The Role of Inhaled Chitosan-Based Nanoparticles in Lung Cancer Therapy
Abstract
1. Introduction
2. Lung Cancer
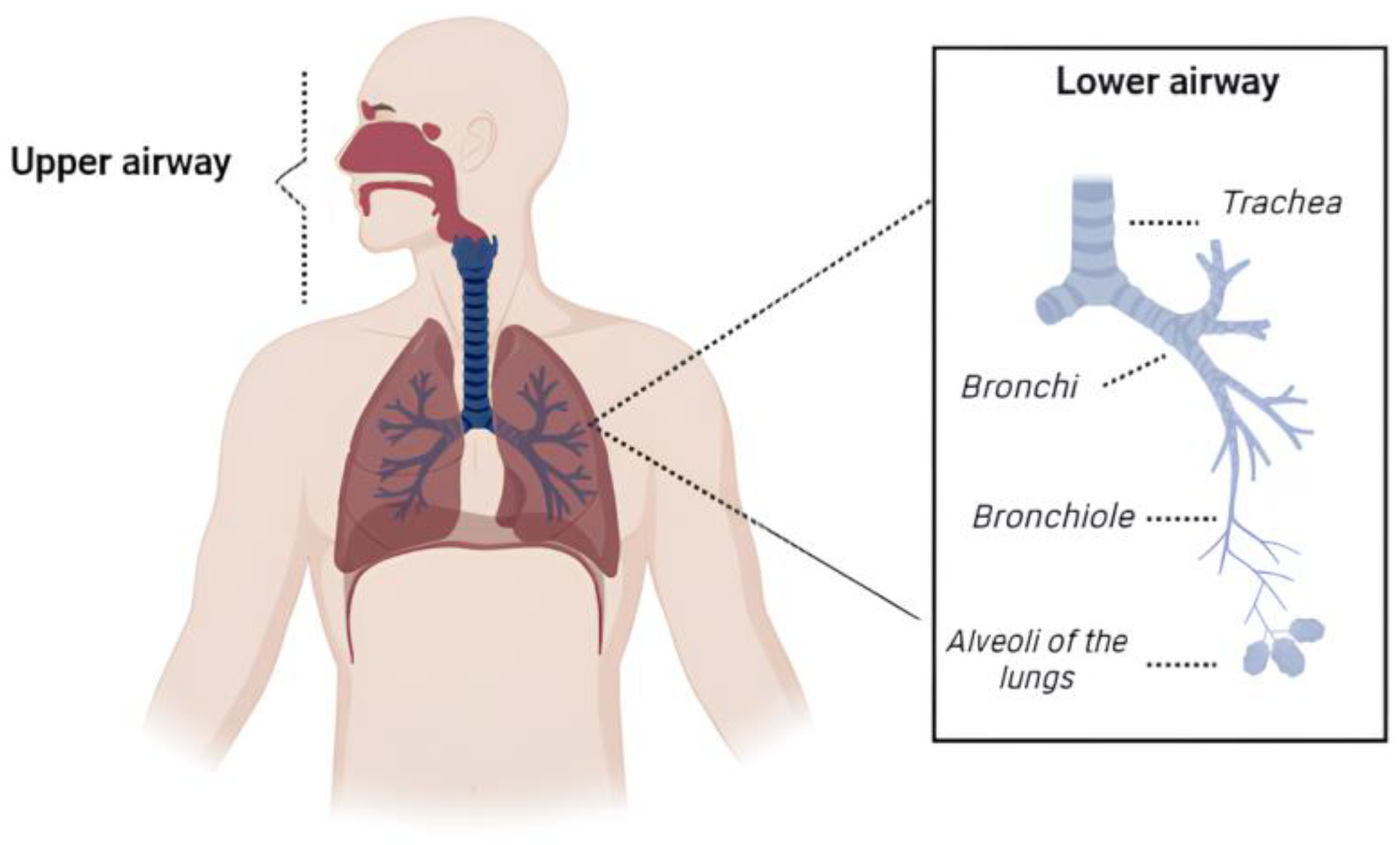
3. Pulmonary Drug Delivery: Perspectives and Challenges
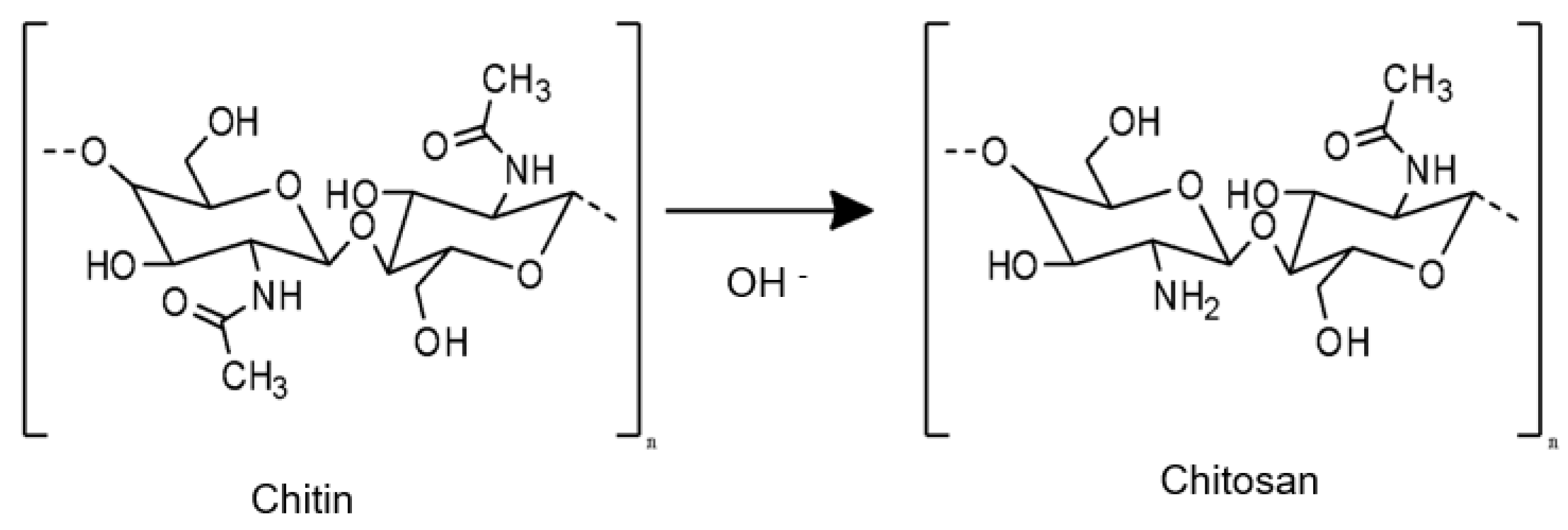
4. CS: A Unique Polymer for Pharmaceutical Applications
5. Preparation of CS Nanoparticles
6. CS-Based Nanoparticles for Pulmonary Delivery in Lung Cancer Therapy
6.1. CS Nanoparticles as an Effective Carrier for Antineoplastic Drugs
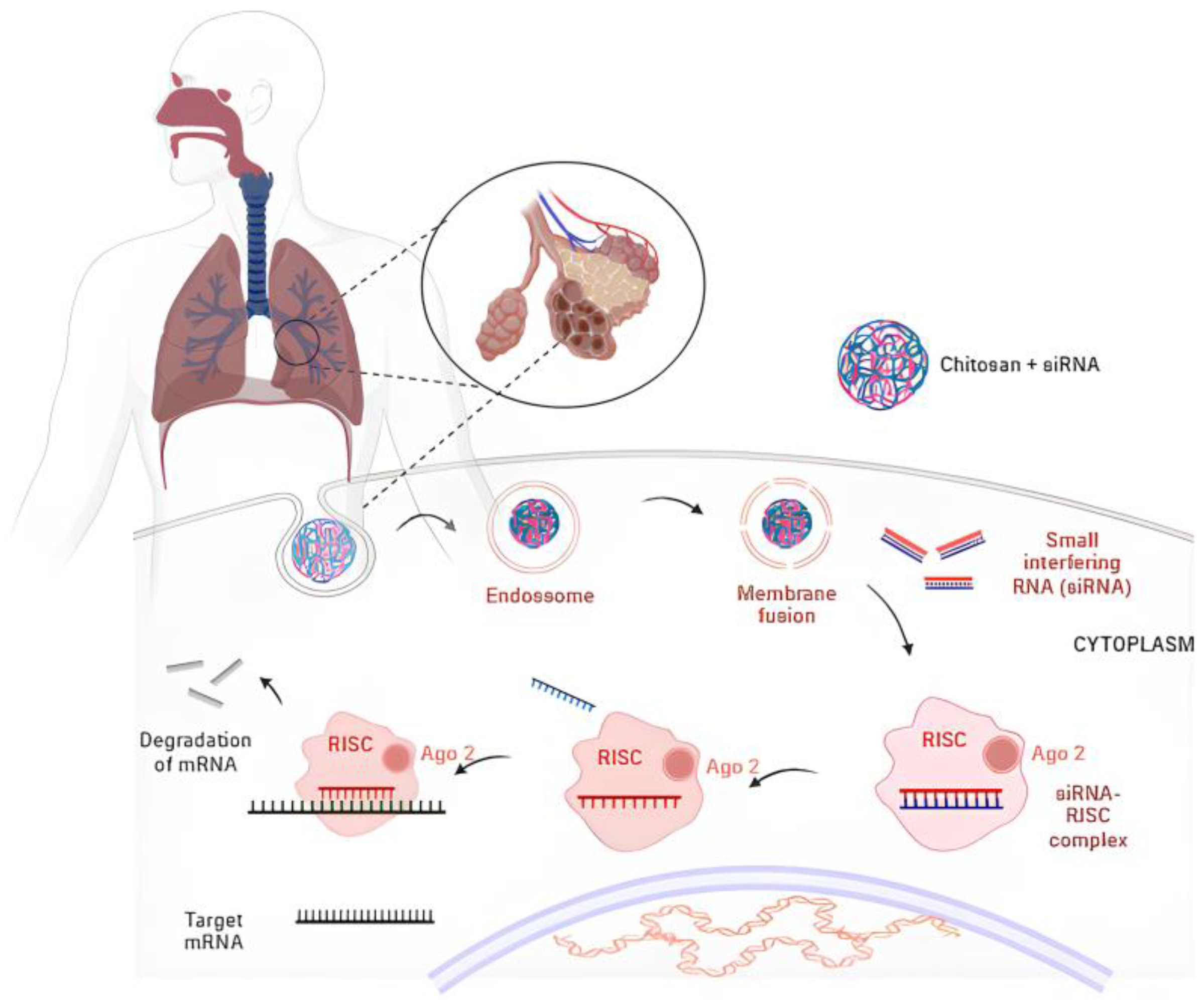
6.2. CS Nanoparticles as an Effective Carrier for siRNA
7. Patent Review
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, M.L.; Choong, P.F.M.; Dass, C.R. Cancer, chitosan nanoparticles and catalytic nucleic acids. J. Pharm. Pharmacol. 2009, 61, 3–12. [Google Scholar] [CrossRef]
- Bukhtoyarov, O.V.; Samarin, D.M. Pathogenesis of cancer: Cancer reparative trap. J. Cancer Ther. 2015, 6, 399–412. [Google Scholar] [CrossRef]
- Refaey, K.; Tripathi, S.; Grewal, S.S.; Bhargav, A.G.; Quinones, D.J.; Chaichana, K.L.; Antwi, S.O.; Cooper, L.T.; Meyer, F.B.; Dronca, R.S.; et al. Cancer mortality rates increasing vs. cardiovascular disease mortality decreasing in the world: Future implications. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 645–653. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Latest Global Cancer Data: Cancer Burden Rises to 19.3 Million New Cases and 10.0 million Cancer Deaths in 2020. Available online: https://www.iarc.who.int/wp-content/uploads/2020/12/pr292_E.pdf (accessed on 15 April 2024).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer progress and priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Vinod, S.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25, 61–71. [Google Scholar] [CrossRef]
- Narmani, A.; Jafari, S.M. Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydr. Polym. 2021, 272, 118464. [Google Scholar] [CrossRef]
- Ding, J.; Guo, Y. Recent advances in chitosan and its derivatives in cancer treatment. Front. Pharmacol. 2022, 13, 888740. [Google Scholar] [CrossRef]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; Luca, M.; Gaofalo, A.; Ragno, G.; Grande, F. Anticancer drugs: Recent strategies to improve stability profile, pharmacokinetic and pharmacodynamic properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-based approved pharmaceuticals for cancer treatment: Present and future challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef]
- Li, Y.; Yan, B.; He, S. Advances and challenges in the treatment of lung cancer. Biomed. Pharmacother. 2023, 169, 15891. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Rizwanullah, M.; Ahmad, J.; Alasmary, M.Y.; Akhter, M.H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing chitosan nanoparticles for cancer therapy. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 602–623. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Tabrez, S.; Ashraf, G.; Shakil, S.; Damanhouri, G.A.; Kamal, M.A. Nanotechnology-based approaches in anticancer research. Int. J. Nanomed. 2012, 7, 4391–4408. [Google Scholar] [CrossRef]
- Bora, R.S.; Gupta, D.; Mukkur, K.S.; Saini, K.S. RNA interference therapeutics for cancer: Challenges and opportunities (Review). Mol. Med. Rep. 2012, 6, 9–15. [Google Scholar] [CrossRef]
