Scalability of API-Loaded Multifilament Yarn Production by Hot-Melt Extrusion and Evaluation of Fiber-Based Dosage Forms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
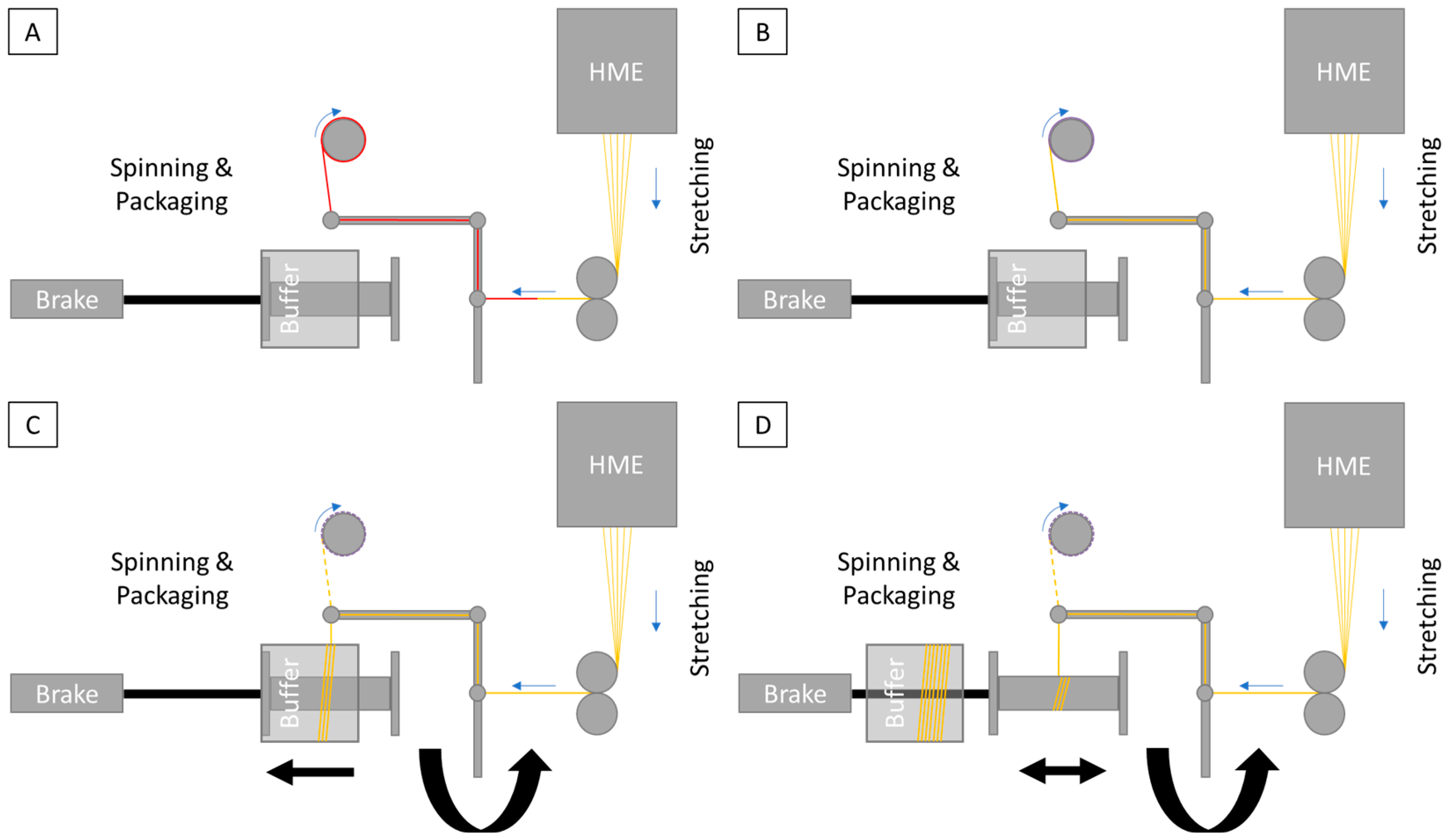
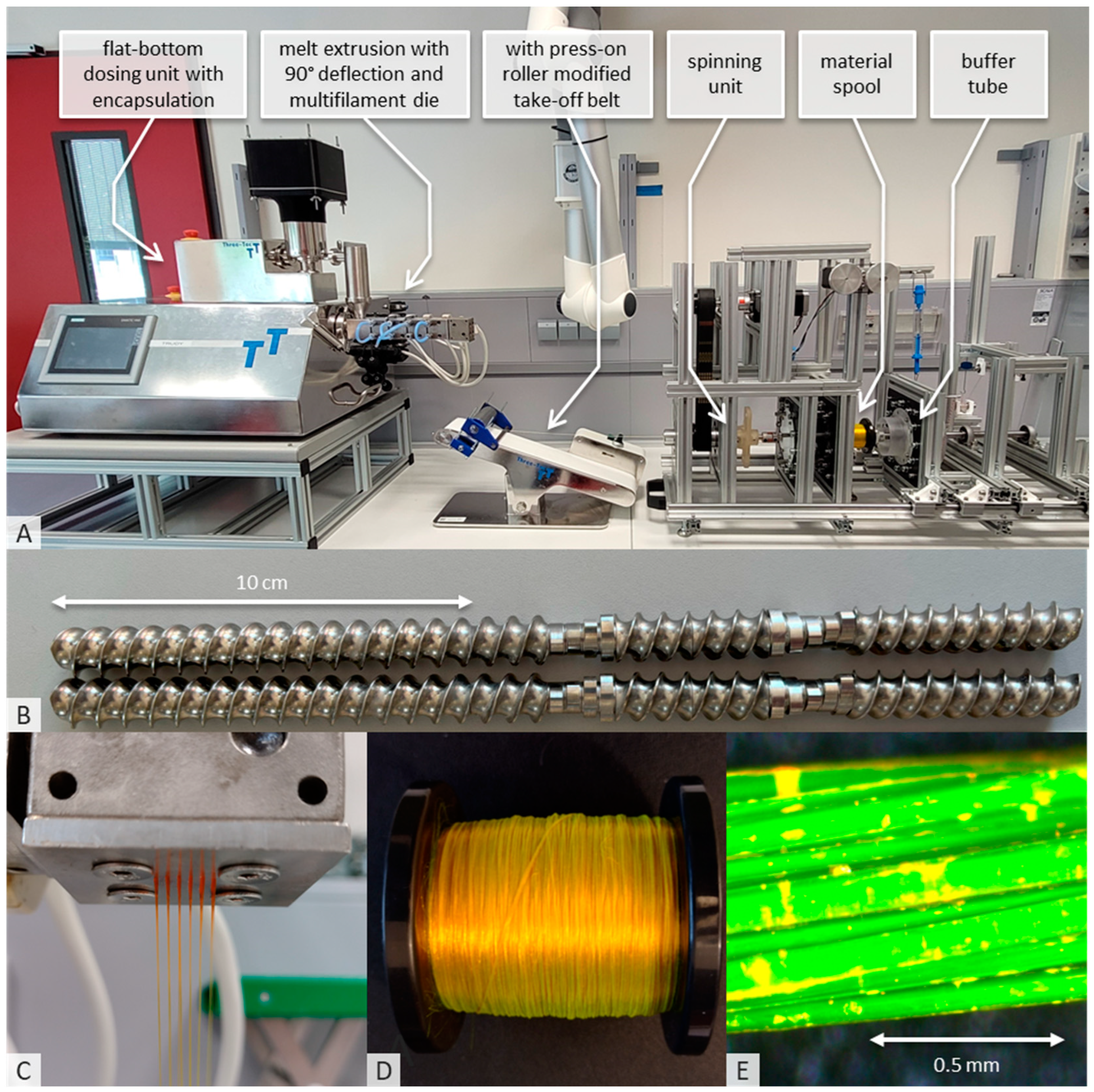
2.2.1. Hot-Melt Extrusion and Spinning Process
2.2.2. Tensile Strength, Elongation, Knot-Pull Tensile Strength
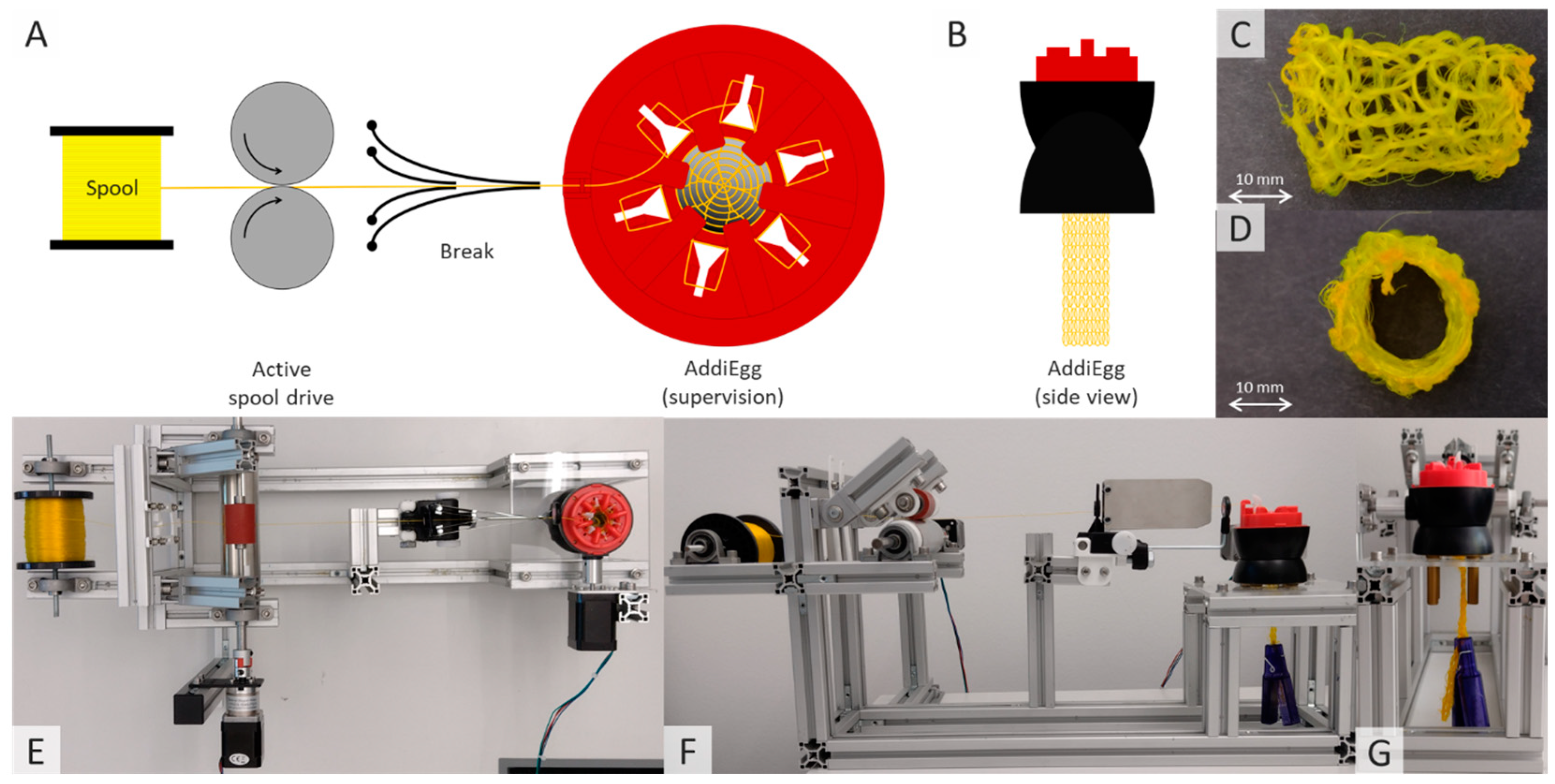
2.2.3. Knitting Processing of Fibers Containing Active Ingredients
2.2.4. Optical Analysis
2.2.5. Modified USP 4 Dissolution Testing
2.2.6. Film Tube Preparation
3. Results and Discussion
3.1. API Ingredient Fiber Production and Spinning
3.2. Tensile Strength as a Measure of Extruded Material Quality and Homogeneity
3.3. Knitting of Fibers Containing Active Ingredients
3.4. Modified USP 4 Dissolution Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humphries, J.; Schneider, B. Spinning the Industrial Revolution. Econ. Hist. Rev. 2019, 72, 126–155. [Google Scholar] [CrossRef]
- Affatato, L.; Carfagna, C. Smart Textiles: A Strategic Perspective of Textile Industry. In Proceedings of the Smart and Interactive Textiles, Terme, Italy, 10–14 June 2012; Volume 80, pp. 1–6. [Google Scholar]
- Zhang, Y.; Ding, J.; Qi, B.; Tao, W.; Wang, J.; Zhao, C.; Peng, H.; Shi, J. Multifunctional Fibers to Shape Future Biomedical Devices. Adv. Funct. Mater. 2019, 29, 1902834. [Google Scholar] [CrossRef]
- Meinel, A.J.; Germershaus, O.; Luhmann, T.; Merkle, H.P.; Meinel, L. Electrospun Matrices for Localized Drug Delivery: Current Technologies and Selected Biomedical Applications. Eur. J. Pharm. Biopharm. 2012, 81, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gierej, A.; Geernaert, T.; Van Vlierberghe, S.; Dubruel, P.; Thienpont, H.; Berghmans, F. Challenges in the Fabrication of Biodegradable and Implantable Optical Fibers for Biomedical Applications. Materials 2021, 14, 1972. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Sooriyarachchi, A.C.; Altural, H.; Montazami, R.; Rylander, M.N.; Hashemi, N. Fiber Based Approaches as Medicine Delivery Systems. ACS Biomater. Sci. Eng. 2016, 2, 1411–1431. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Teoh, X.Y.; Le Hwang, J.; Khong, Z.P.; Sejare, R.; Almashhadani, A.Q.; Assi, R.A.; Chan, S.Y. Electrospinning and Its Potential in Fabricating Pharmaceutical Dosage Form. J. Drug Deliv. Sci. Technol. 2022, 76, 103761. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Sun, S.; Khan, A.R.; Ji, J.; Yang, M.; Zhai, G. Recent Advances in Electrospun for Drug Delivery Purpose. J. Drug Target. 2019, 27, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Ostheller, M.-E.; Balakrishnan, N.K.; Beukenberg, K.; Groten, R.; Seide, G. Pilot-Scale Melt Electrospinning of Polybutylene Succinate Fiber Mats for a Biobased and Biodegradable Face Mask. Polymers 2023, 15, 2936. [Google Scholar] [CrossRef]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-up of Electrospinning Technology: Applications in the Pharmaceutical Industry. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1611. [Google Scholar] [CrossRef]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun Nanofibers for Customized Drug-Delivery Systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Xu, H.; Yagi, S.; Ashour, S.; Du, L.; Hoque, M.E.; Tan, L. A Review on Current Nanofiber Technologies: Electrospinning, Centrifugal Spinning, and Electro-Centrifugal Spinning. Macromol. Mater. Eng. 2023, 308, 2200502. [Google Scholar] [CrossRef]
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-Spun Fibers for Textile Applications. Materials 2020, 13, 4298. [Google Scholar] [CrossRef] [PubMed]
- Kopf, S.; Åkesson, D.; Skrifvars, M. Textile Fiber Production of Biopolymers—A Review of Spinning Techniques for Polyhydroxyalkanoates in Biomedical Applications. Polym. Rev. 2023, 63, 200–245. [Google Scholar] [CrossRef]
