Abstract
3,4-Methylenedioxymethamphetamine (MDMA) is a synthetic amphetamine derivative with notable psychoactive properties and emerging therapeutic potential, particularly for treating post-traumatic stress disorders (PTSD) and substance use disorders. However, its use remains controversial due to inter-individual variability influenced by both environmental and genetic factors. In this context, pharmacogenomics could play a crucial role in guiding MDMA treatment by identifying individuals with genetic predispositions affecting their response to MDMA. Tailoring treatment plans based on individual’s genetic makeup may enhance therapeutic outcomes and minimize adverse effects, leading to safer and more effective use of MDMA in clinical settings. Literature analysis reveals that the influence of genetic variants within genes encoded for enzymes involved in MDMA metabolism and/or pharmacodynamics (PD) targets have been relatively under-investigated in humans. Some studies have pointed out associations between MDMA-induced effects and polymorphisms. For example, the catechol-O-methyltransferase (COMT) Val158Met polymorphism has been associated with cognitive and cardiovascular MDMA-induced effects. Similarly, polymorphisms in the serotonin-linked promoter region (5HTTLPR) have been associated with several MDMA-induced adverse effects including mood disorders. However, despite these findings, only a few associations have been highlighted. Furthermore, some genes encoded for MDMA targets have been only poorly investigated, representing a significant research gap. These observations underscore the need for large-scale, controlled pharmacogenomics studies focusing on a broad panel of genes involved into MDMA pharmacokinetics and PD. Such studies could provide critical insights for optimizing MDMA’s therapeutic use and minimizing its risks.
1. Introduction
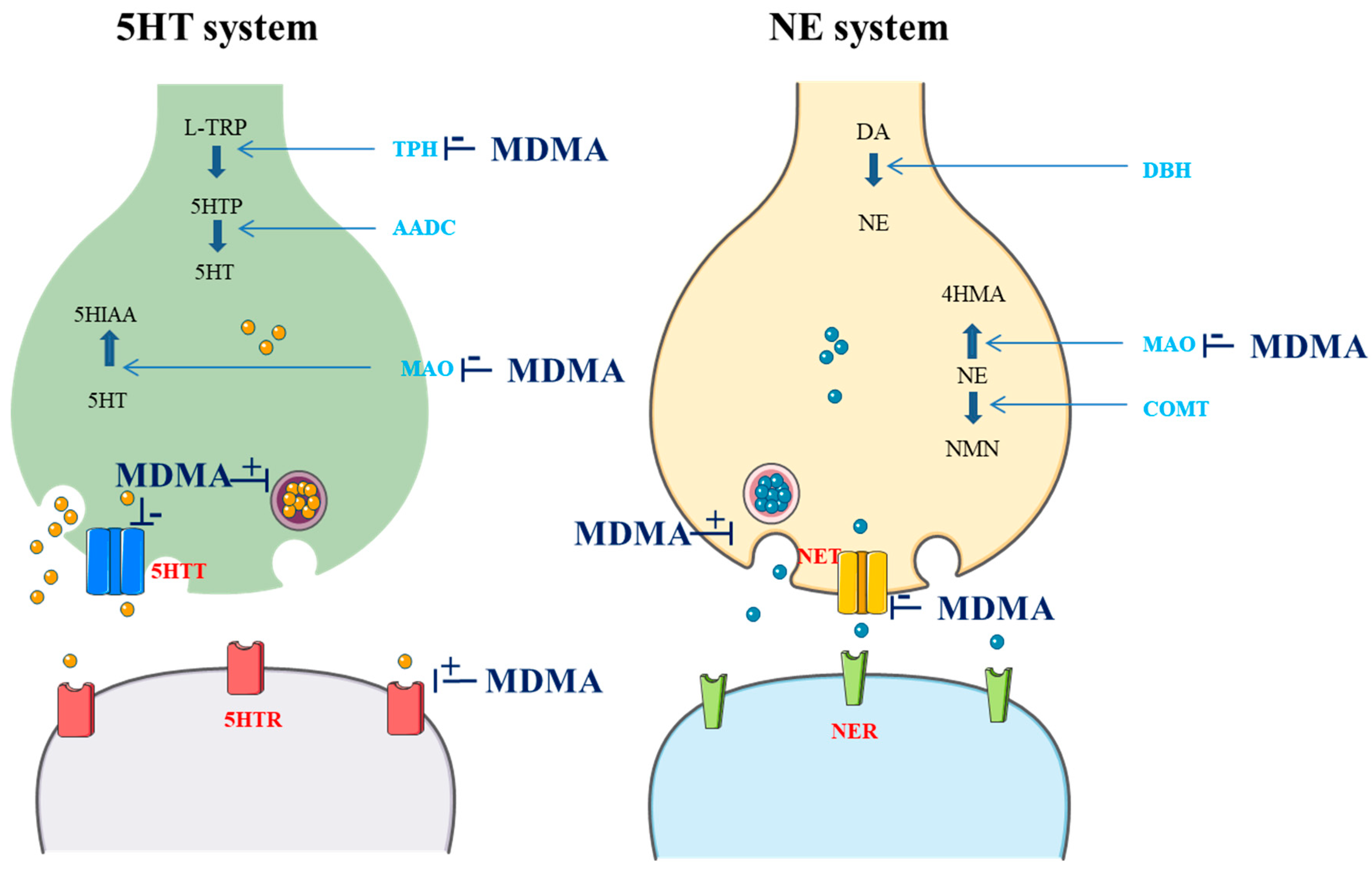
3,4-Methylenedioxymethamphetamine (MDMA) is a ring-substituted amphetamine first synthesized in 1912 by the German pharmaceutical company Merck as an intermediate to the styptic compound methylhydrastitine [1]. MDMA acts as a releaser and reuptake inhibitor of serotonin (5HT), norepinephrine (NE), and, to a lesser extent, dopamine (DA). MDMA can also inhibit both monoamine oxidase (MAO) and L-tryptophan hydroxylase (TPH). Furthermore, it seems to have direct actions on several receptors, including the serotonin 1A (5HTR1A), 1B (5HTR1B), and 2A receptors (5HTR2A), the M1 muscarinic receptor, the H1 histamine receptor, the α- and β-adrenergic receptors, and DA receptors (DR) [2,3,4,5]. Actions on cholinergic, glutamatergic, and gamma-aminobutyric acid receptors have also been reported [4]. Additionally, MDMA stimulates the release of oxytocin from the hypothalamus, increasing its level in the brain and bloodstream. This release is believed to significantly contribute to the enhanced feelings of social connection and empathy that users experience [6]. Overall, typical effects of MDMA can be predominantly attributed to the activation of the 5HT and NE systems [7]. These effects are summarized in the Figure 1.
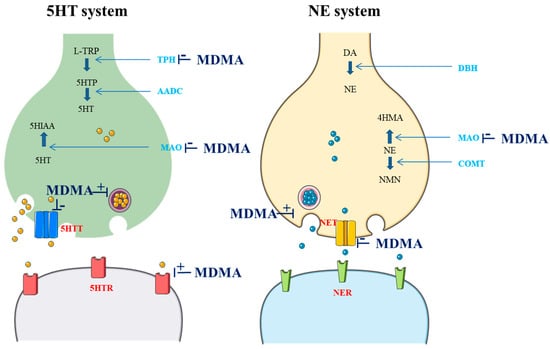
Figure 1.