- Alrushaid, N.; Khan, F.A.; Al-Suhaimi, E.A.; Elaissari, A. Nanotechnology in cancer diagnosis and treatment. Pharmaceutics 2023, 15, 1025. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Park, K. Multicomponent, tumor-homing chitosan nanoparticles for cancer imaging and therapy. Int. J. Mol. Sci. 2017, 18, 594. [Google Scholar] [CrossRef]
- Xu, X.; Dai, F.; Mao, Y.; Zhang, K.; Qin, Y.; Zheng, J. Metallodrugs in the battle against non-small cell lung cancer: Unlocking the potential for improved therapeutic outcomes. Front. Pharmacol. 2023, 14, 1242488. [Google Scholar] [CrossRef]
- Liu, K.; Chen, W.; Yang, T.; Wen, B.; Ding, D.; Keidar, M.; Tang, J.; Zhang, W. Paclitaxel and quercetin nanoparticles co-loaded in microspheres to prolong retention time for pulmonary drug delivery. Int. J. Nanomed. 2017, 12, 8239–8255. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; Deeb, N.M.; Abbas, H. Development of a potential anti-cancer pulmonary nanosystem consisted of chitosan-doped LeciPlex loaded with resveratrol using a machine learning method. J. Drug Deliv. Sci. Technol. 2022, 70, 103259. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, X.; Ye, L.; Zhang, Y.; Ding, R.; Hao, Y.; Zhao, Y.; Zhang, Z.; Zhang, Y. Inhalable microspheres embedding chitosan-coated PLGA nanoparticles for 2-methoxy estradiol. J. Drug Target. 2014, 22, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Byeon, H.J.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Doxorubicin-loaded highly porous large PLGA microparticles as a sustained- release inhalation system for the treatment of metastatic lung cancer. Biomaterials 2012, 33, 5574–5583. [Google Scholar] [CrossRef] [PubMed]
- Anterior, C.M.; Helmy, M.W.; Abdelfattah, E.Z.; Khattab, S.N.; Rahab, D.; Samaha, M.W.; Fang, J.-Y.; Elzoghby, A.O. Inhalable dual-targeted hybrid lipid nanocore-protein shell composites for combined delivery of genistein and all-trans retinoic acid to lung cancer cells. ACS Biomater. Sci. Eng. 2020, 6, 71–87. [Google Scholar] [CrossRef]
- Elbatanony, R.S.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Chauhan, G.; Kunda, N.K.; Gupta, V. Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)-development and in-vitro efficacy. Drug Deliv. Transl. Res. 2021, 11, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Patil, S.M.; Shukla, S.K.; Kulkarni, N.S.; Gupta, V.; Kunda, N.K. Pulmonary delivery of osimertinib liposomes for non-small cell lung cancer treatment: Formulation development and in vitro evaluation. Drug Deliv. Transl. Res. 2022, 12, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E. Progresses in targeted drug delivery systems using chitosan nanoparticles in cancer therapy: A mini-review. J. Drug Deliv. Sci. Technol. 2020, 58, 101813. [Google Scholar] [CrossRef]
- Virmani, T.; Kumar, G.; Sharma, A.; Pathak, K.; Akhtar, S.; Afzal, O.; Altamimi, A.S.A. Amelioration of cancer employing chitosan, its derivatives, and chitosan-based nanoparticles: Recent updates. Polymers 2023, 15, 2928. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.N. Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef]
- Ghadiri, M.; Young, P.M.; Traini, P. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Fact Sheets: Lung Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 2 April 2024).
