Formulation of Polymeric Nanoparticles Loading Baricitinib as a Topical Approach in Ocular Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biological Materials
2.3. Factorial Design
2.4. Preparation of the Selected Nanoparticles Loading BTB
2.5. Physicochemical Characterization of Nanoparticles
2.5.1. Encapsulation Efficiency (EE)
2.5.2. Morphological Study of Nanoparticles
2.5.3. Extensibility of Nanoparticles
2.5.4. Rheological Characterization
2.5.5. Stability of Nanoparticles
2.6. Antimicrobial Challenge Test
2.7. Ex Vivo Cornea Permeation Assays
2.8. In Vitro Tolerance Study
2.8.1. Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM)
2.8.2. Cornea Transparency
2.8.3. Cornea Histology Study
3. Results
3.1. Factorial Design
3.2. Nanoparticle Physicochemical Characterization
3.2.1. Encapsulation Efficiency
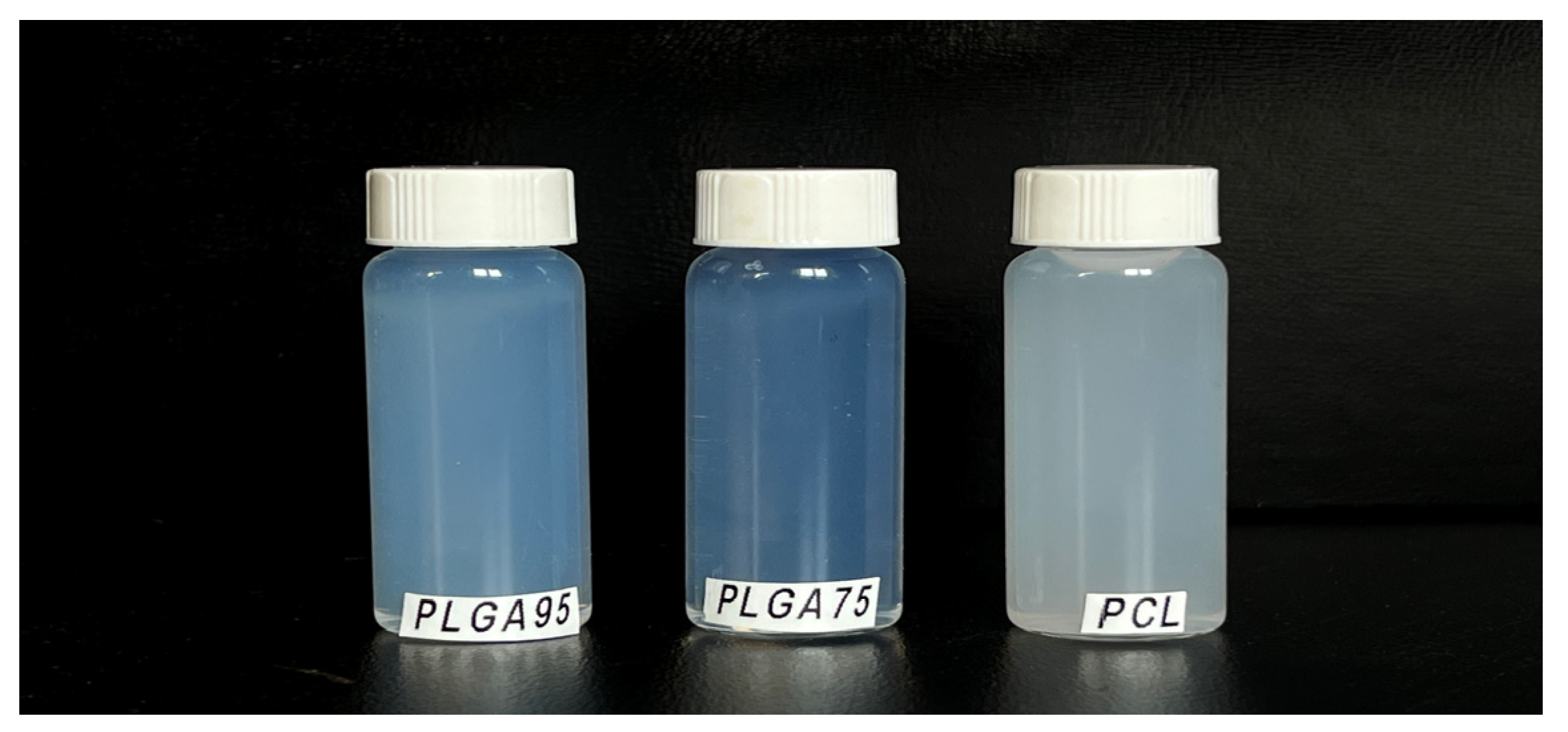
3.2.2. Morphological Study
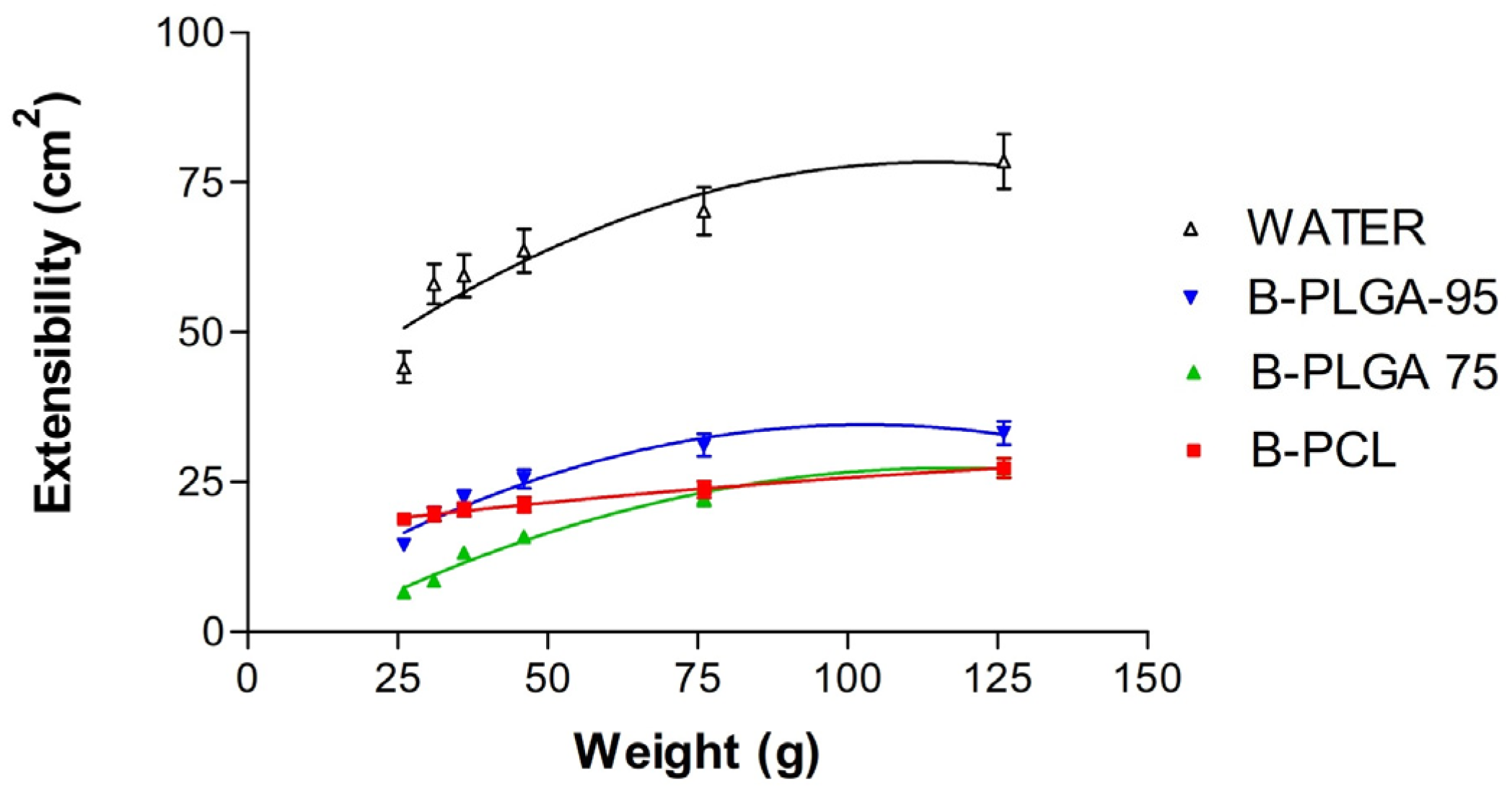
3.2.3. Extensibility Studies
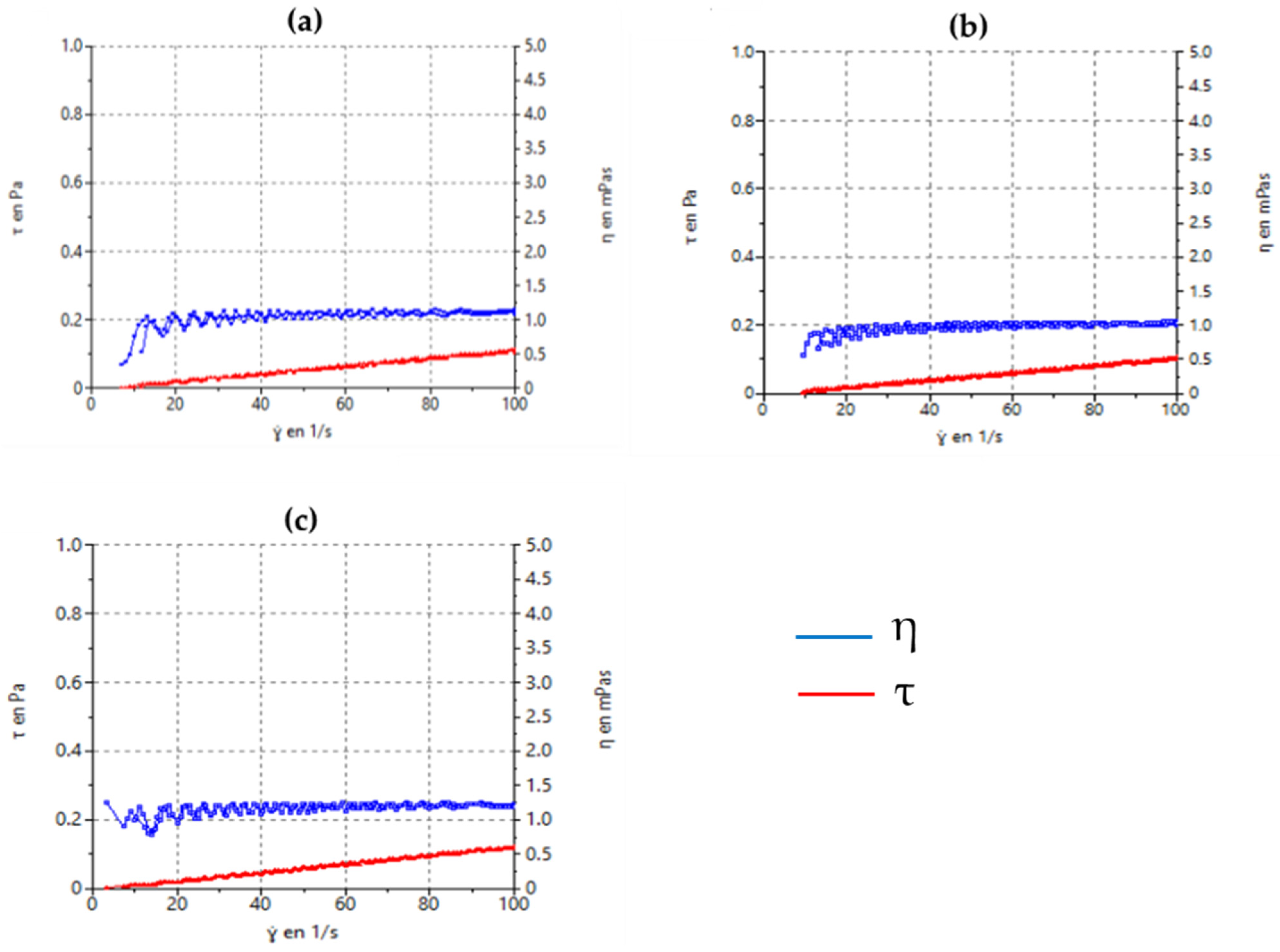
3.2.4. Rheological Characterization
3.2.5. Stability of Nanoparticles
3.3. Antimicrobial Challenge Test
3.4. Ex Vivo Permeation
3.5. In Vitro Tolerance Study
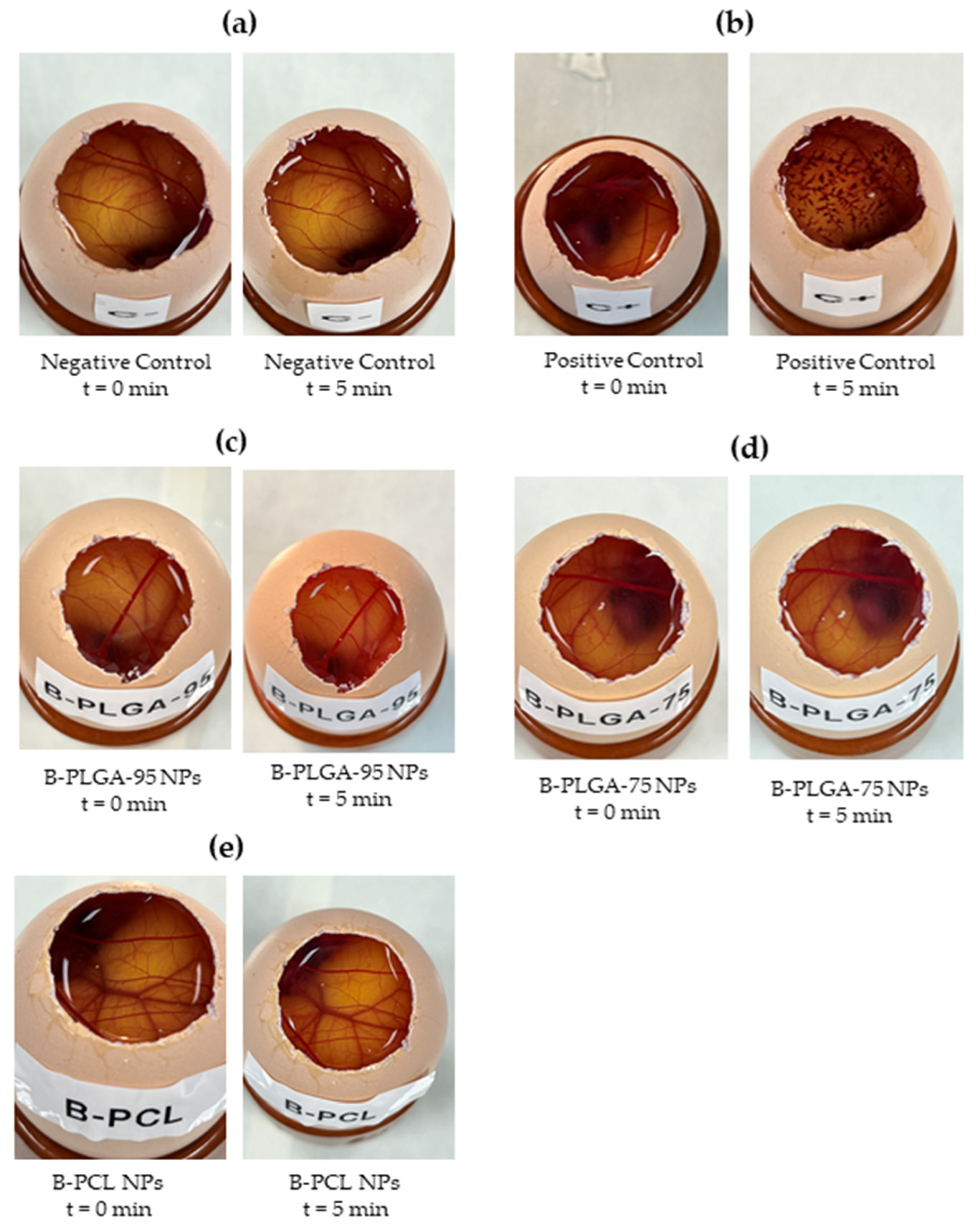
3.5.1. Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM)
3.5.2. Cornea Transparency
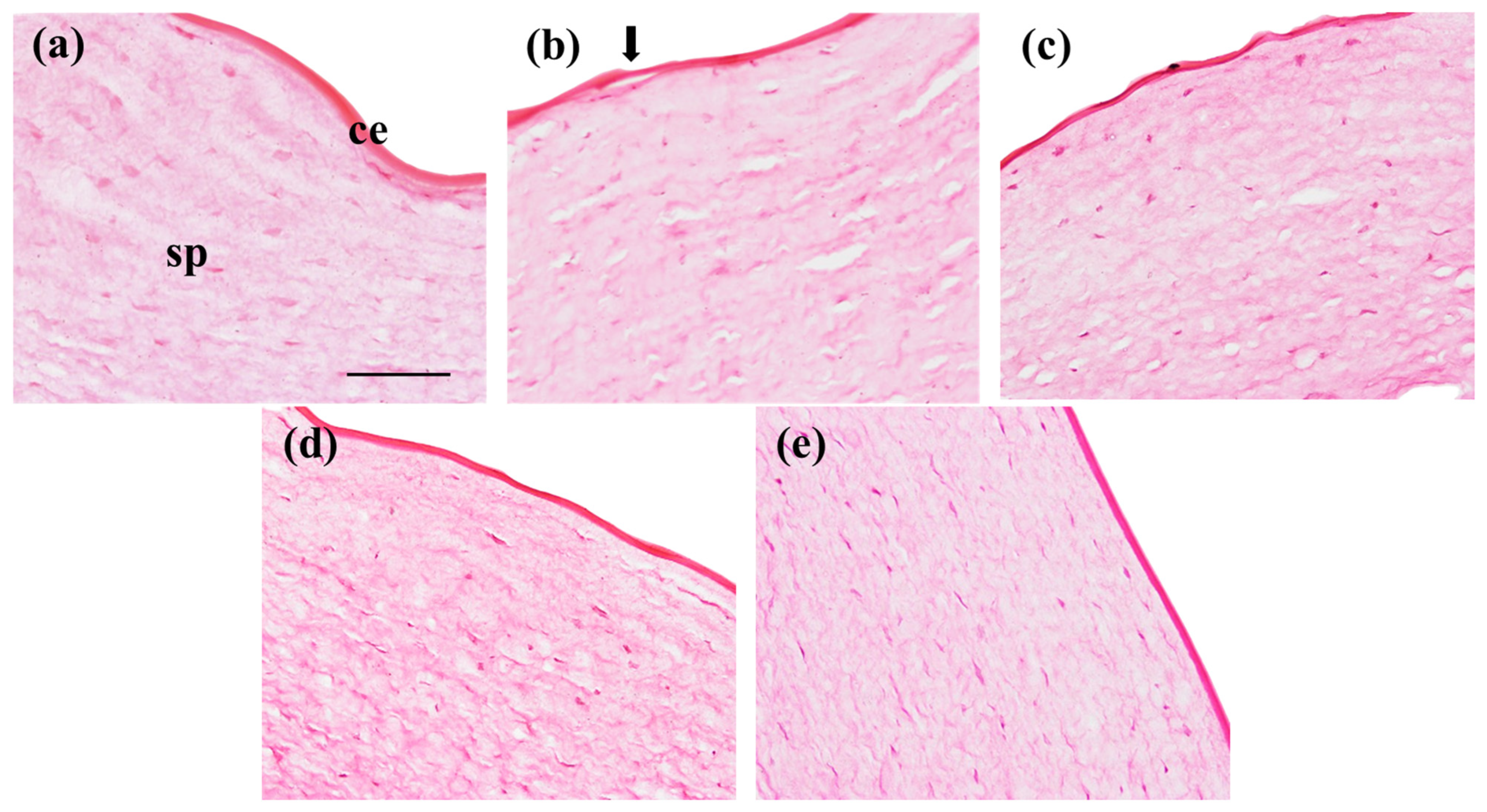
3.5.3. Cornea Histological Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, C.S.; Vitale, A.T. Diagnosis & Treatment of Uveitis; Jaypee Highlinghts, 2nd ed.; Medical Publishers, Inc.: Philadelphia, PA, USA, 2002. [Google Scholar]
