Development of an Intranasal In Situ System for Ribavirin Delivery: In Vitro and In Vivo Evaluation
Abstract
:1. Introduction
1.1. Specifics of Intranasal Administration
1.2. Selection of an Optimal Gelation Stimulus
2. Materials and Methods
2.1. Equipment
2.2. Excipients and Chemicals
2.3. Design of Experiment
2.4. Methods
2.4.1. In Vitro Studies
Determination of In Situ Gelation Ability
pH Measurement
Viscosity Measurement
Gelation Temperature
Gelation pH
Determination of the Completeness of Retention on the Mucosal Surface In Vitro
Spray Torch Measurement
2.4.2. In Vivo “Animal” Studies
Animals
Sample Collection
Pharmacokinetic Study
Statistical Analysis
3. Results
3.1. Preparation of Stimulus-Sensitive Compositions
3.2. In Vitro Studies Results
3.2.1. Gelation Ability of Placebo Compositions
3.2.2. pH of Placebo Compositions
3.2.3. Plastic Viscosity of Placebo Compositions
3.2.4. Gelation Temperature
3.2.5. Completeness of Retention of Placebo Formulations
3.2.6. Spray Torch of Placebo Formulations
3.2.7. Results of In Vitro Studies after API Addition
3.3. In Vivo “Animal” Studies Results
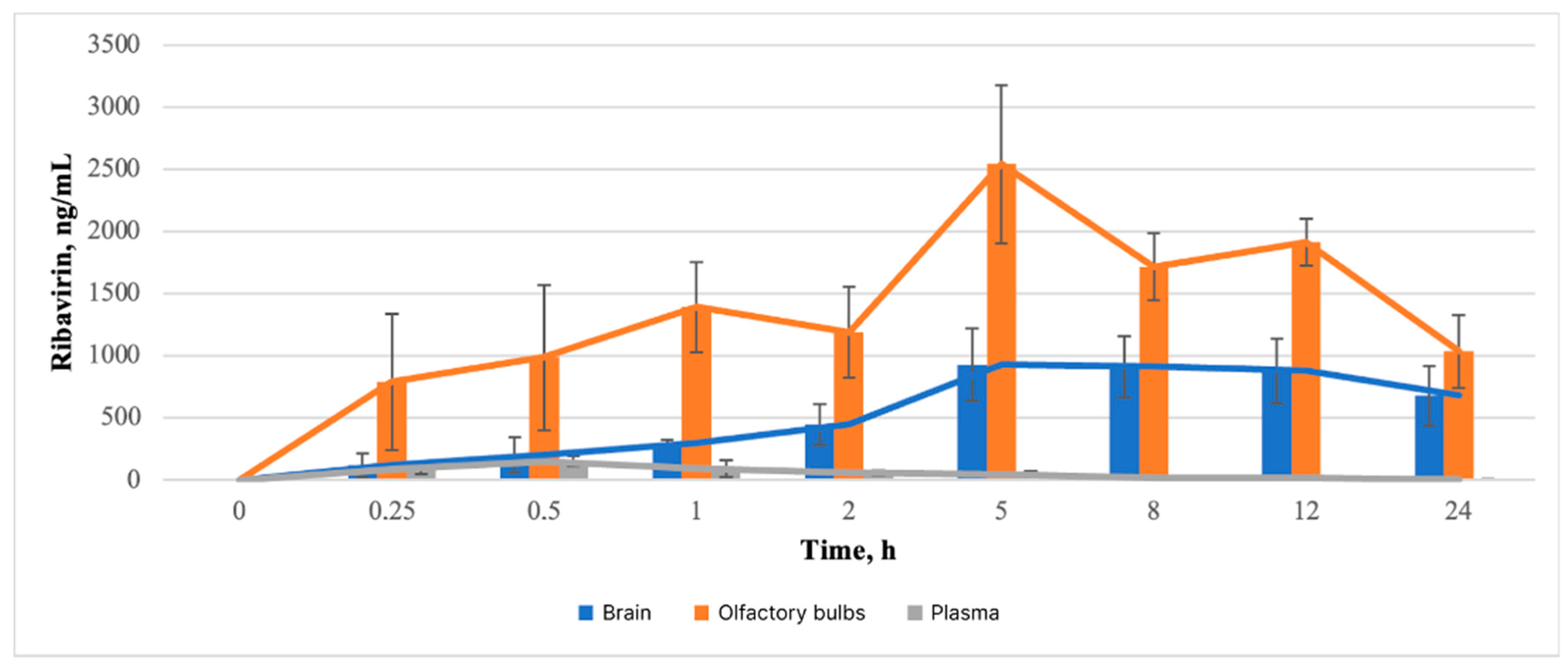
3.3.1. Analytical Study of Brain, Olfactory Bulb, and Blood Plasma Samples
3.3.2. Pharmacokinetic Study of Brain
3.3.3. Pharmacokinetic Study of Olfactory Bulb
3.3.4. Pharmacokinetic Study of Blood Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Reagents
Appendix A.2. Method
| Analyte | Retention Time (min) | Precursor (m/z) | Product (m/z) | Fragmentor (V) | CE (V) |
|---|---|---|---|---|---|
| Ribavirin | 1.57 | 245 | 113 | 80 | 7 |
| 13C5-Ribavirin | 1.57 | 250 | 113 | 80 | 7 |
| Uridine | 2.01 | 245 | 113 | 60 | 5 |
| Cytidine | 2.33 | 244 | 112 | 60 | 5 |
Appendix A.3. Selectivity
Appendix A.4. Linearity
| Analyte | Linear Range (ng/mL) | Slope Mean ± SD | Intercept Mean ± SD | R Mean ± SD |
|---|---|---|---|---|
| Blood plasma | ||||
| Ribavirin | 1–1000 | 1.214 ± 0.017 | 0.065 ± 0.021 | 0.9991 ± 0.0002 |
| Brain | ||||
| Ribavirin | 1–1000 | 1.085 ± 0.045 | 0.047 ± 0.013 | 0.9989 ± 0.0005 |
| Olfactory bulb | ||||
| 1–1000 | 1.101 ± 0.014 | 0.074 ± 0.005 | 0.9998 ± 0.0003 | |
Appendix A.5. Lowest Limit of Quantification
Appendix A.6. Accuracy and Precision
| Analyte | Spiked Concentration (ng/mL) | Calculated Concentration (ng/mL) | RSD (%) | Accuracy, % |
|---|---|---|---|---|
| Ribavirin | 3 | 3.17 ± 0.34 | 10.7 | 105.7 |
| 300 | 310.1 ± 9.4 | 3.0 | 103.4 | |
| 750 | 765 ± 38 | 4.9 | 102.0 |
| Analyte | Spiked Concentration (ng/mL) | Calculated Concentration (ng/mL) | RSD (%) | Accuracy, % |
|---|---|---|---|---|
| Ribavirin | 3 | 3.05 ± 0.14 | 4.6 | 101.7 |
| 300 | 316.6 ± 7.2 | 2.3 | 105.5 | |
| 750 | 785 ± 21 | 2.7 | 104.7 |