- Kuen, C.Y.; Masarudin, M.J. Chitosan nanoparticle-based system: A new insight into the promising controlled release system for lung cancer treatment. Molecules 2022, 27, 473. [Google Scholar] [CrossRef] [PubMed]
- Walser, T.; Cui, X.; Yanagawa, J.; Lee, J.M.; Heinrich, E.; Lee, G.; Sharma, S.; Dubinett, S.M. Smoking and lung cancer. Proc. Am. Thorac. Soc. 2008, 5, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Ercumen, A.; Yuan, Y.; Steinmaus, C.R. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Hubaux, R.; Santos, D.B.; Enfield, K.S.S.; Lam, S.; Lam, W.; Martinez, V. Arsenic, asbestos and radon: Emerging players in lung tumorigenesis. Environ. Health 2012, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Field, R.W.; Withers, B.L. Occupational and environmental causes of lung cancer. Clin. Chest Med. 2012, 33, 23153609. [Google Scholar] [CrossRef] [PubMed]
- Zifodya, J.S.; Crothers, K. Tuberculosis, chronic obstructive lung disease, and lung cancer: The holey upper lobe trinity. Ann. Am. Thorac. Soc. 2022, 19, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Qubo, A.A.; Numan, J.; Snijder, J.; Padilla, M.; Austin, J.H.M.; Capaccione, K.M.; Pernia, M.; Bustamante, J.; O’Connor, T.; Salvatore, M.M. Idiopathic pulmonary fibrosis and lung cancer: Future directions and challenges. Breathe 2022, 18, 220147. [Google Scholar] [CrossRef]
- Park, H.Y.; Kang, D.; Shin, S.H.; Choi, H.; Jang, S.H.; Lee, C.H.; Kim, H.; Kwon, O.J.; Rhee, C.K.; Cho, J. Pulmonary tuberculosis and the incidence of lung cancer among patients with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2022, 19, 640–648. [Google Scholar] [CrossRef]
- Birring, S.S.; Peake, M.D. Symptoms and the early diagnosis of lung cancer. Thorax 2005, 60, 268–269. [Google Scholar] [CrossRef]
- Xing, P.; Zhu, Y.; Wang, L.; Hui, Z.; Liu, S.; Ren, J.; Zhang, Y.; Song, Y.; Liu, C.; Huang, Y.; et al. What are the clinical symptoms and physical signs for non-small cell lung cancer before diagnosis is made? A nation-wide multicenter 10-year retrospective study in China. Cancer Med. 2019, 8, 4055–4069. [Google Scholar] [CrossRef]
- Vázquez, A.C.; Béjar, J.L.R.; García, L.A.; Martos, N.S.; Urquiza, J.L.G.; Cañadas, G.R.; la Fuente, G.A.C.-D. Risk factors for short-term lung cancer survival. J. Clin. Med. 2021, 10, 519. [Google Scholar] [CrossRef]
- Xie, X.; Li, X.; Tang, W.; Xie, P.; Tan, X. Primary tumor location in lung cancer: The evaluation and administration. Chin. Med. J. 2022, 135, 127–136. [Google Scholar] [CrossRef]
- Rojiani, M.V.; Rojiani, A.M. Non-small cell lung cancer—Tumor biology. Cancers 2024, 16, 716. [Google Scholar] [CrossRef]
- Bernhardt, E.B.; Jalal, S. Small cell lung cancer. Cancer Treat. Res. 2016, 170, 301–322. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Fin, C.F.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Prim. 2021, 7, 3. [Google Scholar] [CrossRef]
- Wang, Q.; Gümüş, Z.H.; Colarossi, C.; Memeo, L.; Wang, X.; Kong, C.Y.; Boffetta, P. SCLC: Epidemiology, risk factors, genetic susceptibility, molecular pathology, screening, and early detection. J. Thorac. Oncol. 2023, 18, 31–46. [Google Scholar] [CrossRef]
- Seguin, L.; Durandy, M.; Feral, C.C. Lung adenocarcinoma tumor origin: A guide for personalized medicine. Cancers 2022, 14, 1759. [Google Scholar] [CrossRef]
- Devarakonda, S.; Morgensztern, D.; Govindan, R. Genomic alterations in lung adenocarcinoma. Lancet Oncol. 2015, 16, e342–e351. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.R.; Ma, J.; Khanna, A.; Lyons, G.; Rinsurong, W.; Bassett, R.; Guo, M.; Routbort, M.J.; Zhang, J.; Skoulidis, F.; et al. Simplified molecular classification of lung adenocarcinomas based on EGFR, KRAS, and TP53 mutations. BMC Cancer 2020, 20, 83. [Google Scholar] [CrossRef]
- Gulati, N.; Dua, K.; Dureja, H. Role of chitosan-based nanomedicines in the treatment of chronic respiratory diseases. Int. J. Biol. Macromol. 2021, 185, 20–30. [Google Scholar] [CrossRef]
- Chválová, K.; Brabec, V.; Kašpárková, J. Mechanism of the formation of DNA–protein cross-links by antitumor cisplatin. Nucleic Acids Res. 2007, 35, 1812–1821. [Google Scholar] [CrossRef]
- Salerno, D.; Beretta, G.L.; Zanchetta, G.; Brioschi, S.; Cristofalo, M.; Missana, N.; Nardo, L.; Cassina, V.; Tempestini, A.; Giovannoni, R.; et al. Platinum-Based Drugs and DNA Interactions Studied by Single-Molecule and Bulk Measurements. Biophys. J. 2016, 110, 2151–2161. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; Puente, T.D. A Compressive review about taxol: History and future challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Gubens, M.A.; Wakelee, H.A. Docetaxel in the treatment of non-small cell lung carcinoma: An update and analysis. Lung Cancer 2010, 1, 63–76. [Google Scholar] [CrossRef]
- Schettino, C.; Bareschino, M.A.; Ricci, V.; Ciardiello, F. Erlotinib: An EGF receptor tyrosine kinase inhibitor in non-small-cell lung cancer treatment. Expert Rev. Respir. Med. 2008, 2, 167–178. [Google Scholar] [CrossRef]
- Zubair, T.; Bandyopadhyay, D. Small molecule EGFR inhibitors as anti-cancer agents: Discovery, mechanisms of action, and opportunities. Int. J. Mol. Sci. 2023, 24, 2651. [Google Scholar] [CrossRef]
- Baldwin, E.L.; Osheroff, N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents 2005, 5, 363–372. [Google Scholar] [CrossRef]
- Montecucco, A.; Zanetta, F.; Biamonti, G. Molecular mechanisms of etoposide. EXCLI J. 2015, 14, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Pavlíčková, V.S.; Ruml, T.; Rimpelová, S. Vincristine in combination therapy of cancer: Emerging trends in clinics. Biology 2021, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, Z. Non-Small Cell Lung Cancer Targeted Therapy: Drugs and Mechanisms of Drug Resistance. Int. J. Mol. Sci. 2022, 23, 15056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Z.; Li, Y.; Zhoucorresponding, Q. Development of epidermal growth factor receptor tyrosine kinase inhibitors against EGFR T790M. Mutation in non-small-cell lung carcinoma. Open Med. (Wars) 2016, 11, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Mannani, R.; Heidarnejadmaleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; An update review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Chazan, G.; Solomon, B.J. Optimal first-line treatment for metastatic ALK+ non-small cell lung cancer—A narrative review. Transl. Lung Cancer Res. 2023, 12, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.B.; Hitchen, N.; Chandran, E.; Morris, T.; Manser, R.; Solomon, B.J.; Jordan, V. Targeted therapy for advanced anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer. Cochrane Database Syst. Rev. 2022, 2022, CD013453. [Google Scholar] [CrossRef]
- Fabbri, L.; Federico, A.; Astore, M.; Marchiori, V.; Rejtano, A.; Seminerio, R.; Gelsomino, F.; De Giglio, A. From Development to Place in Therapy of Lorlatinib for the Treatment of ALK and ROS1 Rearranged Non-Small Cell Lung Cancer (NSCLC). Diagnostics 2024, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front. Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef] [PubMed]
- Brazel, D.; Zhang, S.; Nagasaka, M. Spotlight on Tepotinib and Capmatinib for Non-Small Cell Lung Cancer with MET Exon 14 Skipping Mutation. Lung Cancer 2022, 13, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.A.; Subbiah, V. Precision oncology with selective RET inhibitor selpercatinib in RET-rearranged cancers. Ther. Adv. Med. Oncol. 2023, 15, 17588359231177015. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Ferrari, G.; Rio, B.; Novello, S.; Passiglia, F. HER2-Altered Non-Small Cell Lung Cancer: A Journey from Current Approaches to Emerging Strategies. Cancers 2024, 16, 2018. [Google Scholar] [CrossRef] [PubMed]
- Vicary, G.W.; Roman, J. Targeting the Mammalian Target of Rapamycin in Lung Cancer. Am. J. Med. Sci. 2016, 352, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Bhaoighill, M.N.; Dunlop, E.A. Mechanistic target of rapamycin inhibitors: Successes and challenges as cancer therapeutics. Cancer Drug Resist. 2019, 2, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Gyanani, V.; Haley, J.C.; Goswami, R. Challenges of current anticancer treatment approaches with focus on liposomal drug delivery systems. Pharmaceuticals 2021, 14, 835. [Google Scholar] [CrossRef]
