Liposomal Drug Delivery against Helicobacter pylori Using Furazolidone and N-Acetyl Cysteine in Augmented Therapy
Abstract
1. Background
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mucopenetrative Liposomes
2.3. Mucopenetration Testing
2.4. Liposome/Particle Size Analysis
2.5. Zeta Potential Measurement for Mucopenetration Liposomes
2.6. Encapsulation Efficiency of Furazolidone and N-Acetyl Cysteine (NAC) within Mucopenetrative Liposomes
2.7. HPLC Method for N-Acetyl Cysteine (NAC)
2.8. In Vitro Drug (Furazolidone and NAC) Release from Mucopenetrative Liposomes
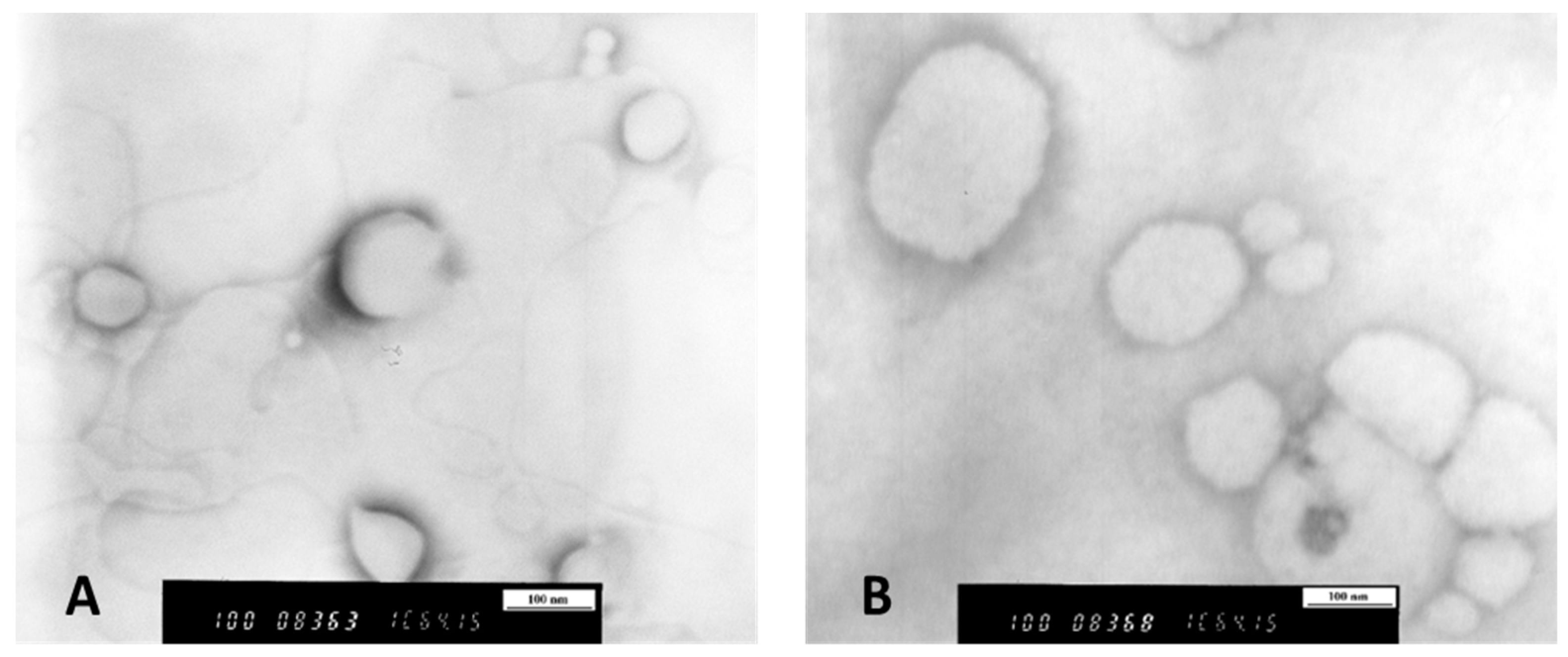
2.9. Transmission Electron Microscopy (TEM)
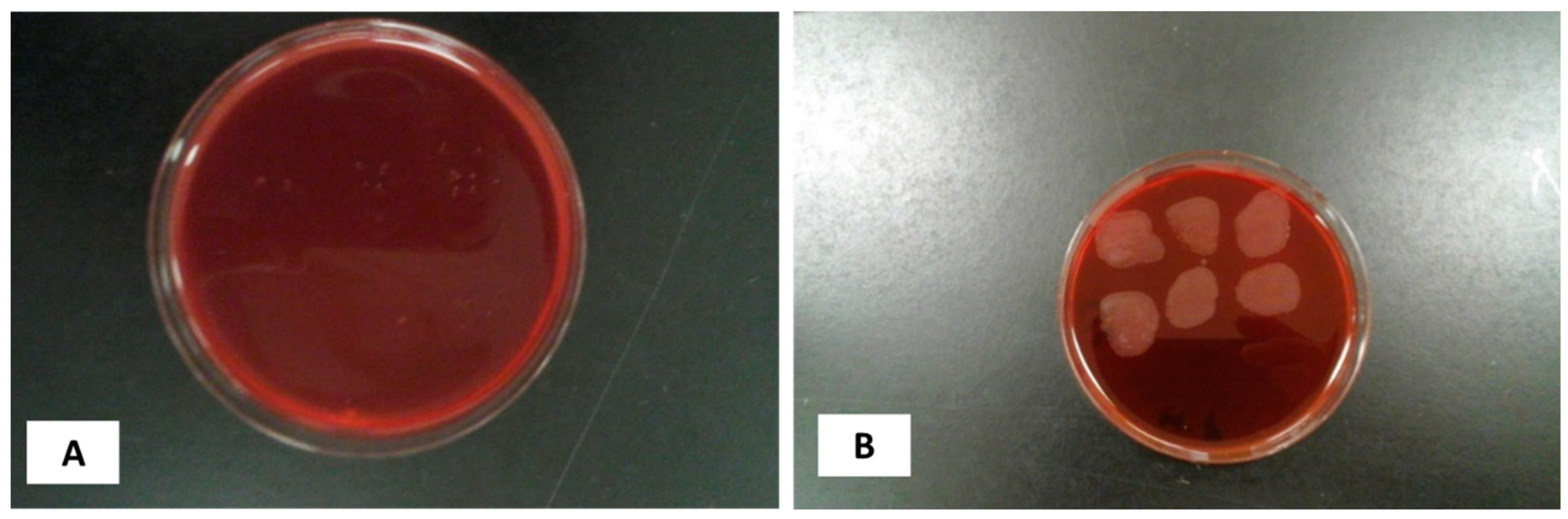
2.10. Reconstitution of Bacterial Culture
2.11. Standard OD Controlled Growth and Inoculum Size
2.12. Time–Kill Curve of Augmented Therapy of Free and Liposomal-Bound Furazolidone with NAC
2.13. Statistic Analysis
3. Results and Discussion
3.1. Characterization of Mucopenetrative Liposomal Formulations Regarding Their Encapsulation Efficiency and Liposome/Particle Morphology
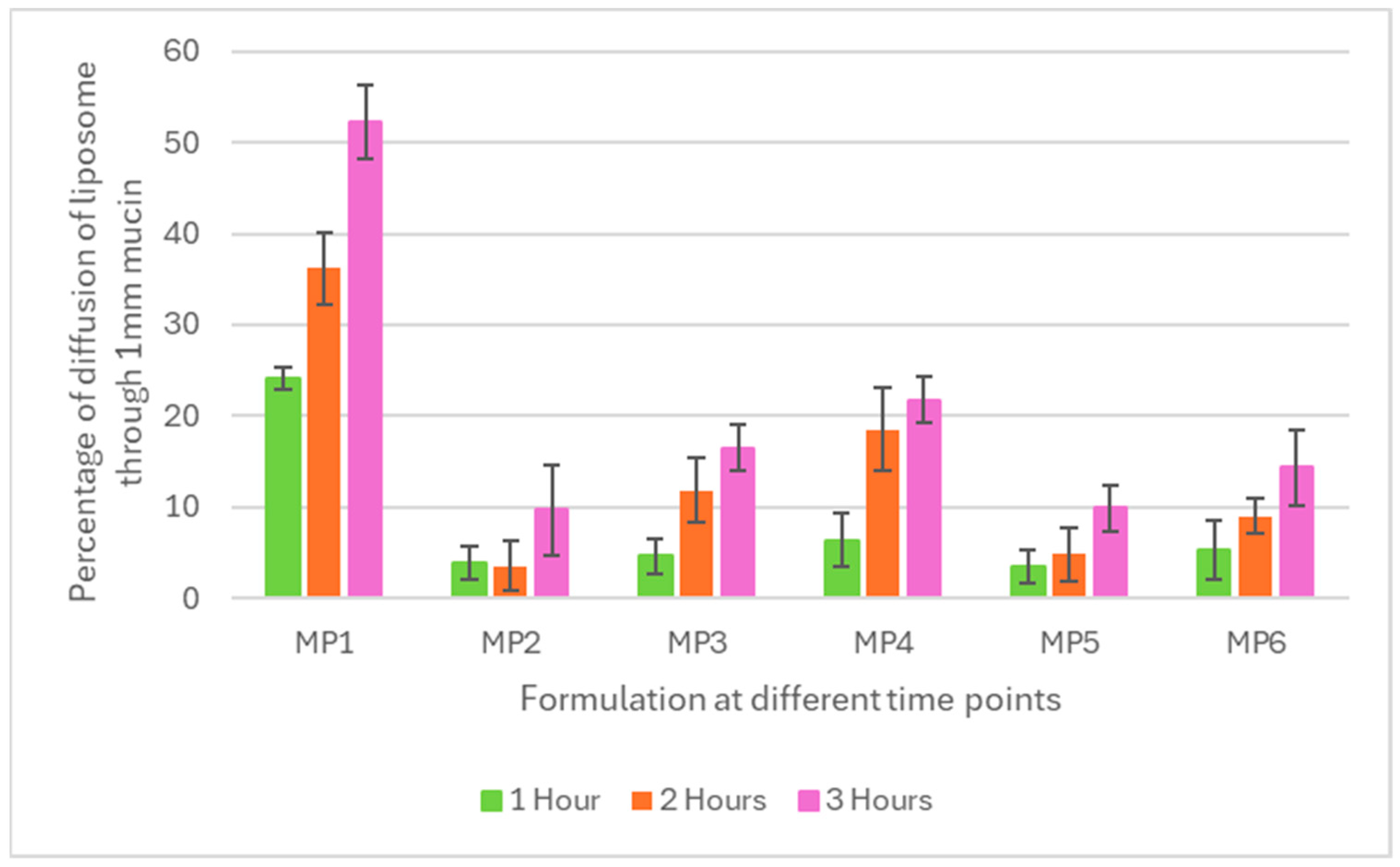
3.2. Mucopenetration
3.3. In Vitro Drug Release of Mucopenetrative Liposomes
3.3.1. In Vitro Drug Release of Furazolidone
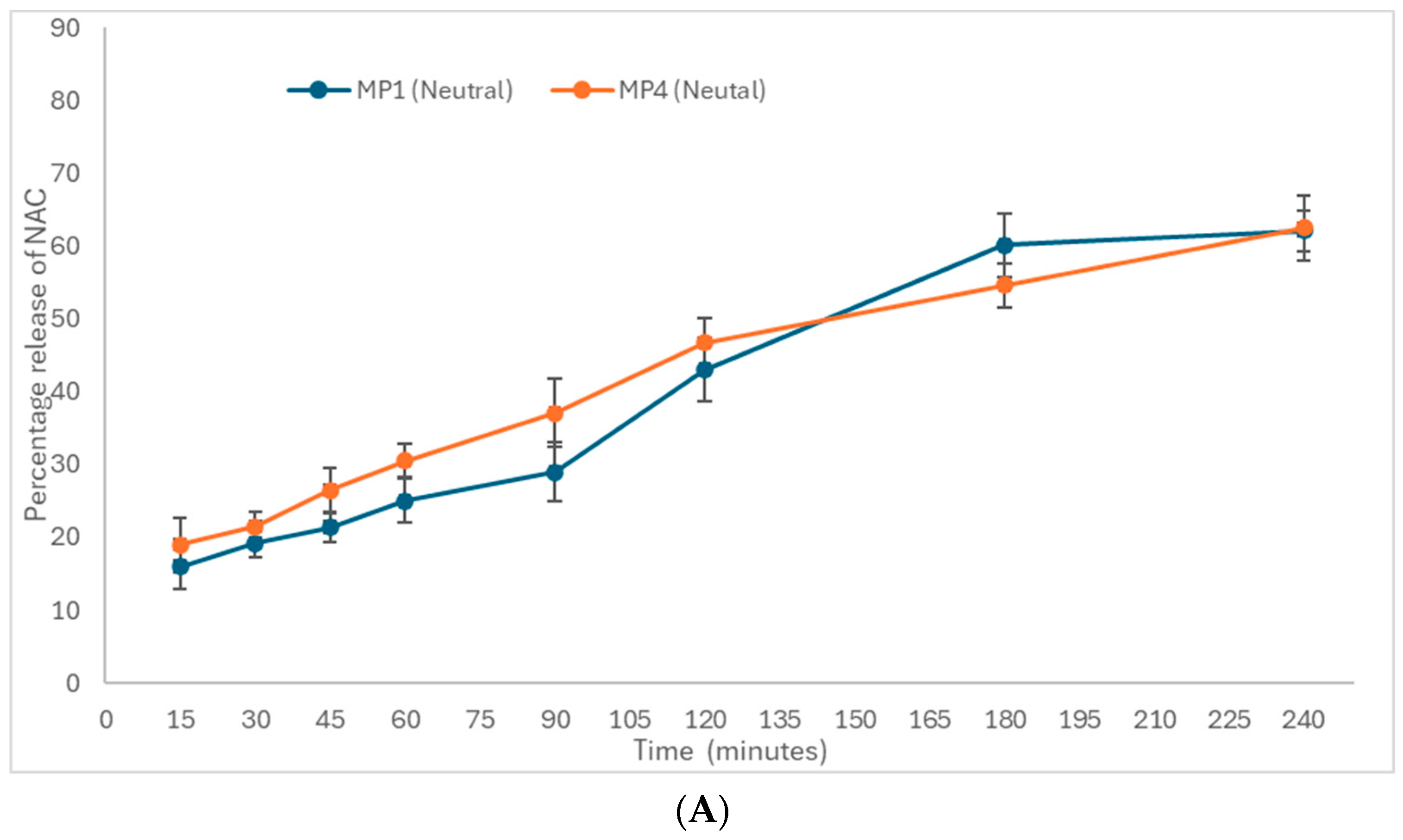
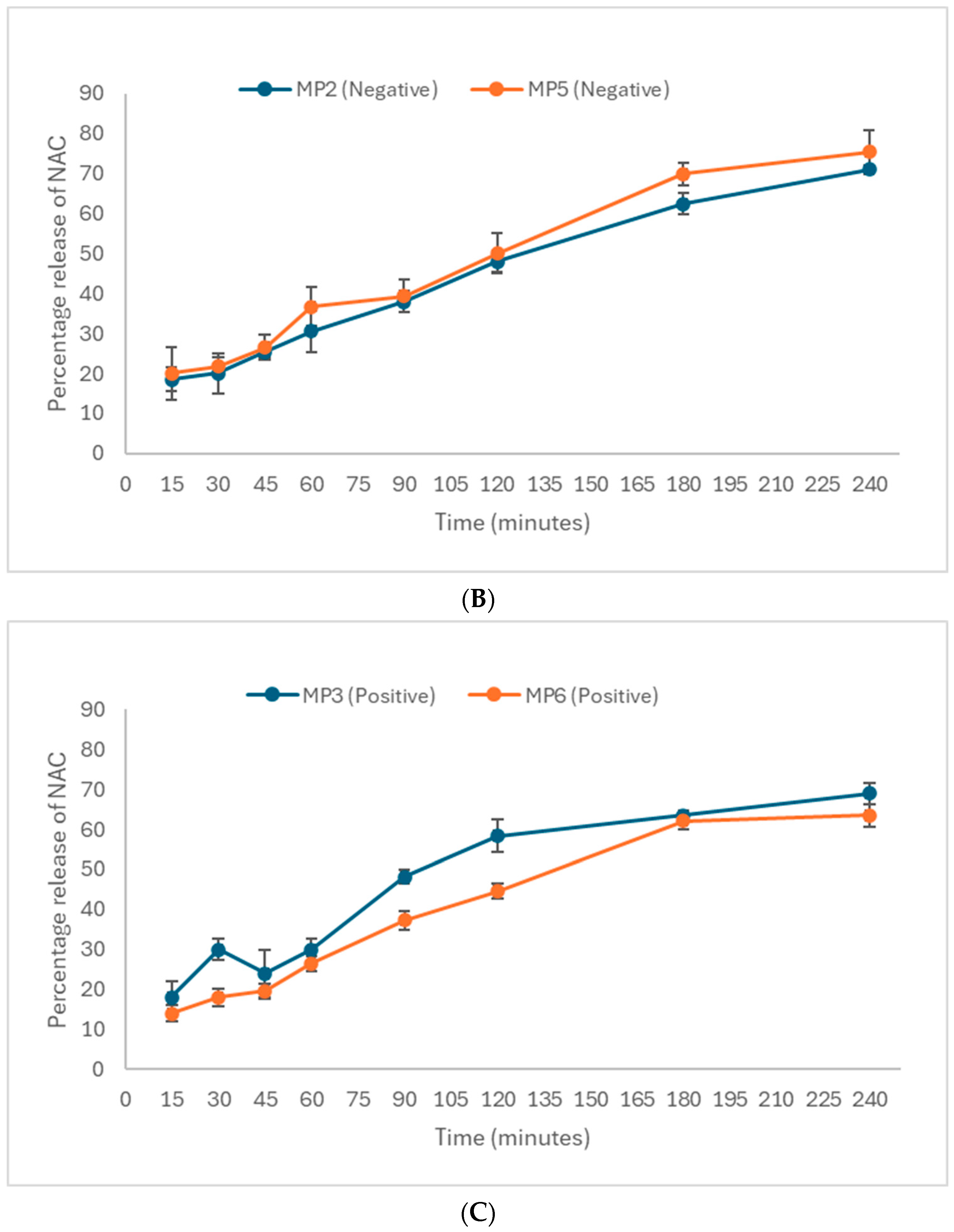
3.3.2. In Vitro Drug Release of N-Acetyl Cysteine, NAC
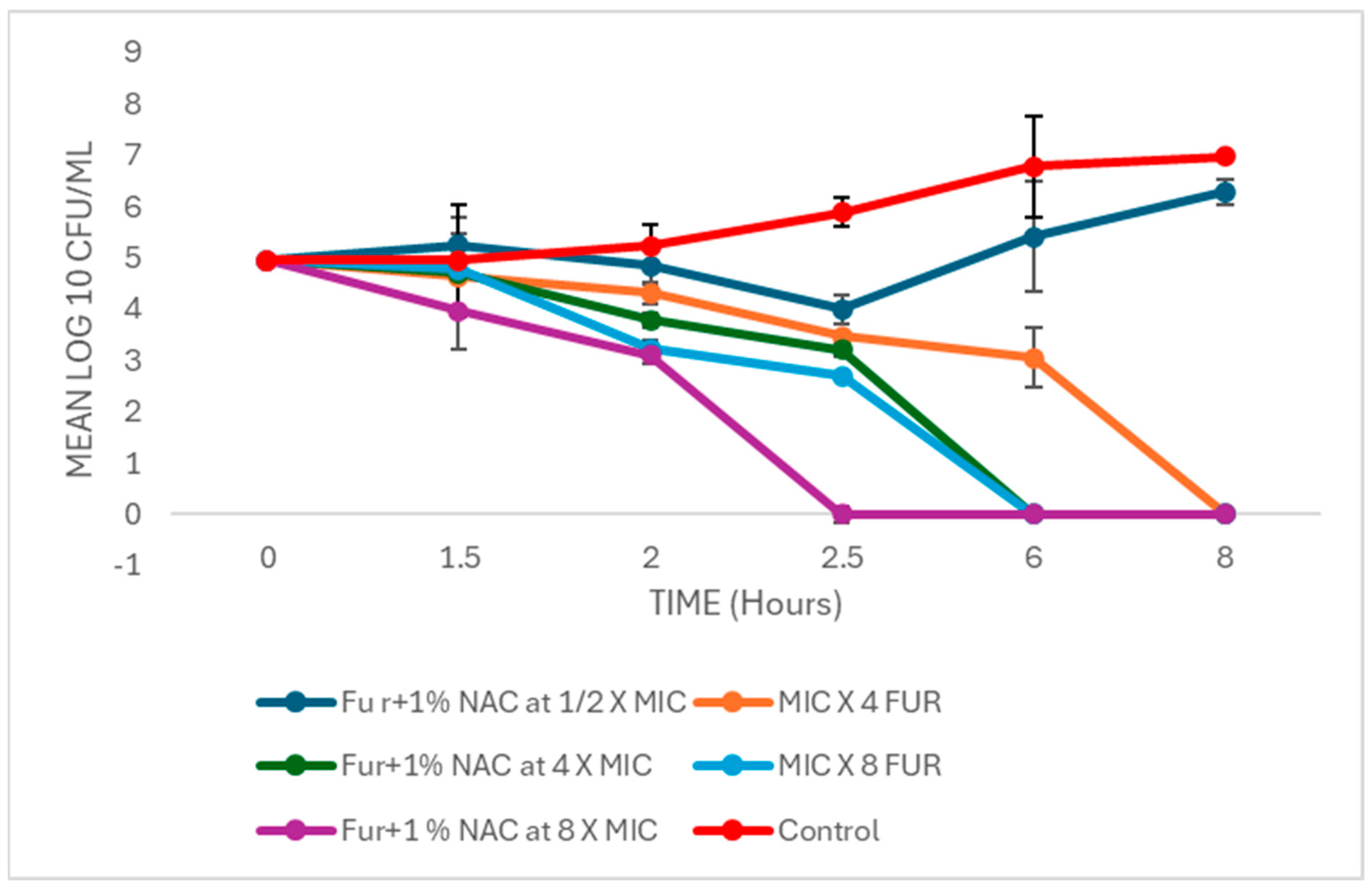
3.4. Time–Kill Curve of Augmented Therapy of Free and Liposomal-Bound Furazolidone with NAC
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Öztekin, M.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Overview of Helicobacter pylori Infection: Clinical Features, Treatment, and Nutritional Aspects. Diseases 2021, 9, 66. [Google Scholar] [CrossRef]
- Ali, A.; AlHussaini, K.I. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms 2024, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Z. Global whole family based-Helicobacter pylori eradication strategy to prevent its related diseases and gastric cancer. World J. Gastroenterol. 2020, 26, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, R.; Smith, S.M. An overview of Helicobacter pylori infection. In Helicobacter pylori; Smith, S.M., Ed.; Humana: Louisville, KY, USA, 2021; Volume 2283, pp. 1–20. [Google Scholar]
