Imaging-Based Drug Penetration Profiling in an Excised Sheep Cornea Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cornea Acquisition
2.2.2. Corneal Characterization
2.2.3. Preparation of Tested Formulations
2.2.4. Penetration Assay and Analysis
2.2.5. Corneal 3D Imaging
2.2.6. Statistics
3. Results
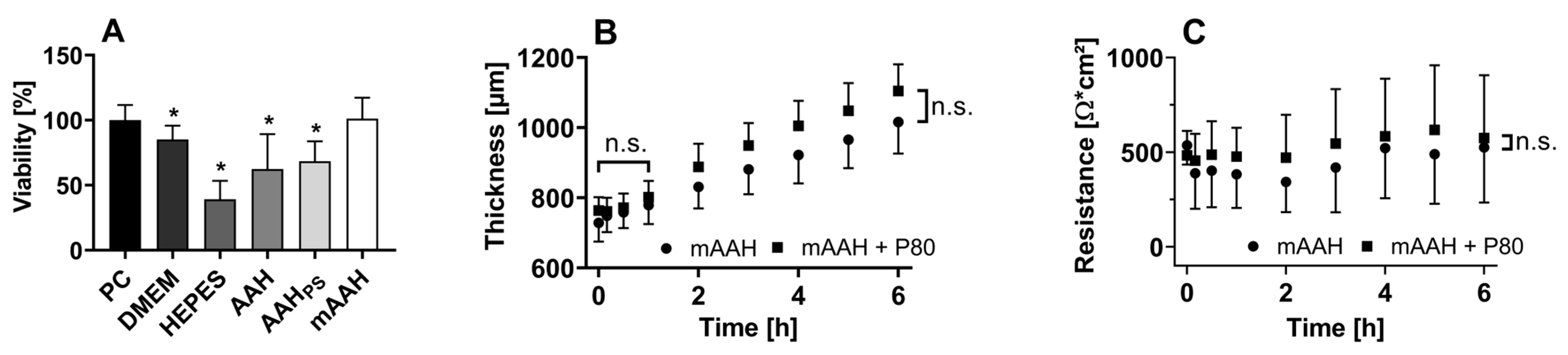
3.1. Corneal Characterization
3.1.1. Viability
3.1.2. Dimensions and Thickness
3.1.3. Electrical Resistance
3.2. Penetration Analysis
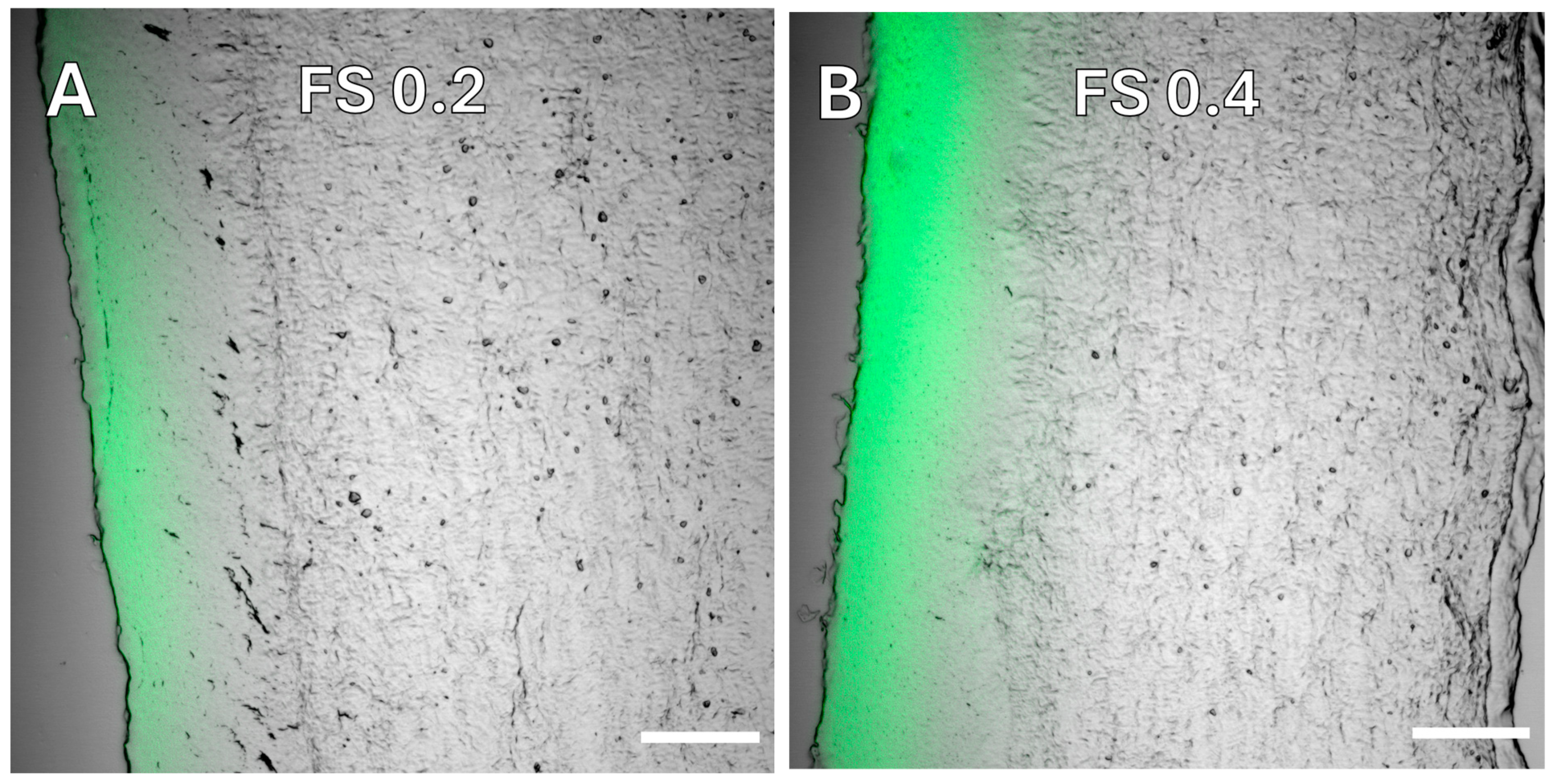
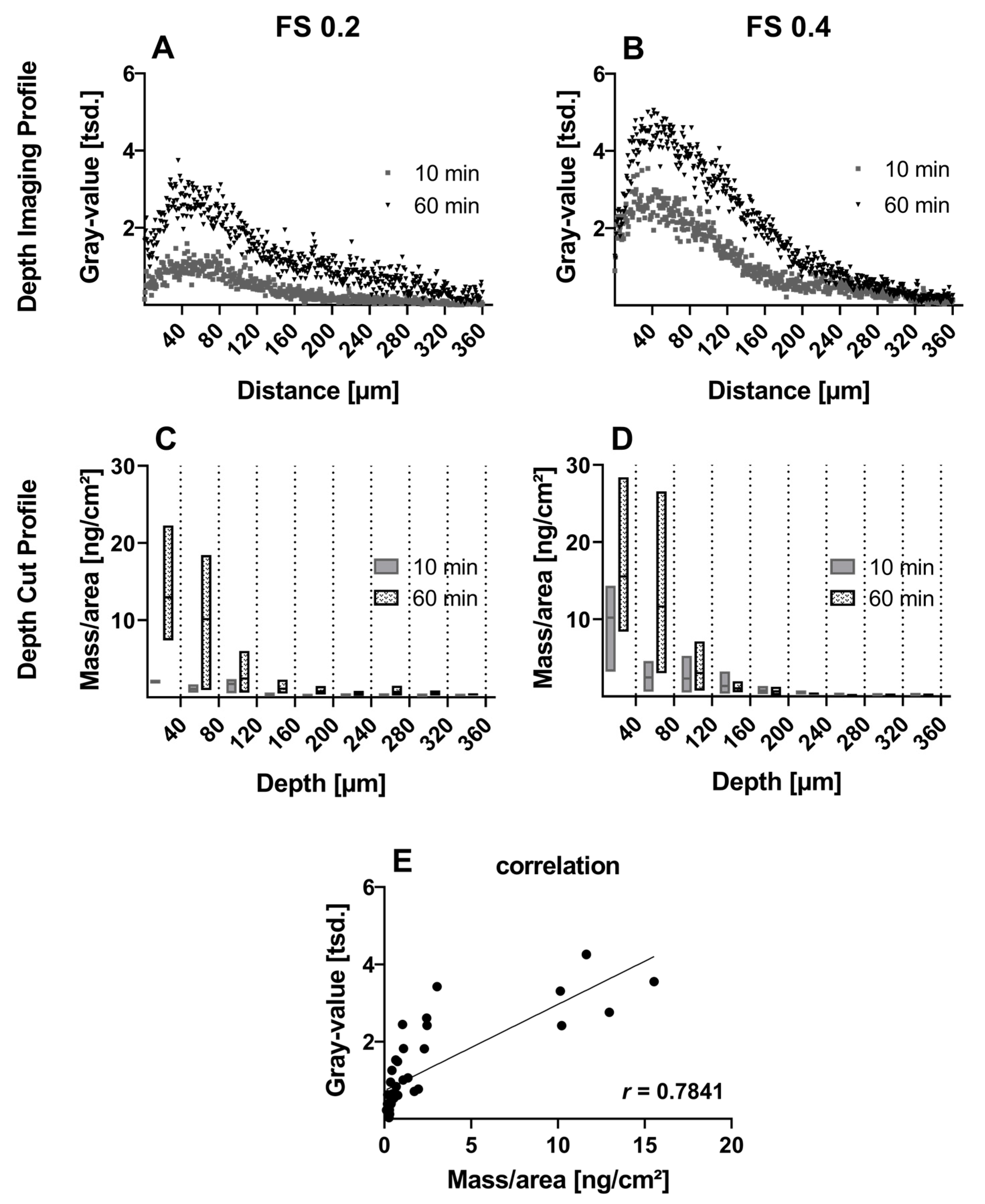
3.2.1. Small Molecule—Fluorescein Sodium
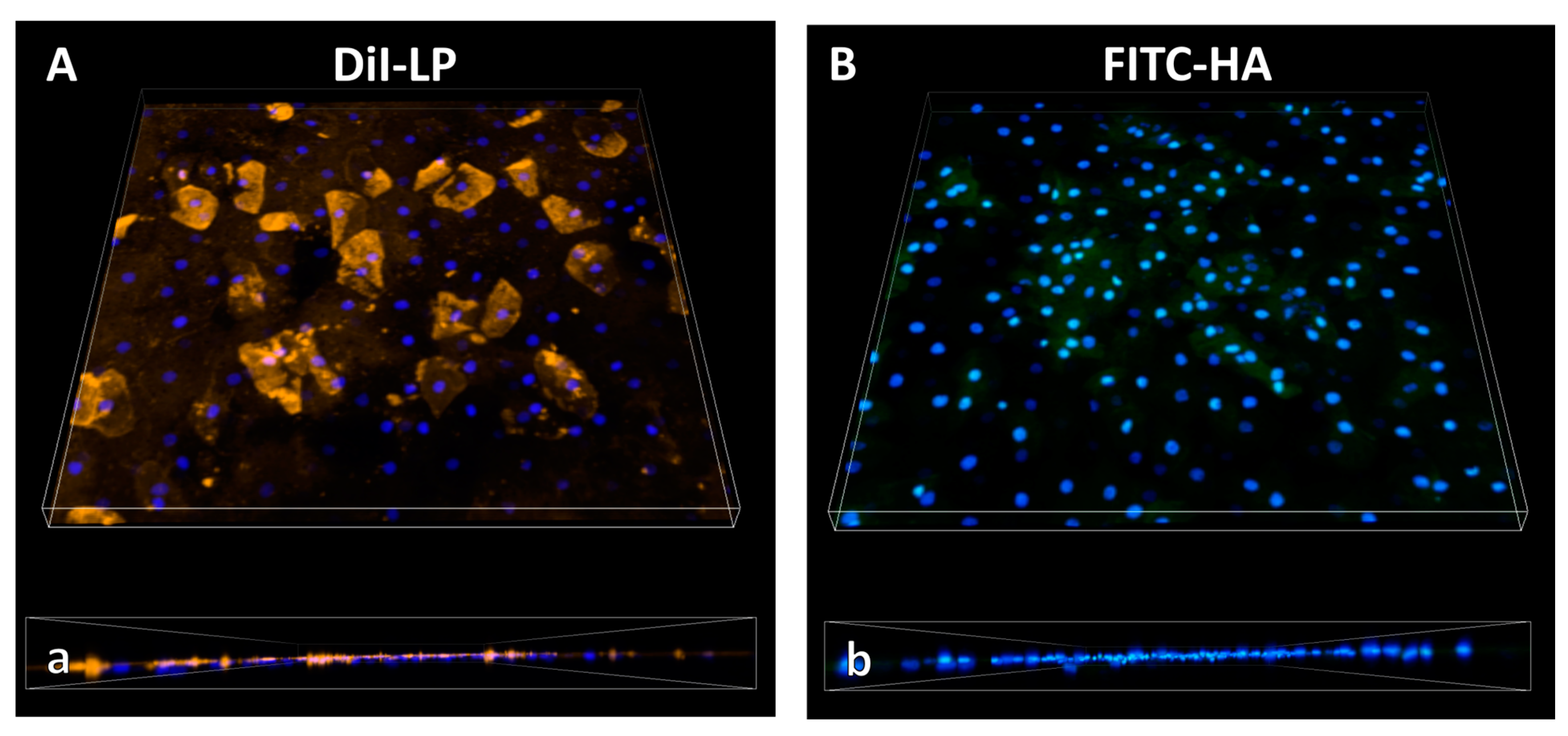
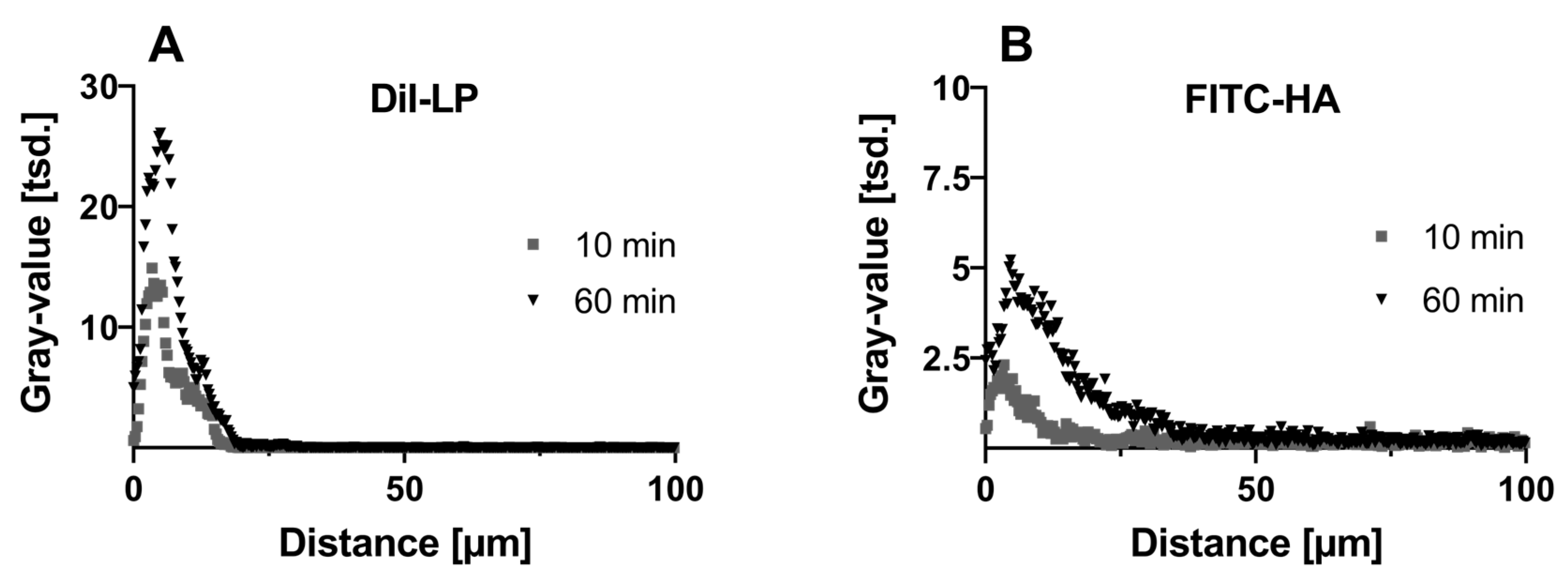
3.2.2. Macromolecules—Hyaluronic Acid—And Lipids from Eyedrop Formulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A Comprehensive Insight on Ocular Pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef] [PubMed]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 134, pp. 7–23. ISBN 978-0-12-801059-4. [Google Scholar]
- Beuerman, R.W.; Pedroza, L. Ultrastructure of the human cornea. Microsc. Res. Tech. 1996, 33, 320–335. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and Physiology of the Cornea. J. Cataract Refract. Surg. 2011, 37, 588. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Shetty, K.H.; Desai, D.T.; Shah, D.O.; Willcox, M.D.P. Recent Advances in Ophthalmic Preparations: Ocular Barriers, Dosage Forms and Routes of Administration. Int. J. Pharm. 2021, 608, 121105. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Lin, J.