Targeting the Adenosine A2A Receptor as a Novel Therapeutic Approach for Renal Cell Carcinoma: Mechanisms and Clinical Trial Review
Abstract
:1. Introduction
2. RCC Epidemiology
2.1. Treatment of Localized RCC
2.2. Treatment of Metastatic RCC
3. General Physiological Role of the Adenosine A2A Receptor
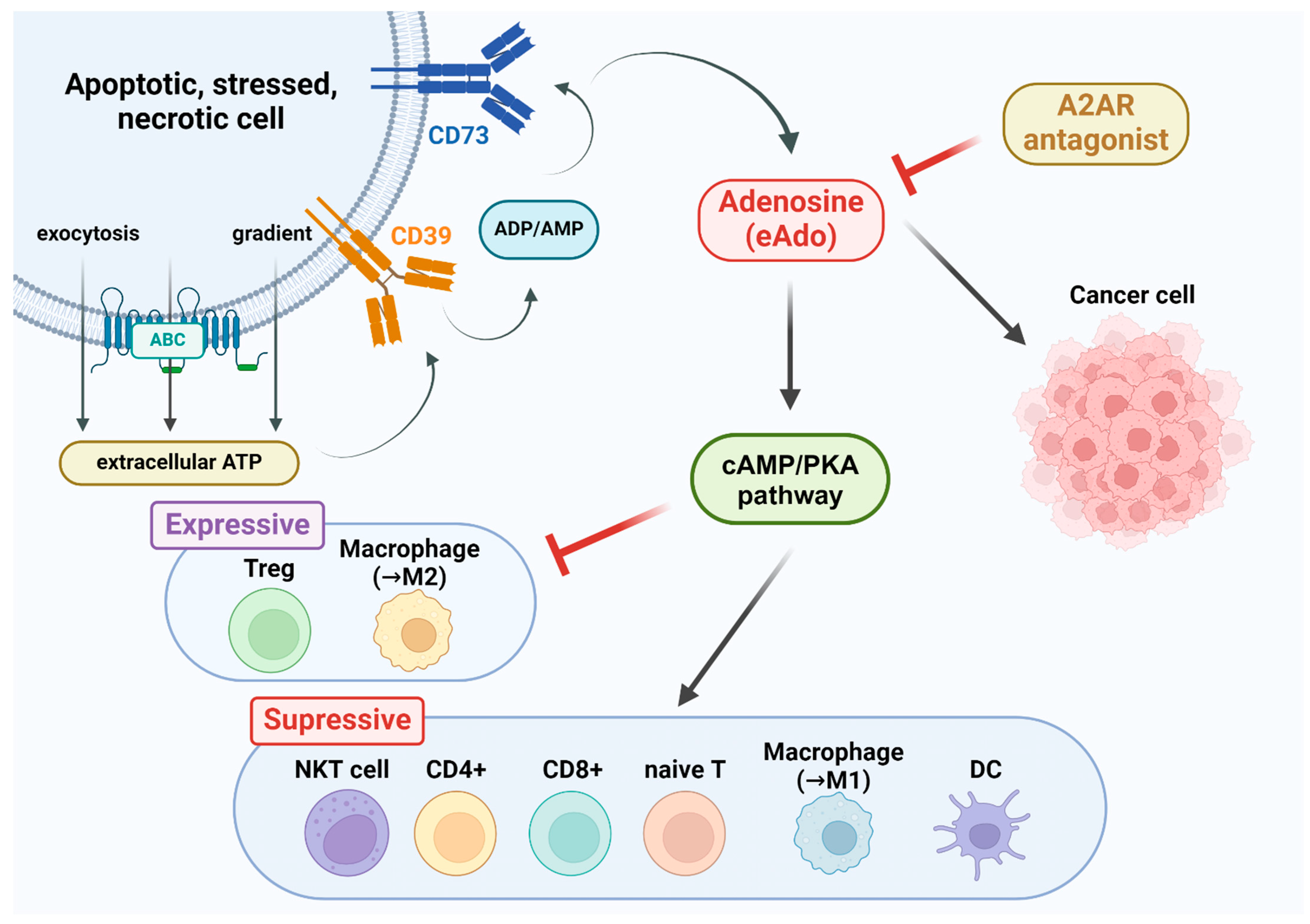
4. Physiological Role of Adenosine A2A Receptors in Tumors
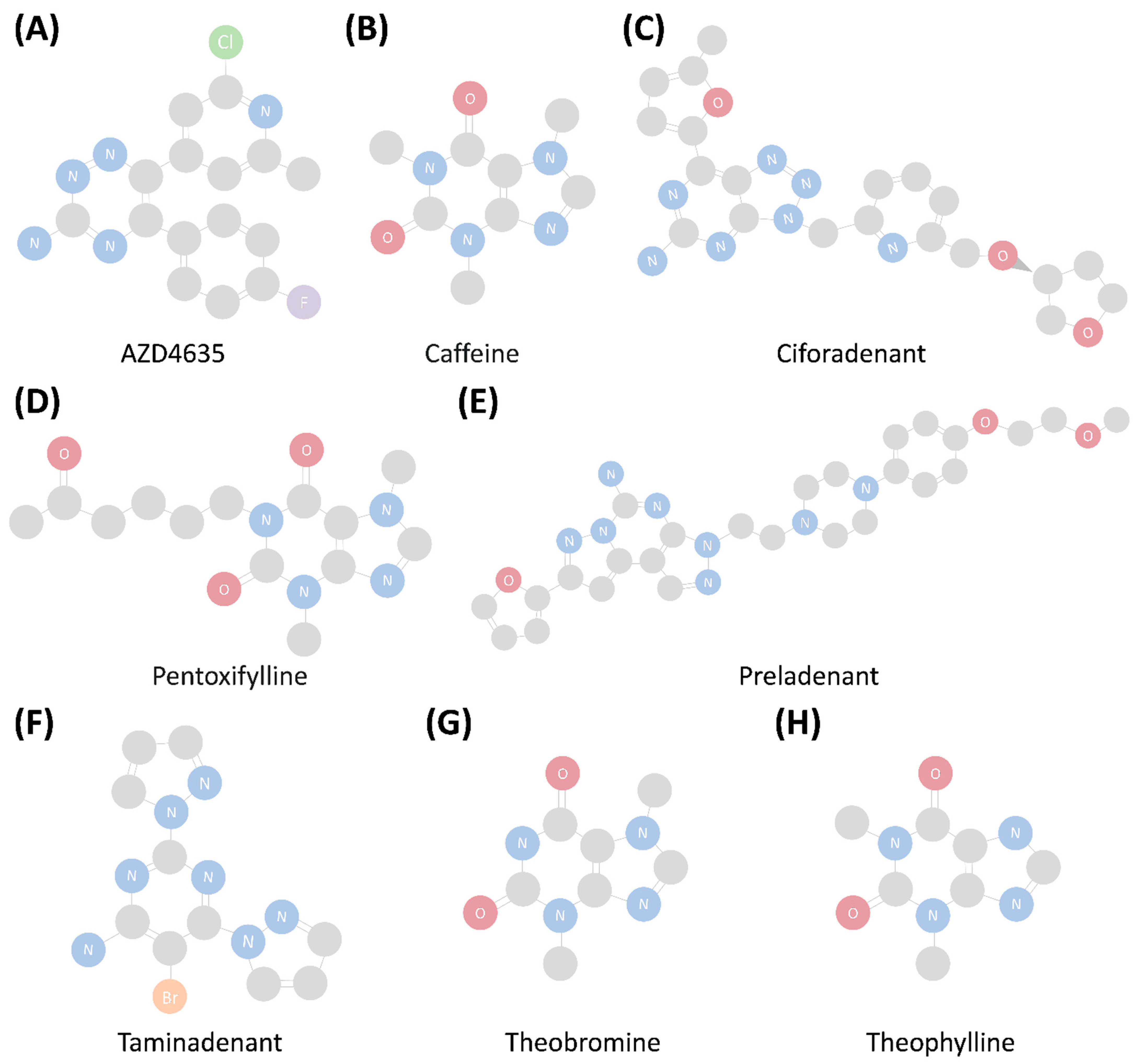
5. Types of Adenosine A2A Receptor Drugs
6. Clinical Trials Investigating Current Adenosine A2A Receptor Drugs in RCC
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rathmell, W.K.; Rumble, R.B.; Van Veldhuizen, P.J.; Al-Ahmadie, H.; Emamekhoo, H.; Hauke, R.J.; Louie, A.V.; Milowsky, M.I.; Molina, A.M.; Rose, T.L.; et al. Management of Metastatic Clear Cell Renal Cell Carcinoma: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2957–2995. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef]
- Makino, T.; Kadomoto, S.; Izumi, K.; Mizokami, A. Epidemiology and Prevention of Renal Cell Carcinoma. Cancers 2022, 14, 4059. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Chen, Y.; Qi, X.; Zhang, D.; Pan, J.; Xie, Z.; Xu, C.; Li, S.; Zhang, X.; Gao, Y.; et al. Temporal trends of kidney cancer incidence and mortality from 1990 to 2016 and projections to 2030. Transl. Androl. Urol. 2020, 9, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.W.; Wang, S.C.; Hsieh, T.Y.; Ho, C.J.; Huang, C.Y.; Kao, Y.L.; Chen, W.J.; Chen, S.L. Favorable mortality-to-incidence ratios of kidney Cancer are associated with advanced health care systems. BMC Cancer 2018, 18, 792. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Pallauf, M.; Ged, Y.; Singla, N. Molecular differences in renal cell carcinoma between males and females. World J. Urol. 2023, 41, 1727–1739. [Google Scholar] [CrossRef]
- Maher, E.R. Hereditary renal cell carcinoma syndromes: Diagnosis, surveillance and management. World J. Urol. 2018, 36, 1891–1898. [Google Scholar] [CrossRef]
- Carlo, M.I.; Hakimi, A.A.; Stewart, G.D.; Bratslavsky, G.; Brugarolas, J.; Chen, Y.B.; Linehan, W.M.; Maher, E.R.; Merino, M.J.; Offit, K.; et al. Familial Kidney Cancer: Implications of New Syndromes and Molecular Insights. Eur. Urol. 2019, 76, 754–764. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Boyle, S.; Carlo, M.I.; Manley, B.; Agarwal, N.; Alva, A.; Beckermann, K.; Choueiri, T.K.; Costello, B.A.; et al. NCCN Guidelines Insights: Kidney Cancer, Version 1.2021. J. Natl. Compr. Cancer Netw. 2020, 18, 1160–1170. [Google Scholar] [CrossRef]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Petejova, N.; Martinek, A. Renal cell carcinoma: Review of etiology, pathophysiology and risk factors. