Etravirine Prevents West Nile Virus and Chikungunya Virus Infection Both In Vitro and In Vivo by Inhibiting Viral Replication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Viruses, and Compounds
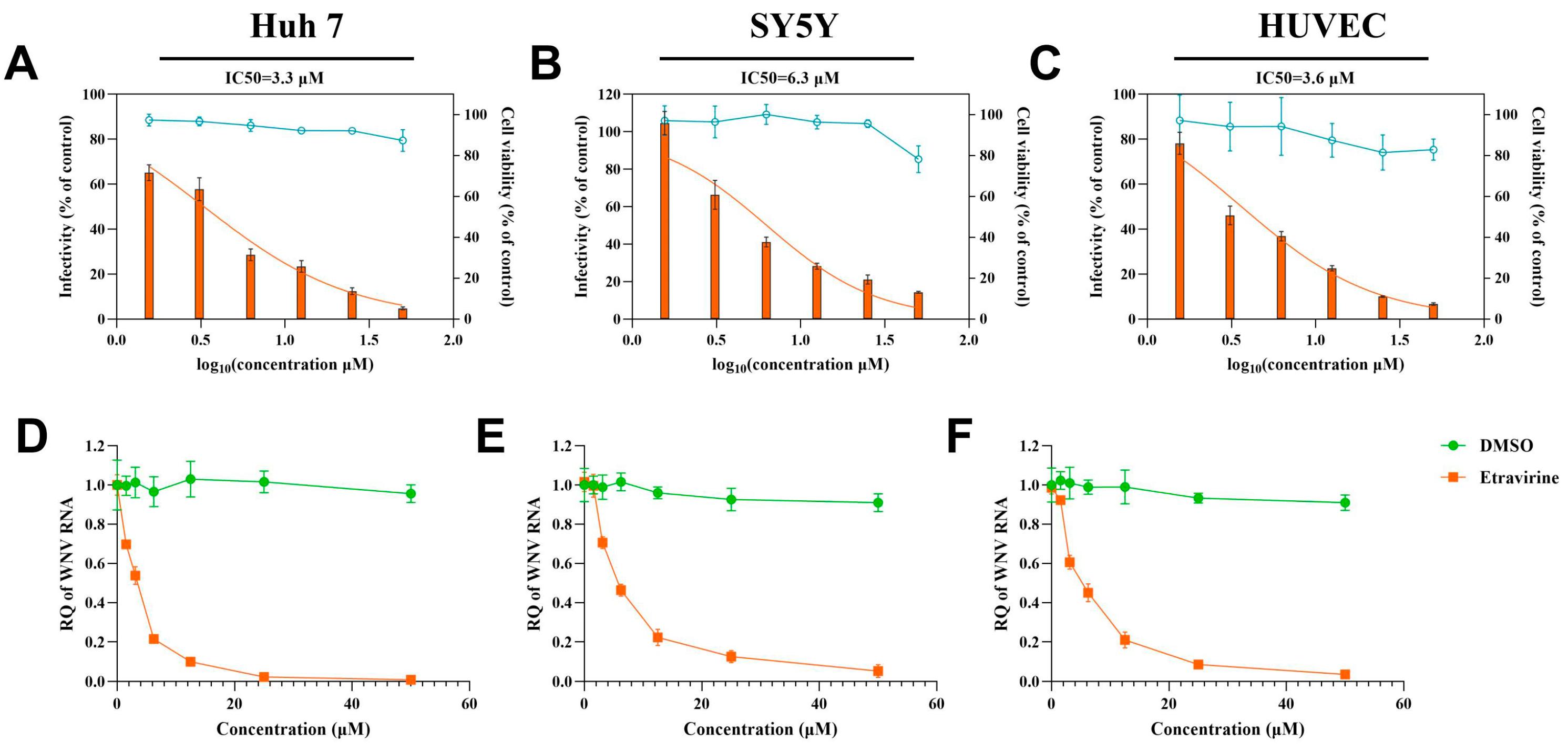
2.2. Drug Inhibition Assay and IC50 Calculation
2.3. Time-of-Drug-Addition Assay
2.4. Immunofluorescence Assay
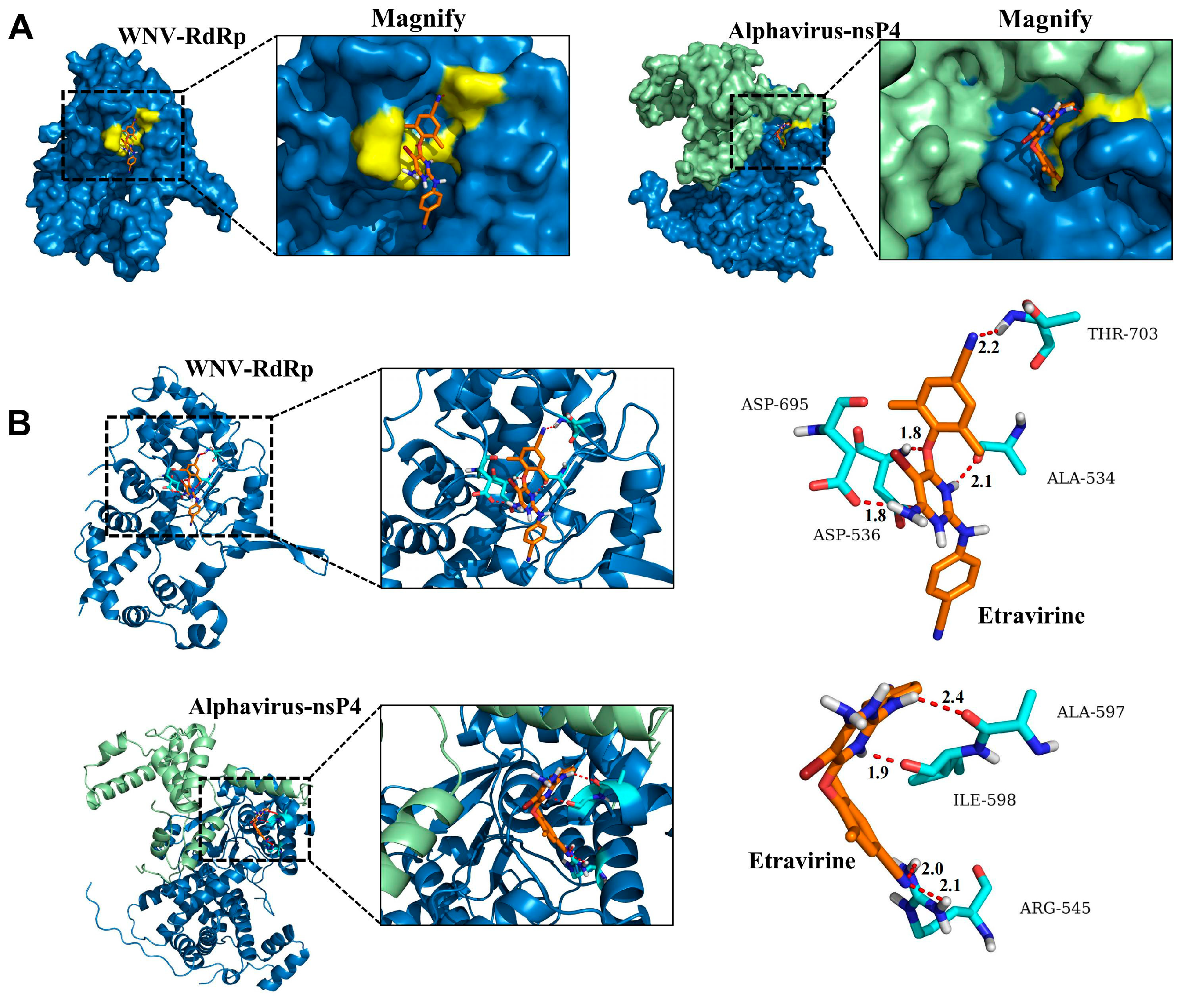
2.5. Molecular Docking
2.6. Cell Viability Assay
2.7. Real-Time Quantitative PCR (RT–qPCR)
2.8. Replication Determination
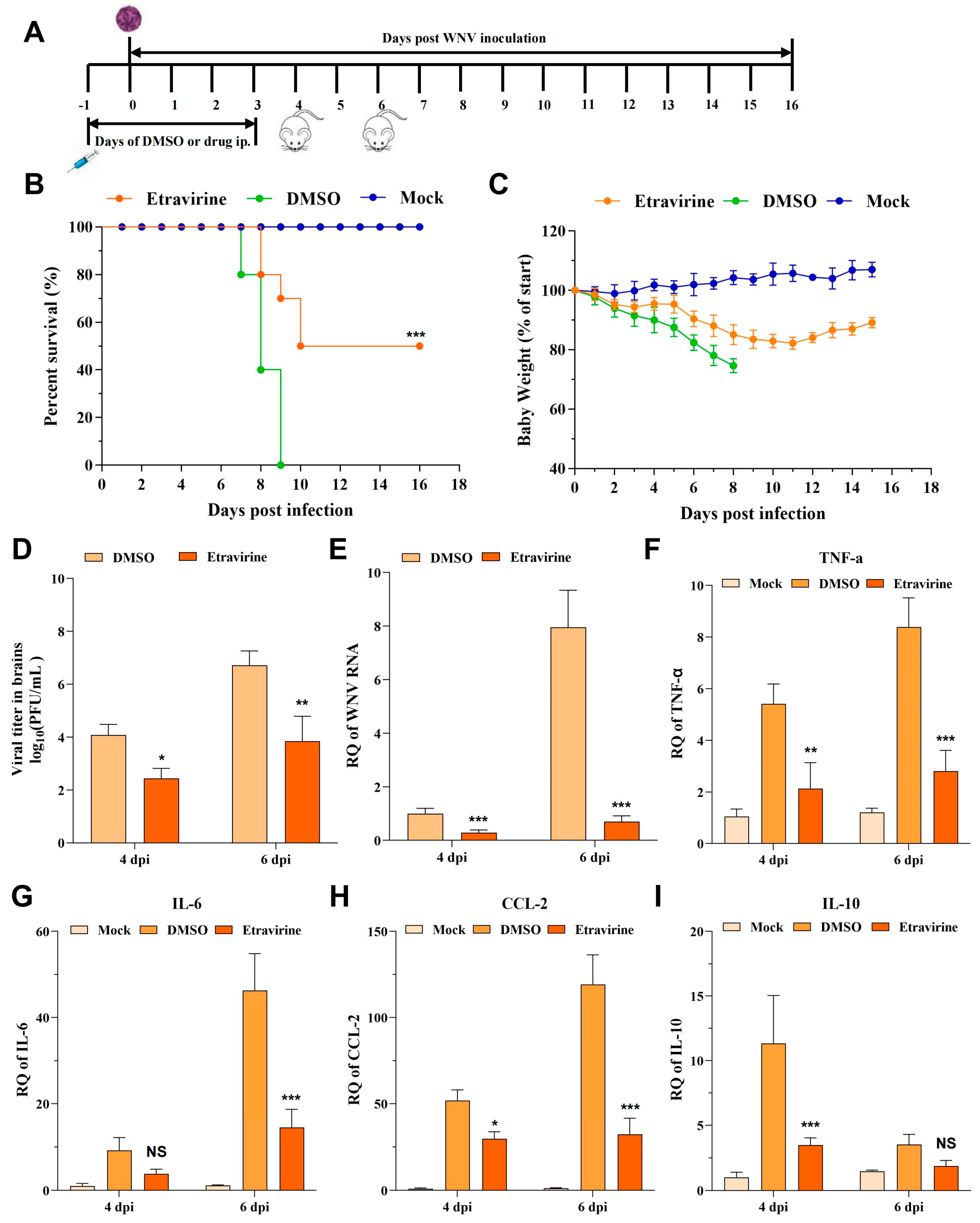
2.9. In Vivo Experiment
2.10. Statistical Analysis
3. Results
3.1. Screening of FDA-Approved Reverse Transcriptase Inhibitors for WNV Infection
3.2. Etravirine Has Dose-Dependent and Cell-Type-Independent Effects on WNV
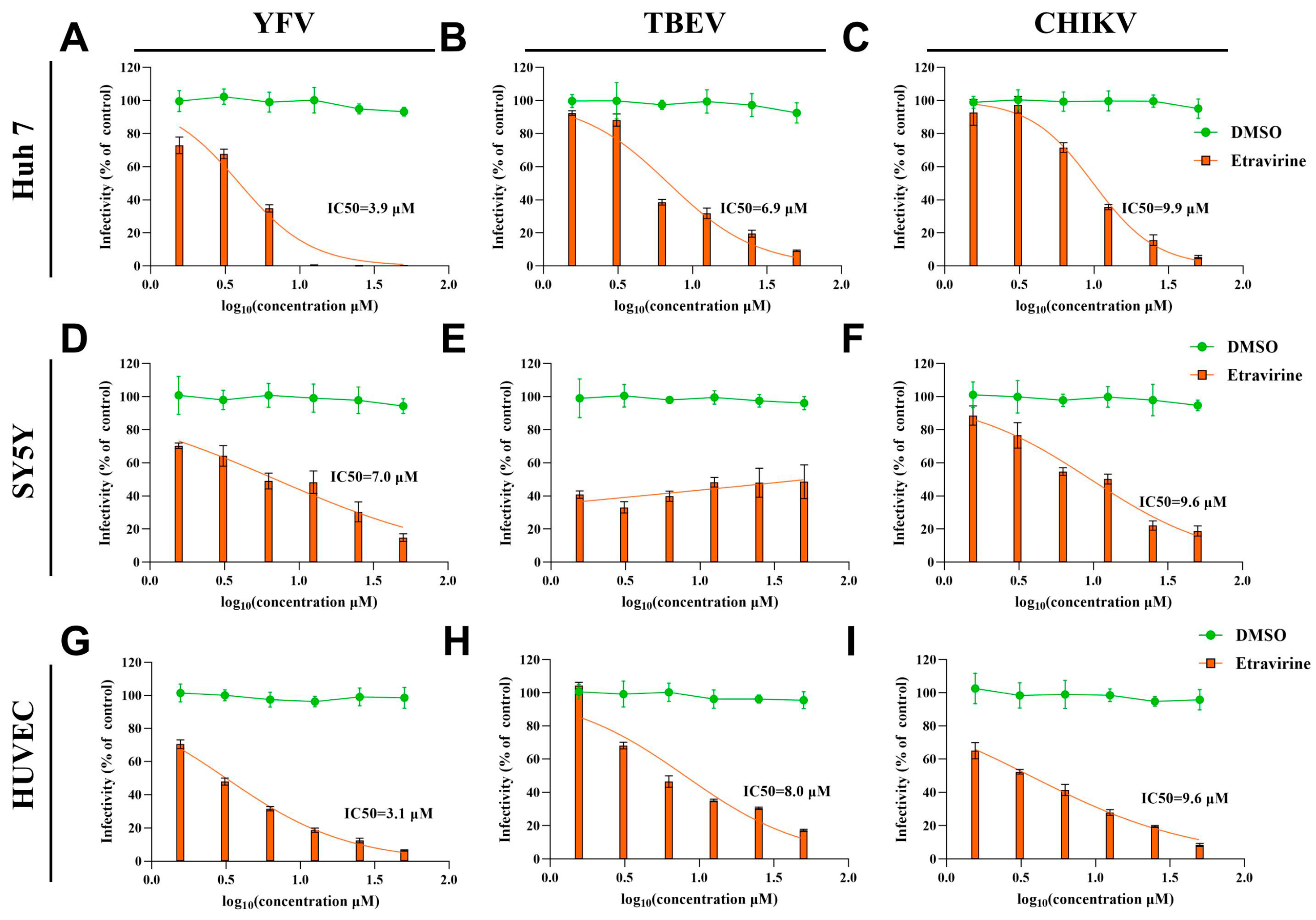
3.3. Etravirine Exhibits Broad Anti-Arboviral Activity
3.4. Etravirine Inhibits Viral Replication
3.5. Etravirine Has the Potential to Interact with RdRp
3.6. Etravirine Protects Mice from WNV Infection-Induced Lethality
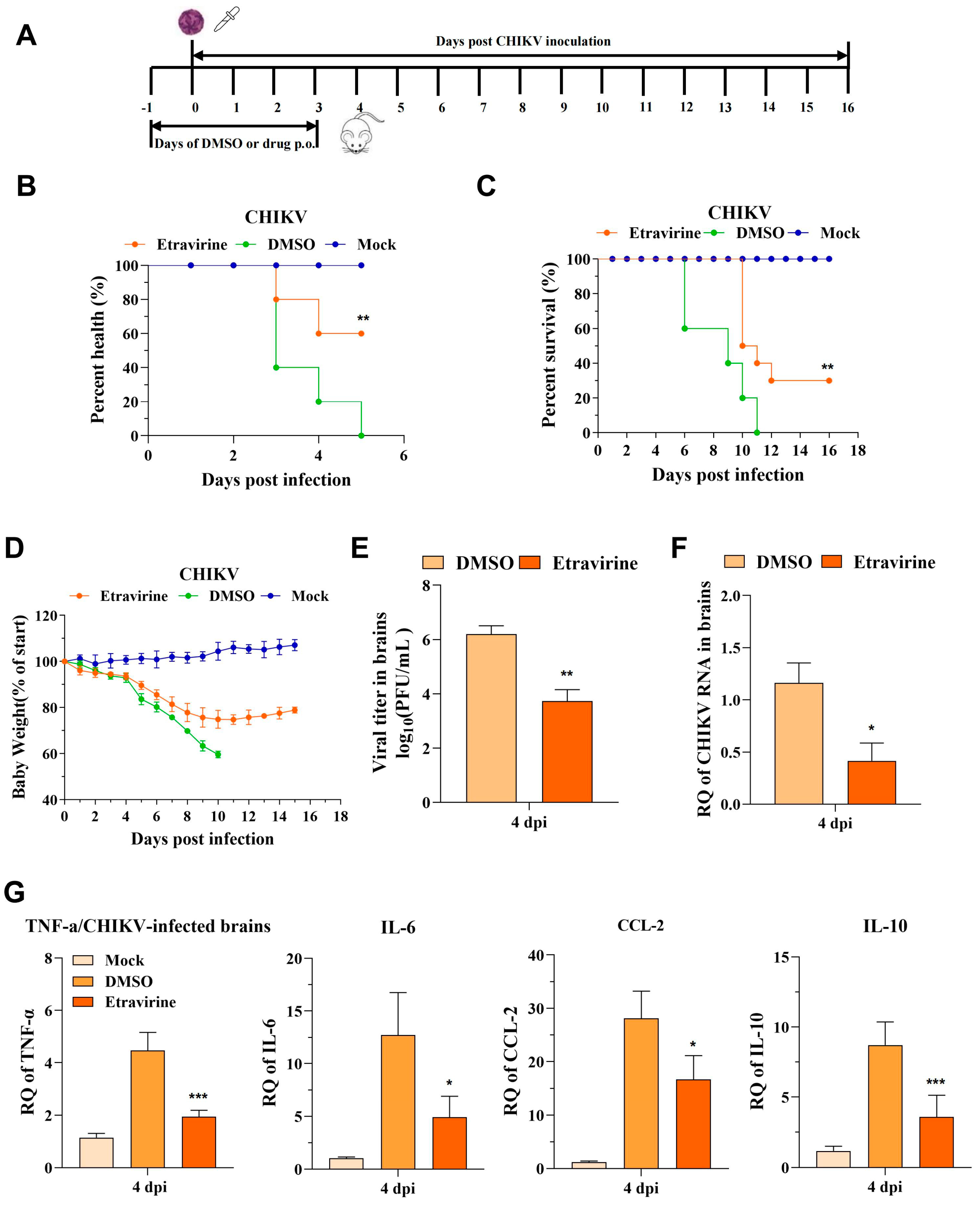
3.7. Etravirine Protects Mice from CHIKV Infection-Induced Lethality and Footpad Swelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antiviral Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.A.; Barrett, A.D.T. Twenty years of progress toward West Nile virus vaccine development. Viruses 2019, 11, 823. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of West Nile virus. Biomed. Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). West Nile Virus Disease Cases and Deaths Reported to CDC by Year and Clinical Presentation, 1999–2020. Available online: https://www.cdc.gov/west-nile-virus/data-maps/index.html (accessed on 18 June 2024.).
- Cassadou, S.; Boucau, S.; Petit-Sinturel, M.; Huc, P.; Leparc-Goffart, I.; Ledrans, M. Emergence of chikungunya fever on the French side of Saint Martin island, October to December 2013. Euro Surveill. 2014, 19, 20752. [Google Scholar] [CrossRef] [PubMed]