- Rosenbaum, C.; Großmann, L.; Neumann, E.; Jungfleisch, P.; Türeli, E.; Weitschies, W. Development of a Hot-Melt-Extrusion-Based Spinning Process to Produce Pharmaceutical Fibers and Yarns. Pharmaceutics 2022, 14, 1229. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Current Manufacturing Processes of Drug-Eluting Sutures. Expert Opin. Drug Deliv. 2017, 14, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.R.M.; Scapellato, S.; Cicciù, M.; Pedullà, E. Antimicrobial Activity of Antibacterial Sutures in Oral Surgery: A Scoping Review. Int. Dent. J. 2024, 74, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Khalid, G.M.; Billa, N. Drug-Eluting Sutures by Hot-Melt Extrusion: Current Trends and Future Potentials. Materials 2023, 16, 7245. [Google Scholar] [CrossRef] [PubMed]
- Zhukovskii, V.A. Problems and Prospects for Development and Production of Surgical Suture Materials. Fibre Chem. 2008, 40, 208–216. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.L.; Katare, R.G.; Ali, M.A. Melt-Extruded Biocompatible Surgical Sutures Loaded with Microspheres Designed for Wound Healing. Biomed. Mater. 2024, 19, 055007. [Google Scholar] [CrossRef]
- Krause, J.; Rosenbaum, C.; Grimm, M.; Rump, A.; Keßler, R.; Hosten, N.; Weitschies, W. The EsoCap-System—An Innovative Platform to Drug Targeting in the Esophagus. J. Control. Release 2020, 327, 1–7. [Google Scholar] [CrossRef]
- Kirsch, K.; Hanke, U.; Weitschies, W. Preparation and Characterization of Gastrointestinal Wafer Formulations. Int. J. Pharm. 2017, 522, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Chandra, A.; Praveen, G.; Snigdha, S.; Roy, S.; Agatemor, C.; Thomas, S.; Provaznik, I. Electrospinning over Solvent Casting: Tuning of Mechanical Properties of Membranes. Sci. Rep. 2018, 8, 5058. [Google Scholar] [CrossRef] [PubMed]