Effects of MDMA on the 5HT and NE system (drawn in part using images from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/; accessed 8 August 2024); L-TRP: L-tryptophan; TPH: L-tryptophan hydroxylase; 5HTP: 5-hydroxytryptophan; AADC: L-aromatic amino acid decarboxylase; 5HT: serotonin; MAO: monoamine oxidase; 5HIAA: 5-hydroxyindoleacetic acid; 5HTT: 5HT transporter; 5HTR: 5HT receptor; DA: dopamine; DBH: dopamine beta-hydroxylase; COMT: catechol; NE: norepinephrine; 4HMA: 4-hydroxymandelic acid; NMN: normetanephrine; NET: NE transporter; NER: NE receptor).
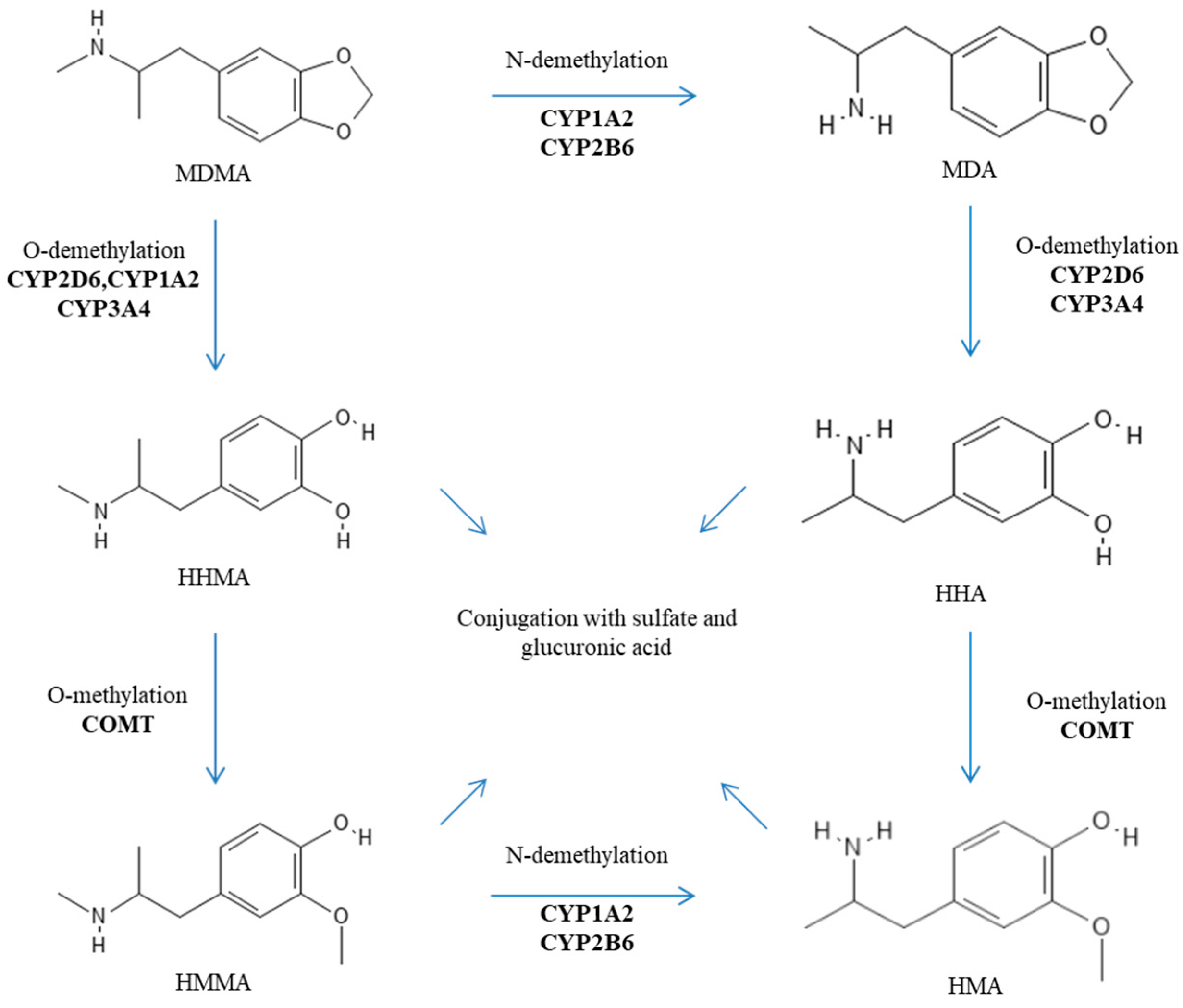
After oral administration, MDMA is rapidly absorbed into the bloodstream (with a Tmax of about 2 h), and its transport is not mediated by P-glycoprotein (also known as MDR1 or ABCB1) [8,9]. MDMA is characterized by nonlinear pharmacokinetics (PK). This nonlinear PK is observed within the normal dosing range and becomes more pronounced at higher, and potentially toxic, doses. Indeed, De la Torre et al. reported nondose-proportional area under the curve (AUC) values for oral doses of 50, 75, 100, 125, and 150 mg, which were 9.15, 17.8, 18.5, 21, and 34.5, respectively [10]. Furthermore, its metabolism is rather complex and includes two main metabolic pathways: (i) O-demethylation to 3,4-dihydroxymethamphetamine (HHMA) followed by conversion to 4-hydroxy-3-methoxyamphetamine (HMA) after several additional steps; and (ii) N-demethylation to 3,4-methylenedioxyamphetamine (MDA) before ending up as HMA [2,3]. MDMA metabolites are eventually excreted in urine as conjugated glucuronide and/or sulfate metabolites [2,3]. These processes appear rather stereoselective with a preference for S-stereoisomers and depend on several enzymes including catechol-O-methyltransferase (COMT), cytochromes P450 (CYPs) (especially the isoforms 2D6, 2B6, 2C19, 3A4, and 1A2), glutathione S-transferase (SULT), and diphospho (UDP)-glucuronosyltransferase (UGT) [4,11,12,13]. The metabolic pathways of MDMA are reported in Figure 2. It is important to note that MDA, HHMA, and HMA are pharmacologically active metabolites [4,11,12,13]. In addition, HHMA (and to a minor extent HHA) can undergo further oxidation to orthoquinones, which, when conjugated to glutathione, display cytotoxicity and contribute to cell apoptosis [4,14,15]. Detoxification of such oxidized metabolites is dependent on glutathione S-transferases (GSTs) [13].
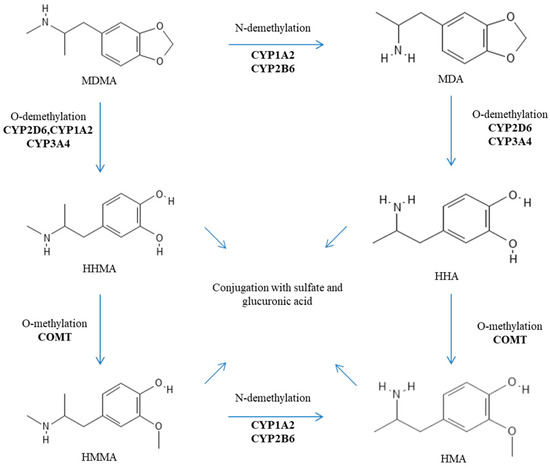
Figure 2.
