Adipose-Derived Stem-Cell-Membrane-Coated PLGA-PEI Nanoparticles Promote Wound Healing via Efficient Delivery of miR-21
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Nanocarriers
2.3. Characterization of Nanocarriers
2.4. Cell Uptake
2.5. Scratch Wound Assay
2.6. Tube Formation Assay
2.7. qRT-PCR
2.8. Western Blot
2.9. Mouse Skin Wound Model and Treatment
2.10. Histochemistry and Immunofluorescence
2.11. Statistical Analysis
3. Results
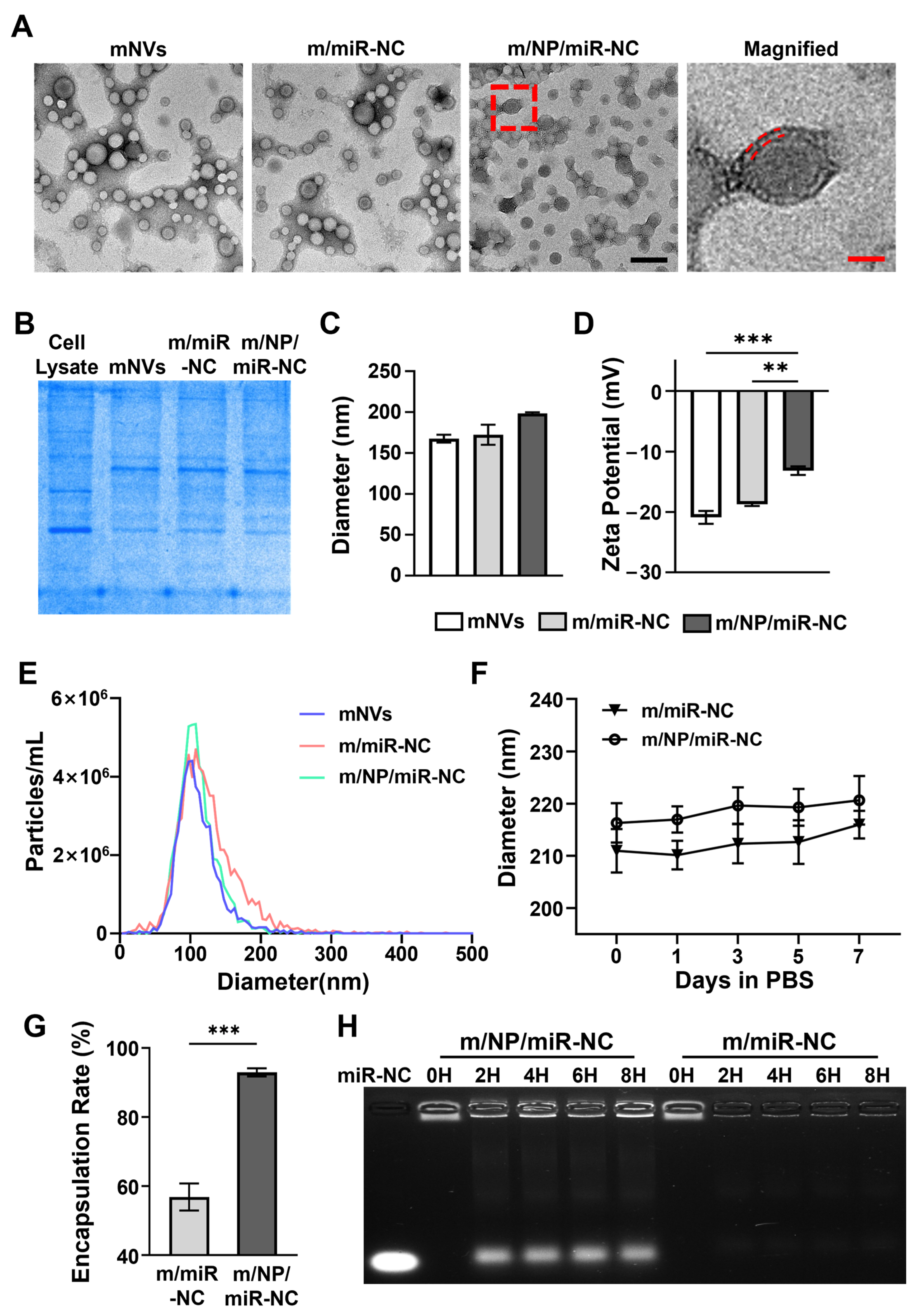
3.1. Preparation and Characteristics of Nanocarriers
3.2. Cellular Uptake Behavior of m/NP/miR-21
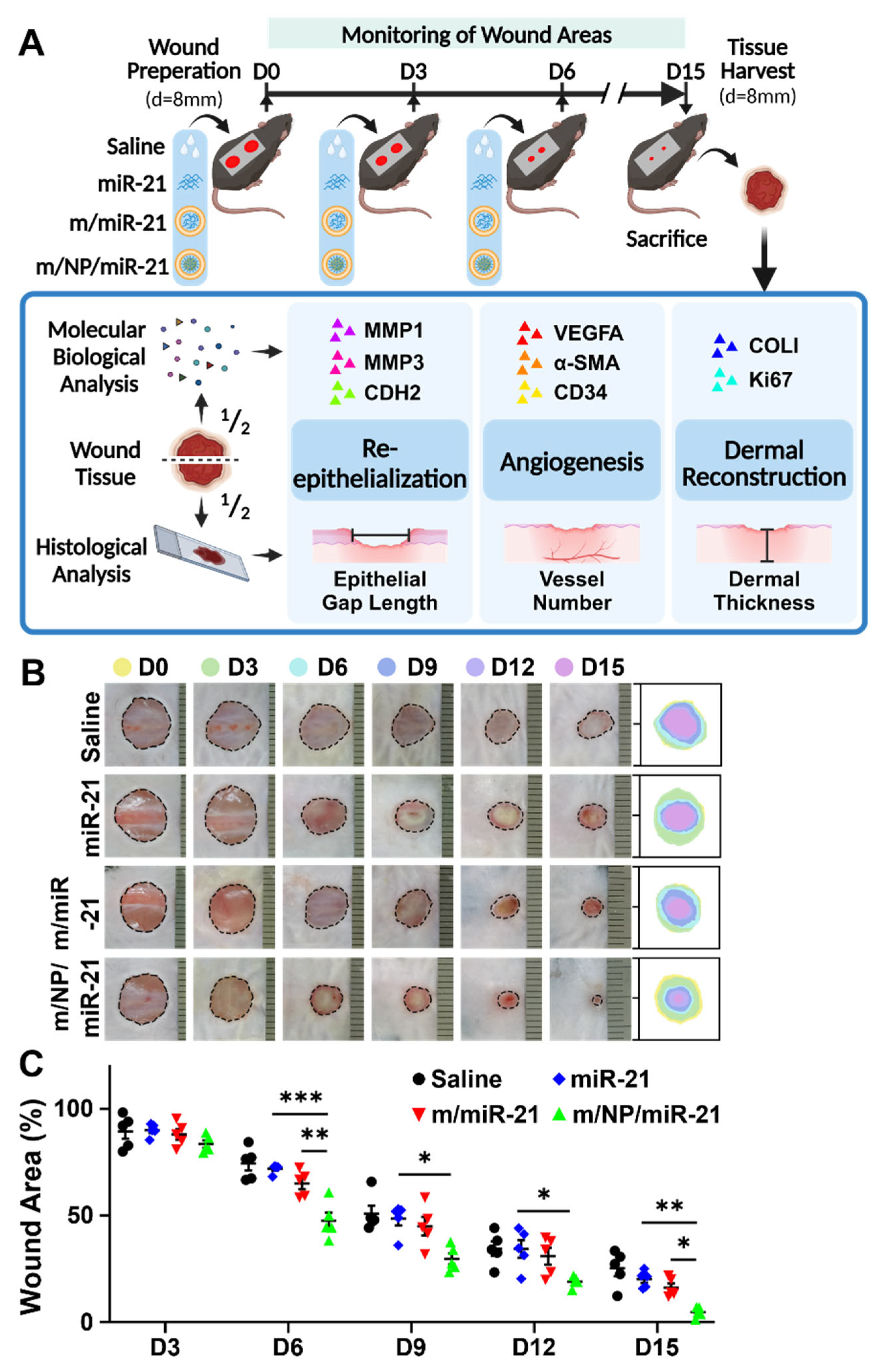
3.3. m/NP/miR-21 Accelerates Mouse Skin Wound Repair
3.4. m/NP/miR-21 Promotes Re-Epithelialization by Facilitating Keratinocyte Migration
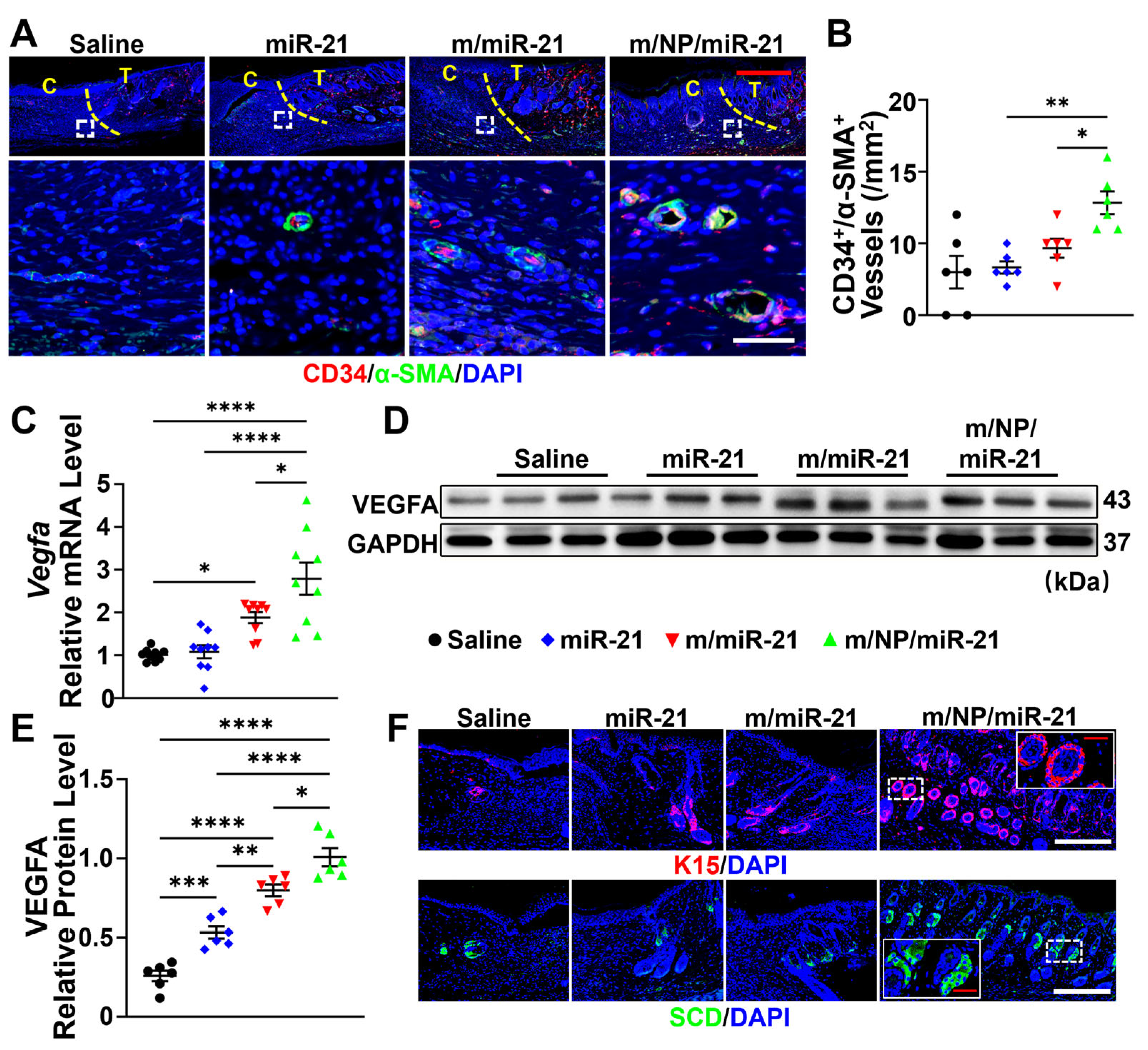
3.5. m/NP/miR-21 Accelerates Angiogenesis
3.6. m/NP/miR-21 Promotes Dermal Reconstruction by Enhancing Fibroblast Function
3.7. m/NP/miR-21 Regulates Skin Repair via the PTEN/Akt Axis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSCs | Adipose mesenchymal stem cells |
| PTEN | Phosphate and tension homology deleted on chromosome ten |
| PLGA-PEI | Poly(d,l-lactide-co-glycolide)-polyethyleneimine |
| NPs | Nanoparticles |
| MSCs | Mesenchymal stem cells |
| RES | Reticuloendothelial system |
| D/H | DMEM high glucose medium |
| D/F | DMEM/F12 |
| FBS | Fetal bovine serum |
| HUVECs | Human umbilical vein endothelial cells |
| ECM | Endothelial culture medium and additive kit |
| hDFs | Human fibroblasts |
| Hacats | Human immortalized keratinocytes |
| m/NP/miR | Membrane nanovesicles coated PLGA-PEI loaded with miRNA |
| NP/miR | PLGA-PEI loaded with miRNA |
| m/miR | Membrane nanovesicles loaded with miRNA |
| mNVs | Membrane nanovesicles |
| DLS | Dynamic light scattering |
| NTA | Nanoparticle tracking analysis |
| TEM | Transmission electron microscopy |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| MMP1 | Matrix metallopeptidase 1 |
| MMP3 | Matrix metallopeptidase 3 |
| CDH2 | Chromodomain helicase DNA binding protein 2 |
| COL1A1 | Collagen type I alpha 1 chain |
| VEGFA | Vascular endothelial growth factor a |