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, A.; Anagreyyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A.; et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics 2023, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.; Katelaris, P. Epidemiology, clinical impacts and current clinical management of Helicobacter pylori infection. Med. J. Aust. 2016, 10, 376–380. [Google Scholar] [CrossRef]
- Kienesberger, S.; Perez-Perez, G.I.; Olivares, A.Z.; Bardhan, P.; Sarker, S.A.; Hasan, K.Z.; Sack, R.B.; Blaser, M.J. When is Helicobacter pylori acquired in populations in developing countries? A birth-cohort study in Bangladeshi children. Gut Microbes 2018, 9, 252–263. [Google Scholar] [CrossRef]
- Guts Charity. Helicobacter pylori. Available online: https://gutscharity.org.uk/advice-and-information/conditions/helicobacter-pylori/ (accessed on 17 July 2024).
- Roberts, L.T.; Issa, P.P.; Sinnathamby, E.S.; Granier, M.; Mayeux, H.; Eubanks, T.N.; Malone, K.; Ahmadzadeh, S.; Cornett, E.M.; Shekoohi, S.; et al. Helicobacter Pylori: A Review of Current Treatment Options in Clinical Practice. Life 2022, 12, 2038. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance—From biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Alam, M.I.; Paget, T.; Elkordy, A.A. Formulation and advantages of furazolidone in liposomal drug delivery systems. Eur. J. Pharm. Sci. 2016, 84, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Resina, E.; Gisbert, J.P. Rescue Therapy with Furazolidone in Patients with at Least Five Eradication Treatment Failures and Multi-Resistant, H. pylori infection. Antibiotics 2021, 10, 1028. [Google Scholar] [CrossRef]
- Hajaghamohammadi, A.; Safiabadi Tali, S.H.; Samimi, R.; Oveisi, S.; Kazemifar, A.M. Low dose furazolidone for eradication of Helicobacter pylori instead of clarithromycin: A clinical trial. Glob. J. Health Sci. 2014, 7, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Secor, W.E.; Le Bras, J.; Clain, J. Mechanisms of resistance to antiparasitic agents. In Antibiotic and Chemotherapy: Anti-Infective Agents and Their Mechanisms of Action; Jorgensen, J.H., Carroll, K.C., Funke, G., Pfaller, M.A., Landry, M.L., Richter, S.S., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 123–136. [Google Scholar]
