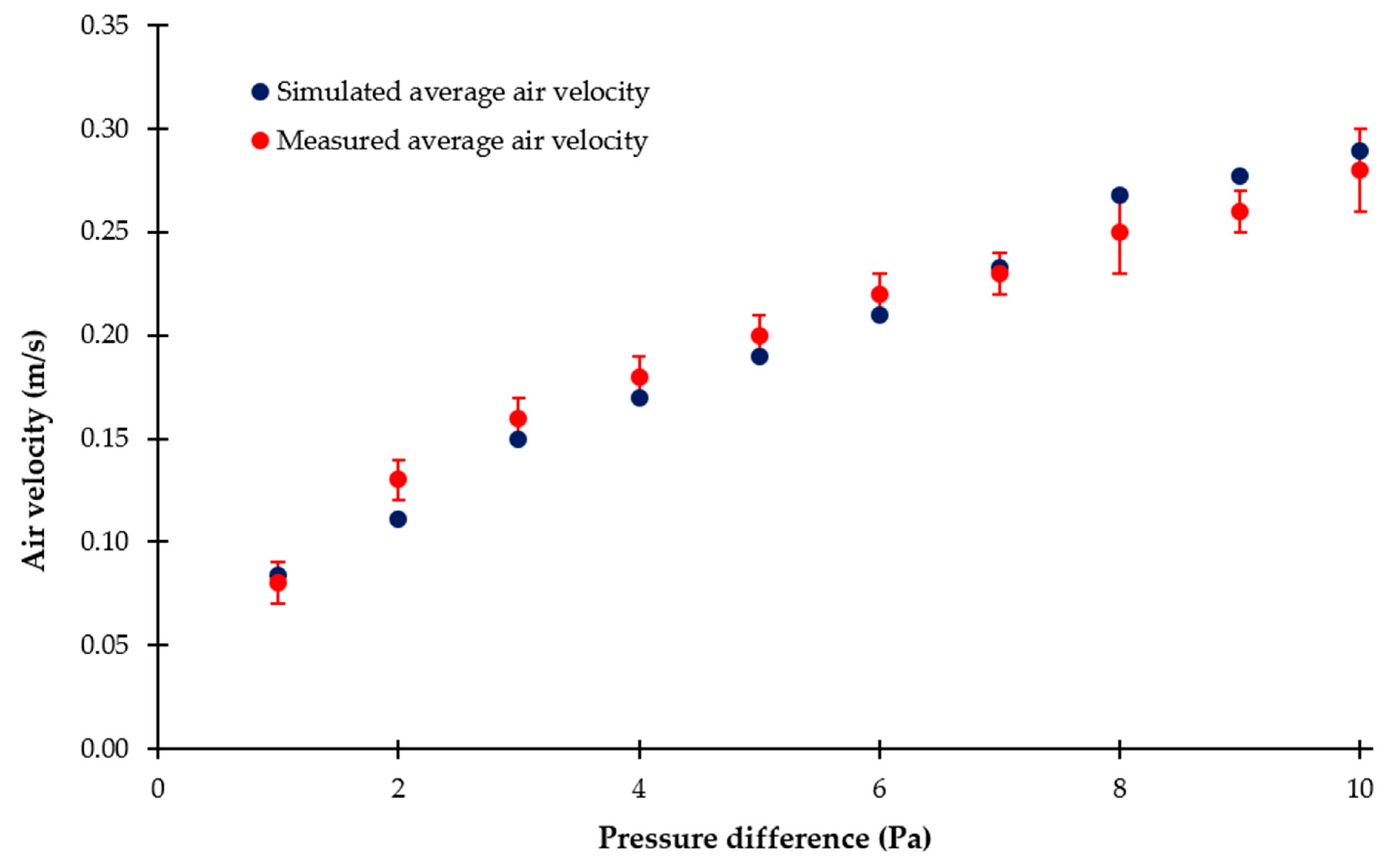
3.2. Air Velocities Resulting from Different Pressure Differences
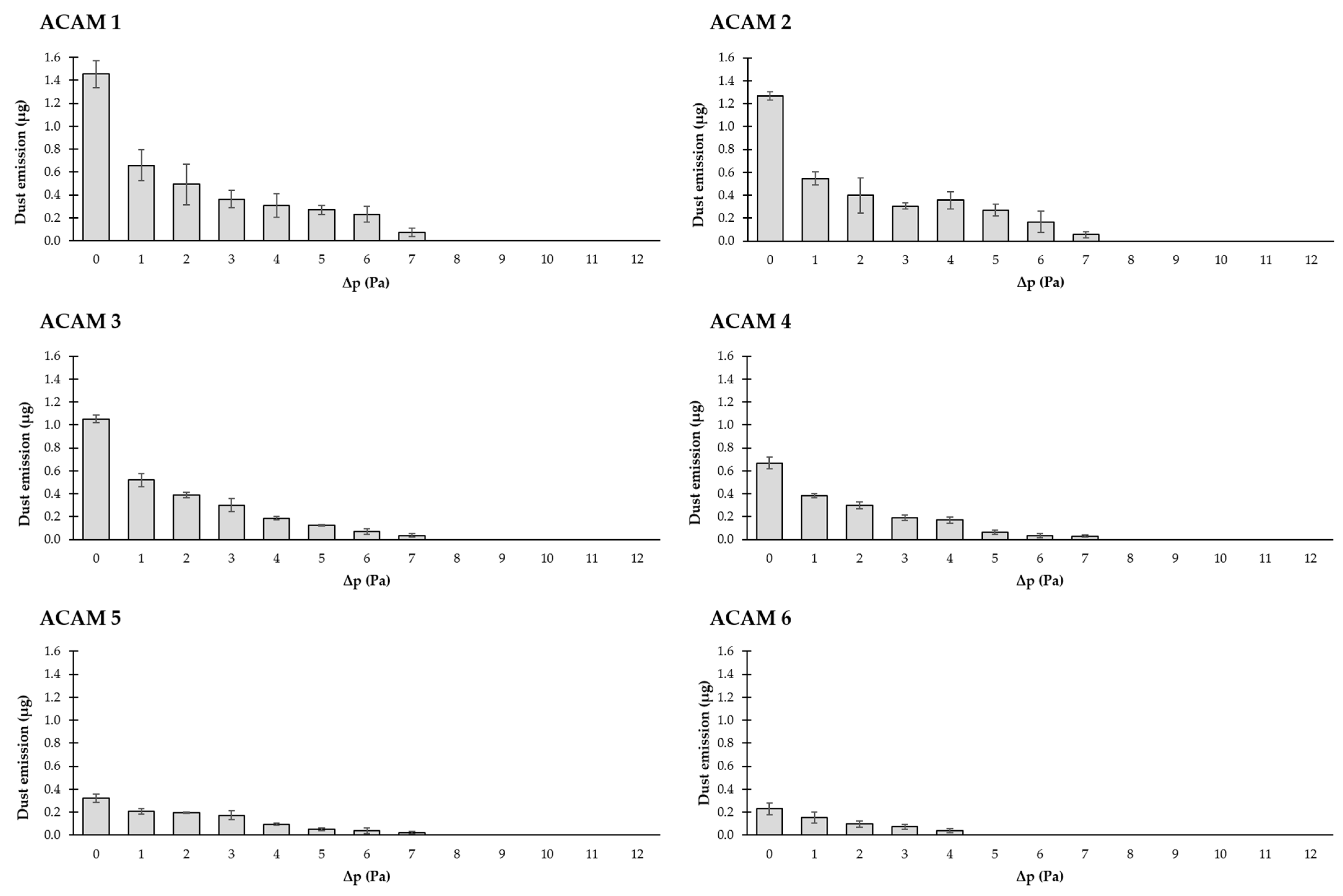
3.3. Dust Emission Depending on the Pressure Difference/Air Velocity and ACAM Particle Size
3.4. ACAM Powder Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Society for Pharmaceutical Engineering (ISPE). ISPE D/A/CH Affiliate: Containment Manual; ISPE D/A/CH: Offenbach, Germany, 2017. [Google Scholar]
- Hristozov, D.; Gottardo, S.; Semenzin, E.; Oomen, A.; Bos, P.; Peijnenburg, W.; van Tongeren, M.; Nowack, B.; Hunt, N.; Brunelli, A.; et al. Frameworks and Tools for Risk Assessment of Manufactured Nanomaterials. Environ. Int. 2016, 95, 36–53. [Google Scholar] [CrossRef]
- Becker, J.; Pohlmann, T. Development and Implementation of a Large-Scale HPAPI Manufacturing Process. Chim. Oggi-Chem. Today 2019, 35, 20–24. [Google Scholar]
- Franco, G. Ramazzini and Workers’ Health. Lancet 1999, 354, 858–861. [Google Scholar] [CrossRef]
- Wollowitz, S. Managing High-Potency Active Pharmaceutical Ingredients—A Drug Sponsor’s Guide. Drug Dev. Res. 2010, 71, 420–428. [Google Scholar] [CrossRef]
- Mari, G.; Moccaldi, A.; Ludovisi, G. Handling of High Potency Drugs: Process and Containment. Trans. Ecol. Environ. 1999, 85, 257–265. [Google Scholar] [CrossRef]
- Dunny, E.; O’Connor, I.; Bones, J. Containment Challenges in HPAPI Manufacture for ADC Generation. Drug Discov. Today 2017, 22, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Barle, E.L.; Winkler, G.C.; Glowienke, S.; Elhajouji, A.; Nunic, J.; Martus, H.J. Setting Occupational Exposure Limits for Genotoxic Substances in the Pharmaceutical Industry. Toxicol. Sci. 2016, 151, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Glogovac, M.; Paulson, C.; Lambert, A.; Winkler, G.C.; Lovsin Barle, E. Disease Area and Mode of Action as Criteria to Asssign a Default Occupational Exposure Limit. Regul. Toxicol. Pharmacol. 2021, 122, 104891. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; FitzGerald, R.; Wilks, M.F.; Barle, E.L.; Hopf, N.B. Use of the Benchmark-Dose (BMD) Approach to Derive Occupational Exposure Limits (OELs) for Genotoxic Carcinogens: N-Nitrosamines. J. Appl. Toxicol. 2023, 43, 1183–1200. [Google Scholar] [CrossRef]
