Au@CuS Nanoshells for Surface-Enhanced Raman Scattering Image-Guided Tumor Photothermal Therapy with Accelerated Hepatobiliary Excretion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Animals
2.3. Preparation of Nanoparticles
2.3.1. Preparation of PEG-AuNPs or PEG-Au-DTTC NPs
2.3.2. Preparation of PEG-Au@CuS NSs or PEG-Au-DTTC@CuS NSs
2.4. Characterization of Different Nanoparticles
2.5. Raman Stability in FBS
2.6. In Vitro Cytotoxicity and Photothermal Ablation Effect of PEG-Au-DTTC@CuS NSs
2.7. Isolation of Primary Mouse Hepatocytes and Kupffer Cells
2.8. Quantification of Au in Mouse Hepatocytes and Kupffer Cells Following Incubation with Different Nanoparticles
2.9. Excretion of Nanoparticles into Gallbladders of Mice
2.10. In Vitro Photothermal Conversion Effect
2.11. Establishment of Tumor Models
2.12. Raman Measurements
2.13. Raman Image-Guided Tumor Photothermal Therapy
2.14. Statistical Analysis
3. Results
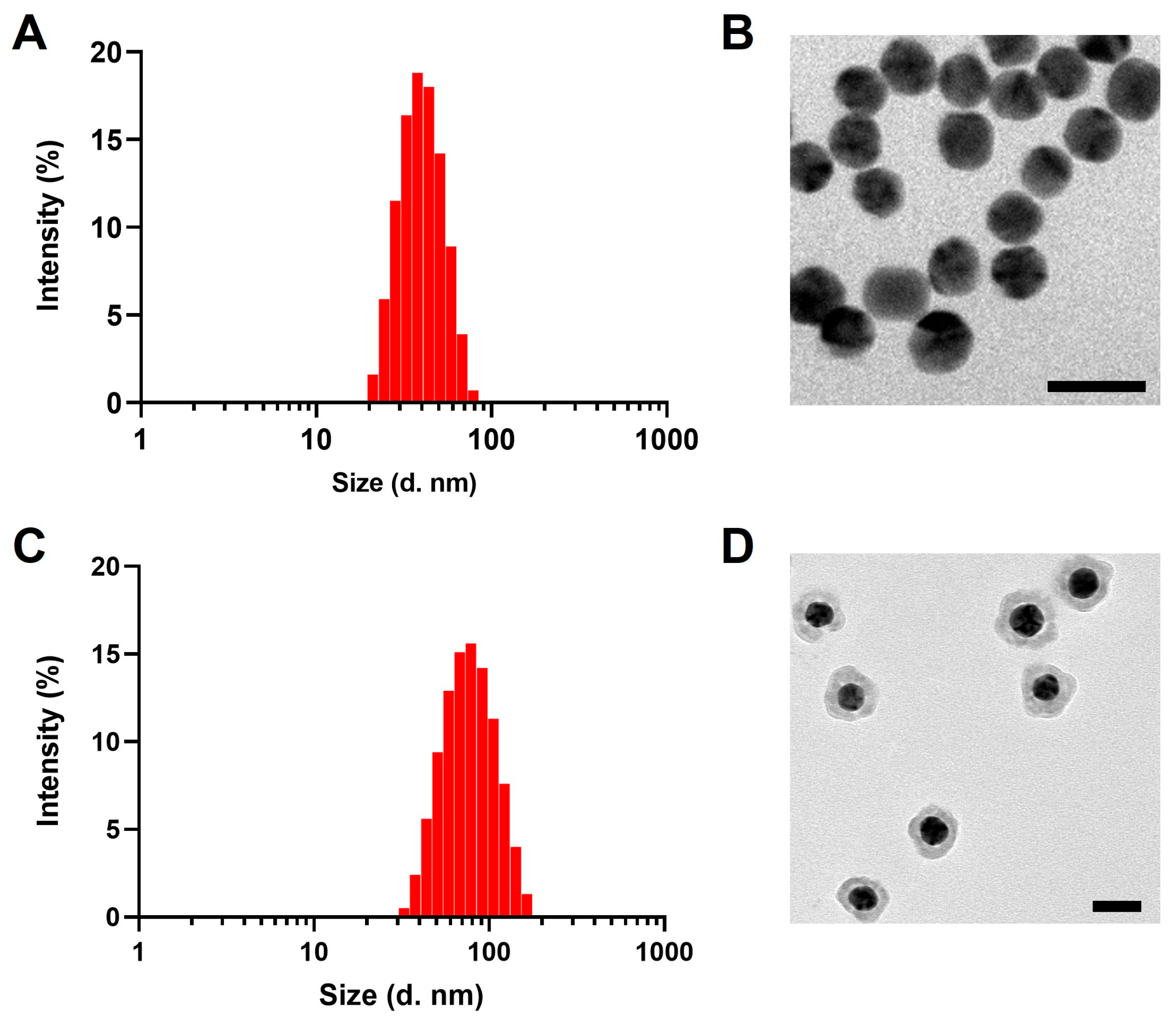
3.1. Characterization of PEG-AuNPs and PEG-Au@CuS NSs
3.2. Hepatobiliary Excretion of PEG-AuNPs and PEG-Au@CuS NSs
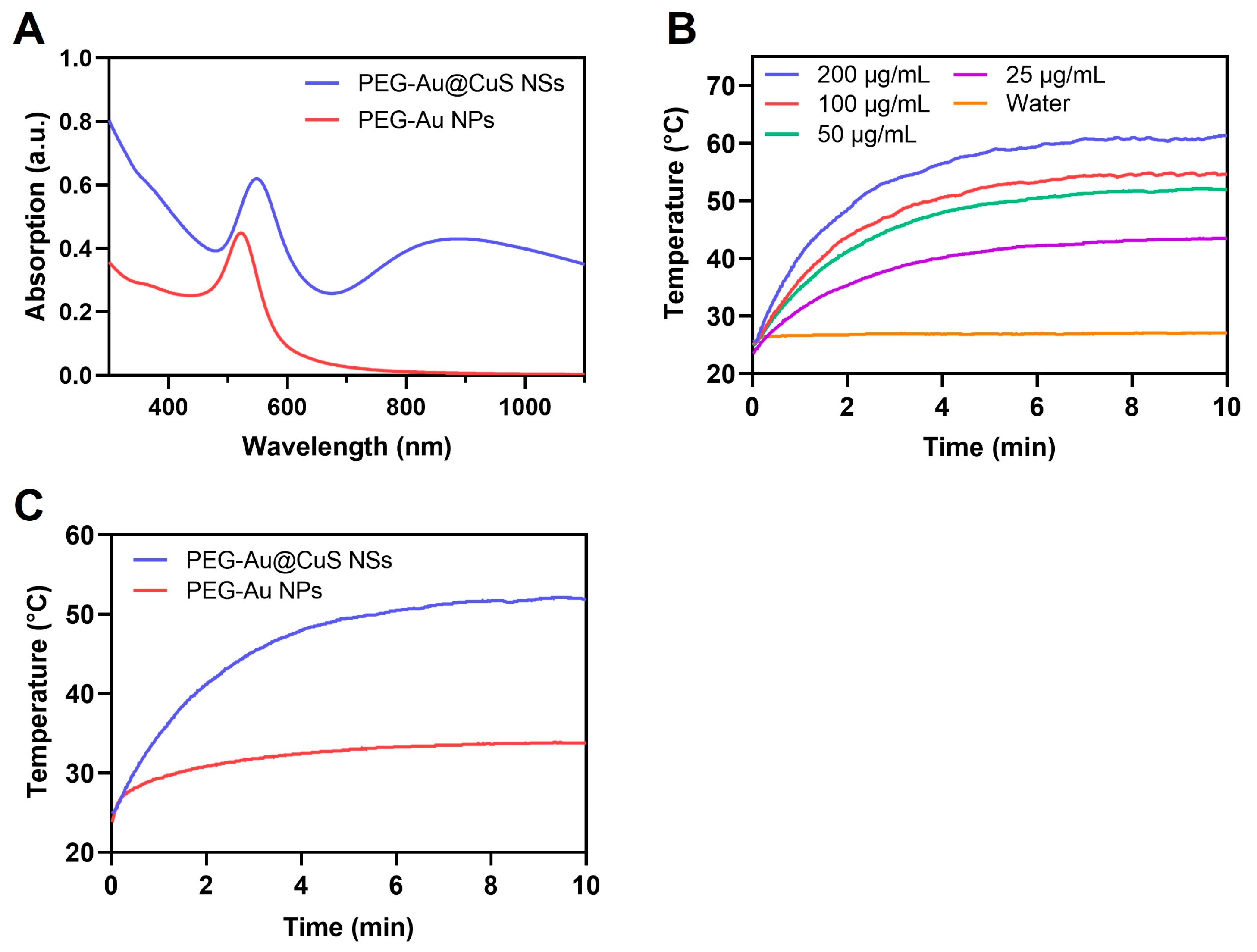
3.3. UV Absorption and In Vitro Photothermal Effect
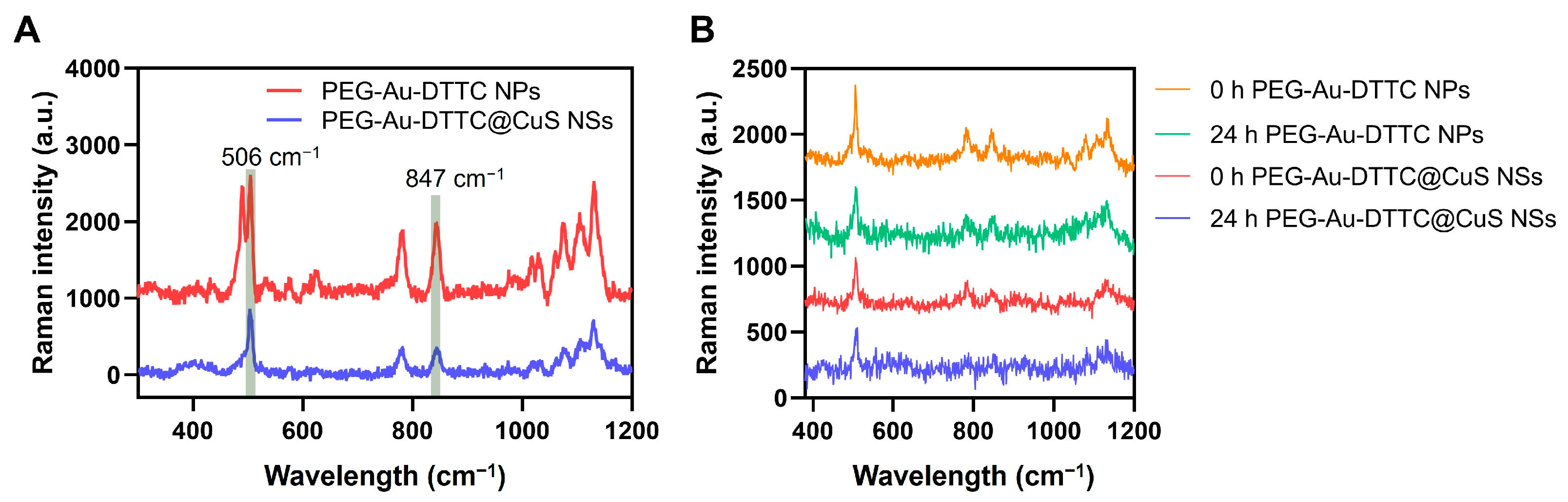
3.4. Raman Spectra of the DTTC-Loaded Nanoparticles
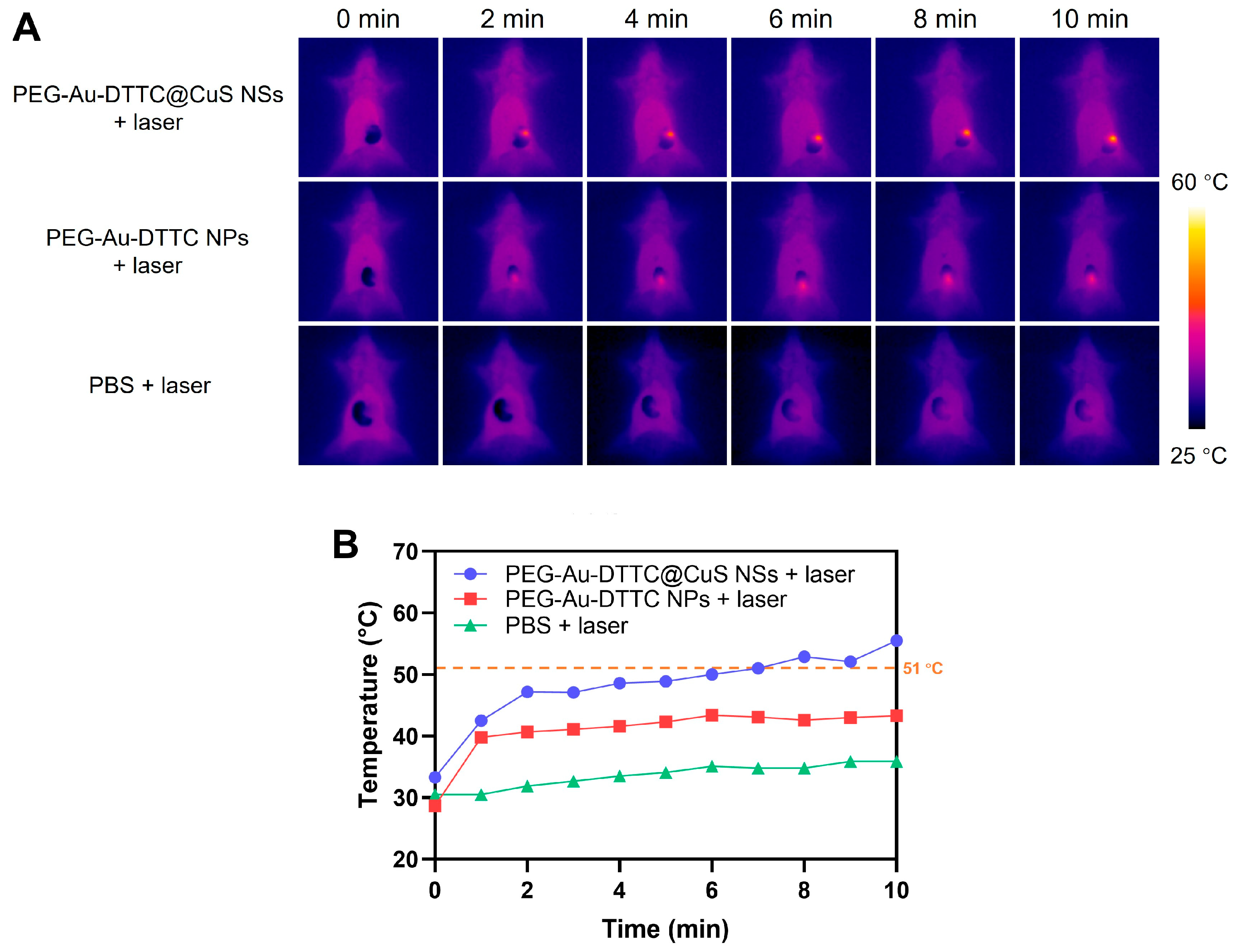
3.5. In Vivo Photothermal Effect
3.6. In Vivo Raman Imaging
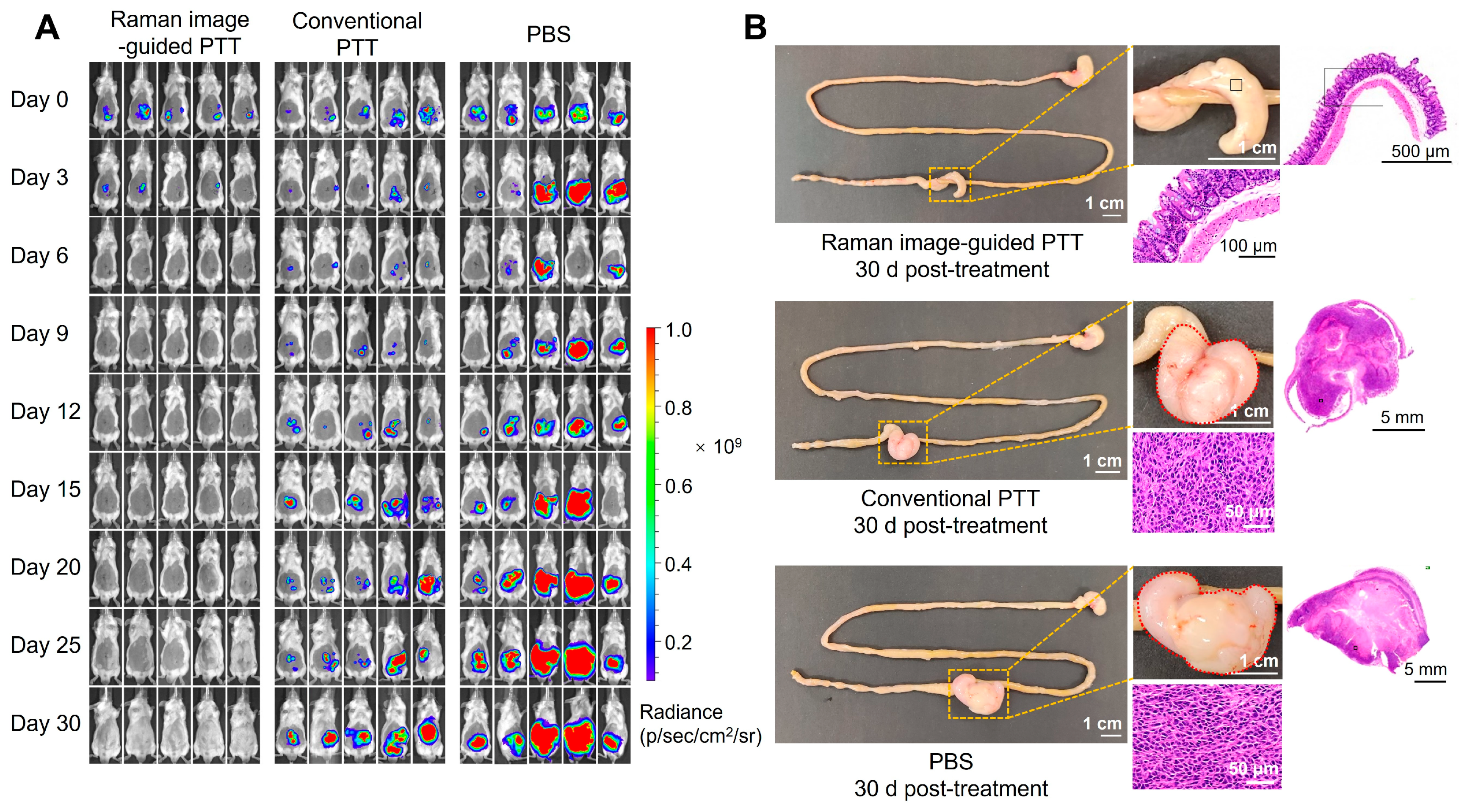
3.7. Therapeutic Efficacy of Intraoperative Raman Image-Guided PTT by PEG-Au-DTTC@CuS NSs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Gao, X.; Xu, C.; Liu, D. SERS tags for biomedical detection and bioimaging. Theranostics 2022, 12, 1870–1903. [Google Scholar] [CrossRef]