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2016, 160, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Vidal, M.J.; Lázaro Quintela, M.; Lainez-Milagro, N.; Perez-Valderrama, B.; Suárez Rodriguez, C.; Arranz Arija, J.; Peláez Fernández, I.; Gallardo Díaz, E.; Lambea Sorrosal, J.; González-Del-Alba, A. SEOM SOGUG clinical guideline for treatment of kidney cancer (2022). Clin. Transl. Oncol. 2023, 25, 2732–2748. [Google Scholar] [CrossRef]
- Van Poppel, H.; Da Pozzo, L.; Albrecht, W.; Matveev, V.; Bono, A.; Borkowski, A.; Colombel, M.; Klotz, L.; Skinner, E.; Keane, T.; et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur. Urol. 2011, 59, 543–552. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Shao, Y.; Wu, K.; Tang, Y.; Ren, S.; Li, X. Radical versus partial nephrectomy for T1 non-clear cell renal cell carcinoma. Eur. J. Surg. Oncol. 2023, 49, 1519–1523. [Google Scholar] [CrossRef]
- Ray, S.; Cheaib, J.G.; Pierorazio, P.M. Active Surveillance for Small Renal Masses. Rev. Urol. 2020, 22, 9–16. [Google Scholar]
- Campbell, S.C.; Uzzo, R.G.; Karam, J.A.; Chang, S.S.; Clark, P.E.; Souter, L. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-up: AUA Guideline: Part II. J. Urol. 2021, 206, 209–218. [Google Scholar] [CrossRef]
- Ali, M.; Mooi, J.; Lawrentschuk, N.; McKay, R.R.; Hannan, R.; Lo, S.S.; Hall, W.A.; Siva, S. The Role of Stereotactic Ablative Body Radiotherapy in Renal Cell Carcinoma. Eur. Urol. 2022, 82, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Hakam, N.; Heidar, N.A.; El-Asmar, J.; Khauli, M.; Degheili, J.; Al-Moussawy, M.; Nasr, R.; El-Hajj, A.; Wazzan, W.; Bulbul, M.; et al. Comparative analysis of partial versus radical nephrectomy for renal cell carcinoma: Is oncologic safety compromised during nephron sparing in higher stage disease? Urol. Ann. 2023, 15, 226–231. [Google Scholar] [PubMed]
- Huang, R.; Zhang, C.; Wang, X.; Hu, H. Partial Nephrectomy Versus Radical Nephrectomy for Clinical T2 or Higher Stage Renal Tumors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 680842. [Google Scholar] [CrossRef]
- Huang, J.J.; Hsieh, J.J. The Therapeutic Landscape of Renal Cell Carcinoma: From the Dark Age to the Golden Age. Semin. Nephrol. 2020, 40, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Liu, L.; Moore, C.; Hsu, E.; Zhang, A.; Ren, Z.; Sun, Z.; Wang, X.; Zhu, J.; Shen, J.; et al. IL-2 delivery by engineered mesenchymal stem cells re-invigorates CD8(+) T cells to overcome immunotherapy resistance in cancer. Nat. Cell Biol. 2022, 24, 1754–1765. [Google Scholar] [CrossRef]
- Gulati, S.; Labaki, C.; Karachaliou, G.S.; Choueiri, T.K.; Zhang, T. First-Line Treatments for Metastatic Clear Cell Renal Cell Carcinoma: An Ever-Enlarging Landscape. Oncologist 2022, 27, 125–134. [Google Scholar] [CrossRef]