| Analyte | Spiked Concentration (ng/mL) | Calculated Concentration (ng/mL) | RSD (%) | Accuracy, % |
|---|---|---|---|---|
| Ribavirin | 3 | 3.21 ± 0.42 | 13.1 | 107.0 |
| 300 | 284.1 ± 22.5 | 7.91 | 94.7 | |
| 750 | 675 ± 54 | 8.0 | 90.0 |
| Analyte | Spiked Concentration (ng/mL) | Calculated Concentration (ng/mL) | RSD (%) | Accuracy, % |
|---|---|---|---|---|
| Ribavirin | 3 | 3.18 ± 0.11 | 3.5 | 106.0 |
| 300 | 297.3 ± 13.5 | 4.5 | 99.1 | |
| 750 | 691 ± 47 | 6.8 | 108.9 |
| Analyte | Spiked Concentration (ng/mL) | Calculated Concentration (ng/mL) | RSD (%) | Accuracy, % |
|---|---|---|---|---|
| Ribavirin | 3 | 3.01 ± 0.14 | 4.71 | 100.3 |
| 300 | 307.2 ± 11.6 | 3.86 | 102.4 | |
| 750 | 849 ± 17 | 2.0 | 113.2 |
| Analyte | Spiked Concentration (ng/mL) | Calculated Concentration (ng/mL) | RSD (%) | Accuracy, % |
|---|---|---|---|---|
| Ribavirin | 3 | 3.00 ± 0.12 | 1.05 | 100.1 |
| 300 | 304.6 ± 9.6 | 3.2 | 101.5 | |
| 750 | 817 ± 26 | 3.1 | 108.9 |
Appendix A.7. Recovery and Matrix Effect
Appendix A.8. Stability
| Name | Sample | Concentration Level | Accuracy, % | Precision, %RSD |
|---|---|---|---|---|
| Short-term stability in working solutions (RT, 6 h) | LQC | 95.1 ± 1.4 | 4.8 | |
| HQC | 94.8 ± 1.3 | 9.2 | ||
| Short-term stability in biological samples (RT, 6 h) | Plasma | LQC | 87.4 ± 4.5 | 3.5 |
| HQC | 101.9 ± 1.9 | 4.8 | ||
| Brain | LQC | 98.4 ± 5.4 | 9.4 | |
| HQC | 102.4 ± 8.8 | 12.3 | ||
| Olfactory bulb | LQC | 104.7 ± 0.8 | 11.8 | |
| HQC | 100.9 ± 1.7 | 10.9 | ||
| Autosampler stability in biological samples (+10 °C, 24 h) | Plasma | LQC | 104.8 ± 5.5 | 8.4 |
| HQC | 104.2 ± 6.4 | 5.6 | ||
| Brain | LQC | 100.1 ± 1.1 | 4.0 | |
| HQC | 97.8 ± 0.4 | 1.2 | ||
| Olfactory bulb | LQC | 97.5 ± 4.8 | 5.9 | |
| HQC | 99.4 ± 6.5 | 4.9 | ||
| Post-preparative stability in biological samples (+4 °C, 24 h) | Plasma | LQC | 95.4 ± 3.2 | 7.8 |
| HQC | 89.5 ± 3.7 | 6.1 | ||
| Brain | LQC | 99.8 ± 2.4 | 10.2 | |
| HQC | 100.8 ± 1.9 | 6.5 | ||
| Olfactory bulb | LQC | 97.7 ± 4.1 | 1.5 | |
| HQC | 97.5 ± 0.8 | 11.9 | ||
| Freeze–thaw stability in biological samples | Plasma | LQC | 94.8 ± 2.4 | 12.8 |
| HQC | 88.7 ± 1.8 | 13.6 | ||
| Brain | LQC | 110.1 ± 6.4 | 1.0 | |
| HQC | 97.6 ± 4.7 | 0.6 | ||
| Olfactory bulb | LQC | 99.4 ± 6.4 | 5.9 | |
| HQC | 101.4 ± 1.1 | 4.8 | ||
| Long-term stability in biological samples (−20 °C, 22 days) | Plasma | LQC | 100.8 ± 9.4 | 1.5 |
| HQC | 87.5 ± 0.4 | 0.5 | ||
| Brain | LQC | 96.8 ± 1.3 | 5.8 | |
| HQC | 96.4 ± 5.4 | 4.9 | ||
| Olfactory bulb | LQC | 95.7 ± 8.1 | 1.9 | |
| HQC | 93.4 ± 0.9 | 2.5 |
References
- Global cancer burden growing, amidst mounting need for services. Available online: https://www.Who.Int/News/Item/01-02-2024-Global-Cancer-Burden-Growing--amidst-Mounting-Need-for-Services (accessed on 7 May 2024).