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale 2021, 13, 11593–11634. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Cheng, Z.; Lv, C.; Wang, R.; Yu, F. Development of bioorthogonal SERS imaging probe in biological and biomedical applications. Chin. Chem. Lett. 2021, 32, 2369–2379. [Google Scholar] [CrossRef]
- Huang, X.; Sheng, B.; Tian, H.; Chen, Q.; Yang, Y.; Bui, B.; Pi, J.; Cai, H.; Chen, S.; Zhang, J.; et al. Real-time SERS monitoring anticancer drug release along with SERS/MR imaging for pH- sensitive chemo-phototherapy. Acta Pharm. Sci. B 2023, 13, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huo, H.; Wu, Y.; Chen, L.; Su, L.; Zhang, X.; Song, J.; Yang, H. Design and synthesis of SERS materials for in vivo molecular imaging and biosensing. Adv. Sci. 2023, 10, 2202051. [Google Scholar] [CrossRef] [PubMed]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Select 2021, 3, 792–828. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, silver, and palladium nanoparticles: A chemical tool for biomedical applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold nanomaterials at work in biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef]
- Chen, B.; Mei, L.; Fan, R.; Chuan, D.; Ren, Y.; Mu, M.; Chen, H.; Zou, B.; Guo, G. Polydopamine-coated i-motif DNA/gold nanoplatforms for synergistic photothermal-chemotherapy. Asian J. Pharm. Sci. 2023, 18, 100781. [Google Scholar] [CrossRef]
