Toward Model-Informed Precision Dosing for Remimazolam: A Population Pharmacokinetic–Pharmacodynamic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Participants
2.1.2. Study Design
2.1.3. Bioassay
2.2. Population Pharmacokinetics and Pharmacodynamics Modeling
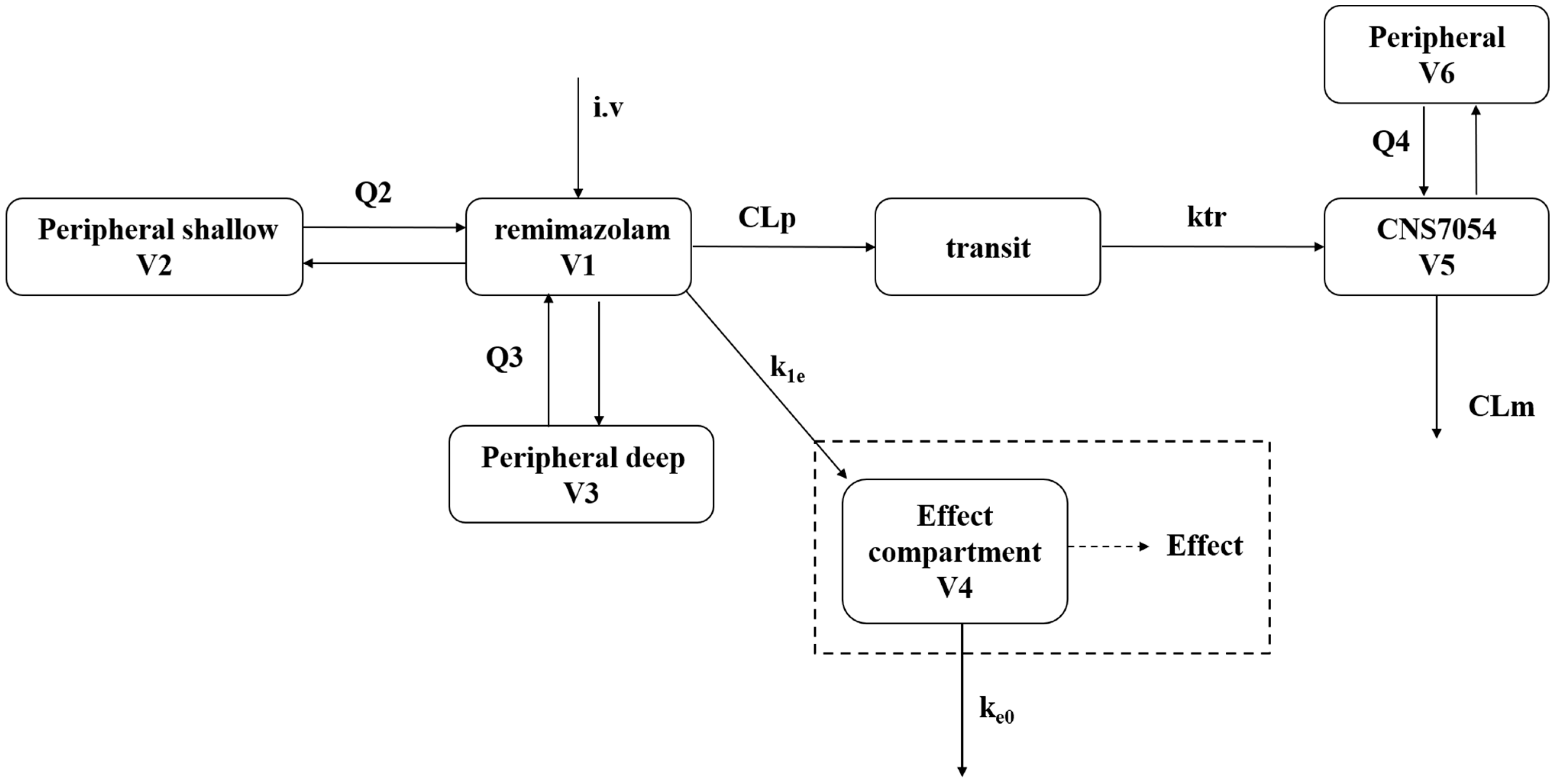
2.2.1. Population Pharmacokinetics Modeling
2.2.2. Population PK/PD Modeling
2.2.3. Model Evaluation
2.3. Dosing Regimen Design
3. Results
3.1. Demographics
3.2. Population Pharmacokinetics and Pharmacodynamics Modeling
3.2.1. Population Pharmacokinetics Modeling
3.2.2. Population PK/PD Modeling
3.2.3. Model Evaluation
3.3. Dosing Regimen Design
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Chen, S.; Huang, Y. Induction and maintenance of procedural sedation in adults: Focus on remimazolam injection. Expert Rev. Clin. Pharmacol. 2021, 14, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.C.; Moss, S.J.; Jurd, R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008, 9, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, A.M.; Zaccagnino, M.P.; Malapero, R.J.; Kaye, A.D.; Urman, R.D. Remimazolam: Pharmacologic Considerations and Clinical Role in Anesthesiology. Pharmacotherapy 2016, 36, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Remimazolam: First Approval. Drugs 2020, 80, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Freyer, N.; Knöspel, F.; Damm, G.; Greuel, S.; Schneider, C.; Seehofer, D.; Stöhr, T.; Petersen, K.U.; Zeilinger, K. Metabolism of remimazolam in primary human hepatocytes during continuous long-term infusion in a 3-D bioreactor system. Drug Des. Devel Ther. 2019, 13, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Antonik, L.J.; Goldwater, D.R.; Kilpatrick, G.J.; Tilbrook, G.S.; Borkett, K.M. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesth. Analg. 2012, 115, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.Y.; Liang, Y.; Yang, X.Y.; Li, L.E.; Ye, X.; Zhao, X.; Cui, Y.M. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur. J. Clin. Pharmacol. 2020, 76, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, P.; Jiang, J. Metabolite characterization of a novel sedative drug, remimazolam in human plasma and urine using ultra high-performance liquid chromatography coupled with synapt high-definition mass spectrometry. J. Pharm. Biomed. Anal. 2017, 137, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Guo, J.; Yuan, J.; Zhao, E.; Yang, J. Quality of Recovery after General Anesthesia with Remimazolam in Patients’ Undergoing Urologic Surgery: A Randomized Controlled Trial Comparing Remimazolam with Propofol. Drug Des. Dev. Ther. 2022, 16, 1199–1209. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, X.; Xu, J.; Ren, L.; Qi, H.; Li, R.; Shu, H.; Zou, X.; Yuan, S.; Yang, X.; et al. Remimazolam besylate versus propofol for deep sedation in critically ill patients: A randomized pilot study. Crit. Care 2023, 27, 474. [Google Scholar] [CrossRef]
- Schüttler, J.; Eisenried, A.; Lerch, M.; Fechner, J.; Jeleazcov, C.; Ihmsen, H. Pharmacokinetics and Pharmacodynamics of Remimazolam (CNS 7056) after Continuous Infusion in Healthy Male Volunteers: Part I. Pharmacokinetics and Clinical Pharmacodynamics. Anesthesiology 2020, 132, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Leonowens, C.; Ivaturi, V.D.; Lohmer, L.L.; Curd, L.; Ossig, J.; Schippers, F.; Petersen, K.U.; Stoehr, T.; Schmith, V. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J. Clin. Anesth. 2020, 66, 109899. [Google Scholar] [CrossRef]
- Savic, R.M.; Jonker, D.M.; Kerbusch, T.; Karlsson, M.O. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J. Pharmacokinet. Pharmacodyn. 2007, 34, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 1978, 6, 165–175. [Google Scholar] [CrossRef]
- Vrieze, S.I. Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol. Methods 2012, 17, 228–243. [Google Scholar] [CrossRef]
- Zhang, L.; Beal, S.L.; Sheiner, L.B. Simultaneous vs. sequential analysis for population PK/PD data I: Best-case performance. J. Pharmacokinet. Pharmacodyn. 2003, 30, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Beal, S.L.; Sheinerz, L.B. Simultaneous vs. sequential analysis for population PK/PD data II: Robustness of methods. J. Pharmacokinet. Pharmacodyn. 2003, 30, 405–416. [Google Scholar] [CrossRef]
- Post, T.M.; Freijer, J.I.; Ploeger, B.A.; Danhof, M. Extensions to the visual predictive check to facilitate model performance evaluation. J. Pharmacokinet. Pharmacodyn. 2008, 35, 185–202. [Google Scholar] [CrossRef]
- Simmons, L.E.; Riker, R.R.; Prato, B.S.; Fraser, G.L. Assessing sedation during intensive care unit mechanical ventilation with the Bispectral Index and the Sedation-Agitation Scale. Crit. Care Med. 1999, 27, 1499–1504. [Google Scholar] [CrossRef]