- Kamar, N.; Abravanel, F.; Behrendt, P.; Hofmann, J.; Pageaux, G.P.; Barbet, C.; Moal, V.; Couzi, L.; Horvatits, T.; De Man, R.A.; et al. Ribavirin for Hepatitis E Virus Infection After Organ Transplantation: A Large European Retrospective Multicenter Study. Clin. Infect. Dis. 2020, 71, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.P.; Duvignaud, A.; Jaspard, M.; Malvy, D.; Carroll, M.; Tarning, J.; Olliaro, P.L.; Horby, P.W. Ribavirin for Treating Lassa Fever: A Systematic Review of Pre-Clinical Studies and Implications for Human Dosing. PLoS Negl. Trop. Dis. 2022, 16, e0010289. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, K.A.; Mischlinger, J.; Jordan, S.; Groger, M.; Günther, S.; Ramharter, M. Ribavirin for the Treatment of Lassa Fever: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2019, 87, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mayor, J.; Engler, O.; Rothenberger, S. Antiviral Efficacy of Ribavirin and Favipiravir against Hantaan Virus. Microorganisms 2021, 9, 1306. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Martinez-Reviejo, R.; Karakoc, H.N.; Peña-López, Y.; Manuel, O.; Rello, J. Ribavirin for Treatment of Subjects with Respiratory Syncytial Virus-Related Infection: A Systematic Review and Meta-Analysis. Adv. Ther. 2022, 39, 4037–4051. [Google Scholar] [CrossRef]
- Poulakou, G.; Barakat, M.; Israel, R.J.; Bacci, M.R. Virazole Collaborator Group for COVID-19 Respiratory Distress Ribavirin Aerosol in Hospitalized Adults with Respiratory Distress and COVID-19: An Open-Label Trial. Clin. Transl. Sci. 2023, 16, 165–174. [Google Scholar] [CrossRef]
- Unal, M.A.; Bitirim, C.V.; Summak, G.Y.; Bereketoglu, S.; Zeytin, I.C.; Besbinar, O.; Gurcan, C.; Aydos, D.; Goksoy, E.; Kocakaya, E.; et al. Ribavirin Shows Antiviral Activity against SARS-CoV-2 and Downregulates the Activity of TMPRSS2 and the Expression of ACE2 in vitro. Can. J. Physiol. Pharmacol. 2021, 99, 449–460. [Google Scholar] [CrossRef]
- Janowski, A.B.; Dudley, H.; Wang, D. Antiviral Activity of Ribavirin and Favipiravir against Human Astroviruses. J. Clin. Virol. 2020, 123, 104247. [Google Scholar] [CrossRef]
- Huq, S.; Casaos, J.; Serra, R.; Peters, M.; Xia, Y.; Ding, A.S.; Ehresman, J.; Kedda, J.N.; Morales, M.; Gorelick, N.L.; et al. Repurposing the FDA-Approved Antiviral Drug Ribavirin as Targeted Therapy for Nasopharyngeal Carcinoma. Mol. Cancer Ther. 2020, 19, 1797–1808. [Google Scholar] [CrossRef]
- Casaos, J.; Gorelick, N.L.; Huq, S.; Choi, J.; Xia, Y.; Serra, R.; Felder, R.; Lott, T.; Kast, R.E.; Suk, I.; et al. The Use of Ribavirin as an Anticancer Therapeutic: Will It Go Viral? Mol. Cancer Ther. 2019, 18, 1185–1194. [Google Scholar] [CrossRef]
- Zhu, S.; Han, X.; Yang, R.; Tian, Y.; Zhang, Q.; Wu, Y.; Dong, S.; Zhang, B. Metabolomics Study of Ribavirin in the Treatment of Orthotopic Lung Cancer Based on UPLC-Q-TOF/MS. Chem. Interact. 2023, 370, 110305. [Google Scholar] [CrossRef]
- Burman, B.; Drutman, S.B.; Fury, M.G.; Wong, R.J.; Katabi, N.; Ho, A.L.; Pfister, D.G. Pharmacodynamic and Therapeutic Pilot Studies of Single-Agent Ribavirin in Patients with Human Papillomavirus–Related Malignancies. Oral Oncol. 2022, 128, 105806. [Google Scholar] [CrossRef] [PubMed]
- Wambecke, A.; Laurent-Issartel, C.; Leroy-Dudal, J.; Giffard, F.; Cosson, F.; Lubin-Germain, N.; Uziel, J.; Kellouche, S.; Carreiras, F. Evaluation of the Potential of a New Ribavirin Analog Impairing the Dissemination of Ovarian Cancer Cells. PLoS ONE 2019, 14, e0225860. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, R.; Tian, Y.; Ge, S.; Nan, X.; Zhu, S.; Dong, S.; Zhang, B. Ribavirin Inhibits Cell Proliferation and Metastasis and Prolongs Survival in Soft Tissue Sarcomas by Downregulating Both Protein Arginine Methyltransferases 1 and 5. Basic Clin. Pharmacol. Toxicol. 2022, 131, 18–33. [Google Scholar] [CrossRef]
- Witkowski, J.T.; Robins, R.K.; Sidwell, R.W.; Simon, L.N. Design, Synthesis, and Broad Spectrum Antiviral Activity of 1-.Beta.-D-Ribofuranosyl-1,2,4-Triazole-3-Carboxamide and Related Nucleosides. J. Med. Chem. 1972, 15, 1150–1154. [Google Scholar] [CrossRef]
- Crotty, S.; Cameron, C.; Andino, R. Ribavirin’s Antiviral Mechanism of Action: Lethal Mutagenesis? J. Mol. Med. 2002, 80, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.H.; Sidwell, R.W.; Khare, G.P.; Witkowski, J.T.; Allen, L.B.; Robins, R.K. In Vitro Effect of 1-β- d -Ribofuranosyl-1,2,4-Triazole-3-Carboxamide (Virazole, ICN 1229) on Deoxyribonucleic Acid and Ribonucleic Acid Viruses. Antimicrob. Agents Chemother. 1973, 3, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xiang, W.; Wu, S.; Wang, M.; Xiao, M.; Deng, A. Targeting eIF4E Signaling with Ribavirin as a Sensitizing Strategy for Ovarian Cancer. Biochem. Biophys. Res. Commun. 2019, 510, 580–586. [Google Scholar] [CrossRef]