- Kempen, J.H.; Gangaputra, S.; Daniel, E.; Levy-Clarke, G.A.; Nussenblatt, R.B.; Rosenbaum, J.T.; Suhler, E.B.; Thorne, J.E.; Foster, C.S.; Jabs, D.A.; et al. Long-Term Risk of Malignancy Among Patients Treated with Immunosuppressive Agents for Ocular Inflammation: A Critical Assessment of the Evidence. Am. J. Ophthalmol. 2008, 146, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Weber-Schöndorfer, C. 2.12—Immunomodulators. In Drugs During Pregnancy and Lactation, 2nd ed.; Schaefer, C., Peters, P., Miller, R.K., Eds.; Academic Press: Oxford, UK, 2007; pp. 321–334. ISBN 978-0-444-52072-2. [Google Scholar]
- Yi, J.; Zhang, Y.; Liang, R.; Zhong, F. Beta-Carotene Chemical Stability in Nanoemulsions Was Improved by Stabilized with Beta-Lactoglobulin—Catechin Conjugates through Free Radical Method. J. Agric. Food Chem. 2015, 63, 297–303. [Google Scholar] [CrossRef]
- Major, J.; Foroncewicz, B.; Paweł, J.; Mucha, K. Immunology and Donor- Specific Antibodies in Corneal Transplantation. Arch. Immunol. Ther. Exp. 2021, 69, 32. [Google Scholar] [CrossRef]
- Coster, D.J.; Williams, K.A. The Impact of Corneal Allograft Rejection on the Long-Term Outcome of Corneal Transplantation. Am. J. Ophthalmol. 2005, 140, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, E.; Laroche, L.; Borderie, V. Le Rejet d’allogreffe de Cornée. J. Fr. Ophtalmol. 2011, 34, 331–348. [Google Scholar] [CrossRef]