- Zeller, H.; Van Bortel, W.; Sudre, B. Chikungunya: Its history in Africa and Asia and its spread to new regions in 2013–2014. J. Infect. Dis. 2016, 214, S436–S440. [Google Scholar] [CrossRef] [PubMed]
- Burt, F.J.; Chen, W.; Miner, J.J.; Lenschow, D.J.; Merits, A.; Schnettler, E.; Kohl, A.; Rudd, P.A.; Taylor, A.; Herrero, L.J.; et al. Chikungunya virus: An update on the biology and pathogenesis of this emerging pathogen. Lancet Infect. Dis. 2017, 17, e107–e117. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef]
- Rangel, M.V.; Catanzaro, N.; Thannickal, S.A.; Crotty, K.A.; Noval, M.G.; Johnson, K.E.E.; Ghedin, E.; Lazear, H.M.; Stapleford, K.A. Structurally conserved domains between flavivirus and alphavirus fusion glycoproteins contribute to replication and infectious-virion production. J. Virol. 2022, 96, e0177421. [Google Scholar] [CrossRef]
- Hansen, M.; Nolan, M.S.; Gorchakov, R.; Hasbun, R.; Murray, K.O.; Ronca, S.E. Unique cytokine response in West Nile virus patients who developed chronic kidney disease: A prospective cohort study. Viruses 2021, 13, 311. [Google Scholar] [CrossRef]
- Karim, S.U.; Bai, F. Introduction to West Nile virus. Methods Mol. Biol. 2023, 2585, 1–7. [Google Scholar]
- Kilpatrick, A.M.; Daszak, P.; Jones, M.J.; Marra, P.P.; Kramer, L.D. Host heterogeneity dominates West Nile virus transmission. Proc. Biol. Sci. 2006, 273, 2327–2333. [Google Scholar] [CrossRef]
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J. Med. Entomol. 2005, 42, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006, 4, e82. [Google Scholar] [CrossRef]
- Couturier, E.; Guillemin, F.; Mura, M.; Léon, L.; Virion, J.M.; Letort, M.J.; De Valk, H.; Simon, F.; Vaillant, V. Impaired quality of life after Chikungunya virus infection: A 2-year follow-up study. Rheumatology 2012, 51, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, J.J.; Jaffar Bandjee, M.C.; Krejbich Trotot, P.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef]