- Corduas, F.; Lamprou, D.A.; Mancuso, E. Next-Generation Surgical Meshes for Drug Delivery and Tissue Engineering Applications: Materials, Design and Emerging Manufacturing Technologies. Bio-Design Manuf. 2021, 4, 278–310. [Google Scholar] [CrossRef]
- Freire, T.F.; Quinaz, T.; Fertuzinhos, A.; Quyền, N.T.; de Moura, M.F.S.M.; Martins, M.; Zille, A.; Dourado, N. Thermal, Mechanical and Chemical Analysis of Poly(Vinyl Alcohol) Multifilament and Braided Yarns. Polymers 2021, 13, 3644. [Google Scholar] [CrossRef]
- Wei, W.; Liu, J.; Huang, J.; Cao, F.; Qian, K.; Yao, Y.; Li, W. Recent Advances and Perspectives of Shape Memory Polymer Fibers. Eur. Polym. J. 2022, 175, 111385. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Liu, Y.; Leng, J. Shape Memory Polymer Fibers: Materials, Structures, and Applications. Adv. Fiber Mater. 2022, 4, 5–23. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.-M.; et al. Electrically Triggered Drug Delivery from Novel Electrospun Poly(Lactic Acid)/Graphene Oxide/Quercetin Fibrous Scaffolds for Wound Dressing Applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef]
- Libanori, A.; Chen, G.; Zhao, X.; Zhou, Y.; Chen, J. Smart Textiles for Personalized Healthcare. Nat. Electron. 2022, 5, 142–156. [Google Scholar] [CrossRef]
- Wulf, K.; Arbeiter, D.; Matschegewski, C.; Teske, M.; Huling, J.; Schmitz, K.P.; Grabow, N.; Kohse, S. Smart Releasing Electrospun Nanofibers—Poly: L.Lactide Fibers as Dual Drug Delivery System for Biomedical Application. Biomed. Mater. 2021, 16, 015022. [Google Scholar] [CrossRef]
- Puiggalí-Jou, A.; Cejudo, A.; Del Valle, L.J.; Alemán, C. Smart Drug Delivery from Electrospun Fibers through Electroresponsive Polymeric Nanoparticles. ACS Appl. Bio Mater. 2018, 1, 1594–1605. [Google Scholar] [CrossRef]