| COL1 | Collagen type I alpha 1 |
| K14 | Keratin 14 |
| K15 | Keratin 15 |
| SCD | Stearoyl-CoA desaturase |
| α-SMA | Actin Alpha 2, smooth muscle |
| EG | Epithelial gap |
| SGs | Sebaceous glands |
| HFs | Hair follicles |
| RBCs | Red blood cells |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| Arranged in order of first appearance in manuscripts. | |
References
- Jiang, Y.Y.; Xu, X.; Xiao, L.; Wang, L.H.; Qiang, S. The Role of microRNA in the Inflammatory Response of Wound Healing. Front. Immunol. 2022, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, D.R.I.; Moselhy, W.A.; Ahmed, R.R.; Al-Muzafar, H.M.; Amin, K.A.; Amin, M.M.; El-Nahass, E.S.; Abdou, K.A.H. Therapeutic effect of mesenchymal stem cells on histopathological, immunohistochemical, and molecular analysis in second-grade burn model. Stem. Cell Res. Ther. 2021, 12, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Tsui, N.B.Y.; Ng, E.K.O.; Lo, Y.M.D. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin. Chem. 2002, 48, 1647–1653. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.Y.; Huang, L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Moon, J.J.; Schwendeman, S.P.; Schwendeman, A. Nanomedicine: Past, present, and future. Adv. Drug Deliv. 2018, 130, 1–2. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Ye, W.H.; Yoon, H.S.; Yang, Y.Y. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat. Mater. 2006, 5, 791–796. [Google Scholar] [CrossRef]
- Kim, I.S.; Lee, S.K.; Park, Y.M.; Lee, Y.B.; Shin, S.C.; Lee, K.C.; Oh, I.J. Physicochemical characterization of poly(l-lactic acid) and poly(d,l-lactide-co-glycolide) nanoparticles with polyethylenimine as gene delivery carrier. Int. J. Pharm. 2005, 298, 255–262. [Google Scholar] [CrossRef]
- Patil, Y.; Panyam, J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int. J. Pharm. 2009, 367, 195–203. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Sustained cytoplasmic delivery of drugs with intracellular receptors using biodegradable nanoparticles. Mol. Pharm. 2004, 1, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Hashida, M. Targeted delivery systems of small interfering RNA by systemic administration. Drug Metab. Pharmacokinet. 2007, 22, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Size matters: Molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999, 45, 268–275. [Google Scholar] [CrossRef]
