Cutting-Edge Biomaterials in Intervertebral Disc Degeneration Tissue Engineering
Abstract
:1. Introduction
2. Structure of IVD and Pathophysiology of IVDD
3. Mechanical Properties of IVD
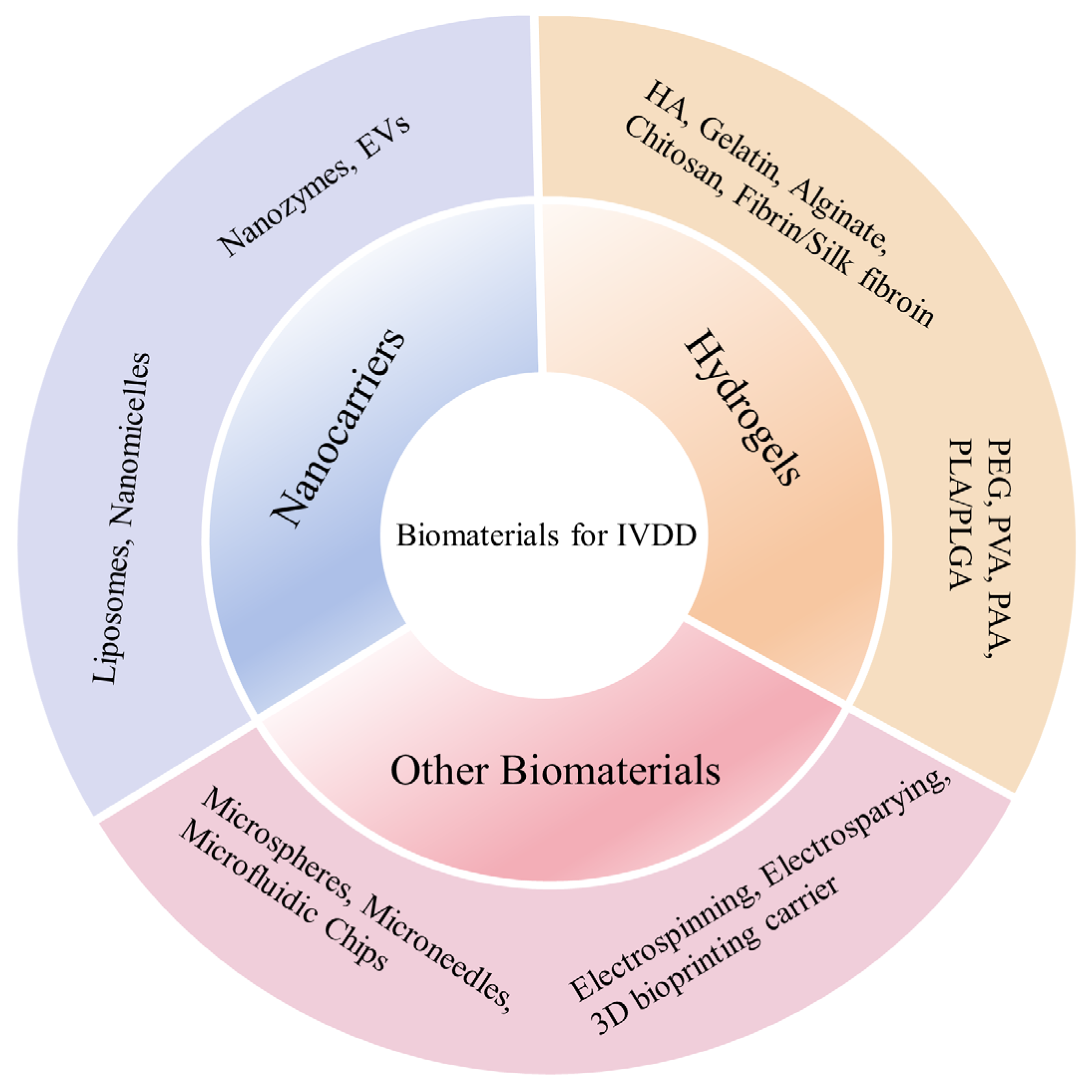
4. Biomaterials in IVD Tissue Engineering
5. Hydrogels
5.1. Natural Hydrogels
5.1.1. Hyaluronic Acid
5.1.2. Collagen and Gelatin
5.1.3. Alginate
5.1.4. Chitosan

5.1.5. Fibrin and Silk Fibroin
5.2. Synthetic Hydrogels
5.2.1. PEG
5.2.2. PVA
5.2.3. PAA
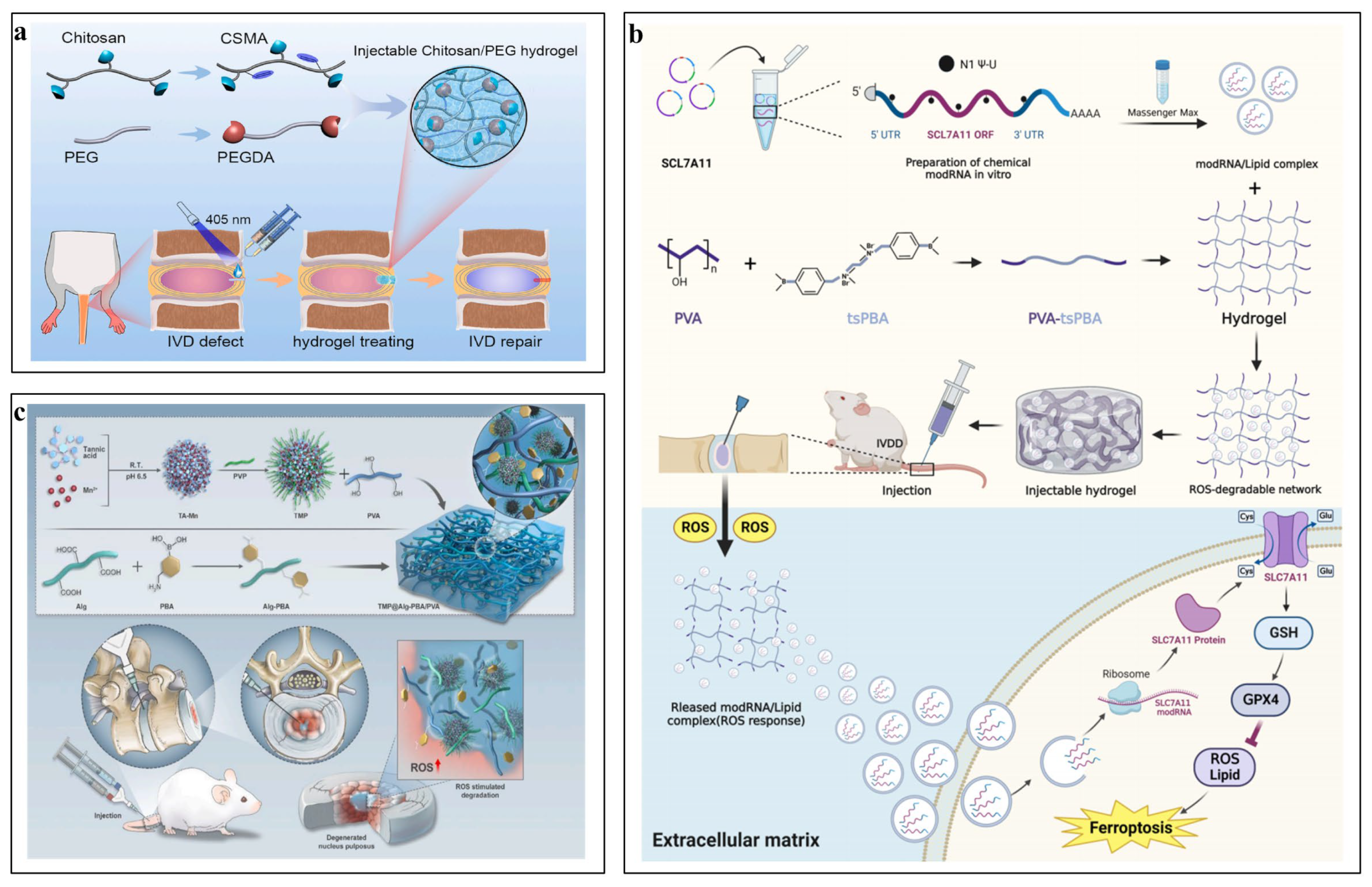
5.2.4. PLA/PLGA
6. Microspheres
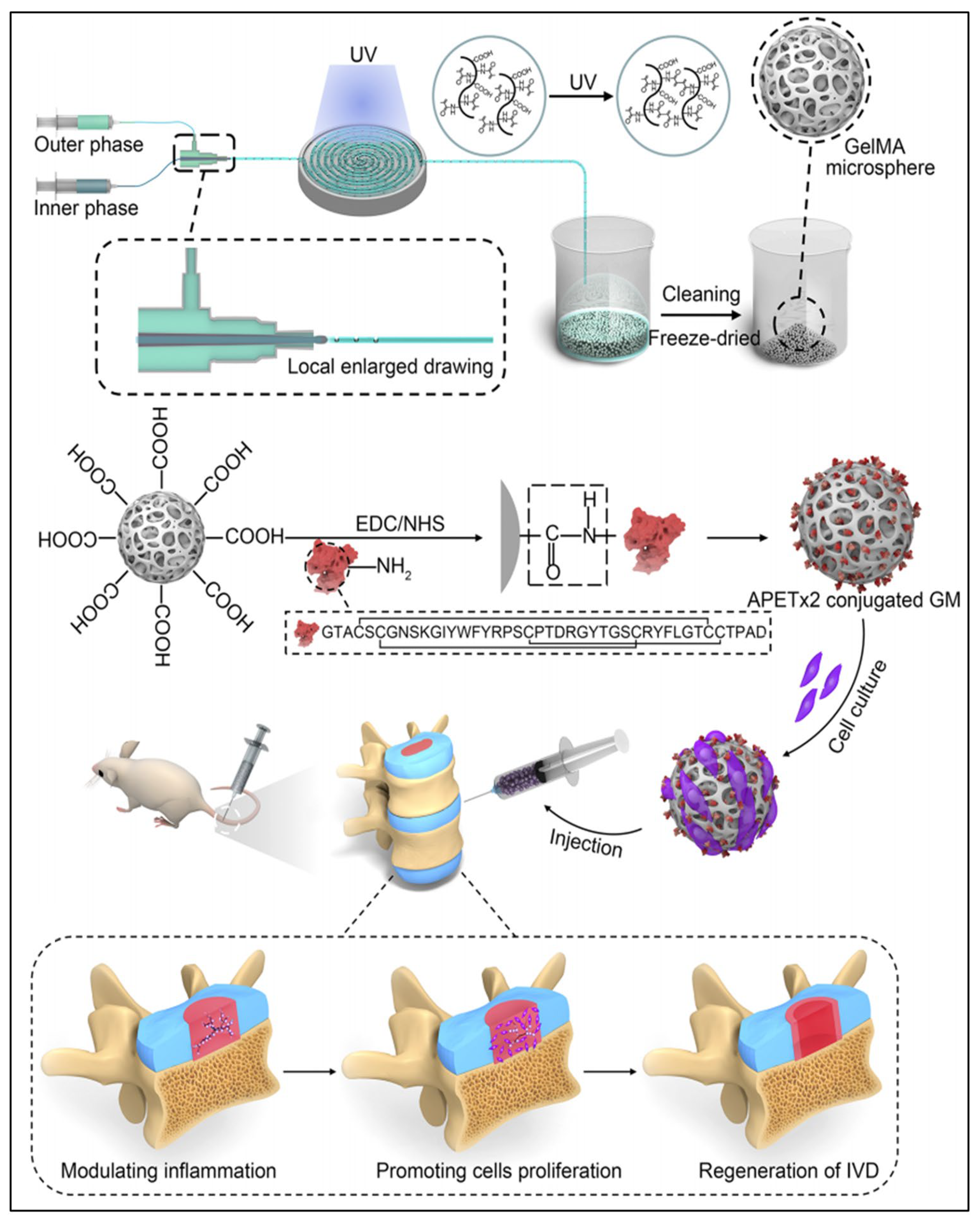
7. Microneedles

8. Microfluidic Chips
9. Electrospinning
10. Electrospraying
11. Three-Dimensional Bioprinting Carrier
12. Nanocarriers
12.1. Liposomes
12.2. Nanomicelles
12.3. Nanozymes
12.4. Extracellular Vesicles

13. Others
14. Conclusions
15. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, K.R. Management of Chronic Low Back Pain. JAMA Intern. Med. 2022, 182, 222–223. [Google Scholar] [CrossRef]
- Meziat-Filho, N.; Fernandez, J.; Castro, J. Cognitive functional therapy for chronic disabling low back pain. Lancet 2023, 401, 1828–1829. [Google Scholar] [CrossRef] [PubMed]
- Traeger, A.C.; Underwood, M.; Ivers, R.; Buchbinder, R. Low back pain in people aged 60 years and over. BMJ 2022, 376, e066928. [Google Scholar] [CrossRef]
- Kadow, T.; Sowa, G.; Vo, N.; Kang, J.D. Molecular basis of intervertebral disc degeneration and herniations: What are the important translational questions? Clin. Orthop. Relat. Res. 2015, 473, 1903–1912. [Google Scholar] [CrossRef]
- Yang, S.; Jing, S.; Wang, S.; Jia, F. From drugs to biomaterials: A review of emerging therapeutic strategies for intervertebral disc inflammation. Front. Cell Infect. Microbiol. 2024, 14, 1303645. [Google Scholar] [CrossRef]
- Abel, F.; Tan, E.T.; Chazen, J.L.; Lebl, D.R.; Sneag, D.B. MRI after Lumbar Spine Decompression and Fusion Surgery: Technical Considerations, Expected Findings, and Complications. Radiology 2023, 308, e222732. [Google Scholar] [CrossRef]
- Ulrich, N.H.; Burgstaller, J.M.; Valeri, F.; Pichierri, G.; Betz, M.; Fekete, T.F.; Wertli, M.M.; Porchet, F.; Steurer, J.; Farshad, M.G. Lumbar Stenosis Outcome Study, Incidence of Revision Surgery after Decompression with vs without Fusion among Patients with Degenerative Lumbar Spinal Stenosis. JAMA Netw. Open 2022, 5, e2223803. [Google Scholar] [CrossRef]
- Genedy, H.H.; Humbert, P.; Laoulaou, B.; Le Moal, B.; Fusellier, M.; Passirani, C.; Le Visage, C.; Guicheux, J.; Lepeltier, E.; Clouet, J. MicroRNA-targeting nanomedicines for the treatment of intervertebral disc degeneration. Adv. Drug Deliv. Rev. 2024, 207, 115214. [Google Scholar] [CrossRef]
- Liu, W.; Ma, Z.; Wang, Y.; Yang, J. Multiple nano-drug delivery systems for intervertebral disc degeneration: Current status and future perspectives. Bioact. Mater. 2023, 23, 274–299. [Google Scholar] [CrossRef]
- Xiang, H.; Zhao, W.; Jiang, K.; He, J.; Chen, L.; Cui, W.; Li, Y. Progress in regulating inflammatory biomaterials for intervertebral disc regeneration. Bioact. Mater. 2024, 33, 506–531. [Google Scholar] [CrossRef]
- Hashmi, S.S.; Seifert, K.D.; Massoud, T.F. Thoracic and Lumbosacral Spine Anatomy. Neuroimaging Clin. N. Am. 2022, 32, 889–902. [Google Scholar] [CrossRef]
- Isa, I.L.M.; Teoh, S.L.; Nor, N.H.M.; Mokhtar, S.A. Discogenic Low Back Pain: Anatomy, Pathophysiology and Treatments of Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022, 24, 208. [Google Scholar] [CrossRef]
- Luo, Z.; Wei, Z.; Zhang, G.; Chen, H.; Li, L.; Kang, X. Achilles’ Heel-The Significance of Maintaining Microenvironmental Homeostasis in the Nucleus Pulposus for Intervertebral Discs. Int. J. Mol. Sci. 2023, 24, 16592. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lyu, M.; Lu, Q.; Cheung, K.; Leung, V. Current Perspectives on Nucleus Pulposus Fibrosis in Disc Degeneration and Repair. Int. J. Mol. Sci. 2022, 23, 6612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Chen, N.; Yan, H.; Wu, K.; Li, J.; Ru, Q.; Deng, R.; Liu, X.; Kang, R. Mechanical Factors Regulate Annulus Fibrosus (AF) Injury Repair and Remodeling: A Review. ACS Biomater. Sci. Eng. 2024, 10, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, H.; Zheng, Z.; Wu, D. Design principles in mechanically adaptable biomaterials for repairing annulus fibrosus rupture: A review. Bioact. Mater. 2024, 31, 422–439. [Google Scholar] [CrossRef]
- Crump, K.B.; Alminnawi, A.; Bermudez-Lekerika, P.; Compte, R.; Gualdi, F.; McSweeney, T.; Munoz-Moya, E.; Nuesch, A.; Geris, L.; Dudli, S.; et al. Cartilaginous endplates: A comprehensive review on a neglected structure in intervertebral disc research. JOR Spine 2023, 6, e1294. [Google Scholar] [CrossRef]