-E.C.; Lee, V.H.L.; Keipert, S. Pilocarpine Permeability across Ocular Tissues and Cell Cultures: Influence of Formulation Parameters. J. Ocul. Pharmacol. Ther. 2002, 18, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Juretić, M.; Jurišić Dukovski, B.; Krtalić, I.; Reichl, S.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Lovrić, J.; Pepić, I. HCE-T Cell-Based Permeability Model: A Well-Maintained or a Highly Variable Barrier Phenotype? Eur. J. Pharm. Sci. 2017, 104, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.D.; Batugo, M.; Gale, D.C.; Zhang, T.; Ye, J.; Li, C.; Zhou, S.; Wu, E.Y.; Zhang, E.Y. Characterization of Human Corneal Epithelial Cell Model As a Surrogate for Corneal Permeability Assessment: Metabolism and Transport. Drug Metab. Dispos. 2009, 37, 992–998. [Google Scholar] [CrossRef]
- Reichl, S. Cell Culture Models of the Human Cornea—A Comparative Evaluation of Their Usefulness to Determine Ocular Drug Absorption In-Vitro. J. Pharm. Pharmacol. 2008, 60, 299–307. [Google Scholar] [CrossRef]
- Kaluzhny, Y.; Klausner, M. In Vitro Reconstructed 3D Corneal Tissue Models for Ocular Toxicology and Ophthalmic Drug Development. In Vitro Cell. Dev. Biol.-Anim. 2021, 57, 207–237. [Google Scholar] [CrossRef]
- De Hoon, I.; Boukherroub, R.; De Smedt, S.C.; Szunerits, S.; Sauvage, F. In Vitro and Ex Vivo Models for Assessing Drug Permeation across the Cornea. Mol. Pharm. 2023, 20, 3298–3319. [Google Scholar] [CrossRef]