- Haigney, S. HPAPI Capacity Challenges. Pharm. Technol. 2019, 2019, 6–10. [Google Scholar]
- Heron, R.J.L.; Pickering, F.C. Health Effects of Exposure to Active Pharmaceutical Ingredients (APIs). Occup. Med. 2003, 53, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Stahl, H. How to Make Tablets from Potent APIs. Pharm. Technol. 2010, 3, 34–39. [Google Scholar]
- Levin, M.; Koponen, I.K.; Jensen, K.A. Exposure Assessment of Four Pharmaceutical Powders Based on Dustiness and Evaluation of Damaged HEPA Filters. J. Occup. Environ. Hyg. 2014, 11, 165–177. [Google Scholar] [CrossRef]
- Abueva, C.; Ryu, H.S.; Bae, J.S.; Kim, J.; Padalhin, A.; Lee, H.Y.; Park, S.Y.; Mo, J.H.; Chung, P.S.; Woo, S.H. Bioaerosol and Fine Dust Protection with Quaternary Trimethyl Chitosan Integration in Polypropylene Filters. Mater. Des. 2024, 238, 112615. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Tang, N.; Zhou, S.; Yu, J.; Ding, B. Spider-Web-Inspired PM0.3 Filters Based on Self-Sustained Electrostatic Nanostructured Networks. Adv. Mater. 2020, 32, 2002361. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.J.; Johnson, D.L.; Lynch, R.A. The Effect of Pressure Differential and Care Provider Movement on Airborne Infectious Isolation Room Containment Effectiveness. Am. J. Infect. Control 2011, 39, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kanjiyangat, V.; Hareendran, M. Engineering Intervention to Reduce API Dust Exposure during Milling Operation. J. Chem. Health Saf. 2018, 25, 36–39. [Google Scholar] [CrossRef]
- Denk, R.; Ag, S.; Flueckinger, A.; Kisaka, H.; Maeck, R.; Restetzki, L.; Schreiner, A.; Schulze, R.; Sachsen, L. Isolators Key to Preventing Cross-Contamination. PDA Lett. 2017, LIII, 34–40. [Google Scholar]
- Tiwari, P.; Chowdhury, S.R. Sustainable Production of Highly Active Pharmaceutical Ingredients (HAPIs). Int. J. Sci. Res. Publ. 2014, 4, 2250–3153. [Google Scholar]
- Lanning, J.; Boundy, M.A.G.; Leith, D. Validating a Model for the Prediction of Dust Generation. Part. Sci. Technol. 1995, 13, 105–116. [Google Scholar] [CrossRef]
- Sethi, S.A. Generation of Small Particles by Gas Fluidization. J. Aerosol Sci. 1997, 28, 539–540. [Google Scholar] [CrossRef]