- Samanta, A.; Lufkin, T.; Kraus, P. Intervertebral disc degeneration-Current therapeutic options and challenges. Front. Public Health 2023, 11, 1156749. [Google Scholar] [CrossRef]
- Xu, J.; Shao, T.; Lou, J.; Zhang, J.; Xia, C. Aging; cell senescence, the pathogenesis and targeted therapies of intervertebral disc degeneration. Front. Pharmacol. 2023, 14, 1172920. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; Wang, T.; Zhang, K.; Zhang, Y.; Kang, X. Oxidative stress in intervertebral disc degeneration: Molecular mechanisms, pathogenesis and treatment. Cell Prolif. 2023, 56, e13448. [Google Scholar] [CrossRef]
- Wen, P.; Zheng, B.; Zhang, B.; Ma, T.; Hao, L.; Zhang, Y. The role of ageing and oxidative stress in intervertebral disc degeneration. Front. Mol. Biosci. 2022, 9, 1052878. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, X.; Han, S.; Wei, L.; Zheng, Z.; Wang, Y.; Xin, J.; Zhang, S. Pathogenesis and therapeutic implications of matrix metalloproteinases in intervertebral disc degeneration: A comprehensive review. Biochimie 2023, 214, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.; Lively, S.; Seguin, C.A.; Perruccio, A.V.; Kapoor, M.; Rampersaud, R. Intervertebral disc degeneration and osteoarthritis: A common molecular disease spectrum. Nat. Rev. Rheumatol. 2023, 19, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhao, Y.; Dong, H.; Mao, Q.; Zhu, L.; Xia, J.; Weng, Z.; Liao, W.; Hu, Z.; Yi, J.; et al. Progress in the study of molecular mechanisms of intervertebral disc degeneration. Biomed. Pharmacother. 2024, 174, 116593. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, H.; Xiong, Z.; Li, C.; Guan, J.; Liu, T.; Yang, Y.; Yu, X. Evaluation of the Efficacy of Stem Cell Therapy in Animal Models of Intervertebral Disc Degeneration Based on Imaging Indicators: A Systematic Review and Meta-Analysis. Stem Cells Int. 2022, 2022, 2482653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.S.; Xu, A.; Ansari, K.; Hardacker, K.; Anderson, G.; Alsoof, D.; Daniels, A.H. Lumbar Disc Herniation: Diagnosis and Management. Am. J. Med. 2023, 136, 645–651. [Google Scholar] [CrossRef]
- Oxland, T.R. Fundamental biomechanics of the spine—What we have learned in the past 25 years and future directions. J. Biomech. 2016, 49, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Feng, M.; Liu, W.; Yu, J.; Wei, X.; Yang, K.; Zhan, J.; Peng, W.; Luo, M.; Han, T.; et al. From Mechanobiology to Mechanical Repair Strategies: A Bibliometric Analysis of Biomechanical Studies of Intervertebral Discs. J. Pain Res. 2022, 15, 2105–2122. [Google Scholar] [CrossRef]
- Cyril, D.; Giugni, A.; Bangar, S.S.; Mirzaeipoueinak, M.; Shrivastav, D.; Sharabi, M.; Tipper, J.L.; Tavakoli, J. Elastic Fibers in the Intervertebral Disc: From Form to Function and toward Regeneration. Int. J. Mol. Sci. 2022, 23, 8931. [Google Scholar] [CrossRef]
- Galbusera, F.; van Rijsbergen, M.; Ito, K.; Huyghe, J.M.; Brayda-Bruno, M.; Wilke, H.J. Ageing and degenerative changes of the intervertebral disc and their impact on spinal flexibility. Eur. Spine J. 2014, 23 (Suppl. 3), S324–S332. [Google Scholar] [CrossRef]
- Newell, N.; Little, J.P.; Christou, A.; Adams, M.A.; Adam, C.J.; Masouros, S.D. Biomechanics of the human intervertebral disc: A review of testing techniques and results. J. Mech. Behav. Biomed. Mater. 2017, 69, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Tendulkar, G.; Chen, T.; Ehnert, S.; Kaps, H.P.; Nussler, A.K. Intervertebral Disc Nucleus Repair: Hype or Hope? Int. J. Mol. Sci. 2019, 20, 3622. [Google Scholar] [CrossRef] [PubMed]
- Harmon, M.D.; Ramos, D.M.; Nithyadevi, D.; Bordett, R.; Rudraiah, S.; Nukavarapu, S.P.; Moss, I.L.; Kumbar, S.G. Growing a backbone—Functional biomaterials and structures for intervertebral disc (IVD) repair and regeneration: Challenges, innovations, and future directions. Biomater. Sci. 2020, 8, 1216–1239. [Google Scholar] [CrossRef]
- Schmitz, T.C.; Salzer, E.; Crispim, J.F.; Fabra, G.T.; LeVisage, C.; Pandit, A.; Tryfonidou, M.; Maitre, C.L.; Ito, K. Characterization of biomaterials intended for use in the nucleus pulposus of degenerated intervertebral discs. Acta Biomater. 2020, 114, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Z.; Guo, C.; Huang, Z.; Zhang, W.; Ma, F.; Wang, Z.; Kong, Q.; Wang, Y. Application and development of hydrogel biomaterials for the treatment of intervertebral disc degeneration: A literature review. Front. Cell Dev. Biol. 2023, 11, 1286223. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Guo, C.; Wu, Y.; Liu, Y.; Kong, Q.; Wang, Y. Therapeutic factors and biomaterial-based delivery tools for degenerative intervertebral disc repair. Front. Cell Dev. Biol. 2024, 12, 1286222. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.U.; Srinivasan, S.S.; Kumbar, S.G.; Moss, I.L. Hydrogel-Based Strategies for Intervertebral Disc Regeneration: Advances, Challenges and Clinical Prospects. Gels 2024, 10, 62. [Google Scholar] [CrossRef]