- Xie, Y.; Aillon, K.L.; Cai, S.; Christian, J.M.; Davies, N.M.; Berkland, C.J.; Forrest, M.L. Pulmonary delivery of cisplatin-hyaluronan conjugates via endotracheal instillation for the treatment of lung cancer. Int. J. Pharm. 2010, 392, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, L.; Casiraghi, M.; Uslenghi, C.; Maiorca, S.; Spaggiari, L. Recent advances in lung cancer research: Unravelling the future of treatment. Updates Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Munshi, A.; Ramesh, R. Combinatorial therapeutic approaches with RNAi and anticancer drugs using nanodrug delivery systems. Drug Dev. Ind. Pharm. 2017, 43, 1391–1401. [Google Scholar] [CrossRef]
- Khan, P.; Siddiqui, J.A.; Lakshmanan, I.; Ganti, A.K.; Salgia, R.; Jain, M.; Batra, S.K.; Nasser, M.W. RNA-based therapies: A cog in the wheel of lung cancer defense. Mol. Cancer 2021, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Garbuzenko, O.B.; Minko, T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine 2008, 3, 761–776. [Google Scholar] [CrossRef]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model. Mol. Pharm. 2014, 11, 3515–3527. [Google Scholar] [CrossRef]
- Shali, H.; Shabanic, M.; Pourgholid, F.; Hajivalilid, M.; Malekid, L.A.; Niaragh, F.J.; Baradaran, B.; Akbari, A.A.M.; Younesi, V.; Yousefi, M. Co-delivery of insulin-like growth factor 1 receptor specific siRNA and doxorubicin using chitosan-based nanoparticles enhanced anticancer efficacy in A549 lung cancer cell line. Artif. Cells Nanomed. Biotechnol. 2018, 46, 293–302. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, Y.; Zhang, Y.; Li, Y.; Bu, Y.; Song, F.; Zhang, C. Silencing of PRR11 suppresses cell proliferation and induces autophagy in NSCLC cells. Genes Dis. 2018, 5, 158–166. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, B.; Gan, C.; Sun, H.; Zhang, J.; Feng, L.A. Comprehensive Review of Small Interfering RNAs (siRNAs): Mechanism, Therapeutic Targets, and Delivery Strategies for Cancer Therapy. Int. J. Nanomed. 2023, 18, 7605–7635. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Muralidharan, A.; Biswas, A.; Kamath, V.; Joseph, A.; Alex, A.T. siRNA therapeutics and its challenges: Recent advances in effective delivery for cancer therapy. OpenNano 2022, 7, 100063. [Google Scholar] [CrossRef]
- Hattab, D.; Gazzali, A.M.; Bakhtiar, A. Clinical Advances of siRNA-Based Nanotherapeutics for Cancer Treatment. Pharmaceutics 2021, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trial: A Study of NBF-006 in Non-Small Cell Lung, Pancreatic, or Colorectal Cancer. Sponsor: Nitto BioPharma, Inc. ID ClinicalTrials.gov: NCT03819387. Available online: https://clinicaltrials.gov/study/NCT03819387#study-overview (accessed on 25 June 2024).
- O’Brien, Z.; Wang, L.; Majeti, B.; Clamme, J.; Baclig, R.; Chu, J.; Fong, S.; Harborth, J.; Ibarra, J.; Yin, H.; et al. Abstract 5917: A novel lipid nanoparticle (NBF-006) encapsulating glutathione S-transferase P (GSTP) siRNA for the treatment of KRAS-driven non-small cell lung cancer. Cancer Res. 2018, 78, 5917. [Google Scholar] [CrossRef]
- Miwata, K.; Okamoto, H.; Nakashima, T.; Ihara, D.; Horimasu, Y.; Masuda, T.; Miyamoto, S.; Iwamoto, H.; Fujitaka, K.; Hamada, H.; et al. Intratracheal Administration of siRNA Dry Powder Targeting Vascular Endothelial Growth Factor Inhibits Lung Tumor Growth in Mice. Mol. Ther. Nucleic Acids 2018, 12, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kronenberger, P.; Teugels, E.; Umelo, I.A.; De Grève, J. Targeting the epidermal growth factor receptor in non-small cell lung cancer cells: The effect of combining RNA interference with tyrosine kinase inhibitors or cetuximab. BMC Med. 2012, 10, 28. [Google Scholar] [CrossRef]
- Srikar, R.; Suresh, D.; Zambre, A.; Taylor, K.; Chapman, S.; Leevy, M.; Upendran, A.; Kannan, R. Targeted nanoconjugate co-delivering siRNA and tyrosine kinase inhibitor to KRAS mutant NSCLC dissociates GAB1-SHP2 post oncogene knockdown. Sci. Rep. 2016, 6, 30245. [Google Scholar] [CrossRef]
- Zoulikha, M.; Xiao, Q.; Boafo, G.F.; Sallam, M.A.; Chen, Z.; He, W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm. Sin. B 2022, 12, 600–620. [Google Scholar] [CrossRef]
- Jain, D.; Prajapati, S.K.; Jain, A.; Singhal, R. Nano-formulated siRNA-based therapeutic approaches for cancer therapy. Nano Trends 2023, 1, 100006. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Liang, W.; Chan, H.K. Pulmonary delivery of therapeutic siRNA. Adv. Drug Deliv. Rev. 2012, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rudzinskia, W.E.; Aminabhavi, T.M. Chitosan as a carrier for targeted delivery of small interfering RNA. Int. J. Pharm. 2010, 399, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Qi, Y.; Yang, L.; Miao, Y.; Ren, W.; Liu, H.; Huang, Y.; Huang, S.; Chen, S.; Shi, Y.; et al. Remodeling the tumor immune microenvironment via siRNA therapy for precision cancer treatment. Asian J. Pharm. Sci. 2023, 18, 100852. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.H.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.H.; Hasan, M.M.; Khan, Y.H.; Hafeez, S.; Massoud, E.E.S.; Rahman, H.; et al. Appraisal for the potential of viral and nonviral vectors in gene therapy: A review. Genes 2022, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Bholakant, R.; Qian, H.; Zhang, J.; Huang, X.; Huang, D.; Feijen, J.; Zhong, Y.; Chen, W. Recent Advances of Polycationic siRNA Vectors for Cancer Therapy. Biomacromolecules 2020, 21, 2966–2982. [Google Scholar] [CrossRef]
- Patwa, A.; Shah, A. Anatomy and physiology of respiratory system relevant to anaesthesia. Indian J. Anaesth. 2015, 59, 533–541. [Google Scholar] [CrossRef]
- Rasul, R.M.; Muniandy, M.T.; Zakaria, Z.; Shah, K.; Chee, C.F.; Dabbagh, A.; Rahman, N.A.; Wong, T.W. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr. Polym. 2020, 250, 116800. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 600–612. [Google Scholar] [CrossRef]
- Zacaron, T.M.; Silva, M.L.S.; Costa, M.P.; Silva, D.M.; Silva, A.C.; Apolônio, A.N.M.; Fabri, R.L.; Pittella, F.; Rocha, H.V.A.; Tavares, G.D. Advancements in chitosan-based nanoparticles for pulmonary drug delivery. Polymers 2023, 15, 3849. [Google Scholar] [CrossRef]
- Khatib, A.O.; El-Tanani, M.; Al-Obaidi, H. Inhaled medicines for targeting non-small cell lung cancer. Pharmaceutics 2023, 15, 2777. [Google Scholar] [CrossRef]
- Garcia, F.M. Nanomedicine and therapy of lung diseases. Einstein 2014, 12, 531–533. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Wang, L.; Yang, Q.; Zhang, Y.; Qinglai, T.; Yang, X.; Xiao, Z.; Lei, L.; Li, S. Pulmonary inhalation for disease treatment: Basic research and clinical translations. Mater. Today Bio 2024, 25, 100966. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Ferro, V. Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Nanoscale 2016, 8, 14341–14358. [Google Scholar] [CrossRef]
- Chaurasiya, B.; Zhao, Y.Y. Dry Powder for pulmonary delivery: A comprehensive review. Pharmaceutics 2021, 13, 31. [Google Scholar] [CrossRef]
- Aryal, S.; Park, S.; Park, H.; Park, C.; Kim, W.C.; Thakur, D.; Won, Y.-J.; Key, J. Clinical trials for oral, inhaled and intravenous drug delivery system for lung cancer and emerging nanomedicine-based approaches. Int. J. Nanomed. 2023, 18, 7865–7888. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Jaipuria, A.; Gupta, N. Inhalable formulations to treat non-small cell lung cancer (NSCLC): Recent therapies and developments. Pharmaceutics 2023, 15, 139. [Google Scholar] [CrossRef]
- Sardeli, C.; Zarogoulidis, P.; Kosmidis, C.; Amaniti, A.; Katsaounis, A.; Giannakidis, D.; Koulouris, C.; Hohenforst-Schmidt, W.; Huang, H.; Bai, C.; et al. Inhaled chemotherapy adverse effects: Mechanisms and protection methods. Lung Cancer Manag. 2019, 8, LMT19. [Google Scholar] [CrossRef]