- Ji, C.R.; Liu, J.; Li, Y.Y.; Guo, C.G.; Qu, J.Y.; Zhang, Y.; Zuo, X. Safety of furazolidone-containing regimen in Helicobacter pylori infection: A systematic review and meta-analysis. BMJ Open 2020, 10, e037375. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.M. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Therap. Adv. Respir. Dis. 2012, 6, 127–135. [Google Scholar] [CrossRef]
- Kalyanaraman, B. NAC, NAC, Knockin’ on Heaven’s door: Interpreting the mechanism of action of N-acetylcysteine in tumor and immune cells. Redox Biol. 2022, 57, 102497. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, C.M. N-acetylcysteine in COPD: Why, how, and when? Multidiscip. Respir. Med. 2016, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Ourique, A.F.; Chaves, P.S.; Souto, G.D.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C. Redispersible liposomal-N-acetylcysteine powder for pulmonary administration: Development, in vitro characterization and antioxidant activity. Eur. J. Pharm. Sci. 2014, 65, 174–182. [Google Scholar] [CrossRef]
- Kundukad, B.; Schussman, M.; Yang, K.; Seviour, T.; Yang, L.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Mechanistic action of weak acid drugs on biofilms. Sci. Rep. 2017, 7, 4783. [Google Scholar] [CrossRef]
- Karbasi, A.; Hossein Hosseini, S.; Shohrati, M.; Amini, M.; Najafian, B. Effect of oral N-acetyl cysteine on eradication of Helicobacter pylori in patients with dyspepsia. Minerva Gastroenterol. Dietol. 2013, 59, 107–112. [Google Scholar]
- Fontes, L.E.S.; Martimbianco, A.L.C.; Zanin, C.; Riera, R. N-acetylcysteine as an adjuvant therapy for Helicobacter pylori eradication. Cochrane Database Syst. Rev. 2019, 2, CD012357. [Google Scholar] [CrossRef]
- Savva, D.A.; Crist, M.; Lardieri, A. N-Acetylcysteine for Gastric Lactobezoars in a 1-Month-Old. J. Pediatr. Pharmacol. Therap. 2019, 24, 247–250. [Google Scholar] [CrossRef]
- Banerjee, S.; McCormack, S. Acetylcysteine for Patients Requiring Mucous Secretion Clearance: A Review of Clinical Effectiveness and Safety [Internet]; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Hamidian, S.M.; Aletaha, N.S.; Taslimi, R.; Montazeri, M. An additive effect of oral N-acetyl cysteine on eradication of Helicobacter pylori. J. Pathog. 2015, 2015, 540271. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.; Krauland, E.; Wirtz, D.; Hanes, J. Transport of polymeric nanoparticle gene carriers in gastric mucus. Biotechnol. Prog. 2004, 20, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Mark, W.S. Drug delivery: Stealth particles give mucus the slip. Nat. Mater. 2009, 8, 11–13. [Google Scholar]
- Khaliq, N.U.; Lee, J.; Kim, S.; Sung, D.; Kim, H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics 2023, 15, 2102. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cai, X.; Huang, Y.; Zhou, Y. Pluronic F127-liposome-encapsulated curcumin activates Nrf2/Keap1 signaling pathway to promote cell migration of HaCaT cells. Mol. Cell. Biochem. 2023, 478, 241–247. [Google Scholar] [CrossRef]
- Su, S.; M Kang, P. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M. Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): Main features and their applications in topical and transdermal administration of drugs. J. Pharm. Pharm. Sci. 2012, 15, 592–605. [Google Scholar] [CrossRef]
- Kim, J.C.; Chungt, Y.I.; Kim, Y.H.; Tae, G. The modulation of the permeability and the cellular uptake of liposome by stable anchoring of lipid-conjugated pluronic on liposome. J. Biomed. Nanotechnol. 2014, 10, 100–108. [Google Scholar] [CrossRef]
- Ferrari, S.; Kitson, C.; Farley, R.; Steel, R.; Marriott, C.; Parkins, D.A.; Scarpa, M.; Wainwright, B.; Evans, M.J.; Colledge, W.H.; et al. Mucus altering agents as adjuncts for nonviral gene transfer to airway epithelium. Gene Ther. 2001, 8, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Fu, V.; Zhu, J.; Lu, D.; Gao, W.; Zhang, L. Nanoparticle-stabilized liposomes for pH-responsive gastric drug delivery. Langmuir ACS J. Surf. Colloids 2013, 29, 12228–12233. [Google Scholar] [CrossRef]
- Neves, L.F.; Duan, J.; Voelker, A.; Khanal, A.; McNally, L.; Steinbach-Rankins, J.; Ceresa, B.P. Preparation and optimisation of anionic liposomes for delivery of small peptides and cDNA to human corneal epithelial cells. J. Microencapsul. 2016, 33, 391–399. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Jonnalagadda, S.B. A comprehensive review on novel liposomal methodologies, commercial formulations, clinical trials and patents. BioNanoScience 2022, 12, 274–291. [Google Scholar] [CrossRef]