- Brissenden, A.J.; Amsden, B.G. In situ forming macroporous biohybrid hydrogel for nucleus pulposus cell delivery. Acta Biomater. 2023, 170, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xia, P.; Meng, F.; Li, X.; Xu, X. Bio-Functional Hydrogel Microspheres for Musculoskeletal Regeneration. Adv. Funct. Mater. 2024, 2024, 2400257. [Google Scholar] [CrossRef]
- Gao, X.D.; Zhang, X.B.; Zhang, R.H.; Yu, D.C.; Chen, X.Y.; Hu, Y.C.; Chen, L.; Zhou, H.Y. Aggressive strategies for regenerating intervertebral discs: Stimulus-responsive composite hydrogels from single to multiscale delivery systems. J. Mater. Chem. B 2022, 10, 5696–5722. [Google Scholar] [CrossRef]
- Choi, S.W.; Guan, W.; Chung, K. Basic principles of hydrogel-based tissue transformation technologies and their applications. Cell 2021, 184, 4115–4136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, H.; Huang, Y.; Wei, Y.; Chen, J. Naturally sourced hydrogels: Emerging fundamental materials for next-generation healthcare sensing. Chem. Soc. Rev. 2023, 52, 2992–3034. [Google Scholar] [CrossRef]
- Yan, C.; Wang, X.; Xiang, C.; Wang, Y.; Pu, C.; Chen, L.; Jiang, K.; Li, Y. Applications of Functionalized Hydrogels in the Regeneration of the Intervertebral Disc. Biomed. Res. Int. 2021, 2021, 2818624. [Google Scholar] [CrossRef]
- Lv, B.; Lu, L.; Hu, L.; Cheng, P.; Hu, Y.; Xie, X.; Dai, G.; Mi, B.; Liu, X.; Liu, G. Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment. Theranostics 2023, 13, 2015–2039. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Graca, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid-Based wound dressings: A review. Polym. C 2020, 241, 116364. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Qin, J.; Gong, J.S.; Ye, Y.H.; Qian, J.Y.; Li, H.; Xu, Z.H.; Shi, J.S. Versatile strategies for bioproduction of hyaluronic acid driven by synthetic biology. Carbohydr. Polym. 2021, 264, 118015. [Google Scholar] [CrossRef]
- Yang, R.; Wang, B.; Zhang, X.; Sun, Y.; Zhang, Y.; Xu, Z.; Yang, Q.; Liu, W. A Nucleobase-Driven Self-Gelled Hyaluronic Acid-Based Injectable Adhesive Hydrogel Enhances Intervertebral Disc Repair. Adv. Funct. Mater. 2024, 2024, 2401232. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, H.; Zhu, Y.; Zhao, C.; Wang, S.; Zheng, Y.; Xie, Z.; Jin, Y.; Song, H.; Yang, L.; et al. Injectable self-healing hydrogel with siRNA delivery property for sustained STING silencing and enhanced therapy of intervertebral disc degeneration. Bioact. Mater. 2022, 9, 29–43. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Oosterlaken, B.M.; Vena, M.P.; de With, G. In Vitro Mineralization of Collagen. Adv. Mater. 2021, 33, e2004418. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomas, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Chawla, D.; Kaur, T.; Joshi, A.; Singh, N. 3D bioprinted alginate-gelatin based scaffolds for soft tissue engineering. Int. J. Biol. Macromol. 2020, 144, 560–567. [Google Scholar] [CrossRef]
- Levato, R.; Jungst, T.; Scheuring, R.G.; Blunk, T.; Groll, J.; Malda, J. From Shape to Function: The Next Step in Bioprinting. Adv. Mater. 2020, 32, e1906423. [Google Scholar] [CrossRef] [PubMed]
- Buie, T.; McCune, J.; Cosgriff-Hernandez, E. Gelatin Matrices for Growth Factor Sequestration. Trends Biotechnol. 2020, 38, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, Y.; Shen, L.; Pan, G.; Yang, H.; Jiang, Z.; Zhu, X.; He, F. Kartogenin-enhanced dynamic hydrogel ameliorates intervertebral disc degeneration via restoration of local redox homeostasis. J. Orthop. Translat. 2023, 42, 15–30. [Google Scholar] [CrossRef]
- Luo, J.; Darai, A.; Pongkulapa, T.; Conley, B.; Yang, L.; Han, I.; Lee, K.B. Injectable bioorthogonal hydrogel (BIOGEL) accelerates tissue regeneration in degenerated intervertebral discs. Bioact. Mater. 2023, 23, 551–562. [Google Scholar] [CrossRef]
- Vasudevan, U.M.; Lee, O.K.; Lee, E.Y. Alginate derived functional oligosaccharides: Recent developments, barriers, and future outlooks. Carbohydr. Polym. 2021, 267, 118158. [Google Scholar] [CrossRef]
- Murab, S.; Gupta, A.; Wlodarczyk-Biegun, M.K.; Kumar, A.; van Rijn, P.; Whitlock, P.; Han, S.S.; Agrawal, G. Alginate based hydrogel inks for 3D bioprinting of engineered orthopedic tissues. Carbohydr. Polym. 2022, 296, 119964. [Google Scholar] [CrossRef]
