Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature
Abstract
:1. Introduction
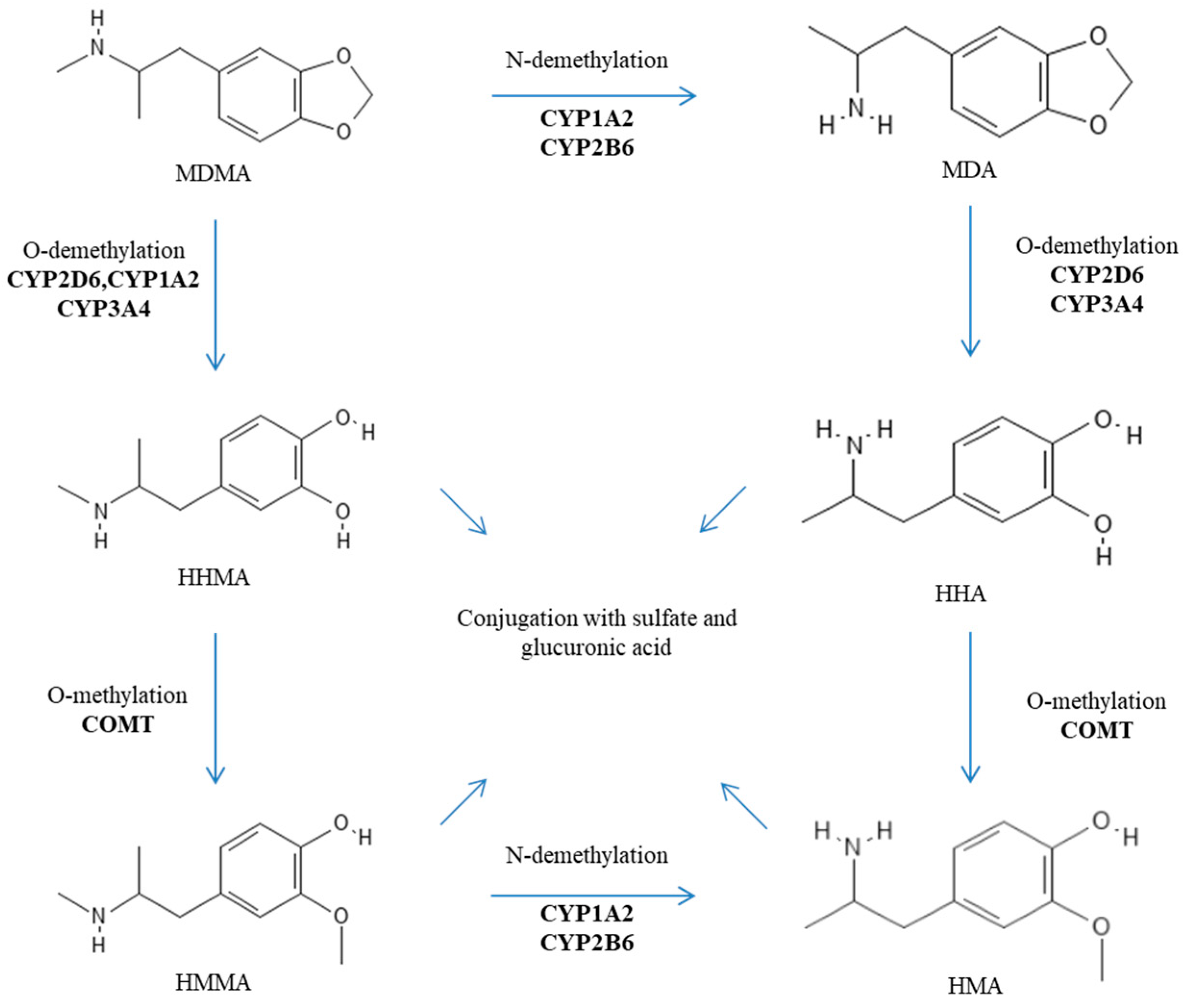
2. Genetic Factors Influencing the Metabolism of MDMA
3. Genetic Factors Influencing Pharmacological MDMA Targets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bernschneider-Reif, S.; Oxler, F.; Freudenmann, R.W. The origin of MDMA (‘ecstasy’)—Separating the facts from the myth. Pharmazie 2006, 61, 966–972. [Google Scholar] [PubMed]
- De La Torre, R.; Farré, M.; Roset, P.N.; Pizarro, N.; Abanades, S.; Segura, M.; Segura, J.; Cami, J. Human pharmacology of MDMA: Pharmacokinetics, metabolism, and disposition. Ther. Drug Monit. 2004, 26, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kalant, H. The pharmacology and toxicology of ‘ecstasy’ (MDMA) and related drugs. Can. Med. Assoc. J. 2001, 165, 917–928. [Google Scholar]
- Rietjens, S.J.; Hondebrink, L.; Westerink, R.H.; Meulenbelt, J. Pharmacokinetics and pharmacodynamics of 3,4-methylenedioxymethamphetamine (MDMA): Interindividual differences due to polymorphisms and drug–drug interactions. Crit. Rev. Toxicol. 2012, 42, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Highgate, Q. Methylenedioxymethamphetamine (MDMA): Serotonergic and dopaminergic mechanisms related to its use and misuse. J. Neurochem. 2021, 157, 1714–1724. [Google Scholar] [CrossRef]
- Dumont, G.J.H.; Sweep, F.C.G.J.; Van der Steen, R.; Hermsen, R.; Donders, A.R.T.; Touw, D.J.; Van Gerven, J.M.A.; Buitelaar, J.K.; Verkes, R.J. Increased oxycotin concentrations and prosocial feelings in human after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc. Neurosci. 2009, 4, 359–366. [Google Scholar] [CrossRef]
- Hysek, C.M.; Simmler, L.D.; Nicola, V.G.; Vischer, N.; Donzelli, M.; Krahenbuhl, S.; Grouzmann, E.; Huwyler, J.; Hoener, M.C.; Liechti, M.E. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS ONE 2012, 7, e36476. [Google Scholar] [CrossRef]
- Kuwayama, K.; Inoue, H.; Kanamori, T.; Tsujikawa, K.; Miyaguchi, H.; Iwata, Y.; Miyauchi, S.; Kamo, N.; Kishi, T. Uptake of 3,4-methylenedioxymethamphetamine and its related compounds by a proton-coupled transport system in Caco-2 cells. Biochim. Biophys. Acta. 2008, 1778, 42–50. [Google Scholar] [CrossRef]
- Bertelsen, K.M.; Greenblatt, D.J.; Von Moltke, L.L. Apparent active transport of MDMA is not mediated by P-glycoprotein: A comparison with MDCK and Caco-2 monolayers. Biopharm. Drug Dispos. 2006, 27, 219–227. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, R.; Farré, M.; Ortuño, J.; Mas, M.; Brenneisen, R.; Roset, P.N.; Segura, J.; Cami, J. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br. J. Clin. Pharmacol. 2000, 49, 104–109. [Google Scholar] [CrossRef]