Metabolic pathways of MDMA.
Acting as a stimulant and hallucinogen drug, MDMA has been pharmacologically characterized as an entactogen by the chemists Alexander Shulgin and David E. Nichols in the 1970s [16,17]. Since, it has been used actively as a psychotherapy tool by the psychedelic community until the early 1980s. However, due to its diversion towards a recreational drug and its abuse in the club scene as “Ecstasy”; the Drug Enforcement Administration (DEA) declared an emergency ban on MDMA in 1985 which, subsequently, became permanent thereafter, placing this compound on the list of Schedule I drugs (defined as substances with no currently accepted medical use and a high potential for abuse) [17]. Nowadays, the therapeutic use of MDMA remains rare or almost nonexistent worldwide [18]. However, MDMA is regaining a certain interest among psychiatrists, and several clinical trials are ongoing to explore whether MDMA has a therapeutic potential, especially in the treatment of post-traumatic stress disorder (PTSD), alcohol use disorder, and anxiety [19]. The data from a recent randomized placebo-controlled phase 3 trial suggest that MDMA-assisted therapy reduced PTSD symptoms and functional impairment in a diverse population with moderate to severe PTSD [20]. Moreover, MDMA assisted therapy could be of potential interest in cases of adult addictions like alcohol use disorder [19,21]. Such a therapeutic use of MDMA raises safety concerns. Indeed, the response to this substance seems to vary greatly among individuals and may be associated with transient neurocognitive effects including verbal and spatial memory deficits, slow processing speeds, and executive functioning impairments [19]. Furthermore, related fatalities have been reported with the illicit used of MDMA [22,23]. Such inter-variability seems to be influenced by both environmental and genetic factors. Environmental factors may include concomitant substance use or individual health status, while genetic factors primarily involve variations within genes encoding for MDMA’s metabolic enzymes and/or pharmacological targets [24,25]. In this context, pharmacogenomics could play a crucial role in guiding MDMA treatment by identifying individuals with genetic predispositions that may affect their response to MDMA. By tailoring treatment plans based on individual’s genetic makeup, it may be possible to enhance therapeutic outcomes and minimize adverse effects, leading to safer and more effective use of MDMA in clinical settings.
The objective of this narrative review is to identify and synthesize existing literature evaluating the pharmacogenomics of MDMA response. Identifying key allelic variants that may impact the PK and pharmacodynamics (PD) of MDMA could help to predict individual response and adapt the treatment, aiming to minimize adverse effects and maximize therapeutic benefits.
2. Genetic Factors Influencing the Metabolism of MDMA
Over the last decades, a certain interest has been focused on the pharmacogenomics of MDMA. Current research predominantly explores genetic factors impacting MDMA metabolism, notably polymorphisms within the genes encoding for COMT and CYP2D6. Tucker et al. first proposed that carrying the poor CYP2D6 metabolizer genotype could predispose individuals to acute MDMA toxicity and related death [26]. Such a postulate seems plausible given the highly polymorphic nature of the CYP2D6 gene, with more than 170 variants described (pharmVAR.org). These variants include single-nucleotide polymorphisms, small insertions/deletions, as well as larger structural variants such as multiplications, deletions, tandem arrangements, and copy number variations (CNV). The frequency of these variants differs across populations, and they significantly influence the drug-metabolizing function of CYP2D6 [27,28,29]. However, this hypothesis was not confirmed by further studies [30,31,32]. Indeed, O’Donohoe et al. aimed to determine whether individuals with poor metabolizer genotypes for the CYP2D6 enzyme were at higher risk of MDMA toxicity [31]. Thus, they retrospectively examined seven cases of toxicity or death thought to be due to MDMA. DNA was extracted from blood samples, amplified using polymerase chain reaction, and analyzed for the presence of CYP2D6 mutations “A” and/or “B”. In this study, none of the seven cases exhibited poor metabolizer genotypes for CYP2D6. According to the authors, such results suggested that CYP2D6 genetic variability did not significantly influence MDMA toxicity [31]. Although the specific CYP2D6 mutations referred to as “A” and “B” were not clearly identified in the study, they likely correspond to the CYP2D6*3 and CYP2D6*4 alleles based on the described genotyping method. Indeed, a polymerase chain reaction (PCR) technique followed by enzyme digestion was performed to detect these specific mutations. Bands at 280 bp and 160 bp indicate the A mutation (CYP2D6*3) while bands at 380 bp and 180 bp indicate the B mutation (CYP2D6*4). These alleles are associated with poor metabolizer phenotype. Nevertheless, the small sample size of only seven cases strongly limits the reliability and generalizability of the conclusions [31]. Further research with a larger cohort is necessary to validate these findings. Similarly, Gilhooly et al. also investigated if CYP2D6 enzyme deficiency could contribute to ecstasy-related fatalities [32]. They selected fifteen individuals believed to have died from ecstasy-related toxicity (defined by the pathologist as the presence of MDMA, MDA, and/or 3,4-methylenedioxy-N-ethylamphetamine). Biological materials (liver or blood) were genetically screened for the two nonfunctional CYP2D6*3 and CYP2D6*4. It is important to note that the study refers to alleles CYP2D6*3 and CYP2D6*4 without specifying the exact rsID numbers. In this study, none of the 15 genotyped samples were predicted to be a poor metabolizer. According to the authors, this result suggested that CYP2D6 deficiency did not appear to be a determinant factor in ecstasy-related fatalities [32]. However, here again, this study has notable limitations. The small sample size of only 15 subjects limits the ability to draw generalizable conclusions. Furthermore, the study focused solely on the CYP2D6*3 and CYP2D6*4 polymorphisms, neglecting other potentially relevant genetic variations that might influence MDMA metabolism and toxicity. Additionally, the complexity of MDMA-related fatalities, which can involve various mechanisms and are often associated with concurrent polydrug use, further limits the extent and applicability of the findings [32].
To date, the real impact of CYP2D6 genetic polymorphisms in explaining inter-individual differences in response to MDMA remains unclear. While some genetic polymorphisms have been shown to influence MDMA metabolism in humans, resulting in altered plasma concentrations of MDMA and/or metabolites (especially MDA and HHMA), their clinical relevance remains a matter of debate [4,33,34,35,36,37,38,39,40,41,42,43,44]. Indeed, some studies have downplayed the potential contribution of CYP2D6 polymorphisms in this response [40,41]. However, others have emphasized their significance [42,43,44]. For instance, low-activity CYP2D6 genotypes have been associated with an increased risk of hyponatremia and increased cortisol production when MDMA is used [42,44]. It is important to note that both studies do not specify rsID numbers for the CYP2D6 polymorphisms but group them into metabolizer status categories (poor, intermediate, extensive, and ultrarapid metabolizers). Conversely, in another study conducted by Cuyas et al. among a cohort of 263 subjects including 60 MDMA users in order to clarify the potential role of genetic polymorphisms in explaining inter-individual differences in cognitive MDMA effects, individuals with a CYP2D6 ultrarapid metabolizer genotype performed worse on semantic fluency tasks compared to a control group [43]. Specifically, those with such a genotype generated significantly fewer words within a set time frame. This study demonstrated an influence of the CYP2D6 ultrarapid metabolizer genotype on MDMA toxicity, indicating that increased enzymatic activity could lead to higher concentrations of neurotoxic metabolites, resulting in cognitive impairments. This result emphasizes the importance of considering genetic factors when assessing the cognitive effects of MDMA use. Of note, here again, specific rsID numbers for CYP2D6 polymorphisms were not mentioned in the study [43]. Overall, these results seem to support the hypothesis that CYP2D6 polymorphisms may modulate MDMA-induced effects. However, further research with larger sample sizes is needed to investigate this question more thoroughly. Indeed, as specified by Papaseit et al., the frequencies of poor metabolizers (7–10%) and ultrarapid metabolizers (less than 5%) in the Caucasian population and approximately 1.4% and 4.5% in the African-American population results in their reduced representation in MDMA clinical trials [45].