- Xu, M.; Qin, M.; Cheng, Y.; Niu, X.; Kong, J.; Zhang, X.; Huang, D.; Wang, H. Alginate microgels as delivery vehicles for cell-based therapies in tissue engineering and regenerative medicine. Carbohydr. Polym. 2021, 266, 118128. [Google Scholar] [CrossRef]
- Yan, K.; Wan, Y.; Xu, F.; Lu, J.; Yang, C.; Li, X.; Lu, Z.; Wang, X.; Wang, D. Ionic crosslinking of alginate/carboxymethyl chitosan fluorescent hydrogel for bacterial detection and sterilization. Carbohydr. Polym. 2023, 302, 120427. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Li, H.; Tan, C.; Ma, S.; Gong, J.; Dong, L.; Huang, W.; Li, X.; Deng, H. Nanofiber reinforced alginate hydrogel for leak-proof delivery and higher stress loading in nucleus pulposus. Carbohydr. Polym. 2023, 299, 120193. [Google Scholar] [CrossRef]
- Wu, R.; Huang, L.; Xia, Q.; Liu, Z.; Huang, Y.; Jiang, Y.; Wang, J.; Ding, H.; Zhu, C.; Song, Y.; et al. Injectable mesoporous bioactive glass/sodium alginate hydrogel loaded with melatonin for intervertebral disc regeneration. Mater. Today Bio 2023, 22, 100731. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Amiri, H.; Basri, S.M.M.; Rastegari, H.; Lam, S.S.; Pan, J.; Gupta, V.K.; Tabatabaei, M. Tuning chitosan’s chemical structure for enhanced biological functions. Trends Biotechnol. 2023, 41, 785–797. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Ma, J.; Zhong, L.; Peng, X.; Xu, Y.; Sun, R. Functional Chitosan-based Materials for Biological Applications. Curr. Med. Chem. 2020, 27, 4660–4672. [Google Scholar] [CrossRef]
- Du, Y.; Li, J.; Tang, X.; Liu, Y.; Bian, G.; Shi, J.; Zhang, Y.; Zhao, B.; Zhao, H.; Sui, K.; et al. The Thermosensitive Injectable Celecoxib-Loaded Chitosan Hydrogel for Repairing Postoperative Intervertebral Disc Defect. Front. Bioeng. Biotechnol. 2022, 10, 876157. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Liu, C.; Zheng, Q.; Chu, G.; Wang, H.; Jin, J.; Wu, H.; Chen, J.; Huang, Q.; Deng, Z.; et al. Exosome-laden injectable self-healing hydrogel based on quaternized chitosan and oxidized starch attenuates disc degeneration by suppressing nucleus pulposus senescence. Int. J. Biol. Macromol. 2023, 232, 123479. [Google Scholar] [CrossRef]
- Heher, P.; Muhleder, S.; Mittermayr, R.; Redl, H.; Slezak, P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 134–147. [Google Scholar] [CrossRef]
- Colombini, A.; Ceriani, C.; Banfi, G.; Brayda-Bruno, M.; Moretti, M. Fibrin in intervertebral disc tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 713–721. [Google Scholar] [CrossRef]
- Panebianco, C.J.; Rao, S.; Hom, W.W.; Meyers, J.H.; Lim, T.Y.; Laudier, D.M.; Hecht, A.C.; Weir, M.D.; Weiser, J.R.; Iatridis, J.C. Genipin-crosslinked fibrin seeded with oxidized alginate microbeads as a novel composite biomaterial strategy for intervertebral disc cell therapy. Biomaterials 2022, 287, 121641. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef]
- Williams, C.K. Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 2007, 36, 1573–1580. [Google Scholar] [CrossRef]
- Agostinacchio, F.; Fitzpatrick, V.; Dire, S.; Kaplan, D.L.; Motta, A. Silk fibroin-based inks for in situ 3D printing using a double crosslinking process. Bioact. Mater. 2024, 35, 122–134. [Google Scholar] [CrossRef]
- Lin, M.; Hu, Y.; An, H.; Guo, T.; Gao, Y.; Peng, K.; Zhao, M.; Zhang, X.; Zhou, H. Silk fibroin-based biomaterials for disc tissue engineering. Biomater. Sci. 2023, 11, 749–776. [Google Scholar] [CrossRef]
- Bhunia, B.K.; Mandal, B.B. Exploring Gelation and Physicochemical Behavior of In Situ Bioresponsive Silk Hydrogels for Disc Degeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 870–886. [Google Scholar] [CrossRef]
- Shi, J.; Yu, L.; Ding, J. PEG-based thermosensitive and biodegradable hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef]
- Bu, Y.; Zhang, L.; Sun, G.; Sun, F.; Liu, J.; Yang, F.; Tang, P.; Wu, D. Tetra-PEG Based Hydrogel Sealants for In Vivo Visceral Hemostasis. Adv. Mater. 2019, 31, e1901580. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Sun, F.; Dou, X.; Liu, J.; Wang, Y.; Li, M.; Gao, J.; Liu, X.; Wang, X.; et al. A Super Tough, Rapidly Biodegradable, Ultrafast Hemostatic Bioglue. Adv. Mater. 2023, 35, e2208622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Xue, Y.; Cheng, J.; Chi, P.; Wang, Z.; Li, B.; Yan, T.; Wu, B.; Wang, Z. Enhanced Hemostatic and Procoagulant Efficacy of PEG/ZnO Hydrogels: A Novel Approach in Traumatic Hemorrhage Management. Gels 2024, 10, 88. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene glycol (PEG): The nature; immunogenicity, and role in the hypersensitivity of PEGylated products. J. Control. Release 2022, 351, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties; formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Huang, L.; Wang, W.; Xian, Y.; Liu, L.; Fan, J.; Liu, H.; Zheng, Z.; Wu, D. Rapidly in situ forming an injectable Chitosan/PEG hydrogel for intervertebral disc repair. Mater. Today Bio 2023, 22, 100752. [Google Scholar] [CrossRef]