- Re, G.L.; Santeufemia, D.A.; Re, F.L.; Bortolus, R.; Doretto, P.; Marus, W.; Buttazzi, L.; Lenardon, O.; Falda, A.; Piazza, R.; et al. Interleukin-2 chronotherapy for metastatic renal cell carcinoma: Results of a phase I-II study. Cytokine 2020, 128, 154984. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, R.Q.; Zhong, Y.M.; Meng, M.Y.; Zhao, Y.Y.; Yang, L.R.; Li, L.; Hou, Z.L. Efficacy of Enhanced Cytokine-Induced Killer Cells as an Adjuvant Immunotherapy for Renal Cell Carcinoma: Preclinical and Clinical Studies. J. Healthc. Eng. 2021, 2021, 5709104. [Google Scholar] [CrossRef]
- Barata, P.C.; Rini, B.I. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J. Clin. 2017, 67, 507–524. [Google Scholar] [CrossRef]
- Kim, H.; Shim, B.Y.; Lee, S.J.; Lee, J.Y.; Lee, H.J.; Kim, I.H. Loss of Von Hippel-Lindau (VHL) Tumor Suppressor Gene Function: VHL-HIF Pathway and Advances in Treatments for Metastatic Renal Cell Carcinoma (RCC). Int. J. Mol. Sci. 2021, 22, 9795. [Google Scholar] [CrossRef]
- Zhao, D.; Pan, C.; Sun, J.; Gilbert, C.; Drews-Elger, K.; Azzam, D.J.; Picon-Ruiz, M.; Kim, M.; Ullmer, W.; El-Ashry, D.; et al. VEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene 2015, 34, 3107–3119. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef]
- Sadeghi Rad, H.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O’Byrne, K.; Rezaei, N.; Kulasinghe, A. Understanding the tumor microenvironment for effective immunotherapy. Med. Res. Rev. 2021, 41, 1474–1498. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Ye, D.; Zhou, A.; Yao, X.; Luo, H.; He, Z.; Wang, Z.; Zhao, Y.; Ji, Z.; Zou, Q.; et al. Efficacy and safety of vorolanib plus everolimus in metastatic renal cell carcinoma: A three-arm, randomised, double-blind, multicentre phase III study (CONCEPT). Eur. J. Cancer 2023, 178, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, R.C.; Salmon, S.E.; Blumenstein, B.A.; Bearman, S.I.; Roy, V.; McGrath, P.C.; Caton, J.R., Jr.; Munshi, N.; Crawford, E.D. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 2001, 345, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Mickisch, G.H.; Garin, A.; van Poppel, H.; de Prijck, L.; Sylvester, R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet 2001, 358, 966–970. [Google Scholar] [CrossRef]
- Das, A.; Shapiro, D.D.; Craig, J.K.; Abel, E.J. Understanding and integrating cytoreductive nephrectomy with immune checkpoint inhibitors in the management of metastatic RCC. Nat. Rev. Urol. 2023, 20, 654–668. [Google Scholar] [CrossRef]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Chevreau, C.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; Guy, L.; et al. Sunitinib Alone or After Nephrectomy for Patients with Metastatic Renal Cell Carcinoma: Is There Still a Role for Cytoreductive Nephrectomy? Eur. Urol. 2021, 80, 417–424. [Google Scholar] [CrossRef]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential. Targeted Therapy in Human Diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef]