- Schilte, C.; Staikowsky, F.; Couderc, T.; Madec, Y.; Carpentier, F.; Kassab, S.; Albert, M.L.; Lecuit, M.; Michault, A. Chikungunya virus-associated long-term arthralgia: A 36-month prospective longitudinal study. PLoS Negl. Trop. Dis. 2013, 7, e2137. [Google Scholar] [CrossRef]
- Gérardin, P.; Couderc, T.; Bintner, M.; Tournebize, P.; Renouil, M.; Lémant, J.; Boisson, V.; Borgherini, G.; Staikowsky, F.; Schramm, F.; et al. Encephalchik Study Group. Chikungunya virus-associated encephalitis: A cohort study on La Réunion Island, 2005-2009. Neurology 2016, 86, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Gérardin, P.; Sampériz, S.; Ramful, D.; Boumahni, B.; Bintner, M.; Alessandri, J.L.; Carbonnier, M.; Tiran-Rajaoefera, I.; Beullier, G.; Boya, I.; et al. Neurocognitive outcome of children exposed to perinatal mother-to-child Chikungunya virus infection: The CHIMERE cohort study on Reunion Island. PLoS Negl. Trop. Dis. 2014, 8, e2996. [Google Scholar] [CrossRef] [PubMed]
- Markoff, L. 5′- and 3′-noncoding regions in flavivirus RNA. Adv. Virus Res. 2003, 59, 177–228. [Google Scholar]
- Biswal, M.; Yao, W.; Lu, J.; Chen, J.; Morrison, J.; Hai, R.; Song, J. A conformational selection mechanism of flavivirus NS5 for species-specific STAT2 inhibition. Commun. Biol. 2024, 7, 76. [Google Scholar] [CrossRef]
- Thurmond, S.; Wang, B.; Song, J.; Hai, R. Suppression of type I interferon signaling by flavivirus NS5. Viruses 2018, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Roby, J.A.; Esser-Nobis, K.; Dewey-Verstelle, E.C.; Fairgrieve, M.R.; Schwerk, J.; Lu, A.Y.; Soveg, F.W.; Hemann, E.A.; Hatfield, L.D.; Keller, B.C.; et al. Flavivirus nonstructural protein NS5 dysregulates HSP90 to broadly inhibit JAK/STAT signaling. Cells 2020, 9, 899. [Google Scholar] [CrossRef]