- Wu, A.; Yang, A.; Tong, Q.; Wei, G.; Zhang, S.; Yu, S.; Zhang, C.; Xu, J.; Lu, W. A rationally designed cancer vaccine based on NIR-II fluorescence image-guided light-triggered remote control of antigen cross-presentation and autophagy. Acta Pharm. Sin. B 2023, 13, 3121–3136. [Google Scholar] [CrossRef] [PubMed]
- Sujai, P.T.; Shamjith, S.; Joseph, M.M.; Maiti, K.K. Elucidating gold-MnO2 core-shell nanoenvelope for real time SERS-guided photothermal therapy on pancreatic cancer cells. ACS Appl. Bio Mater. 2021, 4, 4962–4972. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Xia, Y.; Xing, J.; Li, Z.; Qian, Q.; Wang, Y.; Wu, A.; Zeng, L.; Zhou, Y. Bioconjugation of gold nanobipyramids for SERS detection and targeted photothermal therapy in breast cancer. ACS Biomater. Sci. Eng. 2017, 3, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Oseledchyk, A.; Andreou, C.; Wall, M.A.; Kircher, M.F. Folate-targeted surface-enhanced resonance Raman scattering nanoprobe ratiometry for detection of microscopic ovarian cancer. ACS Nano 2017, 11, 1488–1497. [Google Scholar] [CrossRef]
- Chen, J.; Sheng, Z.; Li, P.; Wu, M.; Zhang, N.; Yu, X.-F.; Wang, Y.; Hu, D.; Zheng, H.; Wang, G.P. Indocyanine green-loaded gold nanostars for sensitive SERS imaging and subcellular monitoring of photothermal therapy. Nanoscale 2017, 9, 11888–11901. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Liang, C. Perspective of future SERS clinical application based on current status of Raman spectroscopy clinical trials. Front. Chem. 2021, 9, 665841. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Q.; Qi, P.; Wang, Z.; Zhang, T.; Zhang, S.; Wu, J.; Guo, Z.; Chen, J.; Zhang, Q. Recent advances in living cell nucleic acid probes based on nanomaterials for early cancer diagnosis. Asian J. Pharm. Sci. 2024, 19, 100910. [Google Scholar] [CrossRef] [PubMed]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz-Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.K.; Jittiwat, J.; Manikandan, J.; Ong, C.N.; Yu, L.E.; Ong, W.Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials 2010, 31, 2034–2042. [Google Scholar] [CrossRef]
- Sadauskas, E.; Danscher, G.; Stoltenberg, M.; Vogel, U.; Larsen, A.; Wallin, H. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine 2009, 5, 162–169. [Google Scholar] [CrossRef]
- Gharatape, A.; Salehi, R. Recent progress in theranostic applications of hybrid gold nanoparticles. Eur. J. Med. Chem. 2017, 138, 221–233. [Google Scholar] [CrossRef]
- Milan, J.; Niemczyk, K.; Kus-Liskiewicz, M. Treasure on the earth-gold nanoparticles and their biomedical applications. Materials 2022, 15, 3355. [Google Scholar] [CrossRef]
- Guo, L.; Panderi, I.; Yan, D.D.; Szulak, K.; Li, Y.; Chen, Y.T.; Ma, H.; Niesen, D.B.; Seeram, N.; Ahmed, A.; et al. A comparative study of hollow copper sulfide nanoparticles and hollow gold nanospheres on degradability and toxicity. ACS Nano 2013, 7, 8780–8793. [Google Scholar] [CrossRef]
- Li, M.; Qiu, Y.; Fan, C.; Cui, K.; Zhang, Y.; Xiao, Z. Design of SERS nanoprobes for Raman imaging: Materials, critical factors and architectures. Acta Pharm. Sin. B 2018, 8, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Wu, Y.; Fan, T.; Xu, Y.; Tang, L.; Ying, Y. Compact shielding of graphene monolayer leads to extraordinary SERS-active substrate with large-area uniformity and long-term stability. Sci. Rep. 2015, 5, 17167. [Google Scholar] [CrossRef] [PubMed]