- Volpin, F.; Casaos, J.; Sesen, J.; Mangraviti, A.; Choi, J.; Gorelick, N.; Frikeche, J.; Lott, T.; Felder, R.; Scotland, S.J.; et al. Use of an Anti-Viral Drug, Ribavirin, as an Anti-Glioblastoma Therapeutic. Oncogene 2017, 36, 3037–3047. [Google Scholar] [CrossRef]
- Ochiai, Y.; Sano, E.; Okamoto, Y.; Yoshimura, S.; Makita, K.; Yamamuro, S.; Ohta, T.; Ogino, A.; Tadakuma, H.; Ueda, T.; et al. Efficacy of Ribavirin against Malignant Glioma Cell Lines: Follow-Up Study. Oncol. Rep. 2017, 39, 537–544. [Google Scholar] [CrossRef]
- Levy, G. Kinetics of Drug Action: An Overview. J. Allergy Clin. Immunol. 1986, 78, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network Pharmacology: The Next Paradigm in Drug Discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Colombo, G.; Lorenzini, L.; Zironi, E.; Galligioni, V.; Sonvico, F.; Balducci, A.G.; Pagliuca, G.; Giuliani, A.; Calzà, L.; Scagliarini, A. Brain Distribution of Ribavirin after Intranasal Administration. Antivir. Res. 2011, 92, 408–414. [Google Scholar] [CrossRef]
- Porfiryeva, N.N.; Semina, I.I.; Moustafine, R.I.; Khutoryanskiy, V.V. Intranasal Administration as a Route to Deliver Drugs to the Brain (Review). Drug Dev. Regist. 2021, 10, 117–127. [Google Scholar] [CrossRef]
- Kurano, T.; Kanazawa, T.; Ooba, A.; Masuyama, Y.; Maruhana, N.; Yamada, M.; Iioka, S.; Ibaraki, H.; Kosuge, Y.; Kondo, H.; et al. Nose-to-Brain/Spinal Cord Delivery Kinetics of Liposomes with Different Surface Properties. J. Control. Release 2022, 344, 225–234. [Google Scholar] [CrossRef]
- Sabir, F.; Ismail, R.; Csoka, I. Nose-to-Brain Delivery of Antiglioblastoma Drugs Embedded into Lipid Nanocarrier Systems: Status Quo and Outlook. Drug Discov. Today 2020, 25, 185–194. [Google Scholar] [CrossRef]
- Shah, V.; Sharma, M.; Pandya, R.; Parikh, R.K.; Bharatiya, B.; Shukla, A.; Tsai, H.-C. Quality by Design Approach for an In Situ Gelling Microemulsion of Lorazepam via Intranasal Route. Mater. Sci. Eng. C 2017, 75, 1231–1241. [Google Scholar] [CrossRef]
- Cao, S.L.; Ren, X.W.; Zhang, Q.Z.; Chen, E.; Xu, F.; Chen, J.; Liu, L.C.; Jiang, X.G. In situ gel based on gellan gum as new carrier for nasal administration of mometasone furoate. Int. J. Pharm. 2009, 365, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Song, X.; Sun, F.; Yang, Z.; Hou, S.; Liu, Z. Formulation and Evaluation of In Situ Gelling Systems for Intranasal Administration of Gastrodin. AAPS PharmSciTech 2011, 12, 1102–1109. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.; et al. Stimuli-Responsive In Situ Gelling System for Nose-to-Brain Drug Delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef] [PubMed]
- Chassenieux, C.; Tsitsilianis, C. Recent Trends in pH/Thermo-Responsive Self-Assembling Hydrogels: From Polyions to Peptide-Based Polymeric Gelators. Soft Matter 2016, 12, 1344–1359. [Google Scholar] [CrossRef]
- Watts, P.; Smith, A. PecSys: In Situ Gelling System for Optimised Nasal Drug Delivery. Expert Opin. Drug Deliv. 2009, 6, 543–552. [Google Scholar] [CrossRef]
- Sakharova, P.S.; Pyzhov, V.S.; Bakhrushina, E.O. Poly(l-Lactide-Co-Glycolide) and Shellac in the Development of Phase-Sensitive in Situ Implants. Aspir. Vestn. Povolzhiya 2022, 22, 51–57. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Mikhel, I.B.; Pyzhov, V.S.; Demina, N.B.; Krasnyuk, I.I., Jr.; Krasnyuk, I.I. Development of in Situ Intranasal System Based on Chitosan Formate. Bull. Exp. Biol. Med. 2022, 174, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Bakhrushina, E.O.; Shulikina, D.S.; Mikhel, I.B.; Demina, N.B.; Krasnyuk, I.I. Development and aprobation of in vitro model of nasal cavity for stanadartisation of in situ drug delivery systems. Proc. Vo-Ronezh State Univ. 2023, 4, 83–91. [Google Scholar]
- Bedford, J.G.; Caminschi, I.; Wakim, L.M. Intranasal Delivery of a Chitosan-Hydrogel Vaccine Generates Nasal Tissue Resident Memory CD8+ T Cells That Are Protective against Influenza Virus Infection. Vaccines 2020, 8, 572. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Díaz, A.G.; Quinteros, D.A.; Gutiérrez, S.E.; Rivero, M.A.; Palma, S.D.; Allemandi, D.A.; Pardo, R.P.; Zylberman, V.; Goldbaum, F.A.; Estein, S.M. Immune Response Induced by Conjunctival Immunization with Polymeric Antigen BLSOmp31 Using a Thermoresponsive and Mucoadhesive in Situ Gel as Vaccine Delivery System for Prevention of Ovine Brucellosis. Vet. Immunol. Immunopathol. 2016, 178, 50–56. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Ahmad, F.J.; Ahmad, W.; Alam, A.; Amir, M.; Ali, A. Poloxamer-Chitosan-Based Naringenin Nanoformulation Used in Brain Targeting for the Treatment of Cerebral Ischemia. Saudi J. Biol. Sci. 2019, 27, 500–517. [Google Scholar] [CrossRef]
- Wang, Q.; Wong, C.-H.; Chan, H.E.; Lee, W.-Y.; Zuo, Z. Statistical Design of Experiment (DoE) Based Development and Optimization of DB213 in Situ Thermosensitive Gel for Intranasal Delivery. Int. J. Pharm. 2018, 539, 50–57. [Google Scholar] [CrossRef]