- Steuer, A.E.; Schmidhauser, C.; Tingelhoff, E.H.; Schmid, Y.; Rickli, A.; Kraemer, T.; Liechti, M.E. Impact of cytochrome P450 2D6 function on the chiral blood plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA) and its phase I and II metabolites in humans. PLoS ONE 2016, 11, e0150955. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Elayan, I.; Hanson, G.R.; Foltz, R.L.; Gibb, J.W.; Lim, H.K. Effects of 3,4-dihydroxymethamphetamine and 2,4,5-trihydroxymethamphetamine, two metabolites of 3,4-methylenedioxyamphetamine, on central serotoninergic and dopaminergic systems. J. Pharmacol. Exp. Ther. 1992, 216, 447–453. [Google Scholar]
- Mueller, M.; Goodwin, A.K.; Ator, N.A.; McCann, U.D.; Ricaurte, G.A. Metabolism and disposition of 3,4-methylenedioxymethamphetamine (“Ecstasy”) in baboons after oral administration: Comparision with humans reveals marked differences. J. Pharmacol. Exp. Ther. 2011, 338, 310–317. [Google Scholar] [CrossRef]
- Pizarro, N.; De La Torre, R.; Joglar, J.; Okumura, N.; Perfetti, X.; Lau, S.S.; Monks, T.J. Serotonergic neurotoxic thioether metabolites of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”): Synthesis, isolation, and characterization of diastereoisomers. Chem. Res. Toxicol. 2008, 21, 2272–2279. [Google Scholar] [CrossRef]
- Baez, S.; Segura-Aguilar, J.; Widersten, M.; Johansson, A.S.; Mannervik, B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem. J. 1997, 324, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Shulgin, A.T.; Nichols, D.E. Characterization of three new psychotomimetics. In The Psychopharmacology of Hallucinogens; Stillman, R.C., Willette, R.E., Eds.; PErgamon Press: New York, NY, USA, 1978; pp. 74–83. [Google Scholar]
- Nichols, D.E. Entactogens: How the name for a novel class of psychoactive agents originated. Front. Psychiatry 2022, 13, 863088. [Google Scholar] [CrossRef]
- Yazar-Klosinski, B.; Mithoefer, M. Potential psychiatric uses for MDMA. Clin. Pharmacol. Ther. 2017, 101, 194–196. [Google Scholar] [CrossRef]
- Sessa, B.; Higbed, L.; Nutt, D. A review of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front. Psychiatry 2019, 10, 138. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Ot’alora, G.M.; Van Der Kolk, B.; Shannon, S.; Bogenschutz, M.; Gelfand, Y.; Paleos, C.; Nicholas, C.R.; Quevedos, S.; Balliett, B.; et al. MDMA-assisted therapy for moderate to severe PTSD: A randomized, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2473–2480. [Google Scholar] [CrossRef]