- Ain, N.U.; Abdul Nasir, J.; Khan, Z.; Butler, I.S.; Rehman, Z. Copper sulfide nanostructures: Synthesis and biological applications. RSC Adv. 2022, 12, 7550–7567. [Google Scholar] [CrossRef]
- Goel, S.; Chen, F.; Cai, W. Synthesis and biomedical applications of copper sulfide nanoparticles: From sensors to theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Synthetic methods of CuS nanoparticles and their applications for imaging and cancer therapy. RSC Adv. 2016, 6, 82596–82615. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Zhang, S.; Chen, Y.; Chen, Y.T.; Zheng, B.; Sun, J.; Qian, Y.; Chen, Y.; Yan, B.; et al. Copper sulfide facilitates hepatobiliary clearance of gold nanoparticles through the copper-transporting ATPase ATP7B. ACS Nano 2019, 13, 5720–5730. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, N.; Wang, J.; Liu, Z.; Han, Y.; Zeng, Y. In vivo synergistic tumor therapies based on copper sulfide photothermal therapeutic nanoplatforms. Exploration 2023, 3, 20220161. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rashidi, L.H.; Yao, M.; Ma, L.; Chen, L.; Zhang, J.; Zhang, Y.; Chen, W. CuS nanoagents for photodynamic and photothermal therapies: Phenomena and possible mechanisms. Photodiagnosis Photodyn. Ther. 2017, 19, 5–14. [Google Scholar] [CrossRef]
- Gao, P.; Li, G.; Wang, Z.; Zhang, H.; Shan, Y.; Yuan, X.; Shi, Q.; Dou, X.; Zhou, Q.; Xu, Q. Protease-loaded CuS nanoparticles with synergistic photothermal/dynamic therapy against f. Nucleatum-induced periodontitis. ACS Appl. Mater. Interfaces 2023, 15, 32215–32225. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir 2004, 20, 6414–6420. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Sun, Q.; Shen, A.-G.; Fan, B.; Hu, J.-M. Nano Au@Cu2−xS with near-infrared photothermal and peroxidase catalytic activities redefines efficient antibiofilm-oriented root canal therapy. Chem. Eng. J. 2021, 422, 130090. [Google Scholar] [CrossRef]
- Damm, G.; Seehofer, D.; Zeilinger, K.; Pfeiffer, E.; Deharde, D.; Kegel, V. Protocol for isolation of primary human hepatocytes and corresponding major populations of non-parenchymal liver cells. J. Vis. Exp. 2016, 109, e53069. [Google Scholar]
- Gao, S.; Wei, G.; Zhang, S.; Zheng, B.; Xu, J.; Chen, G.; Li, M.; Song, S.; Fu, W.; Xiao, Z.; et al. Albumin tailoring fluorescence and photothermal conversion effect of Near-Infrared-II fluorophore with aggregation-induced emission characteristics. Nat. Commun. 2019, 10, 2206. [Google Scholar] [CrossRef]
- Henriques, F.C. Studies of thermal injury; the predictability and the significance of thermally induced rate processes leading to irreversible epidermal injury. Arch. Pathol. 1947, 43, 489–502. [Google Scholar]
- Welch, A.J. The thermal response of laser irradiated tissue. IEEE J. Quantum Electron. 1984, 20, 1471–1481. [Google Scholar] [CrossRef]
- Diederich, C.J. Thermal ablation and high-temperature thermal therapy: Overview of technology and clinical implementation. Int. J. Hyperth. 2005, 21, 745–753. [Google Scholar] [CrossRef]
