Topic Menu
► Topic MenuTopic Editors


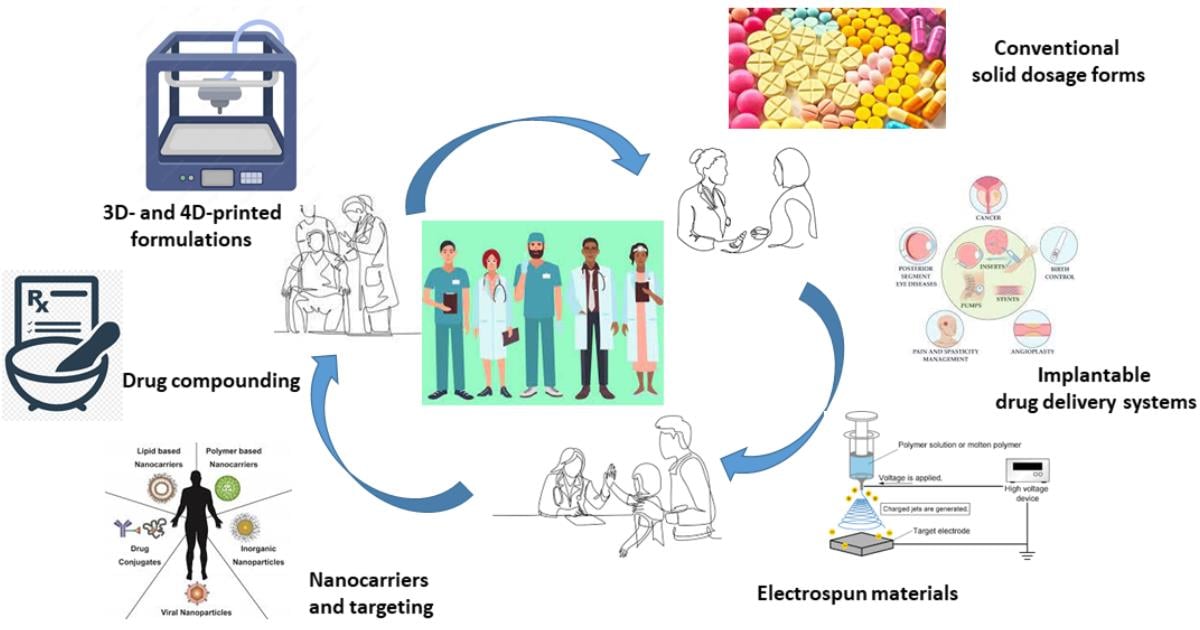
Personalized Drug Formulations
Topic Information
Dear Colleagues,
Personalized medicine, also known as precision medicine, represents a flourishing field of research in the medical and pharmaceutical fields. It refers to the customization of medical treatments to the individual characteristics, needs, and preferences of each person and the choice of appropriate therapies for specific groups of patients affected by the same disease. This approach overcomes the concept of “one-size-fits-all” by proposing the administration of the “right drug” in its “right quantity” for the “right patient” at the “right time”. To achieve this goal, there is a need to develop novel medications tailored to the patient’s condition, which they can be termed as “personalized drug formulations”.
This topic covers any aspect related to the tailored drug therapy regarding formulations and dosage forms in a multidisciplinary context.
We are pleased to invite you to contribute to this topic, which aims to highlight the latest advances in personalized drug formulations, with particular emphasis on innovative approaches that address the current challenges in this research area. All manuscript submissions are welcome.
Dr. Diego Romano Perinelli
Dr. Florentina Lupascu
Topic Editors
Keywords
- drug delivery systems
- tableting
- 3D- and 4D-printing
- electrospinning
- compounding
- controlled release
- nanocarriers
- targeting
- smart formulation
- innovative devices
- pharmacogenomics
- surface modification
- functional polymers
Participating Journals
| Journal Name | Impact Factor | CiteScore | Launched Year | First Decision (median) | APC |
|---|---|---|---|---|---|

Biomedicines
|
3.9 | 5.2 | 2013 | 14.6 Days | CHF 2600 |

Journal of Personalized Medicine
|
- | 4.1 | 2011 | 17.4 Days | CHF 2600 |
Pharmaceuticals
|
4.3 | 6.1 | 2004 | 13.9 Days | CHF 2900 |

Pharmaceutics
|
4.9 | 7.9 | 2009 | 15.5 Days | CHF 2900 |

Preprints.org is a multidisciplinary platform offering a preprint service designed to facilitate the early sharing of your research. It supports and empowers your research journey from the very beginning.
MDPI Topics is collaborating with Preprints.org and has established a direct connection between MDPI journals and the platform. Authors are encouraged to take advantage of this opportunity by posting their preprints at Preprints.org prior to publication:
- Share your research immediately: disseminate your ideas prior to publication and establish priority for your work.
- Safeguard your intellectual contribution: Protect your ideas with a time-stamped preprint that serves as proof of your research timeline.
- Boost visibility and impact: Increase the reach and influence of your research by making it accessible to a global audience.
- Gain early feedback: Receive valuable input and insights from peers before submitting to a journal.
- Ensure broad indexing: Web of Science (Preprint Citation Index), Google Scholar, Crossref, SHARE, PrePubMed, Scilit and Europe PMC.