- Sessa, B. Why MDMA therapy for alcohol use disorder? And why now? Neuropharmacology 2018, 142, 83–88. [Google Scholar] [CrossRef]
- Roxburgh, A.; Sam, B.; Kriikku, P.; Mounteney, J.; Castanera, A.; Dias, M.; Giraudon, I. Trends in MDMA-related mortality across four countries. Addiction 2021, 116, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, A.; Lappin, J. MDMA-related deaths in Australia 2000 to 2018. Int. J. Drug Policy 2020, 76, 102630. [Google Scholar] [CrossRef]
- Vizeli, P.; Liechti, M.E. No influence of dopamine system gene variations on acute effects of MDMA. Front. Psychiatry 2019, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. Role of serotonin transporter and receptor gene variations in the acute effects of MDMA in healthy subjects. ACS Chem. Neurosci. 2019, 10, 3120–3131. [Google Scholar] [CrossRef]
- Tucker, G.T.; Lennard, M.S.; Ellis, S.W.; Woods, H.F.; Cho, A.K.; Lin, L.Y.; Hiratsuka, A.; Schmitz, D.A.; Chu, T.Y.Y. The demethylenation of methylenedioxyamphetamine (“ectasy”) by debrisoquine hydroxylase (CYP2D6). Biochem. Pharmacol. 1994, 7, 1151–1156. [Google Scholar] [CrossRef]
- Leeder, J.S.; Gaedigk, A. CYP2D6 and pharmacogenomics: Where does future research need to focus? Part 2: Clinical aspects. Pharmacogenomics 2014, 15, 1055–1058. [Google Scholar] [CrossRef]
- Taylor, C.; Crosby, I.; Yip, V.; Maguire, P.; Pirmohamed, M.; Turner, R.M. A review of the important role of CYP2D6 in pharmacogenomics. Genes 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Lassen, D.; Damkier, P.; Brøsen, K. The pharmacogenetics of tramadol. Clin. Pharmacokinet. 2015, 54, 825–836. [Google Scholar] [CrossRef]
- Schwab, M.; Griese, U.; Hermle, L.; Gouzoulis, E.; Zanger, U.; Mikus, G. Is there an impact of CYP2D6 genotype on the toxicity of’ecstasy and related designer drugs? Naunyn Schmiedebergs Arch Pharmacol 1998, 4, 163. [Google Scholar]
- O’Donohoe, A.; O’Flynn, K.; Shields, K.; Hawi, Z.; Gill, M. MDMA toxicity: No evidence for a major influence of metabolic genotype at CYP2D6. Addict Biol. 1998, 3, 309–314. [Google Scholar] [CrossRef]
- Gilhooly, T.C.; Daly, A.K. CYP2D6 deficiency, a factor in ecstasy related deaths? Br. J. Clin. Pharmacol. 2002, 54, 69–70. [Google Scholar]