- Feng, W.; Nie, W.; Cheng, Y.H.; Zhou, X.J.; Chen, L.; Qiu, K.X.; Chen, Z.G.; Zhu, M.F.; He, C.L. In vitro and in vivo toxicity studies of copper sulfide nanoplates for potential photothermal applications. Nanomed.-Nanotechnol. 2015, 11, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Hu, H.; Xu, Y.; Zhou, Y.; Xie, R.; Lei, T.; Du, Y.; Yang, W.; He, S.; Huang, Y.; et al. Hollow copper sulfide nanoparticles carrying ISRIB for the sensitized photothermal therapy of breast cancer and brain metastases through inhibiting stress granule formation and reprogramming tumor—Associated macrophages. Acta Pharm. Sin. B 2023, 13, 3471–3488. [Google Scholar] [CrossRef]
- Thakor, A.S.; Luong, R.; Paulmurugan, R.; Lin, F.I.; Kempen, P.; Zavaleta, C.; Chu, P.; Massoud, T.F.; Sinclair, R.; Gambhir, S.S. The fate and toxicity of Raman-active silica-gold nanoparticles in mice. Sci. Transl. Med. 2011, 3, ra33–ra79. [Google Scholar] [CrossRef] [PubMed]
- Saladino, G.M.; Brodin, B.; Kakadiya, R.; Toprak, M.S.; Hertz, H.M. Iterative nanoparticle bioengineering enabled by X-ray fluorescence imaging. Sci. Adv. 2024, 10, eadl2267. [Google Scholar] [CrossRef]
- Chen, F.; Hableel, G.; Zhao, E.R.; Jokerst, J.V. Multifunctional nanomedicine with silica: Role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J. Colloid Interface Sci. 2018, 521, 261–279. [Google Scholar] [CrossRef]
- Zhou, M.Z.; Ge, X.Q.; Ke, D.M.; Tang, H.; Zhang, J.Z.; Calvaresi, M.; Gao, B.; Sun, L.N.; Su, Q.Q.; Wang, H.F. The bioavailability, biodistribution, and toxic effects of silica-coated upconversion nanoparticles in vivo. Front. Chem. 2019, 7, 218. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, M.; Chen, G.; Fang, C.; Li, M.; Gu, X.; Cong, S.; Zhao, Z.; Fu, L.; Fang, X.; et al. Photodegradable CuS SERS probes for lntraoperative residual tumor detection, ablation, and self-clearance. ACS Appl. Mater. Interfaces 2019, 11, 23436–23444. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part i. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- King, F.W.; Van Duyne, R.P.; Schatz, G.C. Theory of Raman scattering by molecules adsorbed on electrode surfaces. J. Chem. Phys. 1978, 69, 4472–4481. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Yu, S.; Sun, J.; Huang, T.; Lin, H.; Li, Z.; Xiao, Z.; Lu, W. Au@CuS Nanoshells for Surface-Enhanced Raman Scattering Image-Guided Tumor Photothermal Therapy with Accelerated Hepatobiliary Excretion. Pharmaceutics 2024, 16, 1089. https://doi.org/10.3390/pharmaceutics16081089
Zhang S, Yu S, Sun J, Huang T, Lin H, Li Z, Xiao Z, Lu W. Au@CuS Nanoshells for Surface-Enhanced Raman Scattering Image-Guided Tumor Photothermal Therapy with Accelerated Hepatobiliary Excretion. Pharmaceutics. 2024; 16(8):1089. https://doi.org/10.3390/pharmaceutics16081089
Chicago/Turabian StyleZhang, Sihang, Sheng Yu, Jingwen Sun, Teng Huang, Hongzheng Lin, Zhe Li, Zeyu Xiao, and Wei Lu. 2024. "Au@CuS Nanoshells for Surface-Enhanced Raman Scattering Image-Guided Tumor Photothermal Therapy with Accelerated Hepatobiliary Excretion" Pharmaceutics 16, no. 8: 1089. https://doi.org/10.3390/pharmaceutics16081089
APA StyleZhang, S., Yu, S., Sun, J., Huang, T., Lin, H., Li, Z., Xiao, Z., & Lu, W. (2024). Au@CuS Nanoshells for Surface-Enhanced Raman Scattering Image-Guided Tumor Photothermal Therapy with Accelerated Hepatobiliary Excretion. Pharmaceutics, 16(8), 1089. https://doi.org/10.3390/pharmaceutics16081089