- Riahinezhad, H.; Amsden, B.G. In situ forming, mechanically resilient hydrogels prepared from 4a-[PEG-b-PTMC-Ac] and thiolated chondroitin sulfate for nucleus pulposus cell delivery. J. Mater. Chem. B 2024, 12, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Tabesh, F.; Hussain, C.M. A new trend of using poly(vinyl alcohol) in 3D and 4D printing technologies: Process and applications. Adv. Colloid Interface Sci. 2022, 301, 102605. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Mahmoudpour, M.; Rahimpour, E.; Jouyban, A. Nanomaterial based PVA nanocomposite hydrogels for biomedical sensing: Advances toward designing the ideal flexible/wearable nanoprobes. Adv. Colloid Interface Sci. 2022, 305, 102705. [Google Scholar] [CrossRef]
- Gao, T.; Xu, G.; Ma, T.; Lu, X.; Chen, K.; Luo, H.; Chen, G.; Song, J.; Ma, X.; Fu, W.; et al. ROS-Responsive Injectable Hydrogel Loaded with SLC7A11-modRNA Inhibits Ferroptosis and Mitigates Intervertebral Disc Degeneration in Rats. Adv. Healthc. Mater. 2024, e2401103. [Google Scholar] [CrossRef]
- Zhou, H.; He, J.; Liu, R.; Cheng, J.; Yuan, Y.; Mao, W.; Zhou, J.; He, H.; Liu, Q.; Tan, W.; et al. Microenvironment-responsive metal-phenolic network release platform with ROS scavenging, anti-pyroptosis, and ECM regeneration for intervertebral disc degeneration. Bioact. Mater. 2024, 37, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zeng, Z.; Li, W. Poly(acrylic acid)-Assisted Intrafibrillar Mineralization of Type I Collagen: A Review. Macromol. Rapid Commun. 2023, 44, e2200827. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.S.; Yildirim, N.; Misir, M.; Misirlioglu, Y.; Celik, E. Synthesis, Characterization of a New Polyacrylic Acid Superabsorbent, Some Heavy Metal Ion Sorption, the Adsorption Isotherms, and Quantum Chemical Investigation. Materials 2020, 13, 4390. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Z.; Zhang, S.; Li, H. Assembled collagen films modified using polyacrylic acid with improved mechanical properties via mineralization. J. Mater. Chem. B 2024, 12, 6643–6653. [Google Scholar] [CrossRef] [PubMed]
- Gaytan, I.; Burelo, M.; Loza-Tavera, H. Current status on the biodegradability of acrylic polymers: Microorganisms, enzymes and metabolic pathways involved. Appl. Microbiol. Biotechnol. 2021, 105, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Chauhan, N.P.S.; Jadoun, S.; Soltani, M.D.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef] [PubMed]
- Prudnikova, K.; Vidal, S.E.L.; Sarkar, S.; Yu, T.; Yucha, R.W.; Ganesh, N.; Penn, L.S.; Han, L.; Schauer, C.L.; Vresilovic, E.J.; et al. Aggrecan-like biomimetic proteoglycans (BPGs) composed of natural chondroitin sulfate bristles grafted onto a poly(acrylic acid) core for molecular engineering of the extracellular matrix. Acta Biomater. 2018, 75, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Kunduru, K.R.; Doppalapudi, S.; Domb, A.J.; Khan, W. Poly(lactic acid) based hydrogels. Adv. Drug Deliv. Rev. 2016, 107, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H. Poly(lactic acid) stereocomplexes: A decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Poly(ethylene glycol)-poly(lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications. J. Control. Release 2013, 172, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels for drug delivery. J. Control. Release 2016, 240, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Xia, Q.; Wu, L.; Chen, F.; Li, L.; Zhu, C.; He, M.; Jiang, Y.; Huang, Y.; et al. Treatment outcomes of injectable thermosensitive hydrogel containing bevacizumab in intervertebral disc degeneration. Front. Bioeng. Biotechnol. 2022, 10, 976706. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.L.; Jacobsen, T.D.; Emsbo, E.; Murali, A.; Anton, K.; Liu, J.Z.; Lu, H.H.; Chahine, N.O. Three-Dimensional-Printed Flexible Scaffolds Have Tunable Biomimetic Mechanical Properties for Intervertebral Disc Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 5836–5849. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chang, B.; Dong, H.; Liu, X. Functional microspheres for tissue regeneration. Bioact. Mater. 2023, 25, 485–499. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Wang, D.; Zhang, J.; Yi, K.; Su, Y.; Luo, J.; Deng, X.; Deng, F. Fabrication of polymeric microspheres for biomedical applications. Mater. Horiz. 2024, 11, 2820–2855. [Google Scholar] [CrossRef]