- Mondello, E.; Siliotti, R.; Noto, G.; Cuzzocrea, E.; Scollo, G.; Trimarchi, G.; Venuti, F.S. Bispectral Index in ICU: Correlation with Ramsay Score on assessment of sedation level. J. Clin. Monit. Comput. 2002, 17, 271–277. [Google Scholar] [CrossRef]
- Zhou, J.; Curd, L.; Lohmer, L.L.; Ossig, J.; Schippers, F.; Stoehr, T.; Schmith, V. Population Pharmacokinetics of Remimazolam in Procedural Sedation with Nonhomogeneously Mixed Arterial and Venous Concentrations. Clin. Transl. Sci. 2021, 14, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, H.R.; Kilpatrick, G.J.; Tilbrook, G.S.; Borkett, K.M. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth. Analg. 2012, 115, 284–296. [Google Scholar] [CrossRef] [PubMed]


| Study Number | Dose Regimen and Number of Subjects | Patient Population with PK/PD Sampling | PK Sampling | PD Sampling |
|---|---|---|---|---|
| 1 | IV bolus: 0.025 mg/kg: 3; 0.05 mg/kg: 3; 0.075 mg/kg: 8; 0.1 mg/kg: 8; 0.2 mg/kg: 8; 0.3 mg/kg: 10; 0.4 mg/kg: 8; | 46 Healthy Adult subjects | 0, 1, 2, 3, 4, 5, 6, 8, 10, 12, 15, 20, 30, 45 min, and 1, 1.5, 2, 3, 4, 8, 12 h post dose | 0, 1, 2, 5, 10, 20, 30, 40, 50, 60 min post dose |
| 2 | IV bolus: 0.2 mg/kg/min for 1 min; IV infusion: 1 mg/kg/h for 2 h; | 9 Healthy Adult subjects | Part I: 0, 1, 15, 45 min, and 1, 1.5, 2 h after IV dose initiation for 2 h infusion; Part II: 1, 2, 3, 4, 5, 6, 8, 10, 12, 15, 20, 30, 45 min, 1, 1.5, 2, 3, 4, 6, 10 h post dose. | Part I: 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 70, 80, 90, 100, 110 min after IV dose initiation for 2 h infusion; Part II: 1, 2, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60 min post dose. |
| Characteristic | Multi-Dose (N = 9) Median (Min, Max) | Single-Dose (N = 46) Median (Min, Max) | Total (N = 55) Median (Min, Max) |
|---|---|---|---|
| Sex (male/female) | 7/2 | ||
| Age (years) | 28 (23, 43) | 28 (19, 41) | 28 (19, 43) |
| BMI (kg/m2) | 22.4 (19.5, 23.8) | 22.3 (19.3, 24) | 22.4 (19.3, 24) |
| Height (cm) | 166 (151, 176) | 169 (154, 185) | 167.5 (151, 185) |
| Weight (kg) | 61.8 (52.5, 67) | 63.2 (52,75) | 62.5 (52, 75) |
| Parameters | Estimate (RSE%) | Shrinkage (%) | Parameters | Estimate (RSE%) | Shrinkage (%) |
|---|---|---|---|---|---|
| Typical value | |||||