- Malet, H.; Massé, N.; Selisko, B.; Romette, J.L.; Alvarez, K.; Guillemot, J.C.; Tolou, H.; Yap, T.L.; Vasudevan, S.; Lescar, J.; et al. The flavivirus polymerase as a target for drug discovery. Antiviral Res. 2008, 80, 23–35. [Google Scholar] [CrossRef]
- García-Zarandieta, M.; Quesada, E.; Martínez-Jiménez, M.I.; Newnes, C.V.; Fernández-Cabello, V.; Sáez-Álvarez, Y.; Blázquez, A.B.; Escribano-Romero, E.; Saiz, J.C.; Del Aguila, C.; et al. Identification of West Nile virus RNA-dependent RNA polymerase non-nucleoside inhibitors by real-time high throughput fluorescence screening. Antiviral Res. 2023, 212, 105568. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Thurmond, S.; Hai, R.; Song, J. Structure and function of Zika virus NS5 protein: Perspectives for drug design. Cell. Mol. Life Sci. 2018, 75, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Elshahawi, H.; Syed Hassan, S.; Balasubramaniam, V. Importance of Zika Virus NS5 Protein for Viral Replication. Pathogens 2019, 8, 169. [Google Scholar] [CrossRef]
- Tian, L.; Qiang, T.; Liang, C.; Ren, X.; Jia, M.; Zhang, J.; Li, J.; Wan, M.; YuWen, X.; Li, H.; et al. RNA-dependent RNA polymerase (RdRp) inhibitors: The current landscape and repurposing for the COVID-19 pandemic. Eur. J. Med. Chem. 2021, 213, 113201. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Reis, P.A.; de Freitas, C.S.; Sacramento, C.Q.; Villas Bôas Hoelz, L.; Bastos, M.M.; Mattos, M.; Rocha, N.; Gomes de Azevedo Quintanilha, I.; da Silva Gouveia Pedrosa, C.; et al. Beyond members of the flaviviridae family, sofosbuvir also inhibits Chikungunya virus replication. Antimicrob. Agents Chemother. 2019, 63, e01389-18. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug. Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Trivedi, J.; Mohan, M.; Byrareddy, S.N. Drug repurposing approaches to combating viral infections. J. Clin. Med. 2020, 9, 3777. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, T. Strategy, progress, and challenges of drug repurposing for efficient antiviral discovery. Front. Pharmacol. 2021, 12, 660710. [Google Scholar] [CrossRef]