- Gupta, V.; Khan, Y.; Berkland, C.J.; Laurencin, C.T.; Detamore, M.S. Microsphere-Based Scaffolds in Regenerative Engineering. Annu. Rev. Biomed. Eng. 2017, 19, 135–161. [Google Scholar] [CrossRef]
- He, Q.; Zhang, J.; Liao, Y.; Alakpa, E.V.; Bunpetch, V.; Zhang, J.; Ouyang, H. Current advances in microsphere based cell culture and tissue engineering. Biotechnol. Adv. 2020, 39, 107459. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Shao, H.; Fan, Y.; Yang, J.; Liu, J.; Deng, Z.; Liu, Z.; Chen, Z.; Zhang, J.; Yi, K.; et al. Superhydrophobic Surface-Assisted Preparation of Microspheres and Supraparticles and Their Applications. Nanomicro Lett. 2024, 16, 68. [Google Scholar] [CrossRef]
- Mamidi, N.; Ijadi, F.; Norahan, M.H. Leveraging the Recent Advancements in GelMA Scaffolds for Bone Tissue Engineering: An Assessment of Challenges and Opportunities. Biomacromolecules 2024, 25, 2075–2113. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, F.; Tang, J.; Xu, Y.; Guo, K.; Xu, Z.; Feng, Y.; Xi, K.; Gu, Y.; Chen, L. Oxygen metabolism-balanced engineered hydrogel microspheres promote the regeneration of the nucleus pulposus by inhibiting acid-sensitive complexes. Bioact. Mater. 2023, 24, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Cai, F.; Chen, H.; Tang, Z.; Xi, K.; Tang, J.; Wu, L.; Xu, Y.; Deng, L.; Gu, Y.; et al. Modulation of Local Overactive Inflammation via Injectable Hydrogel Microspheres. Nano Lett. 2021, 21, 2690–2698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cai, F.; Zhu, H.; Xu, Y.; Tang, J.; Wang, W.; Li, Z.; Wu, J.; Ding, Z.; Xi, K.; et al. Immune-defensive microspheres promote regeneration of the nucleus pulposus by targeted entrapment of the inflammatory cascade during intervertebral disc degeneration. Bioact. Mater. 2024, 37, 132–152. [Google Scholar] [CrossRef]
- Zheng, D.; Chen, W.; Chen, T.; Chen, X.; Liang, J.; Chen, H.; Shen, H.; Deng, L.; Ruan, H.; Cui, W. Hydrogen Ion Capturing Hydrogel Microspheres for Reversing Inflammaging. Adv. Mater. 2024, 36, e2306105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lv, Z.; Zhuang, Y.; Saiding, Q.; Yang, W.; Xiong, W.; Zhang, Z.; Chen, H.; Cui, W.; Zhang, Y. Mechanical Signal-Tailored Hydrogel Microspheres Recruit and Train Stem Cells for Precise Differentiation. Adv. Mater. 2023, 35, e2300180. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, D.; Chen, H.; Ye, T.; Liu, Z.; Qi, J.; Shen, H.; Ruan, H.; Cui, W.; Deng, L. Circadian Clock Regulation via Biomaterials for Nucleus Pulposus. Adv. Mater. 2023, 35, e2301037. [Google Scholar] [CrossRef]
- Chang, H.; Cai, F.; Zhang, Y.; Jiang, M.; Yang, X.; Qi, J.; Wang, L.; Deng, L.; Cui, W.; Liu, X. Silencing Gene-Engineered Injectable Hydrogel Microsphere for Regulation of Extracellular Matrix Metabolism Balance. Small Methods 2022, 6, e2101201. [Google Scholar] [CrossRef]
- Xu, Y.; Gu, Y.; Cai, F.; Xi, K.; Xin, T.; Tang, J.; Wu, L.; Wang, Z.; Wang, F.; Deng, L.; et al. Metabolism Balance Regulation via Antagonist-Functionalized Injectable Microsphere for Nucleus Pulposus Regeneration. Adv. Funct. Mater. 2020, 30, 2006333. [Google Scholar] [CrossRef]
- Economidou, S.N.; Douroumis, D. 3D printing as a transformative tool for microneedle systems: Recent advances, manufacturing considerations and market potential. Adv. Drug Deliv. Rev. 2021, 173, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Jamaledin, R.; Yiu, C.K.Y.; Zare, E.N.; Niu, L.N.; Vecchione, R.; Chen, G.; Gu, Z.; Tay, F.R.; Makvandi, P. Advances in Antimicrobial Microneedle Patches for Combating Infections. Adv. Mater. 2020, 32, e2002129. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Dong, Z.; Xu, X.; Bei, H.P.; Yuen, H.Y.; Cheung, C.W.J.; Wong, M.S.; He, Y.; Zhao, X. Going below and beyond the surface: Microneedle structure, materials, drugs, fabrication, and applications for wound healing and tissue regeneration. Bioact. Mater. 2023, 27, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Xie, E.; Sun, H.; Wang, H.; Li, J.; Liu, Z.; Li, K.; Hu, J.; Chen, Q.; Liu, C.; et al. High-Strength Smart Microneedles with „Offensive and Defensive” Effects for Intervertebral Disc Repair. Adv. Mater. 2024, 36, e2305468. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhu, M.; Xing, H.; Xue, Y.; Li, J.; Wang, Z.; Zhu, Z.; Fang, M.; Li, Z.; Xu, J.; et al. Thread-structural microneedles loaded with engineered exosomes for annulus fibrosus repair by regulating mitophagy recovery and extracellular matrix homeostasis. Bioact. Mater. 2024, 37, 1–13. [Google Scholar] [CrossRef]