- Tang, M.X.; Szoka, F.C. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997, 3, 823–832. [Google Scholar] [CrossRef]
- Yezhelyev, M.V.; Qi, L.; O’Regan, R.M.; Nie, S.; Gao, X. Proton-sponge coated quantum dots for siRNA delivery and intracellular imaging. J. Am. Chem. Soc. 2008, 130, 9006–9012. [Google Scholar] [CrossRef]
- Yuan, X.; Naguib, S.; Wu, Z. Recent advances of siRNA delivery by nanoparticles. Expert Opin. Drug Deliv. 2011, 8, 521–536. [Google Scholar] [CrossRef]
- von Harpe, A.; Petersen, H.; Li, Y.; Kissel, T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J. Control. Release 2000, 69, 309–322. [Google Scholar] [CrossRef]
- Fischer, D.; Bieber, T.; Li, Y.; Elsässer, H.-P.; Kissel, T. A Novel Non-Viral Vector for DNA Delivery Based on Low Molecular Weight, Branched Polyethylenimine: Effect of Molecular Weight on Transfection Efficiency and Cytotoxicity. Pharm. Res. 1999, 16, 1273–1279. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef]
- Fang, R.H.; Gao, W.; Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 2023, 20, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Hu, Y.L.; Fu, Y.H.; Tabata, Y.; Gao, J.Q. Mesenchymal stem cells: A promising targeted-delivery vehicle in cancer gene therapy. J. Control. Release 2010, 147, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Popov, C.; Mutschler, W.; Schieker, M. Human mesenchymal stem cells in contact with their environment: Surface characteristics and the integrin system. J. Cell. Mol. Med. 2007, 11, 21–38. [Google Scholar] [CrossRef]
- Wu, H.H.; Zhou, Y.; Tabata, Y.; Gao, J.Q. Mesenchymal stem cell-based drug delivery strategy: From cells to biomimetic. J. Control. Release 2019, 294, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Ringe, J.; Strassburg, S.; Neumann, K.; Endres, M.; Notter, M.; Burmester, G.R.; Kaps, C.; Sittinger, M. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J. Cell. Biochem. 2007, 101, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xin, Y.; Cao, H.; Li, W.; Hua, Y.; Webster, T.J.; Zhang, C.; Tang, W.; Liu, Z. Recent advances in mesenchymal stem cell membrane-coated nanoparticles for enhanced drug delivery. Biomater. Sci. 2021, 9, 1088–1103. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Jiang, J.; Chen, F.; Fang, X. Doxorubicin Delivered Using Nanoparticles Camouflaged with Mesenchymal Stem Cell Membranes to Treat Colon Cancer. Int. J. Nanomed. 2020, 15, 2873–2884. [Google Scholar] [CrossRef]
- Yu, G.; Wu, X.; Kilroy, G.; Halvorsen, Y.-D.C.; Gimble, J.M.; Floyd, Z.E. Isolation of Murine Adipose-Derived Stem Cells. In Adipose-Derived Stem Cells: Methods and Protocols; Gimble, J.M., Bunnell, B.A., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 29–36. [Google Scholar]
- Davis, J.; Crampton, S.P.; Hughes, C.C.W. Isolation of Human Umbilical Vein Endothelial Cells (HUVEC). JoVE (J. Vis. Exp.) 2007, 3, e183. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, G.; Tan, X.; Qu, L.; Zhang, C.; Ma, L.; Luo, P.; Cao, X.; Yang, F.; Liu, Y.; et al. ID1/ID3 Mediate the Contribution of Skin Fibroblasts to Local Nerve Regeneration Through Itga6 in Wound Repair. Stem. Cells Trans. Med. 2021, 10, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wu, W.; Tang, H.; Jia, X.; Tang, J.; Ruan, X.; Li, F.; Leong, D.T.; Luo, D.; Yang, D. Self-assembly of stem cell membrane-camouflaged nanocomplex for microRNA-mediated repair of myocardial infarction injury. Biomaterials 2020, 257, 120256. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Liu, M.; Wang, J.; Hu, L.; Zeng, D.; Zhou, S.; Zhang, L.; Wang, M.; Xu, X.; Li, C.; et al. Metformin coordinates with mesenchymal cells to promote VEGF-mediated angiogenesis in diabetic wound healing through Akt/mTOR activation. Metabolism 2023, 140, 155398. [Google Scholar] [CrossRef]
- Moon, J.; Lee, S.Y.; Choi, J.W.; Lee, A.R.; Yoo, J.H.; Moon, S.J.; Park, S.H.; Cho, M.L. Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts. J. Transl. Med. 2021, 19, 192. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Copp, J.; Luk, B.T.; Zhang, L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013, 8, 336–340. [Google Scholar] [CrossRef]