- Haufroid, V.; Hantson, P. CYP2D6 genetic polymorphisms and their relevance for poisoning due to amfetamines, opioid analgesics and antidepressants. Clin. Toxicol. 2015, 53, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jamei, M.; Heydari, A.; Yeo, K.R.; De La Torre, R.; Farré, M.; Tucker, G.T.; Rostami-Hodjegan, A. Implications of mechanism-based inhibition of CYP2D6 for the pharmacokinetics and toxicity of MDMA. J. Psychopharmacol. 2006, 20, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Hysek, C.M.; Fink, A.E.; Simmler, L.D.; Donzelli, M.; Grouzmann, E.; Liechti, M.E. α1-adrenergic receptors contribute to the acute effects of 3,4-methylenedioxymethamphetamine in humans. J. Clin. Psychopharmacol. 2013, 5, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Hysek, C.M.; Simmler, L.D.; Schillinger, N.; Meyer, N.; Schmid, Y.; Donzelli, M.; Grouzmann, E.; Lietchti, M.E. Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. Int. J. Neuropsychopharmacol. 2014, 17, 371–381. [Google Scholar] [CrossRef]
- O’Mathúna, B.; Farré, M.; Rostami-Hodjegan, A.; Yang, J.; Cuyàs, E.; Torrens, M.; Pardo, R.; Abanades, S.; Maluf, S.; Tucker, G.T.; et al. The consequences of 3,4-methylenedioxymethamphetamine induced CYP2D6 inhibition in humans. J. Clin. Psychopharmacol. 2008, 28, 523–529. [Google Scholar] [CrossRef]
- De La Torre, R.; Farré, M.; Ó Mathúna, B.; Roset, P.N.; Pizarro, N.; Segura, M.; Torrens, M.; Ortuno, J.; Pujadas, M.; Cami, J. MDMA (ecstasy) pharmacokinetics in a CYP2D6 poor metaboliser and in nine CYP2D6 extensive metabolisers. Eur. J. Clin. Pharmacol. 2005, 61, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Lozano, R.; Farré, M.; Yubero-Lahoz, S.; O’Mathúna, B.; Torrens, M.; Mustata, C.; Pérez-Mana, C.; Langohr, K.; Cuyas, E.; Li Carbo, M.; et al. Clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”): The influence of gender and genetics (CYP2D6, COMT, 5-HTT). PLoS ONE 2012, 7, e47599. [Google Scholar] [CrossRef]
- De La Torre, R.; Yubero-Lahoz, S.; Pardo-Lozano, R.; Farré, M. MDMA, methamphetamine, and CYP2D6 pharmacogenetics: What is clinically relevant? Front. Genet. 2012, 3, 235. [Google Scholar] [CrossRef]
- Schmid, Y.; Vizeli, P.; Hysek, C.M.; Prestin, K.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. CYP2D6 function moderates the pharmacokinetics and pharmacodynamics of 3,4-methylene-dioxymethamphetamine in a controlled study in healthy individuals. Pharmacogenet. Genom. 2016, 26, 397–401. [Google Scholar] [CrossRef]
- Aitchison, K.J.; Tsapakis, E.M.; Huezo-Diaz, P.; Kerwin, R.W.; Forsling, M.L.; Wolff, K. Ecstasy (MDMA)-induced hyponatraemia is associated with genetic variants in CYP2D6 and COMT. J. Psychopharmacol. 2012, 26, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Cuyàs, E.; Verdejo-García, A.; Fagundo, A.B.; Khymenets, O.; Rodríguez, J.; Cuenca, A.; De Sola Llopis, S.; Langhor, K.; Pena-Casanova, J.; Torrens, M.; et al. The influence of genetic and environmental factors among MDMA users in cognitive performance. PLoS ONE 2011, 6, e27206. [Google Scholar] [CrossRef]