- Kanda, T.; Jenner, P. Can adenosine A(2A) receptor antagonists modify motor behavior and dyskinesia in experimental models of Parkinson’s disease? Park. Relat. Disord. 2020, 80 (Suppl. 1), S21–S27. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, J.; Li, X.; Zhou, X.; Hu, Y.M.; Chu, S.F.; Peng, Y.; Chen, N.H. Research progress on adenosine in central nervous system diseases. CNS Neurosci. Ther. 2019, 25, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, S.N.; Fisone, G.; Moresco, R.; Cunha, R.A.; Ferré, S. Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 2007, 83, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, H.; Wei, H.; Gu, S.; Wei, H. Istradefylline, an adenosine A₂A receptor antagonist, for patients with Parkinson’s Disease: A meta-analysis. J. Neurol. Sci. 2013, 324, 21–28. [Google Scholar] [CrossRef]
- Alonso-Andrés, P.; Albasanz, J.L.; Ferrer, I.; Martín, M. Purine-related metabolites and their converting enzymes are altered in frontal, parietal and temporal cortex at early stages of Alzheimer’s disease pathology. Brain Pathol. 2018, 28, 933–946. [Google Scholar] [CrossRef]
- Illes, P.; Ulrich, H.; Chen, J.F.; Tang, Y. Purinergic receptors in cognitive disturbances. Neurobiol. Dis. 2023, 185, 106229. [Google Scholar] [CrossRef]
- Laurent, C.; Burnouf, S.; Ferry, B.; Batalha, V.L.; Coelho, J.E.; Baqi, Y.; Malik, E.; Marciniak, E.; Parrot, S.; Van der Jeugd, A.; et al. A2A adenosine receptor deletion is protective in a mouse model of Tauopathy. Mol. Psychiatry 2016, 21, 149. [Google Scholar] [CrossRef]
- Ganesana, M.; Venton, B.J. Early changes in transient adenosine during cerebral ischemia and reperfusion injury. PLoS ONE 2018, 13, e0196932. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.V.; Kaster, M.P.; Tomé, A.R.; Agostinho, P.M.; Cunha, R.A. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim. Biophys. Acta 2011, 1808, 1380–1399. [Google Scholar] [CrossRef]
- Zhang, Y.; Wernly, B.; Cao, X.; Mustafa, S.J.; Tang, Y.; Zhou, Z. Adenosine and adenosine receptor-mediated action in coronary microcirculation. Basic. Res. Cardiol. 2021, 116, 22. [Google Scholar] [CrossRef]
- Paganelli, F.; Gaudry, M.; Ruf, J.; Guieu, R. Recent advances in the role of the adenosinergic system in coronary artery disease. Cardiovasc. Res. 2021, 117, 1284–1294. [Google Scholar] [CrossRef]