- Copp, J.A.; Fang, R.H.; Luk, B.T.; Hu, C.M.; Gao, W.; Zhang, K.; Zhang, L. Clearance of pathological antibodies using biomimetic nanoparticles. Proc. Natl. Acad Sci. USA 2014, 111, 13481–13486. [Google Scholar] [CrossRef]
- Hu, C.M.; Fang, R.H.; Wang, K.C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhuang, J.; Lee, J.H.; Wang, L.; Fang, R.H.; Gao, W.; Zhang, L. Cell-Membrane-Cloaked Oil Nanosponges Enable Dual-Modal Detoxification. ACS Nano 2019, 13, 7209–7215. [Google Scholar] [CrossRef]
- Hung, W.C.; Lee, M.T.; Chen, F.Y.; Huang, H.W. The condensing effect of cholesterol in lipid bilayers. Biophys. J. 2007, 92, 3960–3967. [Google Scholar] [CrossRef]
- Jia, Y.; Cao, N.; Zhai, J.; Zeng, Q.; Zheng, P.; Su, R.; Liao, T.; Liu, J.; Pei, H.; Fan, Z.; et al. HGF Mediates Clinical-Grade Human Umbilical Cord-Derived Mesenchymal Stem Cells Improved Functional Recovery in a Senescence-Accelerated Mouse Model of Alzheimer’s Disease. Adv. Sci. 2020, 7, 1903809. [Google Scholar] [CrossRef]
- Liesveld, J.L.; Sharma, N.; Aljitawi, O.S. Stem cell homing: From physiology to therapeutics. Stem Cells 2020, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Jiang, Q.; Ding, X.; Li, Y.; Chen, C.; Yang, W.; Chen, S. Highly biosafe biomimetic stem cell membrane-disguised nanovehicles for cartilage regeneration. J. Mater. Chem. B 2020, 8, 8884–8893. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, X. Stem cell membrane-camouflaged targeted delivery system in tumor. Mater. Today Bio 2022, 16, 100377. [Google Scholar] [CrossRef]
- Thanuja, M.Y.; Anupama, C.; Ranganath, S.H. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: So near and yet so far. Adv. Drug Deliv. Rev. 2018, 132, 57–80. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Durham, J.T.; Herman, I.M. Wound Healing Angiogenesis: Innovations and Challenges in Acute and Chronic Wound Healing. Adv. Wound Care 2012, 1, 17–22. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Li, X.; Jiang, C.; Zhao, J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J. Diabetes Complicat. 2016, 30, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 23, 1635–1647. [Google Scholar] [CrossRef]