- Champmartin, C.; Clerc, F. Inhalable Dust Measurements as a First Approach to Assessing Occupational Exposure in the Pharmaceutical Industry. J. Occup. Environ. Hyg. 2014, 11, 85–92. [Google Scholar] [CrossRef]
- Lidén, G. Dustiness Testing of Materials Handled at Workplaces. Ann. Occup. Hyg. 2006, 50, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jog, M.A.; Turkevich, L.A. Computational Fluid Dynamics Simulations of Aerosol Behavior in a High-Speed (Heubach) Rotating Drum Dustiness Tester. Particuology 2023, 72, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Fuchs, T.; Mattern, C. Advanced Milling and Containment Technologies for Superfine Active Pharmaceutical Ingredients. Chem. Eng. Technol. 2010, 33, 1464–1470. [Google Scholar] [CrossRef]
- Setty, P. Optimal Handling of Highly Active Pharmaceutical Ingredients during Milling and Blending Operations; Massachusetts Institute of Technology (Department of Chemical Engineering): Cambridge, MA, USA, 2013. [Google Scholar]
- Phalen, R.F.; Hinds, W.C.; Angeles, L.; John, W.; Researcher, I.; Lioy, P.J. Particle Size-Selective Sampling in the Workplace: Rationale and Recommended Techniques. Ann. Occup. Hyg. 1988, 32, 403–411. [Google Scholar] [CrossRef]
- Armenta, S.; Blanco, M. Ion Mobility Spectrometry: A Comprehensive and Versatile Tool for Occupational Pharmaceutical Exposure Assessment. Anal. Chem. 2012, 84, 4560–4568. [Google Scholar] [CrossRef]
- Calhoun, D.M.; Coler, A.B.; Nieusma, J.L. Strategies for Preventing Occupational Exposure to Potent Compounds. Toxicol. Mech. Methods 2011, 21, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Palakurthi, N.K.; Ghia, U.; Turkevich, L.A. Numerical Investigation of Aerosolization in the Venturi Dustiness Tester: Aerodynamics of a Particle on a Hill. J. Fluids Eng. Trans. ASME 2022, 144, 061113. [Google Scholar] [CrossRef]
- Vincent, J.H.; Mark, D. The Basis of Dust Sampling in Occupational Hygiene: A Critical Review. Ann. Occup. Hyg. 1981, 24, 375–390. [Google Scholar] [CrossRef]
- Ku, R.H. An Overview of Setting Occupational Exposure Limits (OELs) for Pharmaceuticals. Chem. Health Saf. 2000, 7, 34–37. [Google Scholar] [CrossRef]