- Peleli, M.; Carlstrom, M. Adenosine signaling in diabetes mellitus and associated cardiovascular and renal complications. Mol. Aspects Med. 2017, 55, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.S.; Tang, Y.; Song, J.T. ATP and Adenosine in the Retina and Retinal Diseases. Front. Pharmacol. 2021, 12, 654445. [Google Scholar] [CrossRef] [PubMed]
- Cekic, C.; Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells 2020, 9, 2496. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef]
- Yegutkin, G.G.; Boison, D. ATP and Adenosine Metabolism in Cancer: Exploitation for Therapeutic Gain. Pharmacol. Rev. 2022, 74, 797–822. [Google Scholar] [CrossRef]
- Shi, L.; Wu, Z.; Miao, J.; Du, S.; Ai, S.; Xu, E.; Feng, M.; Song, J.; Guan, W. Adenosine interaction with adenosine receptor A2a promotes gastric cancer metastasis by enhancing PI3K-AKT-mTOR signaling. Mol. Biol. Cell 2019, 30, 2527–2534. [Google Scholar] [CrossRef]
- Xia, C.; Yin, S.; To, K.K.W.; Fu, L. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol. Cancer 2023, 22, 44. [Google Scholar] [CrossRef]
- Augustin, R.C.; Leone, R.D.; Naing, A.; Fong, L.; Bao, R.; Luke, J.J. Next steps for clinical translation of adenosine pathway inhibition in cancer immunotherapy. J. Immunother. Cancer 2022, 10, e004089. [Google Scholar] [CrossRef]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef]
- Fredholm, B.B.; AP, I.J.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, S.; Piacentino, D.; Sani, G.; Aromatario, M. Caffeine: Cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Kothari, J.; Hailemariam, A.; Upadhyay, S.; Davidson, L.A.; Chapkin, R.S. Health Benefits of Coffee Consumption for Cancer and Other Diseases and Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 2706. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef]
- Barcz, E.; Sommer, E.; Janik, P.; Marianowski, L.; Skopinska-Rózewska, E. Adenosine receptor antagonism causes inhibition of angiogenic activity of human ovarian cancer cells. Oncol. Rep. 2000, 7, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Lentini, A.; Vidal-Vanaclocha, F.; Facchiano, F.; Caraglia, M.; Abbruzzese, A.; Beninati, S. Theophylline administration markedly reduces hepatic and pulmonary implantation of B16-F10 melanoma cells in mice. Melanoma Res. 2000, 10, 435–443. [Google Scholar] [CrossRef]
- da Silva, J.L.G.; Viana, A.R.; Passos, D.F.; Krause, L.M.F.; Miron, V.V.; Schetinger, M.R.C.; Pillat, M.M.; Palma, T.V.; Leal, D.B.R. Istradefylline modulates purinergic enzymes and reduces malignancy-associated factors in B16F10 melanoma cells. Purinergic Signal. 2023, 19, 633–650. [Google Scholar] [CrossRef]