- Agarwal, P.; Rupenthal, I.D. In Vitro and Ex Vivo Corneal Penetration and Absorption Models. Drug Deliv. Transl. Res. 2016, 6, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug Delivery to the Anterior Segment of the Eye: A Review of Current and Future Treatment Strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef] [PubMed]
- Reichl, S.; Bednarz, J.; Müller-Goymann, C.C. Human Corneal Equivalent as Cell Culture Model for In Vitro Drug Permeation Studies. Br. J. Ophthalmol. 2004, 88, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, T.A.; Suarez, M.F.; Maldonado, C.; Croxatto, J.O.; Insfrán, C.; Urrets-Zavalía, J.A.; Serra, H.M. On the Cornea of Healthy Merino Sheep: A Detailed Ex Vivo Confocal, Histological and Ultrastructural Study. Anat. Histol. Embryol. 2015, 44, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.A.; Misra, S.L.; Lee, H.; McKelvie, J.; Kapadia, K.; McFarlane, R.; McGhee, C.N.J.; Green, C.R.; Sherwin, T. The Sheep Cornea: Structural and Clinical Characteristics. Curr. Eye Res. 2018, 43, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos, A.J.; Asimellis, G. In Vivo Three-Dimensional Corneal Epithelium Imaging in Normal Eyes by Anterior-Segment Optical Coherence Tomography: A Clinical Reference Study. Cornea 2013, 32, 1493. [Google Scholar] [CrossRef]
- LoPinto, A.J.; Pirie, C.G.; Bedenice, D.; Ayres, S.L. Corneal Thickness of Eyes of Healthy Goats, Sheep, and Alpacas Manually Measured by Use of a Portable Spectral-Domain Optical Coherence Tomography Device. Am. J. Vet. Res. 2017, 78, 80–84. [Google Scholar] [CrossRef]