- Ahuja, V.; Krishnappa, M. Approaches for Setting Occupational Exposure Limits in the Pharmaceutical Industry. J. Appl. Toxicol. 2022, 42, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.H.; Herman, D.R.; Markey, T.F.; Wolff, R.K.; Dorato, M.A. A Comparison of Two Dustiness Evaluation Methods. Am. Ind. Hyg. Assoc. J. 1992, 53, 448–454. [Google Scholar] [CrossRef]
- Ohta, T.; Maeda, H.; Kubota, R.; Koga, A.; Terada, K. Establishment of Powder Dustiness Evaluation Method by Dustmeter with Small Amount of Pharmaceutical Ingredients. Int. J. Pharm. 2014, 472, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.; Schmidt, E. Determining the Dustiness of Powders—A Comparison of Three Measuring Devices. Ann. Occup. Hyg. 2008, 52, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Hamelmann, F.; Schmidt, E. Methods for Characterizing the Dustiness Estimation of Powders. Chem. Eng. Technol. 2004, 27, 844–847. [Google Scholar] [CrossRef]
- Dubey, P.; Ghia, U.; Turkevich, L.A. Computational Fluid Dynamics Analysis of the Venturi Dustiness Tester. Powder Technol. 2017, 312, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Klippel, A.; Schmidt, M.; Krause, U. Dustiness in Workplace Safety and Explosion Protection—Review and Outlook. J. Loss Prev. Process Ind. 2015, 34, 22–29. [Google Scholar] [CrossRef]
- Boundy, M.; Leith, D.; Polton, T. Method to Evaluate the Dustiness of Pharmaceutical Powders. Ann. Occup. Hyg. 2006, 50, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.; Eickmann, U.; Schmidt, E. Comparison of Established Systems for Measuring the Dustiness of Powders with the UNC Dustiness Tester Developed Especially for Pharmaceutical Substances. Ann. Occup. Hyg. 2013, 57, 1078–1086. [Google Scholar] [CrossRef]
- Deng, T.; Paul, K.A.; Bradley, M.S.A.; Immins, L.; Preston, C.; Scott, J.F.; Welfare, E.H. Investigations on Air Induced Segregation of Pharmaceutical Powders and Effect of Material Flow Functions. Powder Technol. 2010, 203, 354–358. [Google Scholar] [CrossRef]
- Plinke, M.A.E.; Leith, D.; Boundy, M.G.; Löffler, F. Dust Generation from Handling Powders in Industry. Am. Ind. Hyg. Assoc. J. 1995, 56, 251–257. [Google Scholar] [CrossRef]