- Wolff, K.; Tsapakis, E.M.; Pariante, C.M.; Kerwin, R.W.; Forsling, M.L.; Aitchison, K.J. Pharmacogenetic studies of change in cortisol on ecstasy (MDMA) consumption. J. Psychopharmacol. 2012, 26, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Torrens, M.; Perez-Mana, C.; Muga, R.; Farré, M. Key individual determinants in MDMA pharmacodynamics. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 183–195. [Google Scholar] [CrossRef]
- Vizeli, P.; Schmid, Y.; Prestin, K.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. Pharmacogenetics of ecstasy: CYP1A2, CYP2C19, and CYP2B6 polymorphisms moderate pharmacokinetics of MDMA in healthy subjects. Eur. Neuropsychopharmacol. 2017, 27, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Antolino-Lobo, I.; Meulenbelt, J.; Nijmeijer, S.M.; Scherpenisse, P.; Van Den Berg, M.; Van Duursen, M.B.M. Differential roles of phase I and phase II enzymes in 3,4-methylendioxymethamphetamine-induced cytotoxicity. Drug Metab. Dispos. 2010, 38, 1105–1112. [Google Scholar] [CrossRef]
- Perfetti, X.; O’Mathúna, B.; Pizarro, N.; Cuyàs, E.; Khymenets, O.; Almeida, B.; Pellegrini, M.; Pichini, S.; Lau, S.S.; Monks, T.J.; et al. Neurotoxic thioether adducts of 3,4-methylenedioxymethamphetamine identified in human urine after ecstasy ingestion. Drug Metab. Dispos. 2009, 37, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Fagundo, A.B.; Cuyàs, E.; Verdejo-Garcia, A.; Khymenets, O.; Langohr, K.; Martín-Santos, R.; Farré, M.; De la Torre, R. The influence of 5-HTT and COMT genotypes on verbal fluency in ecstasy users. J. Psychopharmacol. 2010, 24, 1381–1393. [Google Scholar] [CrossRef]
- Bag, H.G.G. Association between COMT gene rs165599 SNP and schizophrenia: A meta-analysis of case-control studies. Mol. Genet. Genom. Med. 2018, 6, 845–854. [Google Scholar]
- Lamb, Y.N.; Thompson, J.M.D.; Murphy, R.; Wall, C.; Kirk, I.J.; Morgan, A.R.; Ferguson, L.R.; Mitchell, E.A.; Waldie, K.E.; ABC Study group. Perceived stress during pregnancy and the catechol-O-methyltransferase (COMT) rs165599 polymorphism impacts on childhood IQ. Cognition 2014, 132, 461–470. [Google Scholar] [CrossRef]
- Bamalan, O.A.; Moore, M.J.; Al Khalili, Y. Physiology, Serotonin. StatPearls Publishing. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545168/ (accessed on 30 July 2023).