- Forrest, L. Current concepts in soft connective tissue wound healing. Br. J. Surg. 1983, 70, 133–140. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–408, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Davidson, J.M.; Kirsner, R.S.; Bornstein, P.; Herman, I.M. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011, 19, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoo, S.M.; Park, H.H.; Lim, H.J.; Kim, Y.L.; Lee, S.; Seo, K.W.; Kang, K.S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef]
- Wu, R.C.; Young, I.C.; Chen, Y.F.; Chuang, S.T.; Toubaji, A.; Wu, M.Y. Identification of the PTEN-ARID4B-PI3K pathway reveals the dependency on ARID4B by PTEN-deficient prostate cancer. Nat. Commun. 2019, 10, 4332. [Google Scholar] [CrossRef]
- Wei, F.; Wang, A.; Wang, Q.; Han, W.; Rong, R.; Wang, L.; Liu, S.; Zhang, Y.; Dong, C.; Li, Y. Plasma endothelial cells-derived extracellular vesicles promote wound healing in diabetes through YAP and the PI3K/Akt/mTOR pathway. Aging 2020, 12, 12002–12018. [Google Scholar] [CrossRef]
- Xie, J.; Wu, W.; Zheng, L.; Lin, X.; Tai, Y.; Wang, Y.; Wang, L. Roles of MicroRNA-21 in Skin Wound Healing: A Comprehensive Review. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, L.Z.; Xiao, R.; Cao, R.; Pan, B.; Lv, X.Y.; Jiao, H.; Zhuang, Q.; Sun, X.J.; Liu, Y.B. Inhibition of microRNA-21-5p reduces keloid fibroblast autophagy and migration by targeting PTEN after electron beam irradiation. Lab. Investig. 2020, 100, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Bamodu, O.A.; Kuo, K.-T.; Fong, I.-H.; Lin, C.-C.; Yeh, C.-T.; Chen, S.-G. Adipose-derived stem cell induced-tissue repair or wound healing is mediated by the concomitant upregulation of miR-21 and miR-29b expression and activation of the AKT signaling pathway. Arch. Biochem. Biophys. 2021, 705, 108895. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, H.; Chen, W.; Huang, P.; Bi, J. Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int. J. Biochem. Cell Biol. 2019, 114, 105570. [Google Scholar] [CrossRef]