- Paramshetti, S.; Angolkar, M.; Talath, S.; Osmani, R.A.M.; Spandana, A.; Al Fatease, A.; Hani, U.; Ramesh, K.V.R.N.S.; Singh, E. Unravelling the in vivo dynamics of liposomes: Insights into biodistribution and cellular membrane interactions. Life Sci. 2024, 346, 122616. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef]
- Watchorn, J.; Clasky, A.J.; Prakash, G.; Johnston, I.A.E.; Chen, P.Z.; Gu, F.X. Untangling Mucosal Drug Delivery: Engineering, Designing, and Testing Nanoparticles to Overcome the Mucus Barrier. ACS Biomater. Sci. Eng. 2022, 8, 1396–1426. [Google Scholar] [CrossRef] [PubMed]
- Witten, J.; Samad, T.; Ribbeck, K. Selective permeability of mucus barriers. Curr. Opin. Biotechnol. 2018, 52, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Mah, K.K.; Seluakumaran, K. Gastric mucosal barrier, Helicobacter pylori. In Defining Physiology: Principles, Themes, Concepts; Springer: Cham, Switzerland, 2020; Volume 2. [Google Scholar]
- Elorza, B.; Elorza, M.A.; Frutos, G.; Chantres, J.R. Characterization of 5-fluorouracil loaded liposomes prepared by reverse-phase evaporation or freezing-thawing extrusion methods: Study of drug release. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1153, 135–142. [Google Scholar] [CrossRef]
- Cortesi, R.; Esposito, E.; Gambarin, S.; Telloli, P.; Menegatti, E.; Nastruzzi, C. Preparation of liposomes by reverse-phase evaporation using alternative organic solvents. J. Microencapsul. 1999, 16, 251–256. [Google Scholar] [CrossRef]
- Handa, T.; Naito, S.; Hiramatsu, M.; Tsuboi, M. Thermal SiO and H13CO+ line observations of the dense molecular cloud G0.11-0.11 in the Galactic Center Region. Astrophys. J. 2006, 636, 261–266. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Pan, H.; He, F.; Liu, Z.; Wu, Q.; Bai, C.; Yu, S.; Yang, X. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci. Rep. 2016, 6, 28796. [Google Scholar] [CrossRef]
- Lai, S.K.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275–282. [Google Scholar] [CrossRef]
- Aljayyoussi, G.; Muthanna, A.; Peter, G.; Gumbleton, M. Pharmaceutical nanoparticles and the mucin biopolymer barrier. BioImpacts 2012, 2, 173–174. [Google Scholar] [PubMed]