- Nie, J.; Fu, J.; He, Y. Hydrogels: The Next Generation Body Materials for Microfluidic Chips? Small 2020, 16, e2003797. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, N.; Qin, L. Recent Advances in Microfluidic Platforms for Programming Cell-Based Living Materials. Adv. Mater. 2021, 33, e2005944. [Google Scholar] [CrossRef]
- Kim, A.G.; Kim, T.W.; Kwon, W.K.; Lee, K.H.; Jeong, S.; Hwang, M.H.; Choi, H. Microfluidic Chip with Low Constant-Current Stimulation (LCCS) Platform: Human Nucleus Pulposus Degeneration In Vitro Model for Symptomatic Intervertebral Disc. Micromachines 2021, 12, 1291. [Google Scholar] [CrossRef]
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Lv, Y.; Liu, Y.; Guo, Q.; Wang, J.; Wang, C.; Hu, P.; Liu, Y. Recent Progress in Electrospun Nanofiber-Based Degenerated Intervertebral Disc Repair. ACS Biomater. Sci. Eng. 2022, 8, 16–31. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Yu, Q.; Chu, G.; Li, J.; Zhu, Z.; Tu, Z.; Liu, C.; Zhang, W.; Zhao, R.; Mao, H.; et al. Multifunctional Nanofibrous Scaffolds with Angle-Ply Microstructure and Co-Delivery Capacity Promote Partial Repair and Total Replacement of Intervertebral Disc. Adv. Healthc. Mater. 2022, 11, e2200895. [Google Scholar] [CrossRef]
- Moreira, A.; Lawson, D.; Onyekuru, L.; Dziemidowicz, K.; Angkawinitwong, U.; Costa, P.F.; Radacsi, N.; Williams, G.R. Protein encapsulation by electrospinning and electrospraying. J. Control. Release 2021, 329, 1172–1197. [Google Scholar] [CrossRef]
- Tanhaei, A.; Mohammadi, M.; Hamishehkar, H.; Hamblin, M.R. Electrospraying as a novel method of particle engineering for drug delivery vehicles. J. Control. Release 2021, 330, 851–865. [Google Scholar] [CrossRef]
- Nosoudi, N.; Hasanzadeh, A.; Hart, M.; Weaver, B. Advancements and Future Perspectives in Cell Electrospinning and Bio-Electrospraying. Adv. Biol. 2023, 7, e2300213. [Google Scholar] [CrossRef] [PubMed]
- Loepfe, M.; Duss, A.; Zafeiropoulou, K.A.; Bjorgvinsdottir, O.; D’Este, M.; Eglin, D.; Fortunato, G.; Klasen, J.; Ferguson, S.J.; Wuertz-Kozak, K.; et al. Electrospray-Based Microencapsulation of Epigallocatechin 3-Gallate for Local Delivery into the Intervertebral Disc. Pharmaceutics 2019, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- De Pieri, A.; Byerley, A.M.; Musumeci, C.R.; Salemizadehparizi, F.; Vanderhorst, M.A.; Wuertz-Kozak, K. Electrospinning and 3D bioprinting for intervertebral disc tissue engineering. JOR Spine 2020, 3, e1117. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Perera, K.; Ivone, R.; Natekin, E.; Wilga, C.A.; Shen, J.; Menon, J.U. 3D Bioprinted Implants for Cartilage Repair in Intervertebral Discs and Knee Menisci. Front. Bioeng. Biotechnol. 2021, 9, 754113. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Lian, M.; Han, Y.; Mo, X.; Jiang, W.; Qiao, Z.; Dai, K. A 3D-Bioprinted dual growth factor-releasing intervertebral disc scaffold induces nucleus pulposus and annulus fibrosus reconstruction. Bioact. Mater. 2021, 6, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, L.; Zhang, W.; Sun, Z.; Li, Y.; Yang, M.; Zeng, D.; Peng, B.; Zheng, W.; Jiang, X.; et al. Reverse Reconstruction and Bioprinting of Bacterial Cellulose-Based Functional Total Intervertebral Disc for Therapeutic Implantation. Small 2018, 14, 1702582. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, R.; Liu, S.; Song, Z.; Wang, H. The Emerging Roles of Nanocarrier Drug Delivery System in Treatment of Intervertebral Disc Degeneration-Current Knowledge, Hot Spots, Challenges and Future Perspectives. Drug Des. Dev. Ther. 2024, 18, 1007–1022. [Google Scholar] [CrossRef]
- Qu, F.; Geng, R.; Liu, Y.; Zhu, J. Advanced nanocarrier- and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics 2022, 12, 3372–3406. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Li, L.; Li, F.; Zhang, J.; Liang, X.J. Lipid Nanoparticles Optimized for Targeting and Release of Nucleic Acid. Adv. Mater. 2024, 36, e2305300. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Banala, R.R.; Vemuri, S.K.; Dar, G.H.; Palanisamy, V.; Penkulinti, M.; Surekha, M.V.; Reddy, A.V.G.; Nalam, M.R.; Subbaiah, G. Efficiency of dual siRNA-mediated gene therapy for intervertebral disc degeneration (IVDD). Spine J. 2019, 19, 896–904. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Xie, R.; Gong, S. A review on core-shell structured unimolecular nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2018, 130, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Haag, R.; Wu, C. Biocatalytic Synthesis Using Self-Assembled Polymeric Nano- and Microreactors. Angew. Chem. Int. Ed. 2022, 61, e202213974. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.S.; Li, D.D.; Wang, C.G.; Ying, L.W.; Wang, J.K.; Yang, B.; Shu, J.W.; Huang, X.P.; Zhang, Y.A.; Yu, C.; et al. An esterase-responsive ibuprofen nano-micelle pre-modified embryo derived nucleus pulposus progenitor cells promote the regeneration of intervertebral disc degeneration. Bioact. Mater. 