| Gene Names | Primer Sequences |
|---|---|
| MMP1 | forward: 5′-ATGAAGCAGCCCAGATGTGGAG-3′ |
| reverse: 5′-TGGTCCACATCTGCTCTTGGCA-3′ | |
| MMP3 | forward: 5′-CACTCACAGACCTGACTCGGTT-3′ |
| reverse: 5′-AAGCAGGATCACAGTTGGCTGG-3′ | |
| CDH2 | forward: 5′-CGAAAACAGGCACTGGACCACT-3′ |
| reverse: 5′-GATGACGACTGTGTCCGCTGAA-3′ | |
| COL1A1 | forward: 5′-GATTCCCTGGACCTAAAGGTGC-3′ |
| reverse: 5′-AGCCTCTCCATCTTTGCCAGCA-3′ | |
| GAPDH | forward: 5′-GTCTCCTCTGACTTCAACAGCG-3′ |
| reverse: 5′-ACCACCCTGTTGCTGTAGCCAA-3′ | |
| Vegfa | forward: 5′-CTGCTGTAACGATGAAGCCCTG-3′ |
| reverse: 5′-GCTGTAGGAAGCTCATCTCTCC-3′ | |
| pten | forward: 5′-TGAGTTCCCTCAGCCGTTACCT-3′ |
| reverse: 5′-GAGGTTTCCTCTGGTCCTGGTA-3′ | |
| U6 | forward: 5′-CTCGCTTCGGCAGCACAT-3′ |
| reverse: 5′-TTTGCGTGTCATCCTTGCG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Du, F.; Wang, J.; Wu, Y.; Wei, Q.; Chen, A.; Duan, Y.; Shi, S.; Zhang, J.; Yu, S. Adipose-Derived Stem-Cell-Membrane-Coated PLGA-PEI Nanoparticles Promote Wound Healing via Efficient Delivery of miR-21. Pharmaceutics 2024, 16, 1113. https://doi.org/10.3390/pharmaceutics16091113
Peng H, Du F, Wang J, Wu Y, Wei Q, Chen A, Duan Y, Shi S, Zhang J, Yu S. Adipose-Derived Stem-Cell-Membrane-Coated PLGA-PEI Nanoparticles Promote Wound Healing via Efficient Delivery of miR-21. Pharmaceutics. 2024; 16(9):1113. https://doi.org/10.3390/pharmaceutics16091113
Chicago/Turabian StylePeng, Huiyu, Fangzhou Du, Jingwen Wang, Yue Wu, Qian Wei, Aoying Chen, Yuhan Duan, Shuaiguang Shi, Jingzhong Zhang, and Shuang Yu. 2024. "Adipose-Derived Stem-Cell-Membrane-Coated PLGA-PEI Nanoparticles Promote Wound Healing via Efficient Delivery of miR-21" Pharmaceutics 16, no. 9: 1113. https://doi.org/10.3390/pharmaceutics16091113
APA StylePeng, H., Du, F., Wang, J., Wu, Y., Wei, Q., Chen, A., Duan, Y., Shi, S., Zhang, J., & Yu, S. (2024). Adipose-Derived Stem-Cell-Membrane-Coated PLGA-PEI Nanoparticles Promote Wound Healing via Efficient Delivery of miR-21. Pharmaceutics, 16(9), 1113. https://doi.org/10.3390/pharmaceutics16091113