- Ensign, L.M.; Lai, S.K.; Wang, Y.Y.; Yang, M.; Mert, O.; Hanes, J.; Cone, R. Pretreatment of human cervicovaginal mucus with pluronic F127 enhances nanoparticle penetration without compromising mucus barrier properties to herpes simplex virus. Biomacromolecules 2014, 15, 4403–4409. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, S.S.; Padgett, J.L.; Yudin, A.I.; Whaley, K.J.; Moench, T.R.; Cone, R.A. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J. 2001, 81, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liang, Y.; Liu, L.; Yin, M.; Wang, A.; Sun, K.; Li, Y.; Shi, Y. Research on the fate of polymeric nanoparticles in the process of intestinal absorption based on model nanoparticles with various characteristics: Size, surface charge and pro-hydrophobics. J. Nanobiotechnol. 2019, 19, 32. [Google Scholar] [CrossRef]
- Yang, M.; Lai, S.K.; Wang, Y.Y.; Zhong, W.; Happe, C.; Zhang, M.; Fu, J.; Hanes, J. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angew. Chem. Int. Ed. 2011, 50, 2597–2600. [Google Scholar] [CrossRef] [PubMed]
- Chater, P.I.; Wilcox, M.D.; Pearson, J.P. Efficacy and safety concerns over the use of mucus modulating agents for drug delivery using nanoscale systems. Adv. Drug Deliv. Rev. 2018, 124, 184–192. [Google Scholar] [CrossRef]
- Henke, M.O.; Ratjen, F. Mucolytics in cystic fibrosis. Paediatr. Respir. Rev. 2007, 8, 24–29. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on human health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Celli, J.; Brian, G.; Bradley, T.; Nezam, H.; Afdhal, R.B.; Hyamsunder, E. Viscoelastic properties and dynamics of porcine gastric mucin. Biomacromolecules 2005, 6, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Lebwohl, B.; Abrams, J.A. The impact of proton pump inhibitors on the human gastrointestinal microbiome. Clin. Lab. Med. 2014, 34, 771–785. [Google Scholar] [CrossRef]
- Hong, Z.; Chasan, B.; Bansil, R.; Turner, B.S.; Bhaskar, K.R.; Afdhal, N.H. Atomic force microscopy reveals aggregation of gastric mucin at low pH. Biomacromolecules 2005, 6, 3458–3466. [Google Scholar] [CrossRef]
- Rubin, B.K.; Thornton, D.J. Dropping acid: Why is cystic fibrosis mucus abnormal? Eur. Respir. J. 2018, 52, 1802057. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.S.; Samuel, K.L.; Nicholas, J.B.; Michelle, R.D.; Michael, P.B.; Justin, H. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine 2011, 6, 365–375. [Google Scholar]