- Yang, H.; Lan, X.; Xiong, Y. In Situ Growth of Zeolitic Imidazolate Framework-L in Macroporous PVA/CMC/PEG Composite Hydrogels with Synergistic Antibacterial and Rapid Hemostatic Functions for Wound Dressing. Gels 2022, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Singh-Joy, S.D.; McLain, V.C. Safety Assessment of Poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, Poloxamer 105 Benzoate, and Poloxamer 182 Dibenzoate as Used in Cosmetics. Int. J. Toxicol. 2008, 27, 93–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, W.; Xian, Y.; Liu, L.; Fan, J.; Liu, H.; Zheng, Z.; Wu, D. Rapidly in Situ Forming an Injectable Chitosan/PEG Hydrogel for Intervertebral Disc Repair. Mater. Today Bio 2023, 22, 100752. [Google Scholar] [CrossRef]
- Toma, I.; Porfire, A.S.; Tefas, L.R.; Berindan-Neagoe, I.; Tomuță, I. A Quality by Design Approach in Pharmaceutical Development of Non-Viral Vectors with a Focus on miRNA. Pharmaceutics 2022, 14, 1482. [Google Scholar] [CrossRef]
- Mardikasari, S.A.; Katona, G.; Budai-Szűcs, M.; Sipos, B.; Orosz, L.; Burián, K.; Rovó, L.; Csóka, I. Quality by Design-Based Optimization of in Situ Ionic-Sensitive Gels of Amoxicillin-Loaded Bovine Serum Albumin Nanoparticles for Enhanced Local Nasal Delivery. Int. J. Pharm. 2023, 645, 123435. [Google Scholar] [CrossRef]
- Nodilo, L.N.; Perkušić, M.; Ugrina, I.; Špoljarić, D.; Brala, C.J.; Klarić, D.A.; Lovrić, J.; Saršon, V.; Kučuk, M.S.; Zadravec, D.; et al. In Situ Gelling Nanosuspension as an Advanced Platform for Fluticasone Propionate Nasal Delivery. Eur. J. Pharm. Biopharm. 2022, 175, 27–42. [Google Scholar] [CrossRef]
- Durgun, M.E.; Mesut, B.; Hacıoğlu, M.; Güngör, S.; Özsoy, Y. Optimization of the Micellar-Based In Situ Gelling Systems Posaconazole with Quality by Design (QbD) Approach and Characterization by In Vitro Studies. Pharmaceutics 2022, 14, 526. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Dubova, A.I.; Nikonenko, M.S.; Grikh, V.V.; Shumkova, M.M.; Korochkina, T.V.; Krasnyuk, I.I.; Krasnyuk, I.I. Thermosensitive Intravitreal In Situ Implant of Cefuroxime Based on Poloxamer 407 and Hyaluronic Acid. Gels 2023, 9, 693. [Google Scholar] [CrossRef]
- Bakhrushina, E.; Khodenok, A.; Pyzhov, V.; Solomatina, P.; Demina, N.; Korochkina, T.; Krasnyuk, I. Study of the Effect of Active Pharmaceutical Ingredients of Various Classes of BCS on the Parameters of Thermosensitive Systems Based on Poloxamers. Saudi Pharm. J. 2023, 31, 101780. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Mikhel, I.B.; Kondratieva, V.M.; Zubareva, I.M.; Kosenkova, S.I.; Belyatskaya, A.V.; Stepanova, O.I.; Krasnyuk, I.I.; Grebennikova, T.V.; Krasnyuk, I.I. Intranasal Ion-Triggered In Situ Delivery System of Virus-like Particles: Development Using the Quality by Design Approach. Polymers 2024, 16, 685. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, M.R.; Matida, E.A.; Kherani, S.; Marsan, J. Creation of a Standardized Geometry of the Human Nasal Cavity. J. Appl. Physiol. 2009, 106, 784–795. [Google Scholar] [CrossRef]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole Animal Perfusion Fixation for Rodents. J. Vis. Exp. 2012, 106, e3564. [Google Scholar] [CrossRef]
- Misra, A.; Kher, G. Drug Delivery Systems from Nose to Brain. Curr. Pharm. Biotechnol. 2012, 13, 2355–2379. [Google Scholar] [CrossRef]
- Giuliani, A.; Balducci, A.G.; Zironi, E.; Colombo, G.; Bortolotti, F.; Lorenzini, L.; Galligioni, V.; Pagliuca, G.; Scagliarini, A.; Calzà, L.; et al. In Vivo Nose-to-Brain Delivery of the Hydrophilic Antiviral Ribavirin by Microparticle Agglomerates. Drug Deliv. 2018, 25, 376–387. [Google Scholar] [CrossRef]
- Said, M.M.; Elmenoufy, G.A. Evaluation of Quetiapine Fumarate and Its Solid Lipid Nanoparticles as Antipsychotic Drug in Rat Model of Schizophrenia. Biomed. Res. Ther. 2017, 4, 1480. [Google Scholar] [CrossRef]


| Factor | Target | Justification |
|---|---|---|
| Route of administration | Intranasal | Directed delivery to the brain |
| Site of effect | Brain | Transport of AFI by cranial nerves (olfactory and trigeminal) to the brain |
| API administration | 100 mg/mL | Test concentration with antitumor effect |
| pH | 5.5–7.5 | Optimal range for comfortable administration |
| Viscosity before gelation | Below 100 mPa·s | Phase transition is due to the selected nebulization system—NEST Pre-filled Disposable Intranasal Atomization Device (China) |
| Gelation stimulus | Temperature, ions (Na+, K+, Ca2+) | Gel formation conditions correspond to the physiologic parameters of the nasal cavity |
| Phase transition temperature | Below 32 °C | Nasal cavity temperature (applicable for thrmoreversible systems) |
| Spray torch | 6–9 cm | Targeting the olfactory bulb |
| Retention on nasal cavity model | >80% | Similar retention percentage to highly adhesive compositions |
| CQA | CMA | CPP |
|---|---|---|
| Gelation stimulus | Smart-polymer concentration | Stirring time |
| Solution viscosity | Additional ingredient | Dispergating speed |
| Spray torch | Concentration of additional ingredient | Dispergating time |