- Vincent, J.H. Aerosol Sampling: Science, Standards, Instrumentation and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- International Society for Pharmaceutical Engineering (ISPE). Good Practice Guide: Assessing the Particulate Containment Performance of Pharmaceutical Equipment; ISPE: North Bethesda, MD, USA, 2012. [Google Scholar]
- Wirth, S.; Schöler, M.; Brügmann, J.; Leopold, C.S. A Novel Two-Chamber Setup for Containment Investigations with Special Focus on the Dustiness of Pharmaceutical Powders Depending on the Airflow. Pharmaceutics 2022, 14, 2387. [Google Scholar] [CrossRef] [PubMed]
- Wirth, S.; Schöler, M.; Leopold, C.S. Investigation on the Dustiness of a Binary Powder Mixture with a Newly Developed Two-Chamber Setup. In Proceedings of the 12th PBP World Meeting 2021, Online, 11–14 May 2021; Volume 320. [Google Scholar]
- Wendt, J. Computational Fluid Dynamics; McGraw-Hill: New York, NY, USA, 1995. [Google Scholar]
- Lavanya, N.; Bhattacharyya, S. Computational Fluid Dynamics-the Futuristic Innovation in Pharmaceutical Industry. Indian J. Pharm. Educ. Res. 2021, 55, 930–938. [Google Scholar] [CrossRef]
- Tong, Z.B.; Zheng, B.; Yang, R.Y.; Yu, A.B.; Chan, H.K. CFD-DEM Investigation of the Dispersion Mechanisms in Commercial Dry Powder Inhalers. Powder Technol. 2013, 240, 19–24. [Google Scholar] [CrossRef]
- European Committee for Standardization (CEN). EN ISO 14664-4: Cleanrooms and Associated Controlled Environments—Part 4: Design, Construction and Start-Up; CEN: Brussels, Belgium, 2001. [Google Scholar]
- American Industrial Hygiene Association; American National Standards Institute. N.S. American National Standard for Laboratory Ventilation; American Industrial Hygiene Association (AIHA): Fairfax, VA, USA, 2003. [Google Scholar]
- Wirth, S.; Schöler, M.; Leopold, C.S. Dust Emissions of Surrogates Substances Resulting from Diffusive and Convective Transport for Containment Investigations with a Newly Developed Two-Chamber Setup. In Proceedings of the PharmSci 360 Annual Meeting 2022, Boston, MA, USA, 16–19 October 2022. [Google Scholar]
- European Pharmacopoeia 10.0; European Directorate for the Quality of Medicines & Health Care (EDQM): Strasbourg, France, 2019.