- Zeng, S.; Meng, X.; Huang, Q.; Lei, N.; Zeng, L.; Jiang, X.; Guo, X. Spiramycin and azithromycin, safe for administration to children, exert antiviral activity against enterovirus A71 in vitro and in vivo. Int. J. Antimicrob. Agents 2019, 53, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Li, Y.H.; Wang, L.L.; Liu, H.Q.; Lu, S.Y.; Liu, Y.; Li, K.; Liu, B.; Li, S.Y.; Shao, F.M.; et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct. Target. Ther. 2021, 6, 414. [Google Scholar] [CrossRef] [PubMed]
- Reina, J.; Iglesias, C. Nirmatrelvir plus ritonavir (Paxlovid) a potent SARS-CoV-2 3CLpro protease inhibitor combination. Rev. Esp. Quimioter. 2022, 35, 236–240. [Google Scholar] [CrossRef]
- Ding, C.; Tang, W.; Xia, B.; Peng, H.; Liu, Y.; Wang, J.; Zheng, X.; Liu, Y.; Zhao, L.; He, Y.; et al. High-throughput screening of FDA-approved drug library reveals ixazomib is a broad-spectrum antiviral agent against arboviruses. Viruses 2022, 14, 1381. [Google Scholar] [CrossRef]
- He, Y.; Pan, Z.; Liu, Y.; Jiang, L.; Peng, H.; Zhao, P.; Qi, Z.; Liu, Y.; Tang, H. Identification of tyrphostin AG879 and A9 inhibiting replication of Chikungunya virus by screening of a kinase inhibitor library. Virology 2023, 588, 109900. [Google Scholar] [CrossRef]
- Nnyigide, O.S.; Nnyigide, T.O.; Lee, S.G.; Hyun, K. Protein repair and analysis server: A web server to repair PDB structures, add missing heavy atoms and hydrogen atoms, and assign secondary structures by amide interactions. J. Chem. Inf. Model. 2022, 62, 4232–4246. [Google Scholar] [CrossRef]
- Bitencourt-Ferreira, G.; Pintro, V.O.; de Azevedo, W.F.J. Docking with AutoDock4. Methods Mol. Biol. 2019, 2053, 125–148. [Google Scholar]