- Ikegame, T.; Hidaka, Y.; Nakachi, Y.; Murata, Y.; Watanabe, R.; Sugawara, H.; Asai, T.; Kiyota, E.; Ikeda, M.; Sasaki, T.; et al. Identification and functional characterization of the extremely long allele of the serotonin transporter-linked polymorphic region. Transl. Psychiatry 2021, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Pungercic, G.; Videtic, A.; Pestotnik, A.; Pajnic, I.Z.; Zupanc, T.; Balazic, J.; Tomori, M.; Komel, R. Serotonin transporter gene promoter (5-HTTLPR) and intron 2 (VNTR) polymorphisms: A study on Slovenian population of suicide victims. Psychiatr. Genet. 2006, 16, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Roiser, J.P.; Cook, L.J.; Cooper, J.D.; Rubinsztein, D.C.; Sahakian, B.J. Association of a functional polymorphism in the serotonin transporter gene with abnormal emotional processing in ecstasy users. Am. J. Psychiatry. 2005, 162, 609–612. [Google Scholar] [CrossRef]
- Martín-Santos, R.; Torrens, M.; Poudevida, S.; Langohr, K.; Cuyás, E.; Pacifici, R.; Farré, M.; Pichini, S.; De la Torre, R. 5-HTTLPR polymorphism, mood disorders and MDMA use in a 3-year follow-up study. Addict. Biol. 2010, 15, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, K.P.C.; De la Torre, R.; Farre, M.; Xicota, L.; De Sousa Ferandes Perna, E.B.; Theunissen, E.L.; Ramakers, J.G. Depressive mood ratings are reduced by MDMA in female polydrug ecstasy users homozygous for the l-allele of serotonin transporter. Sci. Rep. 2018, 8, 1061. [Google Scholar] [CrossRef] [PubMed]
- Reneman, L.; Schilt, T.; De Win, M.M.; Booij, J.; Schmand, B.; Den Brink, W.V.; Bakker, O. Memory function and serotonin transporter promoter gene polymorphism in ecstasy (MDMA) users. J. Psychopharmacol. 2006, 20, 389–399. [Google Scholar] [CrossRef]
- Ates, O.; Karakus, N.; Sezer, S.; Bozkurt, N. Genetic association of 5-HT1A and 5-HT1B gene polymorphisms with migraine in a Turkish population. J. Neurol. Sci. 2013, 326, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, A.C.; Rodrigo, A.H.; Carcone, D.; McMain, S.; Jacobs, G.; Kennedy, J.L. Tryptophan hydroxylase 1 gene polymorphisms alter prefrontal cortex activation during response inhibition. Neuropsychology 2016, 30, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Pan, Z.; Jiao, Z.; Li, F.; Zhao, G.; Wei, Q.; Pan, F.; Evangelou, E. TPH2 gene polymorphisms and major depression—A meta-analysis. PLoS ONE 2012, 7, e36721. [Google Scholar] [CrossRef]
- Genis-Mendoza, A.D.; Ruiz-Ramos, D.; López-Narvaez, M.L.; Tovilla-Zárate, C.A.; García, A.R.; Meda, G.C.; Martinez-Magana, J.J.; Gonzales-Castro, T.B.; Juarez-Rojop, I.E.; Nicolini, H. Genetic association analysis of 5-HTR2A gene variants in eating disorders in a Mexican population. Brain Behav. 2019, 9, e01286. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.C.; MacKillop, J.; Weafer, J.; Hernandez, K.M.; Gao, J.; Palmer, A.A.; De Wit, H. Genetic analysis of impulsive personality traits: Examination of a priori candidates and genome-wide variation. Psychiatry Res. 2018, 259, 398–404. [Google Scholar] [CrossRef]
- Kaur, G.; Singh Chavan, B.; Gupta, D.; Sinhmar, V.; Prasad, R.; Tripathi, A.; Garg, P.D.; Gupta, R.; Khurana, H.; Gautam, S.; et al. An association study of dopaminergic (DRD2) and serotoninergic (5-HT2) gene polymorphism and schizophrenia in a North Indian population. Asian J. Psychiatry 2019, 39, 178–184. [Google Scholar] [CrossRef]