- Garrós, N.; Mallandrich, M.; Beirampour, N.; Mohammadi, R.; Domènech, Ò.; Rodríguez-Lagunas, M.J.; Clares, B.; Colom, H. Baricitinib Liposomes as a New Approach for the Treatment of Sjögren’s Syndrome. Pharmaceutics 2022, 14, 1895. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Murakami, T.; Nishitsuka, K.; Takakubo, Y. Effectiveness of Baricitinib in Refractory Seronegative Rheumatoid Arthritis and Uveitis: A Case Report. Front. Med. 2022, 8, 764067. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. Am. Assoc. Pharm. Sci. 2010, 12, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-Based Ocular Drug Delivery Systems: Recent Advances and Future Prospects. J. Nanobiotechnol. 2023, 21, 232. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in Ocular Applications and Their Potential Toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef]
- Wu, K.Y.; Tan, K.; Akbar, D.; Choulakian, M.Y.; Tran, S.D. A New Era in Ocular Therapeutics: Advanced Drug Delivery Systems for Uveitis and Neuro-Ophthalmologic Conditions. Pharmaceutics 2023, 15, 1952. [Google Scholar] [CrossRef]
- Suri, R.; Nag, T.C.; Mehra, N.; Neupane, Y.R.; Shafi, S.; Sharma, D.; Sharma, K.; Sultana, Y.; Kohli, K. Sirolimus Loaded Chitosan Functionalized PLGA Nanoparticles Protect against Sodium Iodate-Induced Retinal Degeneration. J. Drug Deliv. Sci. Technol. 2023, 82, 104369. [Google Scholar] [CrossRef]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G.P.; Valapala, M.; Kerur, N.; Passaglia, C.L.; et al. Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for Drug Delivery to the Anterior Segment of the Eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J.; Batrakova, E.; Gendelman, H.E. Cell Delivery of Therapeutic Nanoparticles, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 104, ISBN 9780124160200. [Google Scholar]