- Nautscher, N.; Bauer, A.; Steffl, M.; Amselgruber, W.M. Comparative Morphological Evaluation of Domestic Animal Cornea. Vet. Ophthalmol. 2016, 19, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Petroll, W.M.; Møller-Pedersen, T.; Maurer, J.K.; Cavanagh, H.D.; Jester, J.V. Epithelial and Corneal Thickness Measurements by in Vivo Confocal Microscopy through Focusing (CMTF). Curr. Eye Res. 1997, 16, 214–221. [Google Scholar] [CrossRef]
- Baba, K.; Tanaka, Y.; Kubota, A.; Kasai, H.; Yokokura, S.; Nakanishi, H.; Nishida, K. A Method for Enhancing the Ocular Penetration of Eye Drops Using Nanoparticles of Hydrolyzable Dye. J. Control. Release 2011, 153, 278–287. [Google Scholar] [CrossRef]
- Stein, S.; Bogdahn, M.; Rosenbaum, C.; Weitschies, W.; Seidlitz, A. Distribution of Fluorescein Sodium and Triamcinolone Acetonide in the Simulated Liquefied and Vitrectomized Vitreous Model with Simulated Eye Movements. Eur. J. Pharm. Sci. 2017, 109, 233–243. [Google Scholar] [CrossRef]
- Mun, E.A.; Morrison, P.W.J.; Williams, A.C.; Khutoryanskiy, V.V. On the Barrier Properties of the Cornea: A Microscopy Study of the Penetration of Fluorescently Labeled Nanoparticles, Polymers, and Sodium Fluorescein. Mol. Pharm. 2014, 11, 3556–3564. [Google Scholar] [CrossRef]
- Srinivas, S.P.; Maurice, D.M. A Microfluorometer for Measuring Diffusion of Fluorophores across the Cornea. IEEE Trans. Biomed. Eng. 1992, 39, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Chauhan, A.; Srinivas, S.P. Penetration of Fluorescein across the Rabbit Cornea from the Endothelial Surface. Pharm. Res. 2012, 29, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Linetsky, M.; Chemoganskiy, V.G.; Hu, F.; Ortwerth, B.J. Effect