Regarding the other CYPs involved in MDMA metabolism (namely CYP2B6, CYP2C19, CYP3A4, and CYP1A2), the conversion of MDMA to MDA was negatively associated with genotypes known to convey lower CYP2C19 (rs4244285 and rs28399504 single nucleotide polymorphisms [SNP]s) or CYP2B6 (rs3745274 SNP) activities in the study conducted by Vizeli et al. [46]. Moreover, poor CYP2C19 metabolizers (rs4244285 and rs28399504 SNPs) exhibited greater cardiovascular responses to MDMA compared with other CYP2C19 genotypes [46]. In addition, regarding CYP1A2, tobacco smokers with the A/A genotype for the rs762551 SNP exhibited higher MDA blood concentrations compared to all nonsmokers with the A/C and C/C genotypes for the same SNP [46]. At any rate, once again, further research is also needed in the cases of these CYPs.
The impact of genetic COMT polymorphisms among MDMA users has also been well investigated. COMT is involved in the phase II metabolism of MDMA and in the metabolic inactivation of endogenous catecholamines such as NE or DA [4]. The COMT gene displays a major functional polymorphism at codon 158, producing a valine (val) to methionine (met) substitution (Val158Met; rs4680 SNP), resulting in three genotypes: val/val, val/met, and met/met. Carriers of the met allele exhibit lower enzyme activity compared with those homozygous for the val allele [4]. MDMA neurotoxicity and/or hepatotoxicity have been associated with COMT activity level [47,48]. Indeed, according to Perfetti et al., low COMT activity increases the concentration of HHMA, potentially leading to the increased formation of orthoquinones with subsequent consequences in terms of cell damage [48]. COMT activity level has also been associated with MDMA cognitive and cardiovascular effects. Indeed, in the study conducted by Cuyas et al., MDMA users (n = 60) carrying COMT val/val genotype exhibited poorer performance than paired controls on visuospatial attention and/or memory [43]. Furthermore, in another study conducted by Pardo-Lozano et al. (n = 27), individuals carrying COMT Val158Met polymorphism experienced increased cardiovascular effects (especially increased heart rate or even tachycardia) than others [39]. Additionally, COMT activity level has been associated with biological changes among MDMA users. Indeed, as for low CYP2D6 activity genotypes, low COMT activity genotypes have been associated with an increased risk of hyponatremia and increased production of cortisol [42,44]. Otherwise, it is also important to note that a specific polymorphism in COMT (rs165599) seems to modulate MDMA-induced negative effects on verbal fluency among MDMA users [49]. This polymorphism, which involves an A allele being replaced by a G allele, has been the subject of extensive research [50,51]. For example, a study conducted by Lamb et al. highlighted that the G allele is associated with a 24% lower messenger ribonucleic acid (mRNA) expression compared to the A allele [51]. However, the functional consequences of the rs165599 SNP in terms of COMT protein expression and/or enzymatic activity still remain not fully understood [50,51]. Such a lack of clarity underscores the complexity of linking specific genetic polymorphisms to phenotypic outcomes and highlights the necessity for further studies. The influence of metabolic enzyme polymorphisms on MDMA’s effects is summarized in the Table 1.

Table 1.
The influence of metabolic enzyme polymorphisms on MDMA’s effects.
3. Genetic Factors Influencing Pharmacological MDMA Targets
Most studies on genetic variants affecting the PD of MDMA have focused on the 5HT system. 5HT is a neurotransmitter that regulates various activities in humans including behavior, mood, memory, and gastrointestinal homeostasis [52]. 5HT is synthesized in the raphe nuclei of the brain and the enterochromaffin cells of the intestinal mucosa [52]. At a molecular level, synthesis begins with L-tryptophan (L-Trp), which undergoes hydroxylation to 5-hydroxy-L-triptophan and decarboxylation to 5HT. The hydroxylation reaction requires TPH (which is considered the rate limiting enzyme of 5HT production), while the decarboxylation reaction requires aromatic L-amino acid decarboxylase. 5HT activity is regulated by its rate of synthesis, release, and metabolism. 5HT that is recycled back via the 5HT transporter (5HTT) may be stored into vesicles or metabolized by MAO [52]. The 5HT system is a primary target for many medications but also several recreational drugs like MDMA. Indeed, as described above, this compound acts as a releaser and reuptake inhibitor of 5HT. In addition, MDMA acts as a 5HTR2A weak agonist and inhibits both MAO and TPH [2,3] (Figure 1).
Several studies have pointed out associations between MDMA-induced effects and 5HTT expression [43]. 5HTT is encoded by the gene SLC6A4 (located on the human chromosome 17). A variation in the promoter region of this gene (known as serotonin-linked promoter region or 5HTTLPR) influences its transcriptional activity and regulates 5HTT expression [53]. Furthermore, another genetic variation corresponding to a variable number tandem repeat (VNTR) polymorphism in the intron 2 (5HTTVNTR) may also have functional consequences in terms of 5HTT expression [54]. In the study conducted by Roiser et al. (n = 56), MDMA users carrying the 5HTTLPR s/s (low functionality) genotype were the group that scored highest on the Beck Depression Inventory and performed worst in the Go/No-Go affective test, suggesting that the possession of the s allele confers particular vulnerability to disturbances in emotional processing after MDMA use [55]. It is important to note that the 5HTTLPR genotype did not affect outcomes at baseline (i.e., before MDMA use) in this study. There were no significant differences in behavioral measures or genotype frequencies between the comparison groups (cannabis users and healthy volunteers) when analyzed. This result suggests that the 5HTTLPR genotype did not influence initial outcomes prior to MDMA use [55]. Such an association between 5HTTLPR polymorphisms and mood disorders among MDMA users has also been highlighted in other studies [56,57]. Martin-Santos et al. showed a significant association between the 5HTTLPR genotype and lifetime prevalence of primary mood disorders in MDMA users (n = 37). Individuals carrying the 5HTTLPR s/s genotype had a higher prevalence of primary mood disorders (33.3%) compared to those with l/l or l/s genotypes (4%) [56]. In their study, Kuypers et al. observed that MDMA significantly reduced self-rated depressive feelings specifically in females carrying the 5HTTLPR s/s genotype (n = 63). This reduction in depressive feelings was not evident in males or in females carrying the s allele [57]. Moreover, in the study conducted by Fagundo et al. (n = 30), MDMA users carrying the 5HTTLPR s/s genotype performed significantly worse than the others on a verbal fluency assessment [49]. In another study, MDMA users carrying the 5HTTLPR s/s genotype exhibited poorer performance than paired controls on visuospatial attention and/or memory after MDMA consumption [43]. Otherwise, in the study conducted by Pardo-Lozano et al. (n = 27), subjects carrying the 5HTTLPR l/* (high functionality) genotype experienced increased cardiovascular effects (especially increased heart rate or even tachycardia) than others, and the subjects carrying 5HTTLPR s/s genotype exhibited more negative subjective effects (including dizziness, anxiety, and sedation) than others [39]. These results appear to link MDMA-induced effects to 5HTTLPR polymorphisms [58]. These data are summarized in Table 2. Furthermore, it is important to note that such an association has not been demonstrated with HTTVNTR polymorphisms. Similarly, MDMA-induced effects do not seem to depend on genetic variations within the genes encoding for TPH1 (rs1800532 and rs1799913), TPH2 (rs7305115), 5HTR1A (rs6295), 5HTR1B (rs6296), and 5HTR2A (rs6313). This may seem surprising, given the fact that this gene is highly polymorphic and that several polymorphisms have been linked to various neuropsychiatric disorders [59,60,61,62,63,64,65,66]. Otherwise, it is important to note that no study has investigated a potential link between MDMA-induced effects and genetic variations within the gene encoding for MAO.