- El Moussaoui, S.; Abo-horan, I.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-ramírez, M.L.; Clares, B.; Soriano, J.L.; Calpena, A.C.; Fernández-campos, F.; et al. Polymeric Nanoparticles and Chitosan Gel Loading Ketorolac Tromethamine to Alleviate Pain Associated with Condyloma Acuminata during the Pre- and Post-ablation. Pharmaceutics 2021, 13, 1784. [Google Scholar] [CrossRef] [PubMed]
- Anwer, K.; Muharram, M.M.; Mohammad, M. Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-Microbial Activity and Bioavailability of Delafloxacin. Pharmaceutics 2020, 12, 252. [Google Scholar] [CrossRef]
- Andrade-Carrera, B.; Clares, B.; Noé, V.; Mallandrich, M.; Calpena, A.; García, M.; Garduño-Ramírez, M. Cytotoxic Evaluation of (2S)-5,7-Dihydroxy-6-Prenylflavanone Derivatives Loaded PLGA Nanoparticles against MiaPaCa-2 Cells. Molecules 2017, 22, 1553. [Google Scholar] [CrossRef]
- Carvajal-Vidal, P.; González-Pizarro, R.; Araya, C.; Espina, M.; Halbaut, L.; Gómez de Aranda, I.; García, M.L.; Calpena, A.C. Nanostructured Lipid Carriers Loaded with Halobetasol Propionate for Topical Treatment of Inflammation: Development, Characterization, Biopharmaceutical Behavior and Therapeutic Efficacy of Gel Dosage Forms. Int. J. Pharm. 2020, 585, 119480. [Google Scholar] [CrossRef]
- CPMP; ICH. ICH Topic Q 1 A (R2) Stability Testing of New Drug Substances and Products; European Medicines Agency: London, UK, 2003; Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products-scientific-guideline (accessed on 16 May 2022).