2023, 21, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, D.; Wang, C.; Xia, K.; Wang, J.; Zhou, X.; Ying, L.; Shu, J.; Huang, X.; Xu, H.; et al. Injectable kartogenin and apocynin loaded micelle enhances the alleviation of intervertebral disc degeneration by adipose-derived stem cell. Bioact. Mater. 2021, 6, 3568–3579. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, X.; An, S.; Yin, W.; Huang, J.; Jiang, X. Nanozyme-Based Regulation of Cellular Metabolism and Their Applications. Adv. Mater. 2024, 36, e2301810. [Google Scholar] [CrossRef] [PubMed]
- Zandieh, M.; Liu, J. Nanozyme Catalytic Turnover and Self-Limited Reactions. ACS Nano 2021, 15, 15645–15655. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, R.; Liu, Z.; Qi, L.; Xu, H.; Yang, H.; Li, Y.; Liu, L.; Feng, G.; Zhang, L. Core-Shell Structured Nanozyme with PDA-Mediated Enhanced Antioxidant Efficiency to Treat Early Intervertebral Disc Degeneration. ACS Appl. Mater. Interfaces 2024, 16, 5103–5119. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, H.; Chu, D.; Lin, W.; Wang, X.; Wu, Y.; Li, K.; Wang, H.; Li, D.; Xu, Z.; et al. Rescuing Nucleus Pulposus Cells From Senescence via Dual-Functional Greigite Nanozyme to Alleviate Intervertebral Disc Degeneration. Adv. Sci. 2023, 10, e2300988. [Google Scholar] [CrossRef]
- Cheng, L.; Hill, A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- DiStefano, T.J.; Vaso, K.; Danias, G.; Chionuma, H.N.; Weiser, J.R.; Iatridis, J.C. Extracellular Vesicles as an Emerging Treatment Option for Intervertebral Disc Degeneration: Therapeutic Potential, Translational Pathways, and Regulatory Considerations. Adv. Healthc. Mater. 2022, 11, e2100596. [Google Scholar] [CrossRef]
- Qian, H.; He, L.; Ye, Z.; Wei, Z.; Ao, J. Decellularized matrix for repairing intervertebral disc degeneration: Fabrication methods, applications and animal models. Mater. Today Bio 2023, 18, 100523. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Liu, H.; Ma, L.; Lei, J.; Tong, B.; Li, G.; Ke, W.; Wang, K.; Feng, X.; Hua, W.; et al. Engineering Extracellular Vesicles Restore the Impaired Cellular Uptake and Attenuate Intervertebral Disc Degeneration. ACS Nano 2021, 15, 14709–14724. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, L.; Guan, M.; Zheng, Q.; Jin, J.; Kang, X.; Gao, Z.; Deng, X.; Shen, Y.; Chu, G.; et al. A Redox Homeostasis Modulatory Hydrogel with GLRX3+ Extracellular Vesicles Attenuates Disc Degeneration by Suppressing Nucleus Pulposus Cell Senescence. ACS Nano 2023, 17, 13441–13460. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, Y.; Ni, L.; Cao, H.; Shen, H.; Luo, Z.; Niu, J.; Yang, H.; Shi, Q. Biomimetic nanovesicles-based therapeutic strategy for alleviating intervertebral disc degeneration via integration with mechanically responsive miR-1249. Nano Today 2024, 56, 102221. [Google Scholar] [CrossRef]
- Sun, K.; Yan, C.; Dai, X.; Shi, Y.; Li, F.; Chen, L.; Sun, J.; Chen, Y.; Shi, J. Catalytic Nanodots-Driven Pyroptosis Suppression in Nucleus Pulposus for Antioxidant Intervention of Intervertebral Disc Degeneration. Adv. Mater. 2024, 36, e2313248. [Google Scholar] [CrossRef]
- Yu, H.; Teng, Y.; Ge, J.; Yang, M.; Xie, H.; Wu, T.; Yan, Q.; Jia, M.; Zhu, Q.; Shen, Y.; et al. Isoginkgetin-loaded reactive oxygen species scavenging nanoparticles ameliorate intervertebral disc degeneration via enhancing autophagy in nucleus pulposus cells. J. Nanobiotechnology 2023, 21, 99. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, G.; Xie, X.; Yu, W.; Wang, J.; Zang, F.; Yang, C.; Xiao, Q.; Zhang, R.; Wei, L.; et al. Low-dose celecoxib-loaded PCL fibers reverse intervertebral disc degeneration by up-regulating CHSY3 expression. J. Nanobiotechnology 2023, 21, 76. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, C.; Cheng, J.; Yan, T.; He, Q.; Huang, D.; Liu, J.; Wang, Z. Cutting-Edge Biomaterials in Intervertebral Disc Degeneration Tissue Engineering. Pharmaceutics 2024, 16, 979. https://doi.org/10.3390/pharmaceutics16080979
Wang Y, Zhang C, Cheng J, Yan T, He Q, Huang D, Liu J, Wang Z. Cutting-Edge Biomaterials in Intervertebral Disc Degeneration Tissue Engineering. Pharmaceutics. 2024; 16(8):979. https://doi.org/10.3390/pharmaceutics16080979
Chicago/Turabian StyleWang, Yifan, Chuyue Zhang, Junyao Cheng, Taoxu Yan, Qing He, Da Huang, Jianheng Liu, and Zheng Wang. 2024. "Cutting-Edge Biomaterials in Intervertebral Disc Degeneration Tissue Engineering" Pharmaceutics 16, no. 8: 979. https://doi.org/10.3390/pharmaceutics16080979