of UVA Light on the Activity of Several Aged Human Lens Enzymes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Liberda, J.; Tichá, M.; Jona’kova’, V. Preparation of Fluorescein-Labelled and Biotinylated Derivatives of Polysaccharides for Lectin-Saccharide Binding Studies. Biotechnol. Tech. 1997, 11, 265–268. [Google Scholar] [CrossRef]
- Toffoletto, N.; Chauhan, A.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A.P. Asymmetry in Drug Permeability through the Cornea. Pharmaceutics 2021, 13, 694. [Google Scholar] [CrossRef]
- Sebastián-Morelló, M.; Alambiaga-Caravaca, A.M.; Calatayud-Pascual, M.A.; Rodilla, V.; Balaguer-Fernández, C.; Miranda, M.; López-Castellano, A. Ex-Vivo Trans-Corneal and Trans-Scleral Diffusion Studies with Ocular Formulations of Glutathione as an Antioxidant Treatment for Ocular Diseases. Pharmaceutics 2020, 12, 861. [Google Scholar] [CrossRef]
- Wachełko, O.; Zawadzki, M.; Szpot, P. A Novel Procedure for Stabilization of Azide in Biological Samples and Method for Its Determination (HS-GC-FID/FID). Sci. Rep. 2021, 11, 15568. [Google Scholar] [CrossRef]
- Coyo, N.; Peña, M.T.; Costa, D.; Ríos, J.; Lacerda, R.; Leiva, M. Effects of Age and Breed on Corneal Thickness, Density, and Morphology of Corneal Endothelial Cells in Enucleated Sheep Eyes. Vet. Ophthalmol. 2016, 19, 367–372. [Google Scholar] [CrossRef]
- Hodson, S.A. Corneal Stromal Swelling. Prog. Retin. Eye Res. 1997, 16, 99–116. [Google Scholar] [CrossRef]