Table 2.
The influence of 5HTTLPR polymorphisms on MDMA’s effects.
MDMA also interacts with the DA system. Indeed, MDMA is a potent inhibitor of reuptake and a stimulator of DA release [2,3,4,5]. In addition, MDMA has antagonistic effects on DR and, more specifically, DRD2 [4,5]. DA is an endogenous catecholamine, which exerts widespread effects in humans. Within the central nervous system, DA binds to specific membrane receptors and plays a key role in the control of locomotion, learning, memory, cognition, and emotion [66]. The DA system is involved in various neurological and psychiatric disorders such as Parkinson’s disease or schizophrenia and in drug addiction [66]. DA synthesis involves several enzymes and cofactors, but the rate-limiting enzyme appears to be tyrosine hydroxylase. Furthermore, the DA system also includes the DA transporter (DAT1) and several DRs (namely D1, D2, D3, D4, and D5) with various structures and functions [66,67,68]. DAT1 is encoded by the gene SLC6A3 (located on the human chromosome 5). The most commonly investigated genetic variation within this gene is the 3’-UTR VNTR polymorphism, which regulates DAT1 expression. A variation in the number of repeats has been associated with several conditions including attention-deficit hyperactivity disorder, alcohol use disorder, and Parkinson’s disease. DRs are encoded by different genes located in different chromosomes. The gene encoding for DRD2 is located on the human chromosome 11 and appears highly polymorphic [69,70]. To the authors’ knowledge, only one study has explored the influence of variants within genes encoded for the DA system on the effects of MDMA in humans [24]. This randomized, placebo-controlled, crossover study was conducted by Vizeli et al. and investigated several polymorphisms (SNP and VNTR) within genes encoded for key players in the DA system including DAT1 (rs28363170, rs3836790, rs6347, rs11133767, rs11564774, rs460000, and rs463379 SNPs), DRD2/ANKK1 (rs1800497 SNP), DRD2 (rs6277 and rs107959 SNPs), and DRD4 (rs1805186) [24]. However, with the limit of the number of included patients (n = 149), none of the tested genetic polymorphisms were associated with MDMA-induced effects, suggesting that genetic variations within genes encoded for the DA system are unlikely to explain interindividual differences in the effects of MDMA in humans [24]. Importantly, the influence of variants within other genes (namely those encoded for brain-derived neurotrophic factor and the glutamate receptor subunit epsilon-2) has been explored, but no association has been demonstrated [43].
To date, only a few other PD targets of MDMA have been investigated. Bershad et al. have examined the influence of an SNP within the oxytocin receptor (OXTR) gene (rs53576 SNP) on responses to MDMA. In their study, the authors found that a high dose of MDMA (1.5 mg/kg) did not increase sociability in individuals carrying the rs35376 A/A genotype as it did in G allele carriers. Interestingly, the genotypic groups did not differ in response at the lower MDMA dose nor in cardiovascular or other subjective responses [71]. Similarly, Vizeli et al. investigated the impact of OXTR gene variations on the socioemotional effects of MDMA in humans. The study pooled data from eight double-blind, placebo-controlled studies involving 132 healthy subjects. The analysis focused on three SNPs of the OXTR gene (rs53576, rs1042778 and rs2254298 SNPs). The authors found that MDMA produced significantly greater feelings of trust in individuals with the rs1042778 TT genotypes compared with G allele carriers. They concluded that OXTR gene variations might influence certain prosocial effects of MDMA in humans, but this interpretation should be cautious due to the small sample size [72]. Otherwise, Vizeli et al. are the only authors known to have investigated the impact of genotypes within the NE system on MDMA’s effects. This may appear as a significant gap. Indeed, the NE system may play an unexpected role in the actions of MDMA and contributes to its cardiac and psychostimulant effects [73,74]. In this system, the NE transporter (NET) is a crucial player. NET is encoded by the gene SLC6A2, which is located on the human chromosome 16 (locus 16q12.2). This gene is highly polymorphic, and several SNPs have been described [75]. In their study, Vizeli et al. investigated whether the SLC6A2 rs168924, rs47958, rs1861647, rs2242446, and rs36029 SNPs influence MDMA’s cardiovascular and subjective stimulant effects. The study pooled data from eight double-blind, placebo-controlled studies involving 124 healthy subjects. The authors found that three SLC6A2 SNPs (rs1861647, rs2242446, and rs36029 SNPs) seemed to influence the cardiovascular response to MDMA, but the effect sizes were small and did not allow definitive conclusions [74]. Consequently, large-scale, controlled pharmacogenomics studies are needed to investigate the role of the NE system in MDMA’s actions. The influence of pharmacodynamics (PD) target polymorphisms on MDMA’s effects is summarized in the Table 3.

Table 3.
The influence of pharmacodynamics (PD) target polymorphisms on MDMA’s effects.
4. Conclusions
A certain interest has been focused on the pharmacogenomics of MDMA over the last decades. Indeed, the influence of variants within genes encoded for several enzymes involved in MDMA metabolism (especially CYP2D6 and COMT) and/or PD targets (especially the 5HT system) on the effects of MDMA in humans have been investigated. Thereby, some studies have pointed out associations between MDMA-induced effects and polymorphisms. COMT Val158Met polymorphism has, for example, been associated with cognitive and cardiovascular effects. Similarly, the 5HTTLPR s/s genotype has been associated with several MDMA-induced adverse effects, especially mood disorders. However, despite interesting results, only a few associations have been highlighted. Furthermore, some genes, in particular those encoded for MDMA targets, have been only poorly investigated, which appears as a significant gap. This reinforces the need for large-scale, controlled pharmacogenomics studies focused on a large panel of genes involved in MDMA PK and PD. Such an approach appears necessary today due to the re-emergence of psychedelic agents in Western medicine as a promising treatment for several mental conditions including PTSD or major depression disorder.