| pH | Type of solvent | Manufacturing temperature |
| Retention on nasal cavity model | Concentration of API |
| Animals | Groups | Number of Animals | Drug/Dose/Administration Volume | Manipulations |
|---|---|---|---|---|
| Rats, intranasal administration | 0 min | 3 | In situ gel with ribavirin, 10 mg per kg, 15 µL in each nostril per 300 g (rat weight) | Vital manipulations—intranasal injection, blood sampling. After euthanasia—collection of brain and olfactory bulbs with further homogenization and analytical study. |
| 15 min | 3 | |||
| 30 min | 3 | |||
| 1 h | 3 | |||
| 2 h | 3 | |||
| 5 h | 3 | |||
| 8 h | 3 | |||
| 12 h | 3 | |||
| 24 h | 3 | |||
| Control group | ||||
| Rats, intranasal administration | 0 min | 3 | Ribavirin water solution, 10 mg per kg, 15 µL in each nostril per 300 g (rat weight) | Vital manipulations—intranasal injection, blood sampling. After euthanasia—collection of brain and olfactory bulbs with further homogenization and analytical study. |
| 15 min | 3 | |||
| 30 min | 3 | |||
| 1 h | 3 | |||
| 2 h | 3 | |||
| 5 h | 3 | |||
| 8 h | 3 | |||
| 12 h | 3 | |||
| 24 h | 3 | |||
| Time, min | “A” Phase Amount, % | “B” Phase Amount, % |
|---|---|---|
| 0 | 100 | 0 |
| 1.5 | 100 | 0 |
| 2.0 | 10 | 90 |
| 6.0 | 10 | 90 |
| 6.10 | 100 | 0 |
| 8.0 | 100 | 0 |
| Parameter | Value |
|---|---|
| Ionization type | Electrospraying with heated atomizing gas stream |
| Gas Temp | 300 °C |
| Gas Flow | 11 L/min |
| Nebulizer | 35 psi |
| SheathGasHeater | 300 °C |
| SheathGasFlow | 11 L/min |
| Capillary Voltage | 2.5 kV |
| Mode of Analysis | MRM, positive ion |
| № | P407 | P188 | P124 | GG | Ch | PBS | XG | GuG | PVA | HPMC | Pec | Carb | PEG 1500 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 3 | - | - | - | - | - | - | - | - | - | - | - |
| 2 | 16 | 2 | - | - | - | - | - | - | - | - | - | - | - |
| 3 | 18 | 3 | - | - | - | - | - | - | - | - | - | - | - |
| 4 | 18 | 2 | - | - | - | - | - | - | - | - | - | - | - |
| 5 | 16 | - | 2 | - | - | - | - | - | - | - | - | - | - |
| 6 | 16 | - | 1 | - | - | - | - | - | - | - | - | - | - |
| 7 | 18 | - | 2 | - | - | - | - | - | - | - | - | - | - |
| 8 | 18 | - | 1 | - | - | - | - | - | - | - | - | - | - |
| 9 | 16 | - | - | - | - | - | - | - | - | - | - | - | 1 |
| 10 | 16 | - | - | - | - | - | - | - | - | - | - | - | 0.5 |
| 11 | 18 | - | - | - | - | - | - | - | - | - | - | - | 1 |
| 12 | 18 | - | - | - | - | - | - | - | - | - | - | - | 0.5 |
| 13 | 16 | - | - | - | 2 | - | - | - | - | - | - | - | - |
| 14 | 16 | - | - | - | 1 | - | - | - | - | - | - | - | - |
| 15 | 18 | - | - | - | 2 | - | - | - | - | - | - | - | - |
| 16 | 18 | - | - | - | 1 | - | - | - | - | - | - | - | - |
| 17 | 16 | - | - | - | - | - | - | - | - | - | - | - | - |
| 18 | 18 | - | - | - | - | - | - | - | - | - | - | - | - |
| 19 | 16 | - | - | 0.25 | - | - | - | - | - | - | - | - | - |
| 20 | 16 | - | - | 0.5 | - | - | - | - | - | - | - | - | - |
| 21 | 18 | - | - | 0.25 | - | - | - | - | - | - | - | - | - |
| 22 | 18 | - | - | 0.5 | - | - | - | - | - | - | - | - | - |
| 23 | - | - | - | 0.25 | - | - | - | - | - | - | - | - | - |
| 24 | - | - | - | 0.5 | - | - | - | - | - | - | - | - | - |
| 25 | - | - | - | 0.25 | - | 10 | - | - | - | - | - | - | - |
| 26 | - | - | - | 0.25 | - | 15 | - | - | - | - | - | - | - |
| 27 | - | - | - | 0.5 | - | 10 | - | - | - | - | - | - | - |
| 28 | - | - | - | 0.5 | - | 15 | - | - | - | - | - | - | - |
| 29 | - | - | 2 | 0.25 | - | - | - | - | - | - | - | - | - |
| 30 | - | - | 1 | 0.25 | - | - | - | - | - | - | - | - | - |
| 31 | - | - | 2 | 0.5 | - | - | - | - | - | - | - | - | - |
| 32 | - | - | 1 | 0.5 | - | - | - | - | - | - | - | - | - |
| 33 | - | - | - | 0.25 | - | - | 0.25 | - | - | - | - | - | - |
| 34 | - | - | - | 0.25 | - | - | 0.5 | - | - | - | - | - | - |
| 35 | - | - | - | 0.5 | - | - | 0.25 | - | - | - | - | - | - |
| 36 | - | - | - | 0.5 | - | - | 0.5 | - | - | - | - | - | - |
| 37 | - | - | - | 0.25 | - | - | - | 0.25 | - | - | - | - | - |
| 38 | - | - | - | 0.25 | - | - | - | 0.5 | - | - | - | - | - |
| 39 | - | - | - | 0.5 | - | - | - | 0.25 | - | - | - | - | - |
| 40 | - | - | - | 0.5 | - | - | - | 0.5 | - | - | - | - | - |
| 41 | - | - | - | 0.25 | - | - | - | - | 0.25 | - | - | - | - |
| 42 | - | - | - | 0.25 | - | - | - | - | 0.5 | - | - | - | - |
| 43 | - | - | - | 0.5 | - | - | - | - | 0.25 | - | - | - | - |
| 44 | - | - | - | 0.5 | - | - | - | - | 0.5 | - | - | - | - |
| 45 | - | - | - | 0.25 | - | - | - | - | - | 0.1 | - | - | - |
| 46 | - | - | - | 0.25 | - | - | - | - | - | 0.2 | - | - | - |
| 47 | - | - | - | 0.5 | - | - | - | - | - | 0.1 | - | - | - |
| 48 | - | - | - | 0.5 | - | - | - | - | - | 0.2 | - | - | - |
| 49 | - | - | - | 0.25 | - | - | - | - | - | - | - | 0.1 | - |
| 50 | - | - | - | 0.25 | - | - | - | - | - | - | - | 0.2 | - |
| 51 | - | - | - | 0.5 | - | - | - | - | - | - | - | 0.1 | - |
| 52 | - | - | - | 0.5 | - | - | - | - | - | - | - | 0.2 | - |