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Documento de La Real Farmacopea Española Por Internet. Eficacia de La Conservación Antimicrobiana. Available online: https://extranet.boe.es/farmacopea/doc.php?id=50103# (accessed on 21 April 2022).
- Mohammadi-Meyabadi, R.; Beirampour, N.; Garrós, N.; Alvarado, H.L.; Limón, D.; Silva-Abreu, M.; Calpena, A.C.; Mallandrich, M. Assessing the Solubility of Baricitinib and Drug Uptake in Different Tissues Using Absorption and Fluorescence Spectroscopies. Pharmaceutics 2022, 14, 2714. [Google Scholar] [CrossRef]
- ICCVAM-Recommended Test Method Protocol: Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Test Method; NIH Publication: Bethesda, MD, USA, 2010; Available online: https://mdpi-res.com/data/mdpi_references_guide_v9.pdf (accessed on 2 June 2022).
- Gopinathan, U.; Ramakrishna, T.; Willcox, M.; Rao, C.M.; Balasubramanian, D.; Kulkarni, A.; Vemuganti, G.K.; Rao, G.N. Enzymatic, Clinical and Histologic Evaluation of Corneal Tissues in Experimental Fungal Keratitis in Rabbits. Exp. Eye Res. 2001, 72, 433–442. [Google Scholar] [CrossRef]
- Conde Penedo, A.; Díaz Tomé, V.; Fernández Ferreiro, A.; González Barcia, M.; Otero Espinar, F.J. Enhancement in Corneal Permeability of Riboflavin Using Cyclodextrin Derivates Complexes as a Previous Step to Transepithelial Cross-Linking. Eur. J. Pharm. Biopharm. 2021, 162, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.I.; Jadhav, L.S.; Jadhav, R.K.; Kadam, J.V.; Pisal, S.S. Aceclofenac Organogels: In Vitro and In Vivo Characterization. Curr. Drug Deliv. 2009, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Silva-abreu, M.; Calpena, A.C.; Espina, M.; Silva, A.M.; Gimeno, A.; Egea, M.A.; García, M.L. Optimization, Biopharmaceutical Profile and Therapeutic Eff Icacy of Pioglitazone-Loaded PLGA-PEG Nanospheres as a Novel Strategy for Ocular Inflammatory Disorders. Pharm. Res. 2018, 35, 11. [Google Scholar] [CrossRef] [PubMed]
- Laignier Cazedey, E.; Carvalho, F.C.; Másquio Fiorentino, F.A.; Daflon Gremiao, M.P.; Nunes Salgado, H.R. Corrositex®, BCOP and HET-CAM as Alternative Methods to Animal Experimentation. Braz. J. Pharm. Sci. 2009, 45, 759–766. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial Profile of Ocular Infections: A Systematic Review. BMC Ophthalmol. 2017, 17, 212. [Google Scholar] [CrossRef]
- Wiacek, A.E.; Jurak, M.; Ładniak, A.; Szafran, K.; Przykaza, K. Cyclosporine CsA—The Physicochemical Characterization of Liposomal and Colloidal Systems. Colloids Interfaces 2020, 4, 46. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Colloids and Surfaces B: Biointerfaces Factors Affecting Drug Encapsulation and Stability of Lipid—Polymer Hybrid Nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Brandao, A.; Garcia de Oliverira-Mima, E. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology Based Drug Delivery Systems for the Treatment of Anterior Segment Eye Diseases. J. Control. Release 2023, 354, 465–488. [Google Scholar] [CrossRef]
- Han, H.; Li, S.; Xu, M.; Zhong, Y.; Fan, W.; Xu, J.; Zhou, T.; Ji, J.; Ye, J.; Yao, K. Polymer- and Lipid-Based Nanocarriers for Ocular Drug Delivery: Current Status and Future Perspectives. Adv. Drug Deliv. Rev. 2023, 196, 114770. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Suresh, P.K. Drug Delivery to Eye: Special Reference to Nanoparticles. Int. J. Drug Deliv. 2010, 2, 12–21. [Google Scholar] [CrossRef]
- Razavi, M.S.; Ebrahimnejad, P.; Fatahi, Y.; D’Emanuele, A.; Dinarvand, R. Recent Developments of Nanostructures for the Ocular Delivery of Natural Compounds. Front. Chem. 2022, 10, 850757. [Google Scholar] [CrossRef]
- Rahdar, A.; Sargazi, S.; Barani, M.; Shahraki, S.; Sabir, F.; Aboudzadeh, M.A. Lignin-Stabilized Doxorubicin Microemulsions: Synthesis, Physical Characterization, and In Vitro Assessments. Polymers 2021, 13, 641. [Google Scholar] [CrossRef]
- Rahdar, A.; Taboada, P.; Reza, M.; Barani, M.; Beyzaei, H. Effect of Tocopherol on the Properties of Pluronic F127 Microemulsions: Physico-Chemical Characterization and in Vivo Toxicity. J. Mol. Liq. 2019, 277, 624–630. [Google Scholar] [CrossRef]