- Ni, J.; Lu, W.; Wu, Z.; Chen, J.; Yi, Z.; Zhang, C. T102C polymorphism of serotonin 2 A type receptor gene confers susceptibility to (early onset) schizophrenia in Han Chinese: An association study and meta-analysis. Asia Pac. Psychiatry 2013, 5, 24–30. [Google Scholar] [CrossRef]
- Marsden, C.A. Dopamine: The rewarding years. Br. J. Pharmacol. 2006, 147, S136–S144. [Google Scholar] [CrossRef] [PubMed]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.P. The DRD2 gene in psychiatric and neurological disorders and its phenotypes. Pharmacogenomics 2000, 1, 309–333. [Google Scholar] [CrossRef]
- He, M.; Yan, H.; Duan, Z.X.; Qu, W.; Gong, H.Y.; Fan, Z.L.; Kang, J.Y.; Li, B.C.; Wang, J.M. Genetic distribution and association analysis of DRD2 gene polymorphisms with major depressive disorder in the Chinese Han population. Int. J. Clin. Exp. Pathol. 2013, 6, 1142–1149. [Google Scholar]
- Della Torre, O.H.; Paes, L.A.; Henriques, T.B.; De Mello, M.P.; Celeri, E.H.R.V.; Dalgalarrondo, P.; Guerra-Junior, G.; Dos Santos-Junior, A. Dopamine D2 receptor gene polymorphisms and externalizing behaviors in children and adolescents. BMC Med. Genet. 2018, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Bershad, A.K.; Weafer, J.J.; Kirkpatrick, M.G.; Wardle, M.C.; Miller, M.A.; De Wit, H. Oxytocin receptor gene variation predicts subjective response to MDMA. Soc. Neurosci. 2016, 11, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Liechti, M.E. Oxytocin receptor gene variations and socio-emotional effects of MDMA: A pooled analysis of controlled studies in healthy subjects. PLoS ONE 2018, 13, e0199384. [Google Scholar] [CrossRef]
- Newton, T.F. A perhaps unexpected role of noreipenephrine in actions of MDMA. Clin. Pharmacol. Ther. 2011, 90, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Meyer Zu Schwabedissen, H.E.; Liechti, M.E. No major role of norepinephrine transporter gene variations in the cardiostimulant effects of MDMA. Eur. J. Pharmacol. 2018, 74, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Hwang, J.-A.; Lee, H.-J.; Yoon, H.-K.; Ko, Y.-H.; Lee, B.-H.; Jung, H.-Y.; Hahn, S.-W.; Na, K.-S. Association between norepinephrine transporter gene (SLC6A2) polymorphisms and suicide in patients with major depressive disorder. J. Affect. Disord. 2014, 158, 127–132. [Google Scholar] [CrossRef]

| Gene | Polymorphisms (rsId) | Minor Allele Frequency (dbSNP) | Enzyme Activity | Studies | Sample Size | Results/Outcomes | References |
|---|---|---|---|---|---|---|---|
| CYP2B6 | rs3745274 | T = 0.255351/35,719 (ALFA) | Low activity | Vizeli et al. (2017) | 142 | Altered metabolism of MDMA | [46] |
| CYP2C19 | rs4244285 | A = 0.149748/38,985 (ALFA) | Altered metabolism of MDMA Enhanced cardiovascular response | ||||
| rs28399504 | G = 0.00319/863 (ALFA) | ||||||
| CYP1A2 | rs762551 | C = 0.318648/21,670 (ALFA) | High activity | Altered metabolism of MDMA | |||
| CYP2D6 | Not reported | N/A | Low activity | Wolff et al. (2012) | 48 | Increased production of cortisol | [44] |
| Aitchison et al. (2012) | 48 | Increased risk of hyponatremia | [42] | ||||
| De la Torre (2005) | 10 | Altered metabolism of MDMA | [40] | ||||
| Schmid et al. (2016) | 139 | Increased blood pressure Altered metabolism of MDMA | [41] | ||||
| High activity | Cuyas et al. (2011) | 60 | Altered metabolism of MDMA Altered cognitive effects | [43] | |||
| COMT | rs4680 | A = 0.489085/138,095 (ALFA) | Low activity (met/*) | Cuyas et al. (2011) | 60 | Altered metabolism of MDMA Altered cognitive effects | [43] |
| Wolff et al. (2012) | 48 | Increased production of cortisol | [44] | ||||
| Aitchison et al. (2012) | 48 | Increased risk of hyponatremia | [42] | ||||