- Duncan, G.A.; Jung, J.; Hanes, J.; Suk, J.S. The mucus barrier to inhaled gene therapy. Mol. Ther. 2016, 24, 2043–2053. [Google Scholar] [CrossRef]
- Mohamed, M.N.; Labiba, K.E.; Nawal, A.K.; Said, A.K. In vitro release of hydrophilic and hydrophobic drugs from liposomal dispersions and gels. Acta Pharm. 2006, 56, 311–324. [Google Scholar]
- El-Nesr, O.H.; Yahiya, S.A.; El-Gazayerly, O.N. Effect of formulation design and freeze-drying on properties of fluconazole multilamellar liposomes. Saudi Pharm. J. 2010, 18, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Yu, N.; Li, J.; Hong, D.; Li, X.; Li, L.; Bin, H.; Yao, W.; Zhongwei, G. Cholesterol derivatives based charged liposomes for doxorubicin delivery: Preparation, in vitro and in vivo characterization. Theranostics 2012, 2, 1092–1103. [Google Scholar]
- Rukholm, G.; Mugabe, C.; Azghani, A.O.; Omri, A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: A time-kill study. Int. J. Antimicrob. Agents 2006, 27, 247–252. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y. N-acetylcysteine inhibits biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef]
- Goswami, M.; Jawali, N. N-acetylcysteine-mediated modulation of bacterial antibiotic susceptibility. Antimicrob. Agents Chemother. 2010, 54, 3529–3530. [Google Scholar] [CrossRef] [PubMed]
- Kian, M.; Frank, K.F. The potential role of N-acetylcysteine for the treatment of Helicobacter pylori. J. Clin. Gastroenterol. 2011, 45, 841–843. [Google Scholar]
- Biswas, D.P.; Tk, D.S. The efficacy of adjuvant N-acetyl cysteine for the eradication of H. pylori infections: A systematic review and meta-analysis of randomized clinical trials. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101832. [Google Scholar] [PubMed]
- Masaaki, M.; Takafumi, A.; Shin-nosuke, H.; Keizo, T.; Tadao, H.; Dawn, A.I.; Kenji, I.; Kazuo, K.; Hidemi, G.; Michio, O. Effect of glycine on Helicobacter pylori in vitro. Antimicrob. Agents Chemother. 2004, 48, 3782–3788. [Google Scholar]
- Parisa, J.; Mohsen, A.; Abbas, Y.; Rasool, N.; Roghayeh, T. Evaluation inhibitory effect of glycine on Helicobacter pylori in experimental condition. Res. Sq. 2023. advance online publication. [Google Scholar] [CrossRef]