- Matuszewska, A.; Nowak, B.; Niżański, W.; Eberhardt, M.; Domrazek, K.; Nikodem, A.; Wiatrak, B.; Zduniak, K.; Olejnik, K.; Merwid-Ląd, A.; et al. Long-term administration of abacavir and etravirine impairs semen quality and alters redox system and bone metabolism in growing male wistar rats. Oxid. Med. Cell. Longev. 2021, 2021, 5596090. [Google Scholar] [CrossRef]
- Tang, W.D.; Tang, H.L.; Peng, H.R.; Ren, R.W.; Zhao, P.; Zhao, L.J. Inhibition of tick-borne encephalitis virus in cell cultures by ribavirin. Front. Microbiol. 2023, 14, 1182798. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Wu, B.; Tang, H.; Luo, Z.; Xu, Z.; Ouyang, S.; Li, X.; Xie, J.; Yi, Z.; Leng, Q.; et al. Rifapentine is an entry and replication inhibitor against yellow fever virus both in vitro and in vivo. Emerg. Microbes Infect. 2022, 11, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Haussig, J.M.; Young, J.J.; Gossner, C.M.; Mezei, E.; Bella, A.; Sirbu, A.; Pervanidou, D.; Drakulovic, M.B.; Sudre, B. Early start of the West Nile fever transmission season 2018 in Europe. Euro Surveill. 2018, 23, 1800428. [Google Scholar] [CrossRef]
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; Franca, R.F.O. A review on Chikungunya virus epidemiology, pathogenesis and current vaccine development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug repurposing approach to fight COVID-19. Pharmacol. Rep. 2020, 72, 1479–1508. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Vinšová, J. Antiviral activity of substituted salicylanilides—A review. Mini Rev. Med. Chem. 2011, 11, 956–967. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Ren, R.; Liu Yv He, Y.; Qi, Z.; Peng, H.; Zhao, P. Identification of clinical candidates against West Nile virus by activity screening in vitro and effect evaluation in vivo. J. Med. Virol. 2022, 94, 4918–4925. [Google Scholar] [CrossRef]