- Gutknecht da Silva, J.L.; Passos, D.F.; Cabral, F.L.; Miron, V.V.; Schetinger, M.R.C.; Cardoso, A.A.; Dal Piva, C.H.; Gomes, C.O.; Ebone, R.S.; Leal, D.B.R. Istradefylline induces A2A/P2X7 crosstalk expression inducing pro-inflammatory signal, and reduces AKT/mTOR signaling in melanoma-bearing mice. Med. Oncol. 2023, 40, 178. [Google Scholar] [CrossRef]
- Hauser, R.A.; Stocchi, F.; Rascol, O.; Huyck, S.B.; Capece, R.; Ho, T.W.; Sklar, P.; Lines, C.; Michelson, D.; Hewitt, D. Preladenant as an Adjunctive Therapy With Levodopa in Parkinson Disease: Two Randomized Clinical Trials and Lessons Learned. JAMA Neurol. 2015, 72, 1491–1500. [Google Scholar] [CrossRef]
- Yuan, G.; Jankins, T.C.; Patrick, C.G., Jr.; Philbrook, P.; Sears, O.; Hatfield, S.; Sitkovsky, M.; Vasdev, N.; Liang, S.H.; Ondrechen, M.J.; et al. Fluorinated Adenosine A(2A) Receptor Antagonists Inspired by Preladenant as Potential Cancer Immunotherapeutics. Int. J. Med. Chem. 2017, 2017, 4852537. [Google Scholar] [PubMed]
- Mediavilla-Varela, M.; Castro, J.; Chiappori, A.; Noyes, D.; Hernandez, D.C.; Allard, B.; Stagg, J.; Antonia, S.J. A Novel Antagonist of the Immune Checkpoint Protein Adenosine A2a Receptor Restores Tumor-Infiltrating Lymphocyte Activity in the Context of the Tumor Microenvironment. Neoplasia 2017, 19, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Chiappori, A.A.; Creelan, B.; Tanvetyanon, T.; Gray, J.E.; Haura, E.B.; Thapa, R.; Barlow, M.L.; Chen, Z.; Chen, D.T.; Beg, A.A.; et al. Phase I Study of Taminadenant (PBF509/NIR178), an Adenosine 2A Receptor Antagonist, with or without Spartalizumab (PDR001), in Patients with Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2022, 28, 2313–2320. [Google Scholar] [CrossRef]
- Leone, R.D.; Sun, I.M.; Oh, M.H.; Sun, I.H.; Wen, J.; Englert, J.; Powell, J.D. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol. Immunother. 2018, 67, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Ho, P.Y.; Hotson, A.; Hill, C.; Piccione, E.C.; Hsieh, J.; Liu, L.; Buggy, J.J.; McCaffery, I.; Miller, R.A. A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti-PD-(L)1 and Anti-CTLA-4 in Preclinical Models. Cancer Immunol. Res. 2018, 6, 1136–1149. [Google Scholar] [CrossRef]
- Falchook, G.S.; Reeves, J.; Gandhi, S.; Spigel, D.R.; Arrowsmith, E.; George, D.J.; Karlix, J.; Pouliot, G.; Hattersley, M.M.; Gangl, E.T.; et al. A phase 2 study of AZD4635 in combination with durvalumab or oleclumab in patients with metastatic castration-resistant prostate cancer. Cancer Immunol. Immunother. 2024, 73, 72. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, X.; Zheng, J.; Liu, Z.; Yang, Z.; Zhang, X. Overcoming high level adenosine-mediated immunosuppression by DZD2269, a potent and selective A2aR antagonist. J. Exp. Clin. Cancer Res. 2022, 41, 302. [Google Scholar] [CrossRef]