- Chauvet, A.; Lacoulonche, F.; Egea, M.A. PLGA Nanospheres for the Ocular Delivery of Flurbiprofen: Drug Release and Interactions. J. Pharm. Sci. 2008, 97, 5306–5317. [Google Scholar] [CrossRef]
- Amrite, A.C.; Kompella, U.B. Size-Dependent Disposition of Nanoparticles and Microparticles Following Subconjunctival Administration. J. Pharm. Pharmacol. 2005, 57, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Chapter 3—Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. ISBN 978-0-12-814031-4. [Google Scholar]
- Gökçe, E.H.; Sandri, G.; Eğrilmez, S.; Bonferoni, M.C.; Güneri, T.; Caramella, C. Cyclosporine A-Loaded Solid Lipid Nanoparticles: Ocular Tolerance and In Vivo Drug Release in Rabbit Eyes. Curr. Eye Res. 2016, 34, 996–1003. [Google Scholar] [CrossRef]
- Lee, K.H.; Khan, F.N.; Cosby, L.; Yang, G.; Winter, J.O. Polymer Concentration Maximizes Encapsulation Efficiency in Electrohydrodynamic Mixing Nanoprecipitation. Front. Nanotechnol. 2021, 3, 719710. [Google Scholar] [CrossRef]
- Arshinoff, S.A.; Hofmann, I.; Nae, H. Role of Rheology in Tears and Artificial Tears. J. Cataract Refract. Surg. 2021, 47, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, W.; Qin, N.; Zhao, P.; Phan, C.M.; Haines, L.; Jones, L.; Ren, C.L. Shear-Thinning and Temperature-Dependent Viscosity Relationships of Contemporary Ocular Lubricants. Transl. Vis. Sci. Technol. 2022, 11, 1. [Google Scholar] [CrossRef]
- Tiffany, J.M. The Viscosity of Human Tears. Int. Ophthalmol. 1991, 15, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.; Feng, X.; Patel, D.; Mohammad, A.; Kozak, D.; Choi, S.; Ashraf, M.; Xu, X. Factors Affecting the Particle Size Distribution and Rheology of Brinzolamide Ophthalmic Suspensions. Int. J. Pharm. 2020, 586, 119495. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Gulmezian-Sefer, M.; Cheng, Y.; Srikumar, R. Microbiological Considerations for Ophthalmic Products: Sterility, Endotoxin Limits, and Preservatives. In Ophthalmic Product Development: From Bench to Bedside; Neervannan, S., Kompella, U.B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 199–227. ISBN 978-3-030-76367-1. [Google Scholar]
- Wilson, C.G. Ophthalmic Formulation. In Specialised Pharmaceutical Formulation: The Science and Technology of Dosage Forms; Srivastava, V., Pitt, K., Tovey, G.D., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2022; ISBN 978-1-83916-175-9. [Google Scholar]
- Parra, A.; Mallandrich, M.; Clares, B.; Egea, M.A.; Espina, M.; García, M.L.; Calpena, A.C. Design and Elaboration of Freeze-Dried PLGA Nanoparticles for the Transcorneal Permeation of Carprofen: Ocular Anti-Inflammatory Applications. Colloids Surf B Biointerfaces 2015, 136, 935–943. [Google Scholar] [CrossRef]
- Hirenkumar, M.; Steven, S. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2012, 3, 1377–1397. [Google Scholar] [CrossRef]
- Diebold, Y.; Calonge, M. Applications of Nanoparticles in Ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef] [PubMed]





| Factors | ||||||
|---|---|---|---|---|---|---|
| Variables Levels | Polymer Amount (mg) | Surfactant Amount (mg) | Aqueous Phase Volume (mL) | Polymer Amount (mg) | Surfactant Amount (mg) | Aqueous Phase Volume (mL) |
| PCL | PCL | PCL | PLGA | PLGA | PLGA | |
| Low (−1) | 50 | 200 | 38 | 75 | 150 | 50 |
| Centered (0) | 60 | 240 | 44 | 95 | 200 | 60 |
| High (+1) | 70 | 280 | 50 | 115 | 250 | 70 |
| Axial (−0.39) | 46 | 185 | 36.5 | 72 | 134 | 47 |
| Axial (+1.39) | 74 | 295 | 51.5 | 118 | 266 | 73 |
| Levels of Factors | |||
|---|---|---|---|
| Formulations | Polymer Amount (mg) | Surfactant Amount (mg) | Aqueous Phase Volume (mL) |
| F1 | 0 | 0 | 0 |
| F2 | 1 | 0 | 0 |
| F3 | 0 | 1 | 0 |
| F4 | 1 | 1 | 0 |
| F5 | 0 | 0 | 1 |
| F6 | 1 | 0 | 1 |
| F7 | 0 | 1 | 1 |
| F8 | 1 | 1 | 1 |
| F9 | 0.5 | 0.5 | 0.5 |
| F10 | 0.5 | 0.5 | 0.5 |
| F11 | −0.39 | 0.5 | 0.5 |
| F12 | 1.39 | 0.5 | 0.5 |
| F13 | 0.5 | −0.39 | 0.5 |
| F14 | 0.5 | 1.39 | 0.5 |
| F15 | 0.5 | 0.5 | −0.39 |
| F16 | 0.5 | 0.5 | 1.39 |
| F17 | 0.5 | 0.5 | 0.5 |
| F18 | 0.5 | 0.5 | 0.5 |
| F19 | 0.5 | 0.5 | 0.5 |