- Maitland, D.J.; Small IV, W.; Singhal, P.; Hwang, W.; Rodriguez, J.N.; Clubb, F.; Wilson, T.S. Design and Realization of Biomedical Devices Based on Shape Memory Polymers. MRS Proc. 2009, 1190, 1190-NN06-01. [Google Scholar] [CrossRef]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.; Gilkerson, R.; Lozano, K. Past, Present and Future of Surgical Meshes: A Review. Membranes 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Low, S.; Bhise, N.; Yang, J.; Peeke, E.; Schwarz, M.; Wright, J.; Carter, B.; Moorthy, S.; Grant, T.; et al. A Pharmaceutical Answer to Nonadherence: Once Weekly Oral Memantine for Alzheimer’s Disease. J. Control. Release 2019, 303, 34–41. [Google Scholar] [CrossRef]
- Okuda, T.; Tominaga, K.; Kidoaki, S. Time-Programmed Dual Release Formulation by Multilayered Drug-Loaded Nanofiber Meshes. J. Control. Release 2010, 143, 258–264. [Google Scholar] [CrossRef]
- Yang, W.; Lin, S.; Gong, W.; Lin, R.; Jiang, C.; Yang, X.; Hu, Y.; Wang, J.; Xiao, X.; Li, K.; et al. Single Body-Coupled Fiber Enables Chipless Textile Electronics. Science 2024, 384, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of PH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap® System. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef]
- Zhu, L.-M. Mesh Implants: An Overview of Crucial Mesh Parameters. World J. Gastrointest. Surg. 2015, 7, 226. [Google Scholar] [CrossRef]
- Krogstad, E.A.; Woodrow, K.A. Manufacturing Scale-up of Electrospun Poly(Vinyl Alcohol) Fibers Containing Tenofovir for Vaginal Drug Delivery. Int. J. Pharm. 2014, 475, 282–291. [Google Scholar] [CrossRef]
- Panda, B.P.; Dey, N.; Rao, M.E.B. Development of Innovative Orally Fast Disintegrating Film Dosage Forms: A Review. Int. J. Pharm. Sci. Nanotechnol. 2012, 5, 1666–1674. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, J.J.; Williams, G.R.; Zhao, M. Electrospun Amorphous Solid Dispersions of Poorly Water-Soluble Drugs: A Review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef]