| 53 | - | - | - | - | - | - | 0.25 | - | - | - | - | - | - |
| 54 | - | - | - | - | - | - | 0.5 | - | - | - | - | - | - |
| 55 | - | - | - | 0.25 | - | - | - | - | - | - | 0.1 | - | - |
| 56 | - | - | - | 0.25 | - | - | - | - | - | - | 0.2 | - | - |
| 57 | - | - | - | 0.5 | - | - | - | - | - | - | 0.1 | - | - |
| 58 | - | - | - | 0.5 | - | - | - | - | - | - | 0.2 | - | - |
| 59 | - | - | - | - | - | - | - | - | - | - | 0.25 | - | - |
| 60 | - | - | - | - | - | - | - | - | - | - | 0.5 | - | - |
| № | Gelation 1 | № | Gelation 1 | № | Gelation 1 | № | Gelation 1 |
|---|---|---|---|---|---|---|---|
| 1 | +++ | 17 | +++ | 33 | + | 49 | ++ |
| 2 | +++ | 18 | +++ | 34 | ++ | 50 | ++ |
| 3 | +++ | 19 | +++ | 35 | ++ | 51 | +++ |
| 4 | +++ | 20 | +++ | 36 | ++ | 52 | +++ |
| 5 | +++ | 21 | +++ | 37 | - | 53 | - |
| 6 | +++ | 22 | +++ | 38 | + | 54 | - |
| 7 | +++ | 23 | ++ | 39 | + | 55 | - |
| 8 | +++ | 24 | +++ | 40 | - | 56 | + |
| 9 | +++ | 25 | ++ | 41 | + | 57 | - |
| 10 | +++ | 26 | ++ | 42 | + | 58 | + |
| 11 | +++ | 27 | +++ | 43 | ++ | 59 | - |
| 12 | +++ | 28 | +++ | 44 | ++ | 60 | + |
| 13 | +++ | 29 | ++ | 45 | ++ | ||
| 14 | +++ | 30 | ++ | 46 | ++ | ||
| 15 | +++ | 31 | +++ | 47 | +++ | ||
| 16 | +++ | 32 | +++ | 48 | +++ |
| № | pH Value | № | pH Value | № | pH Value | № | pH Value |
|---|---|---|---|---|---|---|---|
| 1 | 6.32 | 17 | 7.52 | 33 | 7.21 | 49 | 4.32 |
| 2 | 6.71 | 18 | 8.04 | 34 | 7.11 | 50 | 4.21 |
| 3 | 6.34 | 19 | 7.92 | 35 | 7.3 | 51 | 4.15 |
| 4 | 6.51 | 20 | 8.1 | 36 | 6.99 | 52 | 4.25 |
| 5 | 6.96 | 21 | 7.89 | 37 | 6.76 | 55 | 6.75 |
| 6 | 6.84 | 22 | 7.3 | 38 | 6.6 | 56 | 6.88 |
| 7 | 6.89 | 23 | 6.93 | 39 | 6.89 | 57 | 6.92 |
| 8 | 7 | 24 | 6.9 | 40 | 6.91 | 58 | 7.07 |
| 9 | 6.7 | 25 | 6.95 | 41 | 6.54 | 59 | 7.04 |
| 10 | 6.44 | 26 | 7.02 | 42 | 6.77 | 60 | 6.89 |
| 11 | 6.58 | 27 | 7.14 | 43 | 6.72 | ||
| 12 | 6.7 | 28 | 7.21 | 44 | 6.81 | ||
| 13 | 6.51 | 29 | 7.04 | 45 | 7.49 | ||
| 14 | 6.1 | 30 | 6.98 | 46 | 7.62 | ||
| 15 | 6.12 | 31 | 6.82 | 47 | 6.44 | ||
| 16 | 6.3 | 32 | 6.91 | 48 | 6.58 |
| № | Viscosity, mPa·s | № | Viscosity, mPa·s | № | Viscosity, mPa·s | № | Viscosity, mPa·s |
|---|---|---|---|---|---|---|---|
| 1 | 45.6 | 17 | 21.2 | 33 | 40.1 | 49 | 27.4 |
| 2 | 41.3 | 18 | 29.2 | 34 | 32.2 | 50 | 27.5 |
| 3 | 69.1 | 19 | 59.9 | 35 | 40.1 | 51 | 28.7 |
| 4 | 62.4 | 20 | 59.1 | 36 | 75.3 | 52 | 29.9 |
| 5 | 45.7 | 21 | 53.3 | 37 | 138.9 | 55 | 22.2 |
| 6 | 42 | 22 | 84.5 | 38 | 134.2 | 56 | 29.7 |
| 7 | 82.6 | 23 | 40.5 | 39 | 129.9 | 57 | 69.7 |
| 8 | 32.8 | 24 | 61.1 | 40 | 131.2 | 58 | 94.6 |
| 9 | 28.2 | 25 | 56.8 | 41 | 59.8 | 59 | 115.1 |
| 10 | 24.3 | 26 | 54.8 | 42 | 59.7 | 60 | 106.9 |
| 11 | 29.3 | 27 | 30.1 | 43 | 54.1 | ||
| 12 | 27.2 | 28 | 33.3 | 44 | 58.2 | ||
| 13 | 41 | 29 | 27.1 | 45 | 39.8 | ||
| 14 | 39.8 | 30 | 28 | 46 | 42.2 | ||
| 15 | 42.3 | 31 | 27.8 | 47 | 39.7 | ||
| 16 | 40.9 | 32 | 59.8 | 48 | 45.6 |
| № | Gelation t, °C | № | Gelation t, °C |
|---|---|---|---|
| 1 | 31.2 | 12 | 36.6 |
| 2 | 30.9 | 13 | 36 |
| 3 | 39.9 | 14 | 31.1 |
| 4 | 33.1 | 15 | 28.1 |
| 5 | 32.3 | 16 | 29.2 |
| 6 | 34.7 | 17 | 27.6 |
| 7 | 35.3 | 18 | 28.5 |
| 8 | 31.2 | 19 | 36.4 |
| 9 | 31.4 | 20 | 32.1 |
| 10 | 35 | 21 | 27.4 |
| 11 | 34.6 | 22 | 28.3 |
| № | Gelation t, °C 1 | № | Gelation t, °C 1 | № | Gelation t, °C 1 | № | Gelation t, °C 1 |
|---|---|---|---|---|---|---|---|
| 1 | + | 17 | + | 33 | - | 49 | - |
| 2 | + | 18 | + | 34 | - | 50 | - |
| 3 | - | 19 | - | 35 | - | 51 | - |
| 4 | - | 20 | - | 36 | - | 52 | - |
| 5 | - | 21 | + | 37 | - | 53 | - |
| 6 | - | 22 | + | 38 | - | 54 | - |
| 7 | - | 23 | - | 39 | - | 55 | - |
| 8 | + | 24 | - | 40 | - | 56 | - |
| 9 | + | 25 | - | 41 | - | 57 | - |
| 10 | - | 26 | - | 42 | - | 58 | - |
| 11 | - | 27 | - | 43 | - | 59 | - |
| 12 | - | 28 | - | 44 | - | 60 | - |
| 13 | - | 29 | - | 45 | - | ||
| 14 | + | 30 | - | 46 | - | ||
| 15 | + | 31 | - | 47 | - | ||
| 16 | + | 32 | - | 48 | - |
| № | Retention, % | № | Retention, % | № | Retention, % | № | Retention, % |
|---|---|---|---|---|---|---|---|
| 1 | 33 | 17 | 35 | 33 | 15 | 49 | 10 |
| 2 | 30 | 18 | 39 | 34 | 25 | 50 | 15 |
| 3 | 10 | 19 | 70 | 35 | 20 | 51 | 15 |
| 4 | 18 | 20 | 72 | 36 | 23 | 52 | 17 |
| 5 | 64 | 21 | 65 | 37 | 29 | 53 | 5 |
| 6 | 68 | 22 | 60 | 38 | 35 | 54 | 10 |
| 7 | 70 | 23 | 67 | 39 | 35 | 55 | 15 |
| 8 | 70 | 24 | 70 | 40 | 40 | 56 | 18 |
| 9 | 39 | 25 | 70 | 41 | 55 | 57 | 20 |
| 10 | 31 | 26 | 77 | 42 | 49 | 58 | 18 |
| 11 | 37 | 27 | 85 | 43 | 65 | 59 | 18 |
| 12 | 35 | 28 | 90 | 44 | 63 | 60 | 20 |
| 13 | 72 | 29 | 80 | 45 | 53 | 61 | 8 |
| 14 | 76 | 30 | 75 | 46 | 87 | 62 | 6 |
| 15 | 85 | 31 | 95 | 47 | 87 | 63 | 20 |
| 16 | 80 | 32 | 90 | 48 | 90 | 64 | 15 |
| № | Spray Torch, cm | № | Spray Torch, cm | № | Spray Torch, cm | № | Spray Torch, cm |
|---|---|---|---|---|---|---|---|
| 1 | 11 | 17 | 8 | 33 | 9 | 49 | 5 |
| 2 | 9 | 18 | 9 | 34 | 11 | 50 | 6 |