- Yong, J.; Shu, H.; Zhang, X.; Yang, K.; Luo, G.; Yu, L.; Li, J.; Huang, H. Natural products-based inhaled formulations for treating pulmonary diseases. Int. J. Nanomed. 2024, 19, 1723–1748. [Google Scholar] [CrossRef] [PubMed]
- Costabile, G.; Conte, G.; Brusco, S.; Savadi, P.; Miro, A.; Quaglia, F.; D’angelo, I.; Ungaro, F. State-of-the-art review on inhalable lipid and polymer nanocarriers: Design and development perspectives. Pharmaceutics 2024, 16, 347. [Google Scholar] [CrossRef] [PubMed]
- Ruge, C.C.; Kirch, J.; Lehr, C.M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Shakil, S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, S.; Uddin, F.; Hasan, A. Using chitosan or chitosan derivatives in cancer therapy. Polysaccharides 2021, 2, 795–816. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Edison, T.N.J.; Oscar, F.L.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan nanopolymers: An overview of drug delivery against cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef]
- Sánchez, L.F.; Cánepa, J.; Kim, S.; Nakamatsu1, J. A simple approach to produce tailor-made chitosans with specific degrees of acetylation and molecular weights. Polymers 2021, 13, 2415. [Google Scholar] [CrossRef] [PubMed]
- Araby, A.E.; Janati, W.; Ullah, R.; Ercisli, S.; Errachidi, F. Chitosan, chitosan derivatives, and chitosan-based nanocomposites: Eco-friendly materials for advanced applications (a review). Front. Chem. 2023, 11, 1327426. [Google Scholar] [CrossRef]
- Melro, E.; Antunes, F.E.; Silva, G.J.; Cruz, I.; Ramos, P.E.; Carvalho, F.; Alves, L. Chitosan films in food applications. tuning film properties by changing acidic dissolution conditions. Polymers 2021, 13, 1. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Maestrelli, F.; Cirri, M.; Mennini, N. Multiple Roles of Chitosan in Mucosal Drug Delivery: An Updated Review. Mar. Drugs 2022, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Sakai, T.; Yamamoto, H.; Takeuchi, H.; Hirashima, N.; Kawashima, Y. Improved cellular uptake of chitosan-modified PLGA nanospheres by A549 cells. Int. J. Pharm. 2009, 382, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Marques, S.C.; Nunes, P.M.; Pedrosa, D.R.; Leandro, S.M. Antifungal and antioxidant properties of chitosan polymers obtained from nontraditional Polybius henslowii sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [PubMed]
- Satitsri, S.; Muanprasat, C. Chitin and chitosan derivatives as biomaterial resources for biological and biomedical applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Harugade, A.; Sherje, A.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, F.; Hu, F. Functional chitosan and its derivative-related drug delivery systems for nano-therapy: Recent advances. Pharmaceutics 2024, 16, 337. [Google Scholar] [CrossRef]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant properties and redox-modulating activity of chitosan and its derivatives: Biomaterials with application in cancer therapy. Biores. Open Access 2020, 9, 64–72. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.; Wang, C.; Ji, H.; Yu, J.; Liu, C.; Liu, A. A novel synthetic chitosan selenate (CS) induces apoptosis in A549 lung cancer cells via the Fas/FasL pathway. Int. J. Biol. Macromol. 2020, 158, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Yu, S.S.; Liu, C.; Liu, A.J. Seleno-Chitosan induces apoptosis of lung cancer cell line SPC-A-1 via Fas/FasL pathway. Bioorg. Chem. 2020, 97, 103701. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Chung, M.J.; Choi, H.N.; Park, Y. Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Shi, D. Cancer cell surface negative charges: A bio-physical manifestation of the warburg effect. Nano Life 2017, 7, 1771001. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Shiratani, M.; Kobayashi, H.; Ouchi, T. Release behavior of 5-fluorouracil from chitosan-gel nanospheres immobilizing 5-fluorouracil coated with polysaccharides and their cell specific cytotoxicity. J. Macromol. Sci. A 1994, 31, 629–642. [Google Scholar] [CrossRef]
- Desai, K.G. Chitosan nanoparticles prepared by ionotropic gelation: An overview of recent advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Uddin, S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Marante, T.; Viegas, C.; Duarte, I.; Macedo, A.S.; Fonte, P. An overview on spray-drying of protein-loaded polymeric nanoparticles for dry powder inhalation. Pharmaceutics 2020, 12, 1032. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xub, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, L.; Zhang, Z.; Chen, J.; Shi, D.D.; Yang, J.; Tang, Z. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng. 2011, 28, 353–362. [Google Scholar] [CrossRef]
- Khalid, M.E.U.; Rashid, A.A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent advances in nanocellulose-based different biomaterials: Types, properties, and emerging applications. J. Mater. Res. 2021, 14, 2601–2623. [Google Scholar] [CrossRef]
- Frenț, O.D.; Duteanu, N.; Teusdea, A.C.; Ciocan, S.; Vicaș, L.; Jurca, T.; Muresan, M.; Pallag, A.; Ianasi, P.; Marian, E. Preparation and characterization of chitosan-alginate microspheres loaded with quercetin. Polymers 2022, 14, 490. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Dawound, M. Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J. Drug Deliv. Sci. Technol. 2021, 62, 102409. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, W.; Zhu, J.; Zhu, J.; Shen, J.; Liu, Z.; Yang, Y.; Chen, Q. Nanoparticle-mediated delivery of inhaled immunotherapeutics for treating lung metastasis. Adv. Mater. 2021, 33, e2007557. [Google Scholar] [CrossRef] [PubMed]
- Raval, M.; Patel, P.; Airao, V.; Bhatt, V.; Sheth, N. Novel Silibinin loaded chitosan-coated PLGA/PCL nanoparticles-based inhalation formulations with improved cytotoxicity and bioavailability for lung cancer. BioNanoScience 2021, 11, 67–83. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Alhakamy, N.A.; Padder, R.; Husain, M.; Shadab, M. Preparation and characterization of chitosan coated PLGA nanoparticles of resveratrol: Improved stability, antioxidant and apoptotic activities in H1299 lung cancer cells. Coatings 2020, 10, 439. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A potential biopolymer in drug delivery and biomedical applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Liechty, W.B.; Peppas, N.A. Expert opinion: Responsive polymer nanoparticles in cancer therapy. Eur. J. Pharm. Biopharm. 2012, 80, 241–246. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.S.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; Reis, A.V.F.; Vieira-Neto, J.B.; Júnior, I.J.d.S.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles. Int. J. Pharm. 2021, 592, 120082. [Google Scholar] [CrossRef]
- Gote, V.; Nookala, A.R.; Bolla, P.K.; Pal, D. Drug Resistance in Metastatic Breast Cancer: Tumor Targeted Nanomedicine to the Rescue. Int. J. Mol. Sci. 2021, 22, 4673. [Google Scholar] [CrossRef]
- Tran, P.; Lee, S.E.; Kim, D.H.; Pyo, Y.C.; Park, J.S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J. Pharm. Investig. 2020, 50, 261–270. [Google Scholar] [CrossRef]
- Abbas, Y.; Azzazy, H.M.; Tammam, S.; Lamprecht, A.; Ali, M.E.; Schmidt, A.; Sollazzo, S.; Mathur, S. Development of an inhalable, stimuli-responsive particulate system for delivery to deep lung tissue. Colloids Surf. B Biointerfaces 2016, 146, 19–30. [Google Scholar] [CrossRef]
- Okuda, T.; Kito, D.; Oiwa, A.; Fukushima, M.; Hira, D.; Okamoto, H. Gene silencing in a mouse lung metastasis model by an inhalable dry small interfering RNA powder prepared using the supercritical carbon dioxide technique. Biol. Pharm. Bull. 2013, 36, 1183–1191. [Google Scholar] [CrossRef]
- Nielsen, E.J.B.; Nielsen, J.M.; Becker, D.; Karlas, A.; Prakash, H.; Glud, S.Z.; Merrison, J.; Besenbacher, F.; Meyer, T.F.; Kjems, J.; et al. Pulmonary gene silencing in transgenic EGFP mice using aerosolized chitosan/siRNA nanoparticles. Pharm. Res. 2010, 27, 2520–2527. [Google Scholar] [CrossRef]