| Pardo-Lozano et al. (2012) | 27 | Increased cardiovascular effects | [39] | ||||
| rs165599 | G = 0.335031/76,442 (ALFA) | Low activity? | Fagundo et al. (2010) | 30 | Impaired language performances | [49] |
| Gene | Genotypes | Studies | Sample Sizes | Results/Outcomes | References |
|---|---|---|---|---|---|
| 5HTTLPR | s/s | Roiser et al. (2005) | 66 | Emotional disturbances | [55] |
| Martin-Santos et al. (2009) | 37 | Mood disorders (comorbid primary mood disorder) | [56] | ||
| Fagundo et al. (2010) | 30 | Impaired cognitive performance (verbal fluency) | [49] | ||
| Cuyas et al. (2011) | 60 | Impaired cognitive performance (visuospatial attention and memory) | [43] | ||
| Pardo-Lozano et al. (2012) | 27 | Emotional disturbances | [39] | ||
| l/l | Kuypers et al. (2018) | 63 | Reduction in self-rated depressive feelings | [57] | |
| l/* | Pardo-Lozano et al. (2012) | 27 | Cardiovascular effects | [39] |
| Genes | Polymorphisms (rsId) | Minor Allele Frequency (dbSNP) | Studies | Sample Size | Results/Outcomes | References |
|---|---|---|---|---|---|---|
| TPH1 | rs1800532 | T = 0.364661/23,040 (ALFA) | Vizeli et al. (2019) | 125 | No significant impact | [25] |
| rs1799913 | T = 0.369055/34,238 (ALFA) | |||||
| TPH2 | rs7305115 | T = 0./0 (ALFA) | ||||
| 5HTR1A | rs6295 | G = 0.479109/8646 (ALFA) | ||||
| 5HTRIB | rs6296 | G = 0.264684/13,303 (ALFA) | ||||
| 5HTR2B | rs6313 | A = 0.420112/145,567 (ALFA) | ||||
| DAT1 | rs28363170 | Not reported | Vizeli et al. (2019) | 149 | [24] | |
| rs3836790 | ||||||
| rs6347 | C = 0.272059/35,631 (ALFA) | |||||
| rs11133767 | T = 0.324161/26,010 (ALFA) | |||||
| rs11564774 | G = 0.230439/4353 (ALFA) | |||||
| rs460000 | C = 0./0 (ALFA) | |||||
| rs463379 | C = 0.151071/2073 (ALFA) | |||||
| DRD2/ANKK1 | rs1800497 | A = 0.204778/41,712 (ALFA) | ||||
| DRD2 | rs6277 | A = 0.48466/33,521 (ALFA) | ||||
| rs107959 | Not reported | |||||
| DRD4 | rs1805186 | Not reported | ||||
| OXTR | rs53576 | A = 0.326459/24,474 (ALFA) | Bershad et al. (2016) | 68 | [72] | |
| Vizeli et al. (2018) | 123 | [73] | ||||
| rs1042778 | C = 0./0 (ALFA) | Greater feelings of trust | ||||
| rs2254298 | A = 0.139783/6522 (ALFA) | No significant impact | ||||
| SLC6A2 | rs168924 | G = 0.147036/22,375 (ALFA) | Vizeli et al. (2018) | 124 | [75] | |
| rs47958 | A = 0.433445/15,578 (ALFA) | |||||
| rs1861647 | A = 0.205928/2668 (ALFA) | Increased cardiovascular response | ||||
| rs2242446 | C = 0.283916/83,373 (ALFA) | |||||
| rs36029 | G = 0.381354/8267 (ALFA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drevin, G.; Pena-Martin, M.; Bauduin, A.; Baudriller, A.; Briet, M.; Abbara, C. Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature. Pharmaceutics 2024, 16, 1091. https://doi.org/10.3390/pharmaceutics16081091
Drevin G, Pena-Martin M, Bauduin A, Baudriller A, Briet M, Abbara C. Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature. Pharmaceutics. 2024; 16(8):1091. https://doi.org/10.3390/pharmaceutics16081091
Chicago/Turabian StyleDrevin, Guillaume, Maria Pena-Martin, Aurélien Bauduin, Antoine Baudriller, Marie Briet, and Chadi Abbara. 2024. "Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature" Pharmaceutics 16, no. 8: 1091. https://doi.org/10.3390/pharmaceutics16081091
APA StyleDrevin, G., Pena-Martin, M., Bauduin, A., Baudriller, A., Briet, M., & Abbara, C. (2024). Pharmacogenomics of 3,4-Methylenedioxymethamphetamine (MDMA): A Narrative Review of the Literature. Pharmaceutics, 16(8), 1091. https://doi.org/10.3390/pharmaceutics16081091