| 3 | 8 | 19 | 5.5 | 35 | 6 | 51 | 5.5 |
| 4 | 10 | 20 | 6 | 36 | 8 | 52 | 4.5 |
| 5 | 9.5 | 21 | 4 | 37 | 2.5 | 53 | 6 |
| 6 | 8.5 | 22 | 3.5 | 38 | 3 | 54 | 8 |
| 7 | 10 | 23 | 12 | 39 | 3 | 55 | 8.5 |
| 8 | 11 | 24 | 13.5 | 40 | 6.5 | 56 | 5 |
| 9 | 11 | 25 | 9 | 41 | 7.5 | 57 | 8 |
| 10 | 10.5 | 26 | 8.5 | 42 | 8.5 | 58 | 3 |
| 11 | 9.5 | 27 | 10 | 43 | 8 | 59 | 3.5 |
| 12 | 13 | 28 | 10.5 | 44 | 9 | 60 | 6 |
| 13 | 11 | 29 | 12 | 45 | 8.5 | ||
| 14 | 9.5 | 30 | 11 | 46 | 7 | ||
| 15 | 8.5 | 31 | 8 | 47 | 8 | ||
| 16 | 9 | 32 | 7.5 | 48 | 9.5 |
| With Ribavirin | ||||||
|---|---|---|---|---|---|---|
| № | Gelation | Gelation t, °C | pH Value | Retention, % | Viscosity, mPa·s | Spray Torch |
| 15 | +++ | 25 | 6.12 | 45 | 27.1 | 12 |
| 16 | +++ | 24.6 | 6.3 | 60 | 39.9 | 9 |
| 27 | +++ | - | 7.14 | 80 | 37.8 | 9.5 |
| 28 | +++ | - | 7.21 | 85 | 30.2 | 10 |
| 31 | +++ | - | 6.82 | 98 | 28.1 | 8.5 |
| 32 | +++ | - | 6.91 | 90 | 24.1 | 9 |
| 46 | ++ | - | 7.62 | 85 | 29.4 | 10.5 |
| 47 | +++ | - | 6.44 | 83 | 35.1 | 7.5 |
| 48 | +++ | - | 6.58 | 85 | 29.9 | 8 |
| Without ribavirin | ||||||
| 15 | +++ | 28.1 | 6.12 | 85 | 42.3 | 8.5 |
| 16 | +++ | 29.2 | 6.3 | 80 | 40.9 | 9 |
| 27 | +++ | - | 7.14 | 85 | 30.1 | 10 |
| 28 | +++ | - | 7.21 | 90 | 33.3 | 10.5 |
| 31 | +++ | - | 6.82 | 95 | 27.8 | 8 |
| 32 | +++ | - | 6.91 | 90 | 59.8 | 7.5 |
| 46 | ++ | - | 7.62 | 87 | 42.2 | 7 |
| 47 | +++ | - | 6.44 | 87 | 39.7 | 8 |
| 48 | +++ | - | 6.58 | 90 | 45.6 | 9.5 |
| Parameter * | Unit | Plasma Value | Brain Value | Olfactory Bulbs Value |
|---|---|---|---|---|
| Experimental group | ||||
| Λ_z | 1/h | 0.1289 | 0.0335 | 0.0378 |
| t1/2 | h | 5.3791 | 20.6782 | 18.3222 |
| Tmax | h | 0.5 | 5 | 5 |
| Cmax | ng/ml | 152.6586 | 931.1437 | 2542.7953 |
| Tlag | h | 0 | 0 | 0 |
| Clast_obs/Cmax | - | 0.0228 | 0.5890 | 0.4073 |
| AUC 0-t | ng/mL*h | 629.5281 | 17,535.4248 | 38,105.3782 |
| AUC 0-inf_obs | ng/mL*h | 656.5046 | 33,897.8019 | 65,483.2040 |
| AUC 0-t/0-inf_obs | - | 0.9589 | 0.5173 | 0.5819 |
| AUMC 0-inf_obs | ng/mL*h2 | 4569.5688 | 1,085,744.771 | 1,796,570.896 |
| MRT 0-inf_obs | h | 6.9605 | 32.0299 | 27.4356 |
| Vz/F_obs | (μg/kg)/(ng/mL) | 0.1182 | 0.0088 | 0.0040 |
| Cl/F_obs | (μg/kg)/(ng/mL)/h | 0.0152 | 0.0003 | 0.0002 |
| Control group | ||||
| Λ_z | 1/h | 0.0960 | 0.0837 | 0.1549 |
| t1/2 | h | 7.2175 | 8.2803 | 4.4754 |
| Tmax | h | 0.25 | 1 | 0.5 |
| Cmax | ng/ml | 154.3622 | 924.4831 | 2062.3333 |
| Tlag | h | 0 | 0 | 0 |
| Clast_obs/Cmax | - | 0.0220 | 0.0857 | 0.0232 |
| AUC 0-t | ng/mL*h | 421.3711 | 7412.3988 | 11,029.7240 |
| AUC 0-inf_obs | ng/mL*h | 456.7740 | 8359.1850 | 11,338.1257 |
| AUC 0-t/0-inf_obs | - | 0.9225 | 0.8867 | 0.9728 |
| AUMC 0-inf_obs | ng/mL*h2 | 3885.3060 | 85,566.0065 | 68,537.7786 |
| MRT 0-inf_obs | h | 8.5060 | 10.2361 | 6.0449 |
| Vz/F_obs | (μg/kg)/(ng/mL) | 0.2280 | 0.0143 | 0.0057 |
| Cl/F_obs | (μg/kg)/(ng/mL)/h | 0.0219 | 0.0012 | 0.0009 |
| CQA/CPP | Stirring Time | Dispergating Speed | Dispergating Time | Manufacturing Temperature | Cross-Linking Time |
|---|---|---|---|---|---|
| Gelation stimulus | Low | Low | Low | High | Low |
| Solution viscosity | Medium | Low | Low | Low | Low |
| Spray torch | Low | Low | Low | Low | Low |
| pH | Low | Low | Low | Low | Low |
| Retention on nasal cavity model | Low | Low | Low | Low | Low |
| CQA/CMA | Smart-Polymer Concentration | Additional Ingredient | Concentration of Additional Ingredient | Type of Solvent | Concentration of API |
|---|---|---|---|---|---|
| Gelation stimulus | High | High | High | High | Low |
| Solution viscosity | High | High | High | High | Medium |
| Spray torch | High | High | High | High | Medium |
| pH | Medium | Medium | Medium | High | Medium |
| Retention on nasal cavity model | High | High | High | High | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhel, I.B.; Bakhrushina, E.O.; Petrusevich, D.A.; Nedorubov, A.A.; Appolonova, S.A.; Moskaleva, N.E.; Demina, N.B.; Kosenkova, S.I.; Parshenkov, M.A.; Krasnyuk, I.I., Jr.; et al. Development of an Intranasal In Situ System for Ribavirin Delivery: In Vitro and In Vivo Evaluation. Pharmaceutics 2024, 16, 1125. https://doi.org/10.3390/pharmaceutics16091125
Mikhel IB, Bakhrushina EO, Petrusevich DA, Nedorubov AA, Appolonova SA, Moskaleva NE, Demina NB, Kosenkova SI, Parshenkov MA, Krasnyuk II Jr., et al. Development of an Intranasal In Situ System for Ribavirin Delivery: In Vitro and In Vivo Evaluation. Pharmaceutics. 2024; 16(9):1125. https://doi.org/10.3390/pharmaceutics16091125
Chicago/Turabian StyleMikhel, Iosif B., Elena O. Bakhrushina, Danila A. Petrusevich, Andrey A. Nedorubov, Svetlana A. Appolonova, Natalia E. Moskaleva, Natalia B. Demina, Svetlana I. Kosenkova, Mikhail A. Parshenkov, Ivan I. Krasnyuk, Jr., and et al. 2024. "Development of an Intranasal In Situ System for Ribavirin Delivery: In Vitro and In Vivo Evaluation" Pharmaceutics 16, no. 9: 1125. https://doi.org/10.3390/pharmaceutics16091125