| Properties | Values |
|---|---|
| Kinematic viscosity | 1.529 × 10−5 m2/s |
| Density | 1.196 kg/m3 |
| Properties | Values |
|---|---|
| Gauge pressure | 0 Pa |
| Δp between inlet and outlet orifice | 1–12 Pa in 1 Pa increments |
| ] | 1.297 × 10−2 m2/s2 |
| ] | 12.49 s−1 |
| Sample | Linear Parameters | Linear R2 | Exponential Parameters | Exponential R2 | Quadratic Parameters | Quadratic R2 |
|---|---|---|---|---|---|---|
| ACAM 1 | m = −0.087 Pa−1 b = 0.820 µg | 0.691 | a = 1.34 µg b = 0.444 Pa−1 | 0.950 | a = 0.014 µg Pa−2 b = −0.253 µg Pa−1 c = 1.124 µg | 0.8804 |
| ACAM 2 | m = −0.076 Pa−1 b = 0.714 µg | 0.689 | a = 1.15 µg b = 0.431 Pa−1 | 0.926 | a = 0.012 µg Pa−2 b = −0.214 µg Pa−1 c = 0.967 µg | 0.8630 |
| ACAM 3 | m = −0.065 Pa−1 b = 0.595 µg | 0.683 | a = 1.00 µg b = 0.472 Pa−1 | 0.982 | a = 0.012 µg Pa−2 b = −0.204 µg Pa−1 c = 0.850 µg | 0.923 |
| ACAM 4 | m = −0.044 Pa−1 b = 0.406 µg | 0.724 | a = 0.65 µg b = 0.423 Pa−1 | 0.987 | a = 0.008 µg Pa−2 b = −0.135 µg Pa−1 c = 0.573 µg | 0.957 |
| ACAM 5 | m = −0.025 Pa−1 b = 0.233 µg | 0.822 | a = 0.324 µg b = 0.321 Pa−1 | 0.958 | a = 0.003 µg Pa−2 b = −0.0633 µg Pa−1 c = 0.304 µg | 0.977 |
| ACAM 6 | m = −0.016 Pa−1 b = 0.138 µg | 0.673 | a = 0.234 µg b = 0.465 Pa−1 | 0.982 | a = 0.003 µg Pa−2 b = −0.052 µg Pa−1 c = 0.206 µg | 0.963 |
| Properties | ACAM 1 | ACAM 2 | ACAM 3 | ACAM 4 | ACAM 5 | ACAM 6 |
|---|---|---|---|---|---|---|
| x10 (µm) | 2.00 ± 0.03 | 4.87 ± 0.59 | 11.70 ± 0.02 | 20.10 ± 1.27 | 27.98 ± 1.00 | 58.80 ± 5.49 |
| x50 (µm) | 9.91 ± 0.21 | 21.99 ± 1.82 | 70.28 ± 0.07 | 126.85 ± 6.83 | 218.32 ± 4.02 | 346.16 ± 7.13 |
| x90 (µm) | 29.98 ± 1.78 | 61.39 ± 3.62 | 139.24 ± 0.20 | 250.43 ± 1.64 | 366.13 ± 2.77 | 574.84 ± 1.75 |
| True density (g/cm3) | 1.300 ± 0.009 | 1.295 ± 0.002 | 1.299 ± 0.002 | 1.289 ± 0.006 | 1.288 ± 0.005 | 1.288 ± 0.006 |
| Bulk density (g/cm3) | 0.35 ± 0.02 | 0.46 ± 0.00 | 0.53 ± 0.00 | 0.65 ± 0.00 | 0.67 ± 0.01 | 0.70 ± 0.00 |
| Tapped density (g/cm3) | 0.57 ± 0.01 | 0.69 ± 0.00 | 0.76 ± 0.01 | 0.78 ± 0.00 | 0.78 ± 0.01 | 0.82 ± 0.01 |
| Hausner ratio | 1.61 ± 0.08 | 1.52 ± 0.01 | 1.43 ± 0.00 | 1.20 ± 0.00 | 1.16 ± 0.00 | 1.16 ± 0.01 |
| Compressibility index (%) | 37.62 ± 3.20 | 34.09 ± 0.27 | 29.88 ± 0.21 | 16.57 ± 0.17 | 13.95 ± 0.00 | 13.84 ± 1.09 |
| Residual moisture content (%) | 0.14 ± 0.02 | 0.14 ± 0.01 | 0.15 ± 0.02 | 0.14 ± 0.02 | 0.14 ± 0.03 | 0.15 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirth, S.; Schöler, M.; Brügmann, J.; Leopold, C.S. An Investigation on the Relationship between Dust Emission and Air Flow as Well as Particle Size with a Novel Containment Two-Chamber Setup. Pharmaceutics 2024, 16, 1088. https://doi.org/10.3390/pharmaceutics16081088
Wirth S, Schöler M, Brügmann J, Leopold CS. An Investigation on the Relationship between Dust Emission and Air Flow as Well as Particle Size with a Novel Containment Two-Chamber Setup. Pharmaceutics. 2024; 16(8):1088. https://doi.org/10.3390/pharmaceutics16081088
Chicago/Turabian StyleWirth, Steffen, Martin Schöler, Jonas Brügmann, and Claudia S. Leopold. 2024. "An Investigation on the Relationship between Dust Emission and Air Flow as Well as Particle Size with a Novel Containment Two-Chamber Setup" Pharmaceutics 16, no. 8: 1088. https://doi.org/10.3390/pharmaceutics16081088