Author Contributions
Conceptualization, G.D. and M.B.; methodology, G.D., C.A. and M.B.; software, validation, C.A. and M.B.; investigation, G.D., M.P.-M., A.B. (Aurélien Bauduin) and A.B. (Antoine Baudriller); writing—original draft preparation, G.D., M.P.-M. and A.B. (Aurélien Bauduin); writing—review and editing, C.A. and M.B.; supervision, M.B. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bernschneider-Reif, S.; Oxler, F.; Freudenmann, R.W. The origin of MDMA (‘ecstasy’)—Separating the facts from the myth. Pharmazie 2006, 61, 966–972. [Google Scholar] [PubMed]
- De La Torre, R.; Farré, M.; Roset, P.N.; Pizarro, N.; Abanades, S.; Segura, M.; Segura, J.; Cami, J. Human pharmacology of MDMA: Pharmacokinetics, metabolism, and disposition. Ther. Drug Monit. 2004, 26, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kalant, H. The pharmacology and toxicology of ‘ecstasy’ (MDMA) and related drugs. Can. Med. Assoc. J. 2001, 165, 917–928. [Google Scholar]
- Rietjens, S.J.; Hondebrink, L.; Westerink, R.H.; Meulenbelt, J. Pharmacokinetics and pharmacodynamics of 3,4-methylenedioxymethamphetamine (MDMA): Interindividual differences due to polymorphisms and drug–drug interactions. Crit. Rev. Toxicol. 2012, 42, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Highgate, Q. Methylenedioxymethamphetamine (MDMA): Serotonergic and dopaminergic mechanisms related to its use and misuse. J. Neurochem. 2021, 157, 1714–1724. [Google Scholar] [CrossRef]
- Dumont, G.J.H.; Sweep, F.C.G.J.; Van der Steen, R.; Hermsen, R.; Donders, A.R.T.; Touw, D.J.; Van Gerven, J.M.A.; Buitelaar, J.K.; Verkes, R.J. Increased oxycotin concentrations and prosocial feelings in human after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc. Neurosci. 2009, 4, 359–366. [Google Scholar] [CrossRef]
- Hysek, C.M.; Simmler, L.D.; Nicola, V.G.; Vischer, N.; Donzelli, M.; Krahenbuhl, S.; Grouzmann, E.; Huwyler, J.; Hoener, M.C.; Liechti, M.E. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS ONE 2012, 7, e36476. [Google Scholar] [CrossRef]
- Kuwayama, K.; Inoue, H.; Kanamori, T.; Tsujikawa, K.; Miyaguchi, H.; Iwata, Y.; Miyauchi, S.; Kamo, N.; Kishi, T. Uptake of 3,4-methylenedioxymethamphetamine and its related compounds by a proton-coupled transport system in Caco-2 cells. Biochim. Biophys. Acta. 2008, 1778, 42–50. [Google Scholar] [CrossRef]
- Bertelsen, K.M.; Greenblatt, D.J.; Von Moltke, L.L. Apparent active transport of MDMA is not mediated by P-glycoprotein: A comparison with MDCK and Caco-2 monolayers. Biopharm. Drug Dispos. 2006, 27, 219–227. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, R.; Farré, M.; Ortuño, J.; Mas, M.; Brenneisen, R.; Roset, P.N.; Segura, J.; Cami, J. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br. J. Clin. Pharmacol. 2000, 49, 104–109. [Google Scholar] [CrossRef]
- Steuer, A.E.; Schmidhauser, C.; Tingelhoff, E.H.; Schmid, Y.; Rickli, A.; Kraemer, T.; Liechti, M.E. Impact of cytochrome P450 2D6 function on the chiral blood plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA) and its phase I and II metabolites in humans. PLoS ONE 2016, 11, e0150955. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Elayan, I.; Hanson, G.R.; Foltz, R.L.; Gibb, J.W.; Lim, H.K. Effects of 3,4-dihydroxymethamphetamine and 2,4,5-trihydroxymethamphetamine, two metabolites of 3,4-methylenedioxyamphetamine, on central serotoninergic and dopaminergic systems. J. Pharmacol. Exp. Ther. 1992, 216, 447–453. [Google Scholar]
- Mueller, M.; Goodwin, A.K.; Ator, N.A.; McCann, U.D.; Ricaurte, G.A. Metabolism and disposition of 3,4-methylenedioxymethamphetamine (“Ecstasy”) in baboons after oral administration: Comparision with humans reveals marked differences. J. Pharmacol. Exp. Ther. 2011, 338, 310–317. [Google Scholar] [CrossRef]
- Pizarro, N.; De La Torre, R.; Joglar, J.; Okumura, N.; Perfetti, X.; Lau, S.S.; Monks, T.J. Serotonergic neurotoxic thioether metabolites of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”): Synthesis, isolation, and characterization of diastereoisomers. Chem. Res. Toxicol. 2008, 21, 2272–2279. [Google Scholar] [CrossRef]
- Baez, S.; Segura-Aguilar, J.; Widersten, M.; Johansson, A.S.; Mannervik, B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem. J. 1997, 324, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Shulgin, A.T.; Nichols, D.E. Characterization of three new psychotomimetics. In The Psychopharmacology of Hallucinogens; Stillman, R.C., Willette, R.E., Eds.; PErgamon Press: New York, NY, USA, 1978; pp. 74–83. [Google Scholar]
- Nichols, D.E. Entactogens: How the name for a novel class of psychoactive agents originated. Front. Psychiatry 2022, 13, 863088. [Google Scholar] [CrossRef]
- Yazar-Klosinski, B.; Mithoefer, M. Potential psychiatric uses for MDMA. Clin. Pharmacol. Ther. 2017, 101, 194–196. [Google Scholar] [CrossRef]
- Sessa, B.; Higbed, L.; Nutt, D. A review of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front. Psychiatry 2019, 10, 138. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Ot’alora, G.M.; Van Der Kolk, B.; Shannon, S.; Bogenschutz, M.; Gelfand, Y.; Paleos, C.; Nicholas, C.R.; Quevedos, S.; Balliett, B.; et al. MDMA-assisted therapy for moderate to severe PTSD: A randomized, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2473–2480. [Google Scholar] [CrossRef]
- Sessa, B. Why MDMA therapy for alcohol use disorder? And why now? Neuropharmacology 2018, 142, 83–88. [Google Scholar] [CrossRef]
- Roxburgh, A.; Sam, B.; Kriikku, P.; Mounteney, J.; Castanera, A.; Dias, M.; Giraudon, I. Trends in MDMA-related mortality across four countries. Addiction 2021, 116, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, A.; Lappin, J. MDMA-related deaths in Australia 2000 to 2018. Int. J. Drug Policy 2020, 76, 102630. [Google Scholar] [CrossRef]
- Vizeli, P.; Liechti, M.E. No influence of dopamine system gene variations on acute effects of MDMA. Front. Psychiatry 2019, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. Role of serotonin transporter and receptor gene variations in the acute effects of MDMA in healthy subjects. ACS Chem. Neurosci. 2019, 10, 3120–3131. [Google Scholar] [CrossRef]
- Tucker, G.T.; Lennard, M.S.; Ellis, S.W.; Woods, H.F.; Cho, A.K.; Lin, L.Y.; Hiratsuka, A.; Schmitz, D.A.; Chu, T.Y.Y. The demethylenation of methylenedioxyamphetamine (“ectasy”) by debrisoquine hydroxylase (CYP2D6). Biochem. Pharmacol. 1994, 7, 1151–1156. [Google Scholar] [CrossRef]