- Howard, K.A.; Rahbek, U.L.; Liu, X.; Damgaard, C.K.; Glud, S.Z.; Andersen, M.; Hovgaard, M.B.; Schmitz, A.; Nyengaard, J.R.; Besenbacher, F.; et al. RNA Interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol. Ther. 2006, 14, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.Y.; Kankala, R.K.; Pan, Y.J.; Yuan, H.; Wang, S.B.; Chen, A.Z. Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int. J. Nanomed. 2018, 13, 4685–4698. [Google Scholar] [CrossRef]
- Storti, C.; Noci, V.L.; Sommariva, M.; Tagliabue, E.; Balsari, A.; Sfondrini, L. Aerosol delivery in the treatment of lung cancer. Curr. Cancer Drug Targets 2015, 15, 604–612. [Google Scholar] [CrossRef]
- Tatsumura, T.; Koyama, S.; Tsujimoto, M.; Kitagawa, M.; Kagamimori, S. Further study of nebulization chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: Fundamental and clinical. Br. J. Cancer 1993, 68, 1146–1149. [Google Scholar] [CrossRef]
- Gautam, A.; Koshkina, N. Paclitaxel (Taxol) and taxoid derivates for lung cancer treatment: Potential for aerosol delivery. Curr. Cancer Drug Targets 2003, 3, 287–296. [Google Scholar] [CrossRef]
- Shanmugam, T.; Joshi, N.; Kaviratna, A.; Ahamad, N.; Bhatia, E.; Banerjee, R. Aerosol delivery of paclitaxel-containing self-assembled nanocochleates for treating pulmonary metastasis: An approach supporting pulmonary mechanics. ACS Biomater. Sci. Eng. 2020, 7, 144–156. [Google Scholar] [CrossRef]
- Ma, P.; Mumper, R.J. Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J. Nanomed. Nanotechnol. 2013, 4, 1000164. [Google Scholar] [CrossRef] [PubMed]
- Najafiyan, B.; Hosseini, Z.B.; Esmaelian, S.; Firuzpour, F.; Anaraki, S.R.; Kalantari, L.; Hheidari, A.; Mesgari, H.; Nabi-Afjadi, M. Unveiling the potential effects of resveratrol in lung cancer treatment: Mechanisms and nanoparticle-based drug delivery strategies. Biomed. Pharmacother. 2024, 172, 116207. [Google Scholar] [CrossRef] [PubMed]
- Vuuren, R.J.V.; Botes, M.; Jurgens, T.; Joubert, A.M.; Bout, I.V. Novel sulphamoylated 2-methoxy estradiol derivatives inhibit breast cancer migration by disrupting microtubule turnover and organization. Cancer Cell Int. 2019, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lv, X.; Le, Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef]
- Durán, V.; Yasar, H.; Becker, J.; Thiyagarajan, D.; Loretz, B.; Kalinke, U.; Lehr, C.-M. Preferential uptake of chitosan-coated PLGA nanoparticles by primary human antigen presenting cells. Nanomedicine 2019, 21, 102073. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiao, K. Nanoparticles-based strategies to improve the delivery of therapeutic small interfering RNA in precision oncology. Pharmaceutics 2022, 14, 1586. [Google Scholar] [CrossRef]
- Dua, K.; Wadhwa, R.; Singhvi, G.; Rapalli, V.; Shukla, S.D.; Shastri, M.D.; Gupta, G.; Satija, S.; Mehta, M.; Khurana, N.; et al. The potential of siRNA based drug delivery in respiratory disorders: Recent advances and progress. Drug Dev. Res. 2019, 80, 714–730. [Google Scholar] [CrossRef]
- Chalbatani, G.M.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rad, M.R.; Marmari, V. Small interfering RNAs (siRNAs) in cancer therapy: A nano-based approach. Int. J. Nanomed. 2019, 14, 3111–3128. [Google Scholar] [CrossRef]
- Charbe, N.B.; Amnerkar, N.D.; Ramesh, B.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.A.; Khadse, S.C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small interfering RNA for cancer treatment: Overcoming hurdles in delivery. Acta Pharm. Sin. B 2020, 10, 2075–2109. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Y.; Sun, Q.; Li, B.; Yue, C.; Liu, Y.; de la Fuente, J.M.; Cui, D. Dual-targeted lung cancer therapy via inhalation delivery of UCNP-siRNA-AS1411 nanocages. Cancer Biol. Med. 2022, 19, 1047–1060. [Google Scholar] [CrossRef]
- Mehta, A.; Vedove, E.D.; Isert, L.; Merkel, O.M. Targeting KRAS mutant lung cancer cells with sirna-loaded bovine serum albumin nanoparticles. Pharm. Res. 2019, 36, 133. [Google Scholar] [CrossRef] [PubMed]
- Bäumer, N.; Tiemann, J.; Scheller, A.; Meyer, T.; Wittmann, L.; Ezequiel, M.; Greune, L.; Peipp, M.; Kellmann, N.; Gumnior, A.; et al. Targeted siRNA nanocarrier: A platform technology for cancer treatment. Oncogene 2022, 41, 2210–2224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ho, W.; Chu, J.; Xiong, X.; Hu, B.; Yiadom, K.O.; Xu, X.; Zhang, X.-Q. Inhalable siRNA nanoparticles for enhanced tumor-targeting treatment of kras-mutant non-small-cell lung cancer. ACS Appl. Mater. Interfaces 2023, 15, 31273–31284. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, J.; Fang, Q.; Du, Y.; Zhang, X. Gold nanoparticle delivery of glut1 sirna facilitates glucose starvation therapy in lung cancer. Chembiochem 2024, 25, e202400239. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wong, S.W.; Forgham, H.; Esser, L.; Lai, M.; Leiske, M.N.; Kempe, K.; Sharbeen, G.; Youkhana, J.; Mansfeld, F.; et al. Aerosol delivery of star polymer-siRNA nanoparticles as a therapeutic strategy to inhibit lung tumor growth. Biomaterials 2022, 285, 121539. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, V.; Vilwanathan, R. Silencing Sirtuin 6 induces cell cycle arrest and apoptosis in non-small cell lung cancer cell lines. Genomics 2020, 112, 3703–3712. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, I.H.; Choi, Y.K.; Lee, Y.K.; Moon, E.; Huh, Y.H.; Im, W.; Jin, J.; Kwak, M.; Lee, P.C. Suppression of Lung Cancer Malignancy by Micellized siRNA through Cell Cycle Arrest. Adv. Healthc. Mater. 2023, 12, e2202358. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, L.; Li, T.; Zhang, J.; Zhang, D.; Li, J.; Xia, Y.; Liu, Y.; Tan, W. Beyond Blocking: Engineering RNAi-Mediated Targeted Immune Checkpoint Nanoblocker Enables T-Cell-Independent Cancer Treatment. ACS Nano 2020, 14, 17524–17534. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Faraj, A.A. siRNA conjugated nanoparticles—A next generation strategy to treat lung cancer. Int. J. Mol. Sci. 2019, 20, 6088. [Google Scholar] [CrossRef]
- Sajid, M.I.; Moazzam, M.; Kato, S.; Cho, K.Y.; Tiwari, R.K. Overcoming barriers for siRNA therapeutics: From bench to bedside. Pharmaceuticals 2020, 13, 294. [Google Scholar] [CrossRef]
- Gupta, N.; Rai, D.B.; Jangid, A.K.; Pooja, D.; Kulhari, H. Nanomaterials-based siRNA delivery: Routes of administration, hurdles and role of nanocarriers. In Nanotechnology in Modern Animal Biotechnology; Springer: Singapore, 2019; pp. 67–114. [Google Scholar] [CrossRef]
- Li, N.; Sun, Y.; Fu, Y.; Sun, K. RNA Drug delivery using biogenic nanovehicles for cancer therapy. Front. Pharmacol. 2021, 12, 734443. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent advances in chitosan-based carriers for gene delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Park, T.; Singh, B.; Maharjan, S.; Choi, Y.; Choung, P.; Arote, R.B.; Cho, C.-S. Nanoparticle-mediated delivery of siRNA for effective lung cancer therapy. Nanomedicine 2015, 10, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Xia, J.; Wu, J. Functional chitosan nanoparticles in cancer treatment. J. Biomed. Nanotechnol. 2016, 12, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Akter, Z.; Modareszadeh, P.; Modareszadeh, P.; Berisha, E.; Alemi, P.S.; Castro, M.d.C.C.; Deese, A.R.; Zhang, L. Current landscape of therapeutic resistance in lung cancer and promising strategies to overcome resistance. Cancers 2022, 14, 4562. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting drug chemo-resistance in cancer using natural products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Zureigat, H.; Karaki, A.A.; Kadash, A.A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.A.; Awidi, A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef]
- Güliz, A.K. Nano-Delivery System for Inhaled Chemotherapy. Patent WO2022119528A1, 9 June 2022. [Google Scholar]
- Weifen, Z.; Kang, L.; Jinbão, T.; Zengjuan, Z. Quercetin and Paclitaxel Co-Transportation Pulmonary Inhaled Nanometer Targeted Porous Polymer Particle and Preparation Method Thereof. Patent CN106309411A, 26 June 2015. [Google Scholar]