| CL for remimazolam (L/min) | 1.21 (3%) | / | CL for CNS7054 (L/min) | 0.0637 (3%) | / |
| V1 for remimazolam (L) | 16 (8%) | / | V5 for CNS7054 (L) | 3.72 (5%) | / |
| V2 for remimazolam (L) | 22.6 (3%) | / | Q4 for CNS7054 (L/min) | 0.166 (5%) | / |
| Q2 for remimazolam (L/min) | 2.61 (4%) | / | V6 for CNS7054 (L) | 5.15 (4%) | / |
| Q3 for remimazolam (L/min) | 0.227 (14%) | / | |||
| V3 for remimazolam (L) | 23.5 (6%) | / | |||
| Ktr for remimazolam (1/min) | 0.447 (8%) | / | |||
| Inter-individual variation (IIV) | |||||
| IIV_CL for remimazolam (%) | 20 (10%) | 2 | IIV_CL for CNS7054 (%) | 22.1 (10%) | 2 |
| IIV_V1 for remimazolam (%) | 55 (10%) | 3 | IIV_V5 for CNS7054 (%) | 30.1 (12%) | 7 |
| IIV_Q2 for remimazolam (%) | 24.3 (26%) | 30 | IIV_V6 for CNS7054 (%) | 17.5 (15%) | 22 |
| IIV_V2 for remimazolam (%) | 32.1 (13%) | 9 | |||
| IIV_Ktr for remimazolam (%) | 40.1 (14%) | 17 | |||
| Residual unexplained variability (RUV) | |||||
| prop RUV for remimazolam (%) | 23.2 (1%) | 7 | prop RUV for CNS7054 (%) | 6.4 (0%) | 14 |
| COR (remimazolam _CNS7054) | 0.0066 | / | add RUV for CNS 7054 | 43.13 (8%) | 8 |
| Parameters | Estimate (RSE%) | Shrinkage (%) |
|---|---|---|
| Typical value | ||
| 54.5 (6%) | / | |
| (ng/mL) | 504 (9%) | / |
| BIS_baseline | 92.5 (1%) | / |
| (min−1) | 1.38 (26%) | / |
| Hill coefficient | 1.44 (10%) | / |
| Inter-individual variation (IIV) | ||
| IIV_ (%) | 27.2 (16%) | 28 |
| IIV_HILL (%) | 48.8 (13%) | 17 |
| Residual unexplained variability (RUV) | ||
| prop RUV pd (%) | 11.2 (2.4%) | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Gong, C.; Liu, F.; Jiao, Z.; Zheng, X. Toward Model-Informed Precision Dosing for Remimazolam: A Population Pharmacokinetic–Pharmacodynamic Analysis. Pharmaceutics 2024, 16, 1122. https://doi.org/10.3390/pharmaceutics16091122
Chen Y, Gong C, Liu F, Jiao Z, Zheng X. Toward Model-Informed Precision Dosing for Remimazolam: A Population Pharmacokinetic–Pharmacodynamic Analysis. Pharmaceutics. 2024; 16(9):1122. https://doi.org/10.3390/pharmaceutics16091122
Chicago/Turabian StyleChen, Yueting, Cansheng Gong, Feng Liu, Zheng Jiao, and Xiaochun Zheng. 2024. "Toward Model-Informed Precision Dosing for Remimazolam: A Population Pharmacokinetic–Pharmacodynamic Analysis" Pharmaceutics 16, no. 9: 1122. https://doi.org/10.3390/pharmaceutics16091122