- Meek, K.M.; Leonard, D.W.; Connon, C.J.; Dennis, S.; Khan, S. Transparency, Swelling and Scarring in the Corneal Stroma. Eye 2003, 17, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Nakamura, T.; Teshima, M.; To, H.; Uematsu, M.; Kitaoka, T.; Taniyama, K.; Nishida, K.; Nakamura, J.; Sasaki, H. Breakdown Evaluation of Corneal Epithelial Barrier Caused by Antiallergic Eyedrops Using an Electrophysiologic Method. J. Ocul. Pharmacol. Ther. 2008, 24, 43–51. [Google Scholar] [CrossRef]
- Morrison, P.W.J.; Khutoryanskiy, V.V. Enhancement in Corneal Permeability of Riboflavin Using Calcium Sequestering Compounds. Int. J. Pharm. 2014, 472, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Toropainen, E.; Ranta, V.-P.; Vellonen, K.-S.; Palmgrén, J.; Talvitie, A.; Laavola, M.; Suhonen, P.; Hämäläinen, K.M.; Auriola, S.; Urtti, A. Paracellular and Passive Transcellular Permeability in Immortalized Human Corneal Epithelial Cell Culture Model. Eur. J. Pharm. Sci. 2003, 20, 99–106. [Google Scholar] [CrossRef]
- Gupta, C.; Chauhan, A.; Mutharasan, R.; Srinivas, S.P. Measurement and Modeling of Diffusion Kinetics of a Lipophilic Molecule across Rabbit Cornea. Pharm. Res. 2010, 27, 699–711. [Google Scholar] [CrossRef]
- Rege, B.D.; Kao, J.P.Y.; Polli, J.E. Effects of Nonionic Surfactants on Membrane Transporters in Caco-2 Cell Monolayers. Eur. J. Pharm. Sci. 2002, 16, 237–246. [Google Scholar] [CrossRef]
- Pacella, E.; Pacella, F.; De Paolis, G.; Parisella, F.R.; Turchetti, P.; Anello, G.; Cavallotti, C. Glycosaminoglycans in the Human Cornea: Age-Related Changes. Ophthalmol. Eye Dis. 2015, 7, OED.S17204. [Google Scholar] [CrossRef]