- Kumar, A.; Mohapatra, T.S.S.; Cameron, D. Nanoparticle Targeted Drug Delivery to the Lungs Using Extra-Testicular Sertoli Cells. U.S. Patent US2019038574A1, 9 October 2018. [Google Scholar]
- Aizheng, C.; Shibin, W.; Hufan, S.; Yuangang, L.; Wenguo, W. Method for Preparing Micro Nano Porous Microspheres carrying Gene and Polypeptide Drugs through Supercritical Fluid Technology. Patent CN105963714A, 16 June 2016. [Google Scholar]
- Ascione, R.; Qi, S. Composition of Tumor-Associated Proliferative Peptides and Related Anti-Cancer Immunogen for the Treatment of Lung Cancers and Other Cancers. Patent CN111491660A, 21 August 2018. [Google Scholar]
- Zhibin, L.; Xuefeng, Y. Medicine-Carried Black Phosphorus Shell Glycan Composite Nanospheres and Preparation Method and Application Thereof. Patent CN110090307A, 4 June 2019. [Google Scholar]
- Mohapatra, S.S.; Xu, W.; Kong, X.; Wang, X.; Mohapatra, S.S. Methods and Compositions for Reducing Activity of the Atrial Natriuretic Peptide Receptor and for Treatment of Diseases. Patent CA2707444A1, 26 November 2008. [Google Scholar]
- Rocha, S.; Wu, L. Nanoparticles and Porous Particles and Methods of Making the Same. Patent WO2010057214A2, 20 May 2010. [Google Scholar]
- Raju, G.G.; Sudhakar, K.; Raju, R.R.; Raju, G.V.K.R.; Venkateswarlu, S.; Triurtulu, G.; Kiran, B.; Krishanu, S.; Raju, A.V.K. Anti-Cancer Drugs, and Uses Relating for Malignant Melanoma and Other Cancers. Patent CN102438449A, 26 June 2010. [Google Scholar]
- Gao, J.; Hsu, E.; Cheung, A. Dually Derivatized Chitosan Nanoparticles and Methods of Making and Using the Same. U.S. Patent US2015051265A1, 15 March 2013. [Google Scholar]
- Cheung, A.; Lora, J. Localized Expression of Therapeutic Nucleic Acids in Lung Epithelial Cells. U.S. Patent US2023210995A1, 22 January 2021. [Google Scholar]



| Drug(s) | Class | Mechanism of Action |
|---|---|---|
| Cisplatin Carboplatin | Alkylating agents | Promotes platinum covalent bonds with purine bases, resulting in damage to DNA replication and transcription due to inter- and intra-chain crosslinking [54,55]. |
| Docetaxel Paclitaxel | Taxanes | The alteration of the balance between the formation and degradation of tubulin results in the disruption of the dynamics of microtubules, which in turn affects the process of cell mitosis [56,57]. |
| Erlotinibe | Tyrosine kinase inhibitor | The irreversible inhibition of tyrosine kinase activity in the epidermal growth factor receptor (EGFR) results in impaired autophosphorylation of EGFR-associated tyrosine residues and impaired cell signaling and proliferation [58,59]. |
| Etoposide | Topoisomerase inhibitor | Inhibiting topoisomerase II results in the compromise of the transient breaks in the DNA molecule that occur during cell replication [60,61]. |
| Vincristine | Vinca alkaloids | The compound destabilizes microtubules, which impairs the formation of mitotic spindles. This, in turn, results in the interruption of the cell cycle in the G2/M phase [62] |
| Erlotinib Gefitinib Afatinib | EGFR-directed tyrosine kinase inhibitors (TKI) | Promotes selective and irreversible inhibition of the epidermal growth factor receptor (EGFR), resulting in inhibition of tumor cell growth and progression [63,64]. |
| Crizotinib Ceritinib Alectinib Lorlatinib | ALK-directed tyrosine kinase inhibitors | Selectively inhibits the ALK tyrosine kinase receptor and its variables, interfering with the survival and proliferation of cancer cells [63,65,66,67]. |
| Ciritinib Lorlatinib Entrectinib | ROS1-directed Therapy | Inhibits the tyrosine kinase ALK and ROS1, reducing the resistance mechanism associated with previous treatment with ALK inhibitor [63,65,68]. |
| Vemurafenib Dabrafenib | BRAF V600E | It selectively inhibits BRAF serine–threonine kinase, interfering with constitutive activation of the RAS/RAF/MEK/ERK signaling pathway and consequently suppressing cell differentiation and proliferation in BRAF V600 mutation-positive tumor cells [63,65,69]. |
| Tepotinib Capmatinib | MET inhibitors | Inhibits the binding of adenosine triphosphate (ATP) to the MET tyrosine kinase receptor, jeopardizing phosphorylation of MET and its downstream effects, thus inhibiting tumor proliferation and inducing apoptosis in MET-dependent tumor cell lines [63,65,70]. |
| Selpercatinib Pralsetinib | RET inhibitors | Selectively inhibits RET kinase small molecules via the ATP-competitive mechanism, compromising the activation of multiple downstream cell signaling pathways, including RAS/MAPK/ERK, PI3K/AKT, and JAK/STAT, and consequently reduces cell proliferation and differentiation [63,65,71]. |
| Sotorasib Adagrasib | KRAS G12C inhibitors | Selectively inhibits the KRAS G12C gene (tumor-restricted oncogenic mutant form of KRAS) by interacting with a surface groove of the histidine 95 next to the cysteine 12 switch II pocket, keeping GDP-inactive, compromising oncogenic pathways and uncontrolled cell growth [63,65,72]. |
| T-DXd | HER2-targeted Therapy | Inhibits HER2 overexpression, compromising various proliferative or apoptotic signaling pathways, including MAPK, PI3K/AKT, and JAK/STAT [63,65,73]. |
| Larotrectinib Entrectinib | NTRK inhibitors | Inhibits the kinase portion of the tropomyosin receptor (TRK), thereby impairing cell proliferation [63,65]. |
| Everolimus, Rapamycin Temsirolimus | Immunotherapy and mTOR inhibitors. | It inhibits the proliferation, migration, and survival of cancer cells by forming a complex with the FKBP12 protein, which inhibits the mTOR1 kinase [65,74,75]. |
| Methods | Main Characteristics | Advantages | Limitations | Refs. |
|---|---|---|---|---|
| Covalent crosslinking | Based on covalent crosslinking between the amino group of CS and the aldehyde group of a crosslinking agent, such as glutaraldehyde. | Obtaining nanoparticles of small size and narrow size distribution. | Glutaraldehyde can cause toxicity and compromise the integrity of the drug. | [140,148] |
| Ionic gelation | The ionic crosslinking occurs in the presence of the protonated amino groups of the CS and the negatively charged groups of the polyanion, such as sodium tripolyphosphate (TPP). | Simplicity, low cost, and potential scalability. Environmentally friendly preparation techniques. | Destabilization of the system when pH changes occur. The production of nanoparticles with a large size (100–400 nm) and a high degree of polydispersity. | [104,143,144,149,150] |
| Reverse micellar method (microemulsion) | Based on covalent crosslinking between the aqueous phase composed of CS and glutaraldehyde and an organic phase composed of a lipophilic surfactant (cetyltrimethylammonium bromide or sodium 1,4-bis-2-ethylhexylsulfosuccinate) and an organic solvent (n-hexane). | Obtaining nanoparticles with a size of less than 100 nm and with a narrow size distribution. | The utilization of the organic phase and glutaraldehyde has been limited to biomedical applications. The process is lengthy. | [140,150,151] |
| Coacervation | It results from the colloidal interaction between CS and oppositely charged macromolecules (e.g., sodium alginate) forming insoluble complexes due to solvent repulsion and the consequent separation of the phases. | Simplicity, no organic solvent, mild temperature conditions during processing. | Challenge of precisely controlling the size and size distribution of nanoparticles. | [140,152] |
| Spray-drying | CS is dissolved in aqueous acetic acid and subsequently mixed with the crosslinking agent. The nanoparticles are formed by passing through a stream of hot air (120 °C to 150 °C). | The procedure is straightforward and does not utilize toxic solvents. Furthermore, it does not necessitate additional separation and drying steps. | Obtaining nanoparticles with a large size. Unsuitable for thermosensitive drugs. Nanoparticle characteristics are highly influenced by operating parameters, including nozzle size, flow rate, and inlet and outlet temperatures. | [104,146,153] |
| Drug or siRNA | Justification | Carrier | Evaluation of Nanoparticles | Main Results | Ref. |
|---|---|---|---|---|---|