- Sariyer, I.K.; Gordon, J.; Burdo, T.H.; Wollebo, H.S.; Gianti, E.; Donadoni, M.; Bellizzi, A.; Cicalese, S.; Loomis, R.; Robinson, J.A.; et al. Suppression of Zika Virus infection in the brain by the antiretroviral drug Rilpivirine. Mol. Ther. 2019, 27, 2067–2079. [Google Scholar] [CrossRef]
- Kityo, C.; Thompson, J.; Nankya, I.; Hoppe, A.; Ndashimye, E.; Warambwa, C.; Mambule, I.; van Oosterhout, J.J.; Wools-Kaloustian, K.; Bertagnolio, S.; et al. Europe Africa research network for evaluation of second-line therapy (EARNEST) trial team. HIV drug resistance mutations in non-B subtypes after prolonged virological failure on NNRTI-based first-line regimens in sub-saharan Africa. J. Acquir. Immune Defic. Syndr. 2017, 75, e45–e54. [Google Scholar] [CrossRef]
- Peng, S.; Wang, H.; Wang, Z.; Wang, Q. Progression of antiviral agents targeting viral polymerases. Molecules 2022, 27, 7370. [Google Scholar] [CrossRef]
- Usach, I.; Melis, V.; Peris, J.E. Non-nucleoside reverse transcriptase inhibitors: A review on pharmacokinetics, pharmacodynamics, safety and tolerability. J. Int. AIDS Soc. 2013, 16, 1–14. [Google Scholar] [CrossRef]
- Namasivayam, V.; Vanangamudi, M.; Kramer, V.G.; Kurup, S.; Zhan, P.; Liu, X.; Kongsted, J.; Byrareddy, S.N. The Journey of HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) from Lab to Clinic. J. Med. Chem. 2019, 62, 4851–4883. [Google Scholar] [CrossRef] [PubMed]
- Indu, P.; Rameshkumar, M.R.; Arunagirinathan, N.; Al-Dhabi, N.A.; Valan Arasu, M.; Ignacimuthu, S. Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine against main protease and RNA-dependent RNA polymerase of SARS-CoV-2: A molecular docking and drug repurposing approach. J. Infect. Public Health 2020, 13, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, D.; McKimmie, C.S.; Pingen, M.; Haxton, B.; Mansfield, K.; Johnson, N.; Fooks, A.R.; Graham, G.J. Defining the chemokine basis for leukocyte recruitment during viral encephalitis. J. Virol. 