| Formulation | Polymer Amount (%) | Aqueous Phase (%) | Surfactant Amount P188 (%) |
|---|---|---|---|
| B-PLGA-95 | 0.157 | 99.511 | 0.332 |
| B-PLGA-75 | 0.107 | 99.609 | 0.284 |
| B-PCL | 0.100 | 99.502 | 0.398 |
| Test Microorganisms | Microorganism Concentration | |||
|---|---|---|---|---|
| B-PCL NPs (CFU/mL) | B-PLGA-95 and B-PLGA-75 NPs (CFU/mL) | |||
| Bacteria | Pseudomonas aeruginosa | ATCC 9027 (NCIMB 8626, CIP 82.118) | 1.5 × 108 | 2.3 × 108 |
| Staphylococcus aureus | ATCC 6538, (NCTC 10788, NCIMB 9518) | 2.1 × 108 | 2.3 × 108 | |
| Fungus | Candida albicans | ATCC 10231 (NCPF 3179, IP 48.72) | 1 × 108 | 1 × 108 |
| Aspergillus brasiliensis | ATCC 16404 (IMI 149007, IP 1431.83) | 2 × 107 | 2 × 107 | |
| Log Reduction (CFU/mL) | ||||||
|---|---|---|---|---|---|---|
| Criteria | 6 h | 24 h | 7 d | 14 d | 28 d | |
| Bacteria | A | 2 | 3 | - | - | NR |
| B | - | 1 | 3 | - | NI | |
| Fungus | A | - | - | 2 | - | NI |
| B | - | - | - | 1 | NI | |
| Irritation Score | Irritation Classification |
|---|---|
| 0–0.9 | Non-Irritant |
| 1–4.9 | Slight Irritant |
| 5–8.9 | Moderate Irritant |
| 9–21 | Severe Irritant |
| Levels of Factors | Responses | ||||
|---|---|---|---|---|---|
| Formulations | PLGA Amount (mg) | P188Amount (mg) | Aqueous Phase Volume (mL) | Z-Ave ± SD (nm) | PDI ± SD |
| F1 | 75 | 150 | 50 | 206.65 ± 2.9 | 0.303 ± 0.016 |
| F2 | 115 | 150 | 50 | 245.80 ± 2.1 | 0.177 ± 0.035 |
| F3 | 75 | 250 | 50 | 201.50 ± 1.8 | 0.265 ± 0.049 |
| F4 | 115 | 250 | 50 | 237.55 ± 5.0 | 0.150 ± 0.001 |
| F5 | 75 | 150 | 70 | 180.80 ± 4.9 | 0.213 ± 0.050 |
| F6 | 115 | 150 | 70 | 209.90 ± 3.0 | 0.231 ± 0.035 |
| F7 | 75 | 250 | 70 | 197.25 ± 1.2 | 0.217 ± 0.053 |
| F8 | 115 | 250 | 70 | 233.05 ± 1.3 | 0.166 ± 0.079 |
| F9 | 95 | 200 | 60 | 98.20 ± 0.0 | 0.090 ± 0.056 |
| F10 | 95 | 200 | 60 | 118.70 ± 0.6 | 0.168 ± 0.017 |
| F11 | 72 | 200 | 60 | 209.55 ± 1.8 | 0.103 ± 0.028 |
| F12 | 118 | 200 | 60 | 288.20 ± 4.5 | 0.091 ± 0.051 |
| F13 | 95 | 134 | 60 | 214.75 ± 3.6 | 0.218 ± 0.022 |
| F14 | 95 | 266 | 60 | 225.25 ± 3.0 | 0.117 ± 0.009 |
| F15 | 95 | 200 | 47 | 217.55 ± 2.2 | 0.251 ± 0.008 |
| F16 | 95 | 200 | 73 | 206.40 ± 1.6 | 0.101 ± 0.038 |
| F17 | 95 | 200 | 60 | 116.60 ± 0.7 | 0.184 ± 0.002 |
| F18 | 95 | 200 | 60 | 104.90 ± 0.0 | 0.191 ± 0.016 |
| F19 | 95 | 200 | 60 | 159.75 ± 0.8 | 0.056 ± 0.010 |
| Levels of Factors | Responses | ||||
|---|---|---|---|---|---|
| Formulations | PCL Amount (mg) | P188 Amount (mg) | Aqueous Phase Volume (mL) | Z-Ave ± SD (nm) | PDI ± SD |
| F1 | 50 | 200 | 38 | 104.20 ± 1.70 | 0.137 ± 0.004 |
| F2 | 70 | 200 | 38 | 138.30 ± 5.94 | 0.165 ± 0.016 |
| F3 | 50 | 280 | 38 | 109.35 ± 0.78 | 0.174 ± 0.006 |
| F4 | 70 | 280 | 38 | 120.00 ± 6.93 | 0.247 ± 0.062 |
| F5 | 50 | 200 | 50 | 127.50 ± 1.98 | 0.107 ± 0.037 |
| F6 | 70 | 200 | 50 | 398.65 ± 15.34 | 0.159 ± 0.047 |
| F7 | 50 | 280 | 50 | 366.40 ± 4.38 | 0.160 ± 0.065 |
| F8 | 70 | 280 | 50 | 339.75 ± 2.76 | 0.197 ± 0.009 |
| F9 | 60 | 240 | 44 | 347.50 ± 2.55 | 0.223 ± 0.015 |
| F10 | 60 | 240 | 44 | 426.60 ± 6.79 | 0.029 ± 0.030 |
| F11 | 46 | 240 | 44 | 403.90 ± 1.56 | 0.096 ± 0.053 |
| F12 | 118 | 240 | 44 | 335.75 ± 3.32 | 0.355 ± 0.066 |
| F13 | 60 | 185 | 44 | 370.60 ± 4.53 | 0.158 ± 0.031 |
| F14 | 60 | 295 | 44 | 349.95 ± 6.43 | 0.182 ± 0.040 |
| F15 | 60 | 240 | 36.5 | 401.35 ± 7.85 | 0.215 ± 0.011 |
| F16 | 60 | 240 | 51.5 | 374.95 ± 1.06 | 0.200 ± 0.013 |
| F17 | 60 | 240 | 44 | 458.10 ± 4.95 | 0.203 ± 0.027 |
| F18 | 60 | 240 | 44 | 356.90 ± 2.83 | 0.181 ± 0.034 |
| F19 | 60 | 240 | 44 | 309.70 ± 6.08 | 0.147 ± 0.048 |
| Formulation | Z-Ave ± SD | PDI ± SD | ZP (mV) ± SD | pH ± SD | EE (%) ± SD |
|---|---|---|---|---|---|
| (NPs) | (nm) | ||||
| B-PLGA-95 | 84.50 ± 0.72 | 0.10 ± 0.01 | −25.70 ± 2.01 | 6.9 ± 0.3 | 65.63 ± 3.87 |
| B-PLGA-75 | 77.65 ± 0.03 | 0.04 ± 0.02 | −28.20 ± 0.14 | 6.8 ± 0.3 | 27.39 ± 2.05 |
| B-PCL | 138.36 ± 0.92 | 0.09 ± 0.02 | −20.55 ± 0.07 | 6.8 ± 0.2 | 77.82 ± 5.33 |
| Best-Fit Values | B-PCL | B-PLGA-75 | B-PLGA-95 | Water |
|---|---|---|---|---|
| A | 16.03 | −5.633 | 2.408 | 31.99 |
| B | 0.125 | 0.562 | 0.624 | 0.812 |