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic Acid in the Treatment of Dry Eye Disease. Acta Ophthalmol. 2022, 100, 844–860. [Google Scholar] [CrossRef]
- Garrigue, J.-S.; Amrane, M.; Faure, M.-O.; Holopainen, J.M.; Tong, L. Relevance of Lipid-Based Products in the Management of Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2017, 33, 647–661. [Google Scholar] [CrossRef]
- Chaiyasan, W.; Praputbut, S.; Kompella, U.B.; Srinivas, S.P.; Tiyaboonchai, W. Penetration of Mucoadhesive Chitosan-Dextran Sulfate Nanoparticles into the Porcine Cornea. Colloids Surf. B Biointerfaces 2017, 149, 288–296. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viehmeister, K.; Manuelli, A.; Guerin, C.; Kappes, S.; Lamprecht, A. Imaging-Based Drug Penetration Profiling in an Excised Sheep Cornea Model. Pharmaceutics 2024, 16, 1126. https://doi.org/10.3390/pharmaceutics16091126
Viehmeister K, Manuelli A, Guerin C, Kappes S, Lamprecht A. Imaging-Based Drug Penetration Profiling in an Excised Sheep Cornea Model. Pharmaceutics. 2024; 16(9):1126. https://doi.org/10.3390/pharmaceutics16091126
Chicago/Turabian StyleViehmeister, Karla, Aurélie Manuelli, Camille Guerin, Sebastian Kappes, and Alf Lamprecht. 2024. "Imaging-Based Drug Penetration Profiling in an Excised Sheep Cornea Model" Pharmaceutics 16, no. 9: 1126. https://doi.org/10.3390/pharmaceutics16091126
APA StyleViehmeister, K., Manuelli, A., Guerin, C., Kappes, S., & Lamprecht, A. (2024). Imaging-Based Drug Penetration Profiling in an Excised Sheep Cornea Model. Pharmaceutics, 16(9), 1126. https://doi.org/10.3390/pharmaceutics16091126