| Paclitaxel or quercetin | Increase drug retention in lung tissue and reduce resistance mechanisms. | Oleic acid-conjugated CS nanoparticles | Analysis of particle-size, zeta potential, and aerodynamic diameter of polymeric microspheres. In vivo pharmacokinetic study and tissue distribution (rats). | Physicochemical properties ideal for lung deposition; increased bioavailability and pulmonary retention of paclitaxel following inhalation administration. | [22] |
| Resveratrol | Improving therapeutic efficacy in the treatment of lung cancer. | Cationic nanocarrier of CS and lecithin | In vitro anticancer activity (A549 cell line). Drug uptake analysis using flow cytometry; Selectivity index. | Enhanced anticancer activity in lung cells; improved selectivity in human adenocarcinoma cells (A549). | [23] |
| Silibinin | Optimize lung tissue targeting and antitumor activity. | PLGA/PCL nanoparticles coated with CS | In vitro pulmonary deposition and anticancer activity assay (A549 cell line). In vivo Pharmacokinetic study (rats) | Increased bioavailability and enhanced cell inhibition. | [156] |
| 2-ME | Targeted delivery to the lung and improved clinical efficacy. | PLGA nanoparticles coated with CS nanoparticles | In vitro cytotoxicity and cellular uptake (SPC-A1 and A549 cell lines). In vivo lung deposition and histological examination (rats). | Optimized intracellular drug uptake; improved antitumor activity in A549 cells without promoting an inflammatory response; Promoted deep pulmonary deposition of 2-ME. | [24] |
| Drug or magnetic nanoparticle | Optimize targeted, non-invasive delivery to lung tissue. | CS nanoparticles | Evaluation of aerodynamic properties; In vitro cellular uptake (A549 cell line) and cell interaction (L929 and A549 cell lines); Determination of drug release profile from nanoparticles. | Physicochemical properties optimized targeted delivery to lung tissue with cellular uptake in A549 cell lines and sustained release profile. | [165] |
| siRNA-luciferase | Evaluate the biodistribution and gene silencing efficacy of siRNA through the carrier. | CS nanoparticles | In vivo biodistribution of siRNA following pulmonary delivery of the siRNA/chitosan and gene silencing. | Increased retention in lung tissue; effective and specific gene silencing. of metastatic tumor cells in mouse lungs. | [166] |
| siRNA-EGFP | Optimize lung distribution and gene silencing. | CS nanoparticles | Flow cytometric (H1299 cell line); In vivo Pulmonary deposition assay and RNA Interference (mice). | Delivery system demonstrated safe dosing profile and enhanced gene silencing. | [167] |
| siRNA-EGFP | Evaluate the efficiency of the siRNA delivery system. | CS nanoparticles | In vivo pulmonary RNA interference in the transgenic EGFP mouse assay. | Enhanced silencing of EGFP expression in vivo in the lower and upper regions of lung tissue. | [168] |
| a-PDL-1 | Enable efficient transmucosal delivery in the treatment of lung metastases. | CS nanoparticles | In vivo transmucosal absorption and permeability assay and in vivo immune responses induced by CS/aPD-L1 nanocomplex assay. | Increased absorption capacity and transmucosal penetration; activation of the immune system resulting in apoptosis of cancer cells. | [155] |
| Doxorubicin and siRNA-MRP-1 | Increase therapeutic efficacy in lung cancer to overcome multidrug resistance. | CS nanoparticles | In vitro anticancer study (H69AR cell line) | Enhanced antiproliferative effect of doxorubicin mediated by multidrug resistance gene silencing. | [169] |
| Patent Name | Patent Number | Country | Chitosan Function | Active Pharmaceutical Ingredient | Ref. |
|---|---|---|---|---|---|
| Nano-delivery system for inhaled chemotherapy. | WO2022119528A1 | Turkey | Attach the drug to the mucosa of the respiratory system. | Doxorubicin | [203] |
| Quercetin and paclitaxel co-transportation pulmonary inhaled nanometer targeted porous polymer particle and preparation method thereof. | CN106309411A | China | Providing bioadhesion to the pulmonary mucosa with a sustained release effect; safety by reducing the dosage of conventional chemotherapies and extending the scope of treatment. Provide simultaneous drug delivery. | Quercetin Paclitaxel | [204] |
| Nanoparticle targeted drug delivery to the lungs using to the extra-testicular Sertoli cells. | WO2009105278A2 | United States of America | Drug carrier | Curcumin | [205] |
| Method for preparing micro nano porous microspheres carrying gene and polypeptide drugs through supercritical fluid technology | CN105963714A | China | Increase the stability of encapsulated agents | siRNA-OD. Polypeptide GLP-1 (glucagon-like peptide-1). | [206] |
| Composition of tumor-associated proliferative peptides and related anticancer immunogen for the treatment of lung cancers and other cancers. | CN111491660A | China | Immunogen transporter linked to the mimetic peptide. | (A) one or more mimetic peptides, selected from the group consisting of sequences SEQ ID NO 1 to SEQ ID NO 40, consisting of an equal mixture of the following amino acid sequences: CYS-pro-pro-pro -SER-SER-GLN-PRO-LYS-ALA-LEU-GLY-ASN-GLN-GLN-PRO-SER-TRP-ASP-SER-GLU-ASP-SER-SER-ASN-PHE-LYS-ASP(ONKO-5a) (SEQ ID NO: 1) and Cys-pro-pro-pro-pro-SER-SER-TYR-PRO-ARG-GLY-ASN-HIS-TRP-ALA-VAL-GLY-HIS-LEU-MET-NH2 (SEQ ID NO: 9). | [207] |
| Medicine-carried black phosphorus shell glycan composite nanospheres and preparation method and application thereof. | CN110090307A | China | Chitosan provides the ability to adhere to the lung mucosa and antibacterial capacity. | Not specified | [208] |
| Methods and compositions for reducing activity of the atrial natriuretic peptide receptor and for treatment of diseases. | CA2707444A1 | Canada | Administer the polynucleotide complex intranasally or by nebulization. | siRNA-NPRA (natriuretic peptide receptor A) si-NPRC (natriuretic peptide receptor C) | [209] |
| Nanopaticles and porous particles anda methods of making the same. | WO2010057214A2 | United States of America | Improve the bioavailability of the drug and reduce the frequency of dosing. | Budesonide. Salbutamol sulfate. | [210] |
| Anticancer drugs, and uses relating for malignant melanoma and other cancers | CN102438449A | India | Increase the bioavailability of silephenol and silephenol triazene. | Selephenoll Selenophenol triazene. | [211] |
| Dually Derivatized Chitosan Nanoparticles and Methods of Making and Using the Same | US2015051265A1 | United States of America | Non-viral nucleic acid delivery. | Therapeutic RNA (antisense RNA, siRNA, short hairpin RNA, micro-RNA, and enzymatic RNA). | [212] |
| Localized expression of therapeutic nucleic acids in lung epithelial cells | US2023210995A1 | United States of America | Nucleic acid transport. | Therapeutic proteins and therapeutic RNA. | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.C.; Costa, M.P.; Zacaron, T.M.; Ferreira, K.C.B.; Braz, W.R.; Fabri, R.L.; Frézard, F.J.G.; Pittella, F.; Tavares, G.D. The Role of Inhaled Chitosan-Based Nanoparticles in Lung Cancer Therapy. Pharmaceutics 2024, 16, 969. https://doi.org/10.3390/pharmaceutics16080969
Silva AC, Costa MP, Zacaron TM, Ferreira KCB, Braz WR, Fabri RL, Frézard FJG, Pittella F, Tavares GD. The Role of Inhaled Chitosan-Based Nanoparticles in Lung Cancer Therapy. Pharmaceutics. 2024; 16(8):969. https://doi.org/10.3390/pharmaceutics16080969
Chicago/Turabian StyleSilva, Allana Carvalho, Mirsiane Pascoal Costa, Thiago Medeiros Zacaron, Kézia Cristine Barbosa Ferreira, Wilson Rodrigues Braz, Rodrigo Luiz Fabri, Frédéric Jean Georges Frézard, Frederico Pittella, and Guilherme Diniz Tavares. 2024. "The Role of Inhaled Chitosan-Based Nanoparticles in Lung Cancer Therapy" Pharmaceutics 16, no. 8: 969. https://doi.org/10.3390/pharmaceutics16080969
APA StyleSilva, A. C., Costa, M. P., Zacaron, T. M., Ferreira, K. C. B., Braz, W. R., Fabri, R. L., Frézard, F. J. G., Pittella, F., & Tavares, G. D. (2024). The Role of Inhaled Chitosan-Based Nanoparticles in Lung Cancer Therapy. Pharmaceutics, 16(8), 969. https://doi.org/10.3390/pharmaceutics16080969