- Rajak, P.; Bhattacharya, N.; Sharma, N.; Sarma, M.K.; Kataki, R. Gastro-retentive floating drug delivery system—An approach in gastroretentive drug delivery. Int. J. Pharm. Sci. 2011, 3, 9–16. [Google Scholar]



| Liposomes | Coding | Lipid/DSPC | Cholesterol | Total Lipid | DDAB | DCP | Furazolidone | NAC | Pluronic F-127 |
|---|---|---|---|---|---|---|---|---|---|
| Neutral | MP1 | 58.5 | 6.5 | 65 | - | - | 6 | 14 | 5 |
| Neutral | MP4 | 58.5 | 6.5 | 65 | - | - | 6 | 14 | - |
| Negative | MP2 | 57.5 | 6.5 | 65 | - | 1 | 6 | 14 | 5 |
| Negative | MP5 | 57.5 | 6.5 | 65 | - | 1 | 6 | 14 | - |
| Positive | MP3 | 57.5 | 6.5 | 65 | 1 | - | 6 | 14 | 5 |
| Positive | MP6 | 57.5 | 6.5 | 65 | 1 | - | 6 | 14 | - |
| Formulation | Encapsulation Efficiency (%) | Zeta Potential (mV) | Particle Size (nm) | |
|---|---|---|---|---|
| Furazolidone | NAC | |||
| MP1 | 58.9 ± 3.4 | 51.41 ± 1.2 | +3.5 | 490 |
| MP2 | 62.2 ± 3.2 | 52.35 ± 0.9 | −19.1 | 520 |
| MP3 | 67.9 ± 3.8 | 54.25 ± 1.5 | +10.3 | 600 |
| MP4 | 56.0 ± 4.2 | 50.53 ± 2.1 | −1.4 | 530 |
| MP5 | 63.9 ± 6.5 | 59.52 ± 1.2 | −36.1 | 570 |
| MP6 | 65.6 ± 7.5 | 62.45 ± 2.3 | +7.2 | 740 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.I.; Paget, T.; Moosa, N.Y.; Alghurairy, H.; Elkordy, A.A. Liposomal Drug Delivery against Helicobacter pylori Using Furazolidone and N-Acetyl Cysteine in Augmented Therapy. Pharmaceutics 2024, 16, 1123. https://doi.org/10.3390/pharmaceutics16091123
Alam MI, Paget T, Moosa NY, Alghurairy H, Elkordy AA. Liposomal Drug Delivery against Helicobacter pylori Using Furazolidone and N-Acetyl Cysteine in Augmented Therapy. Pharmaceutics. 2024; 16(9):1123. https://doi.org/10.3390/pharmaceutics16091123
Chicago/Turabian StyleAlam, Muhammad Irfan, Timothy Paget, Najla Yussuf Moosa, Husein Alghurairy, and Amal Ali Elkordy. 2024. "Liposomal Drug Delivery against Helicobacter pylori Using Furazolidone and N-Acetyl Cysteine in Augmented Therapy" Pharmaceutics 16, no. 9: 1123. https://doi.org/10.3390/pharmaceutics16091123
APA StyleAlam, M. I., Paget, T., Moosa, N. Y., Alghurairy, H., & Elkordy, A. A. (2024). Liposomal Drug Delivery against Helicobacter pylori Using Furazolidone and N-Acetyl Cysteine in Augmented Therapy. Pharmaceutics, 16(9), 1123. https://doi.org/10.3390/pharmaceutics16091123