- Ardalkar, S.; Mathure, D.; Pawar, A.; Awasthi, R. Formulation and Optimization of Electrospun Nanofiber Films for Ultrafast Delivery of Domperidone: In Vitro and in-Vivo Characterization. J. Drug Deliv. Sci. Technol. 2024, 95, 105645. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Balogh, A.; Vajna, B.; Farkas, A.; Patyi, G.; Kramarics, Á.; Marosi, G. Comparison of Electrospun and Extruded Soluplus®-Based Solid Dosage Forms of Improved Dissolution. J. Pharm. Sci. 2012, 101, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Dott, C.; Tyagi, C.; Tomar, L.K.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Pillay, V. A Mucoadhesive Electrospun Nanofibrous Matrix for Rapid Oramucosal Drug Delivery. J. Nanomater. 2013, 2013, 924947. [Google Scholar] [CrossRef]
- Pyo, S.; Lee, J.; Kim, W.; Jo, E.; Kim, J. Multi-Layered, Hierarchical Fabric-Based Tactile Sensors with High Sensitivity and Linearity in Ultrawide Pressure Range. Adv. Funct. Mater. 2019, 29, 1902484. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenbaum, C.; Gerds, N.; Hack, L.; Weitschies, W. Scalability of API-Loaded Multifilament Yarn Production by Hot-Melt Extrusion and Evaluation of Fiber-Based Dosage Forms. Pharmaceutics 2024, 16, 1103. https://doi.org/10.3390/pharmaceutics16081103
Rosenbaum C, Gerds N, Hack L, Weitschies W. Scalability of API-Loaded Multifilament Yarn Production by Hot-Melt Extrusion and Evaluation of Fiber-Based Dosage Forms. Pharmaceutics. 2024; 16(8):1103. https://doi.org/10.3390/pharmaceutics16081103
Chicago/Turabian StyleRosenbaum, Christoph, Naemi Gerds, Liliane Hack, and Werner Weitschies. 2024. "Scalability of API-Loaded Multifilament Yarn Production by Hot-Melt Extrusion and Evaluation of Fiber-Based Dosage Forms" Pharmaceutics 16, no. 8: 1103. https://doi.org/10.3390/pharmaceutics16081103