- Leeder, J.S.; Gaedigk, A. CYP2D6 and pharmacogenomics: Where does future research need to focus? Part 2: Clinical aspects. Pharmacogenomics 2014, 15, 1055–1058. [Google Scholar] [CrossRef]
- Taylor, C.; Crosby, I.; Yip, V.; Maguire, P.; Pirmohamed, M.; Turner, R.M. A review of the important role of CYP2D6 in pharmacogenomics. Genes 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Lassen, D.; Damkier, P.; Brøsen, K. The pharmacogenetics of tramadol. Clin. Pharmacokinet. 2015, 54, 825–836. [Google Scholar] [CrossRef]
- Schwab, M.; Griese, U.; Hermle, L.; Gouzoulis, E.; Zanger, U.; Mikus, G. Is there an impact of CYP2D6 genotype on the toxicity of’ecstasy and related designer drugs? Naunyn Schmiedebergs Arch Pharmacol 1998, 4, 163. [Google Scholar]
- O’Donohoe, A.; O’Flynn, K.; Shields, K.; Hawi, Z.; Gill, M. MDMA toxicity: No evidence for a major influence of metabolic genotype at CYP2D6. Addict Biol. 1998, 3, 309–314. [Google Scholar] [CrossRef]
- Gilhooly, T.C.; Daly, A.K. CYP2D6 deficiency, a factor in ecstasy related deaths? Br. J. Clin. Pharmacol. 2002, 54, 69–70. [Google Scholar]
- Haufroid, V.; Hantson, P. CYP2D6 genetic polymorphisms and their relevance for poisoning due to amfetamines, opioid analgesics and antidepressants. Clin. Toxicol. 2015, 53, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jamei, M.; Heydari, A.; Yeo, K.R.; De La Torre, R.; Farré, M.; Tucker, G.T.; Rostami-Hodjegan, A. Implications of mechanism-based inhibition of CYP2D6 for the pharmacokinetics and toxicity of MDMA. J. Psychopharmacol. 2006, 20, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Hysek, C.M.; Fink, A.E.; Simmler, L.D.; Donzelli, M.; Grouzmann, E.; Liechti, M.E. α1-adrenergic receptors contribute to the acute effects of 3,4-methylenedioxymethamphetamine in humans. J. Clin. Psychopharmacol. 2013, 5, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Hysek, C.M.; Simmler, L.D.; Schillinger, N.; Meyer, N.; Schmid, Y.; Donzelli, M.; Grouzmann, E.; Lietchti, M.E. Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. Int. J. Neuropsychopharmacol. 2014, 17, 371–381. [Google Scholar] [CrossRef]
- O’Mathúna, B.; Farré, M.; Rostami-Hodjegan, A.; Yang, J.; Cuyàs, E.; Torrens, M.; Pardo, R.; Abanades, S.; Maluf, S.; Tucker, G.T.; et al. The consequences of 3,4-methylenedioxymethamphetamine induced CYP2D6 inhibition in humans. J. Clin. Psychopharmacol. 2008, 28, 523–529. [Google Scholar] [CrossRef]
- De La Torre, R.; Farré, M.; Ó Mathúna, B.; Roset, P.N.; Pizarro, N.; Segura, M.; Torrens, M.; Ortuno, J.; Pujadas, M.; Cami, J. MDMA (ecstasy) pharmacokinetics in a CYP2D6 poor metaboliser and in nine CYP2D6 extensive metabolisers. Eur. J. Clin. Pharmacol. 2005, 61, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Lozano, R.; Farré, M.; Yubero-Lahoz, S.; O’Mathúna, B.; Torrens, M.; Mustata, C.; Pérez-Mana, C.; Langohr, K.; Cuyas, E.; Li Carbo, M.; et al. Clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”): The influence of gender and genetics (CYP2D6, COMT, 5-HTT). PLoS ONE 2012, 7, e47599. [Google Scholar] [CrossRef]
- De La Torre, R.; Yubero-Lahoz, S.; Pardo-Lozano, R.; Farré, M. MDMA, methamphetamine, and CYP2D6 pharmacogenetics: What is clinically relevant? Front. Genet. 2012, 3, 235. [Google Scholar] [CrossRef]
- Schmid, Y.; Vizeli, P.; Hysek, C.M.; Prestin, K.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. CYP2D6 function moderates the pharmacokinetics and pharmacodynamics of 3,4-methylene-dioxymethamphetamine in a controlled study in healthy individuals. Pharmacogenet. Genom. 2016, 26, 397–401. [Google Scholar] [CrossRef]
- Aitchison, K.J.; Tsapakis, E.M.; Huezo-Diaz, P.; Kerwin, R.W.; Forsling, M.L.; Wolff, K. Ecstasy (MDMA)-induced hyponatraemia is associated with genetic variants in CYP2D6 and COMT. J. Psychopharmacol. 2012, 26, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Cuyàs, E.; Verdejo-García, A.; Fagundo, A.B.; Khymenets, O.; Rodríguez, J.; Cuenca, A.; De Sola Llopis, S.; Langhor, K.; Pena-Casanova, J.; Torrens, M.; et al. The influence of genetic and environmental factors among MDMA users in cognitive performance. PLoS ONE 2011, 6, e27206. [Google Scholar] [CrossRef]
- Wolff, K.; Tsapakis, E.M.; Pariante, C.M.; Kerwin, R.W.; Forsling, M.L.; Aitchison, K.J. Pharmacogenetic studies of change in cortisol on ecstasy (MDMA) consumption. J. Psychopharmacol. 2012, 26, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Torrens, M.; Perez-Mana, C.; Muga, R.; Farré, M. Key individual determinants in MDMA pharmacodynamics. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 183–195. [Google Scholar] [CrossRef]
- Vizeli, P.; Schmid, Y.; Prestin, K.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. Pharmacogenetics of ecstasy: CYP1A2, CYP2C19, and CYP2B6 polymorphisms moderate pharmacokinetics of MDMA in healthy subjects. Eur. Neuropsychopharmacol. 2017, 27, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Antolino-Lobo, I.; Meulenbelt, J.; Nijmeijer, S.M.; Scherpenisse, P.; Van Den Berg, M.; Van Duursen, M.B.M. Differential roles of phase I and phase II enzymes in 3,4-methylendioxymethamphetamine-induced cytotoxicity. Drug Metab. Dispos. 2010, 38, 1105–1112. [Google Scholar] [CrossRef]
- Perfetti, X.; O’Mathúna, B.; Pizarro, N.; Cuyàs, E.; Khymenets, O.; Almeida, B.; Pellegrini, M.; Pichini, S.; Lau, S.S.; Monks, T.J.; et al. Neurotoxic thioether adducts of 3,4-methylenedioxymethamphetamine identified in human urine after ecstasy ingestion. Drug Metab. Dispos. 2009, 37, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Fagundo, A.B.; Cuyàs, E.; Verdejo-Garcia, A.; Khymenets, O.; Langohr, K.; Martín-Santos, R.; Farré, M.; De la Torre, R. The influence of 5-HTT and COMT genotypes on verbal fluency in ecstasy users. J. Psychopharmacol. 2010, 24, 1381–1393. [Google Scholar] [CrossRef]
- Bag, H.G.G. Association between COMT gene rs165599 SNP and schizophrenia: A meta-analysis of case-control studies. Mol. Genet. Genom. Med. 2018, 6, 845–854. [Google Scholar]
- Lamb, Y.N.; Thompson, J.M.D.; Murphy, R.; Wall, C.; Kirk, I.J.; Morgan, A.R.; Ferguson, L.R.; Mitchell, E.A.; Waldie, K.E.; ABC Study group. Perceived stress during pregnancy and the catechol-O-methyltransferase (COMT) rs165599 polymorphism impacts on childhood IQ. Cognition 2014, 132, 461–470. [Google Scholar] [CrossRef]
- Bamalan, O.A.; Moore, M.J.; Al Khalili, Y. Physiology, Serotonin. StatPearls Publishing. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545168/ (accessed on 30 July 2023).