| C | −0.000279 | −0.002398 | −0.003030 | −0.003556 |
| Std. Error | ||||
| A | 0.456 | 2.849 | 3.556 | 10.720 |
| B | 0.016 | 0.097 | 0.121 | 0.365 |
| C | 0.000101 | 0.000629 | 0.000785 | 0.002366 |
| Goodness of Fit | ||||
| R2 | 0.999 | 0.994 | 0.988 | 0.960 |
| NPs | Storage Condition (°C) | Z-Ave (nm) | PDI | ||||
|---|---|---|---|---|---|---|---|
| Time Assayed (Days) | |||||||
| 0 | 7 | 30 | 0 | 7 | 30 | ||
| B-PLGA-95 | 25 | 80.56 ± 0.72 | 81.72 ± 0.22 | 83.03 ± 2.90 | 0.03 ± 0.01 | 0.07 ± 0.02 * | 0.07 ± 0.05 * |
| 4 | 82.24 ± 0.54 | 85.48 ± 0.72 | 91.07 ± 4.85 * | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.07 ± 0.08 * | |
| B-PLGA-75 | 25 | 71.43 ± 0.01 | 74.75 ± 2.24 | 81.42 ± 5.42 * | 0.05 ± 0.01 | 0.07 ± 0.02 | 0.08 ± 0.04 |
| 4 | 75.23 ± 0.15 | 77.58 ± 0.24 | 86.00 ± 5.24 * | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.06 ± 0.01 * | |
| B-PCL | 25 | 130.36 ± 1.82 | 134.8 ± 2.12 | 141.5 ± 5.03 * | 0.03 ± 0.01 | 0.07 ± 0.04 | 0.12 ± 0.08 * |
| 4 | 128.30 ± 1.23 | 131.30 ± 2.82 | 152.5 ± 7.79 * | 0.06 ± 0.02 | 0.09 ± 0.07 | 0.02 ± 0.04 * | |
| Microorganism | Nanoparticle | Log Reduction (CFU/mL) | ||||
|---|---|---|---|---|---|---|
| 6 h | 24 h | 7 d | 14 d | 28 d | ||
| P. aeruginosa | B-PLGA-95 | 1 | 3 | NR | NR | |
| B-PLGA-75 | NR | NR | - | - | NR | |
| B-PCL | 1 | NI ** | 3 | - | NR | |
| S. aureus | B-PLGA-95 | 1 | NR * | NR | NR | |
| B-PLGA-75 | NR * | NR | - | - | NR | |
| B-PCL | - | 1 | NI (105) | - | NR | |
| C. albicans | B-PLGA-95 | - | - | NI | 1 | NI |
| B-PLGA-75 | - | - | 3 | NI ** | NI | |
| B-PCL | - | - | NI (106) | ~1 | NI | |
| A. brasiliensis | B-PLGA-95 | - | - | 1 | NI (1) | NI |
| B-PLGA-75 | NR | NR | NR | |||
| B-PCL | - | - | NI (105) | NI (105) | NI | |
| B-PLGA-95 | B-PLGA-75 | B-PCL | B-Solution | ||
|---|---|---|---|---|---|
| Parameters | Units | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Flux (J/cm2) | µg/h/cm2 | 20.90 ± 2.47 | 10.12 ± 1.23 A | 16.12 ± 1.92 A,B | 0.07 ± 0.01 A,B,C |
| Permeability coefficient (Kp) | cm/h | 0.126 ± 0.014 | 0.071 ± 0.008 A | 0.081 ± 0.009 A | 0.004 ± 0.000 A,B,C |
| Lag Time (Tl) | h | 2.90 ± 0.53 | 2.75 ± 0.37 | 2.84 ± 0.24 | 0.83 ± 0.07 A,B,C |
| Partition coefficient (P1) | cm | 2.19 ± 0.02 | 1.17 ± 0.02 A | 1.38 ± 0.01 A,B | 0.03 ± 0.00 A,B,C |
| Diffusion coefficient (P2) | 1/h | 0.057 ± 0.007 | 0.061 ± 0.005 | 0.059 ± 0.005 | 0.139 ± 0.014 A,B,C |
| Amount of BTB in the skin (Qret) | µg/cm2 | 3.35 ± 0.29 | 0.40 ± 0.04 A | 6.09 ± 0.57 A,B | b.l.q. |
| Enhancement ratio (ER) | - | 27.7 | 15.6 | 17.8 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beirampour, N.; Bustos-Salgado, P.; Garrós, N.; Mohammadi-Meyabadi, R.; Domènech, Ò.; Suñer-Carbó, J.; Rodríguez-Lagunas, M.J.; Kapravelou, G.; Montes, M.J.; Calpena, A.; et al. Formulation of Polymeric Nanoparticles Loading Baricitinib as a Topical Approach in Ocular Application. Pharmaceutics 2024, 16, 1092. https://doi.org/10.3390/pharmaceutics16081092
Beirampour N, Bustos-Salgado P, Garrós N, Mohammadi-Meyabadi R, Domènech Ò, Suñer-Carbó J, Rodríguez-Lagunas MJ, Kapravelou G, Montes MJ, Calpena A, et al. Formulation of Polymeric Nanoparticles Loading Baricitinib as a Topical Approach in Ocular Application. Pharmaceutics. 2024; 16(8):1092. https://doi.org/10.3390/pharmaceutics16081092
Chicago/Turabian StyleBeirampour, Negar, Paola Bustos-Salgado, Núria Garrós, Roya Mohammadi-Meyabadi, Òscar Domènech, Joaquim Suñer-Carbó, María José Rodríguez-Lagunas, Garyfallia Kapravelou, María Jesús Montes, Ana Calpena, and et al. 2024. "Formulation of Polymeric Nanoparticles Loading Baricitinib as a Topical Approach in Ocular Application" Pharmaceutics 16, no. 8: 1092. https://doi.org/10.3390/pharmaceutics16081092
APA StyleBeirampour, N., Bustos-Salgado, P., Garrós, N., Mohammadi-Meyabadi, R., Domènech, Ò., Suñer-Carbó, J., Rodríguez-Lagunas, M. J., Kapravelou, G., Montes, M. J., Calpena, A., & Mallandrich, M. (2024). Formulation of Polymeric Nanoparticles Loading Baricitinib as a Topical Approach in Ocular Application. Pharmaceutics, 16(8), 1092. https://doi.org/10.3390/pharmaceutics16081092