2014, 88, 9553–9567. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Town, T.; Qian, F.; Wang, P.; Kamanaka, M.; Connolly, T.M.; Gate, D.; Montgomery, R.R.; Flavell, R.A.; Fikrig, E. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009, 5, e1000610. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Ghosh, S.; Keshry, S.S.; Mahish, C.; Mohapatra, C.; Guru, A.; Mamidi, P.; Datey, A.; Pani, S.S.; Vasudevan, D.; et al. MBZM-N-IBT, a novel small molecule, restricts Chikungunya virus infection by targeting nsP2 protease activity in Vitro, in vivo, and ex vivo. Antimicrob. Agents Chemother. 2022, 66, e0046322. [Google Scholar] [CrossRef]
- Renault, P.; Josseran, L.; Pierre, V. Chikungunya-related fatality rates, Mauritius, India, and Reunion Island. Emerg. Infect. Dis. 2008, 14, 1327. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; He, Y.; Xia, B.; Tang, W.; Zhang, C.; Wang, D.; Tang, H.; Zhao, P.; Peng, H.; Liu, Y. Etravirine Prevents West Nile Virus and Chikungunya Virus Infection Both In Vitro and In Vivo by Inhibiting Viral Replication. Pharmaceutics 2024, 16, 1111. https://doi.org/10.3390/pharmaceutics16091111
Zheng X, He Y, Xia B, Tang W, Zhang C, Wang D, Tang H, Zhao P, Peng H, Liu Y. Etravirine Prevents West Nile Virus and Chikungunya Virus Infection Both In Vitro and In Vivo by Inhibiting Viral Replication. Pharmaceutics. 2024; 16(9):1111. https://doi.org/10.3390/pharmaceutics16091111
Chicago/Turabian StyleZheng, Xu, Yanhua He, Binghui Xia, Wanda Tang, Congcong Zhang, Dawei Wang, Hailin Tang, Ping Zhao, Haoran Peng, and Yangang Liu. 2024. "Etravirine Prevents West Nile Virus and Chikungunya Virus Infection Both In Vitro and In Vivo by Inhibiting Viral Replication" Pharmaceutics 16, no. 9: 1111. https://doi.org/10.3390/pharmaceutics16091111