- Ikegame, T.; Hidaka, Y.; Nakachi, Y.; Murata, Y.; Watanabe, R.; Sugawara, H.; Asai, T.; Kiyota, E.; Ikeda, M.; Sasaki, T.; et al. Identification and functional characterization of the extremely long allele of the serotonin transporter-linked polymorphic region. Transl. Psychiatry 2021, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Pungercic, G.; Videtic, A.; Pestotnik, A.; Pajnic, I.Z.; Zupanc, T.; Balazic, J.; Tomori, M.; Komel, R. Serotonin transporter gene promoter (5-HTTLPR) and intron 2 (VNTR) polymorphisms: A study on Slovenian population of suicide victims. Psychiatr. Genet. 2006, 16, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Roiser, J.P.; Cook, L.J.; Cooper, J.D.; Rubinsztein, D.C.; Sahakian, B.J. Association of a functional polymorphism in the serotonin transporter gene with abnormal emotional processing in ecstasy users. Am. J. Psychiatry. 2005, 162, 609–612. [Google Scholar] [CrossRef]
- Martín-Santos, R.; Torrens, M.; Poudevida, S.; Langohr, K.; Cuyás, E.; Pacifici, R.; Farré, M.; Pichini, S.; De la Torre, R. 5-HTTLPR polymorphism, mood disorders and MDMA use in a 3-year follow-up study. Addict. Biol. 2010, 15, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, K.P.C.; De la Torre, R.; Farre, M.; Xicota, L.; De Sousa Ferandes Perna, E.B.; Theunissen, E.L.; Ramakers, J.G. Depressive mood ratings are reduced by MDMA in female polydrug ecstasy users homozygous for the l-allele of serotonin transporter. Sci. Rep. 2018, 8, 1061. [Google Scholar] [CrossRef] [PubMed]
- Reneman, L.; Schilt, T.; De Win, M.M.; Booij, J.; Schmand, B.; Den Brink, W.V.; Bakker, O. Memory function and serotonin transporter promoter gene polymorphism in ecstasy (MDMA) users. J. Psychopharmacol. 2006, 20, 389–399. [Google Scholar] [CrossRef]
- Ates, O.; Karakus, N.; Sezer, S.; Bozkurt, N. Genetic association of 5-HT1A and 5-HT1B gene polymorphisms with migraine in a Turkish population. J. Neurol. Sci. 2013, 326, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, A.C.; Rodrigo, A.H.; Carcone, D.; McMain, S.; Jacobs, G.; Kennedy, J.L. Tryptophan hydroxylase 1 gene polymorphisms alter prefrontal cortex activation during response inhibition. Neuropsychology 2016, 30, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Pan, Z.; Jiao, Z.; Li, F.; Zhao, G.; Wei, Q.; Pan, F.; Evangelou, E. TPH2 gene polymorphisms and major depression—A meta-analysis. PLoS ONE 2012, 7, e36721. [Google Scholar] [CrossRef]
- Genis-Mendoza, A.D.; Ruiz-Ramos, D.; López-Narvaez, M.L.; Tovilla-Zárate, C.A.; García, A.R.; Meda, G.C.; Martinez-Magana, J.J.; Gonzales-Castro, T.B.; Juarez-Rojop, I.E.; Nicolini, H. Genetic association analysis of 5-HTR2A gene variants in eating disorders in a Mexican population. Brain Behav. 2019, 9, e01286. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.C.; MacKillop, J.; Weafer, J.; Hernandez, K.M.; Gao, J.; Palmer, A.A.; De Wit, H. Genetic analysis of impulsive personality traits: Examination of a priori candidates and genome-wide variation. Psychiatry Res. 2018, 259, 398–404. [Google Scholar] [CrossRef]
- Kaur, G.; Singh Chavan, B.; Gupta, D.; Sinhmar, V.; Prasad, R.; Tripathi, A.; Garg, P.D.; Gupta, R.; Khurana, H.; Gautam, S.; et al. An association study of dopaminergic (DRD2) and serotoninergic (5-HT2) gene polymorphism and schizophrenia in a North Indian population. Asian J. Psychiatry 2019, 39, 178–184. [Google Scholar] [CrossRef]
- Ni, J.; Lu, W.; Wu, Z.; Chen, J.; Yi, Z.; Zhang, C. T102C polymorphism of serotonin 2 A type receptor gene confers susceptibility to (early onset) schizophrenia in Han Chinese: An association study and meta-analysis. Asia Pac. Psychiatry 2013, 5, 24–30. [Google Scholar] [CrossRef]
- Marsden, C.A. Dopamine: The rewarding years. Br. J. Pharmacol. 2006, 147, S136–S144. [Google Scholar] [CrossRef] [PubMed]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.P. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics 2000, 1, 309–333. [Google Scholar] [CrossRef]
- He, M.; Yan, H.; Duan, Z.X.; Qu, W.; Gong, H.Y.; Fan, Z.L.; Kang, J.Y.; Li, B.C.; Wang, J.M. Genetic distribution and association analysis of DRD2 gene polymorphisms with major depressive disorder in the Chinese Han population. Int. J. Clin. Exp. Pathol. 2013, 6, 1142–1149. [Google Scholar]
- Della Torre, O.H.; Paes, L.A.; Henriques, T.B.; De Mello, M.P.; Celeri, E.H.R.V.; Dalgalarrondo, P.; Guerra-Junior, G.; Dos Santos-Junior, A. Dopamine D2 receptor gene polymorphisms and externalizing behaviors in children and adolescents. BMC Med. Genet. 2018, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Bershad, A.K.; Weafer, J.J.; Kirkpatrick, M.G.; Wardle, M.C.; Miller, M.A.; De Wit, H. Oxytocin receptor gene variation predicts subjective response to MDMA. Soc. Neurosci. 2016, 11, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Liechti, M.E. Oxytocin receptor gene variations and socio-emotional effects of MDMA: A pooled analysis of controlled studies in healthy subjects. PLoS ONE 2018, 13, e0199384. [Google Scholar] [CrossRef]
- Newton, T.F. A perhaps unexpected role of noreipenephrine in actions of MDMA. Clin. Pharmacol. Ther. 2011, 90, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. No major role of norepinephrine transporter gene variations in the cardiostimulant effects of MDMA. Eur. J. Pharmacol. 2018, 74, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Hwang, J.-A.; Lee, H.-J.; Yoon, H.-K.; Ko, Y.-H.; Lee, B.-H.; Jung, H.-Y.; Hahn, S.-W.; Na, K.-S. Association between norepinephrine transporter gene (SLC6A2) polymorphisms and suicide in patients with major depressive disorder. J. Affect. Disord. 2014, 158, 127–132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).