- Khoury, W.; Trus, R.; Chen, X.; Baghaie, L.; Clark, M.; Szewczuk, M.R.; El-Diasty, M. Parsimonious Effect of Pentoxifylline on Angiogenesis: A Novel Pentoxifylline-Biased Adenosine G Protein-Coupled Receptor Signaling Platform. Cells 2023, 12, 1199. [Google Scholar] [CrossRef]
- Nathan, J.R.; Lakshmanan, G.; Michael, F.M.; Seppan, P.; Ragunathan, M. Expression of adenosine receptors and vegf during angiogenesis and its inhibition by pentoxifylline-A study using zebrafish model. Biomed. Pharmacother. 2016, 84, 1406–1418. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Procopio, G.; Astore, S.; Cannella, M.A.; Maratta, M.G.; Rizzo, M.; Verzoni, E.; Porta, C.; Tortora, G. Current evidence for second-line treatment in metastatic renal cell carcinoma after progression to immune-based combinations. Cancer Treat. Rev. 2022, 105, 102379. [Google Scholar] [CrossRef]
- Pastore, D.R.E.; Mookhtiar, K.; Schwartz, B.; Kumar, S.; Nagaraj, R.; Meru, A.V. Adenosine receptor antagonists A2AR (TT-10) and A2BR (TT-4) demonstrate anti-tumor activity in 4T1-induced syngeneic breast cancer mouse model. Cancer Res. 2022, 82, 3454. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Fay, A.P.; Gagnon, R.; Lin, Y.; Bahamon, B.; Brown, V.; Rosenberg, J.E.; Hutson, T.E.; Baker-Neblett, K.L.; Carpenter, C.; et al. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin. Cancer Res. 2013, 19, 5218–5226. [Google Scholar] [CrossRef] [PubMed]
| Drug | Trial | Phase | Number of Patients | Drugs Involved | Condition or Disease | Status |
|---|---|---|---|---|---|---|
| Ciforadenant (CPI-444) | NCT02655822 | I/Ib | 502 | Atezolizumab | Renal cell cancer, metastatic castration-resistant prostate cancer | Completed |
| NCT05501054 | Ib/II | 24 | Ipilimumab, Nivolumab | Renal cell carcinoma | Recruiting | |
| TT-10 | NCT04969315 | I/II | 90 | Renal cell cancer, castration-resistant prostate cancer, non-small cell lung cancer, head and neck squamous cell carcinoma | Active, not recruiting | |
| NIR178 (PBF509/Taminadenant) | NCT04895748 | I/Ib | 40 | DFF332, RAD001, PDR001 | Renal cell carcinoma | Active, not recruiting |
| NCT03549000 | I/Ib | 127 | NZV930, PDR001 | Renal cell carcinoma, non-small cell lung cancer, triple-negative breast cancer, pancreatic ductal adenocarcinoma, colorectal cancer microsatellite stable, ovarian cancer, metastatic castration-resistant prostate cancer | Terminated | |
| NCT03207867 | II | 315 | PDR001 | Renal cell cancer, non-small cell lung cancer, pancreatic cancer, urothelial cancer, head and neck cancer, diffused large B cell lymphoma, microsatellite stable colon cancer, triple-negative breast cancer, melanoma, metastatic castration-resistant prostate cancer | Terminated | |
| Etrumadenant (AB928) | NCT03629756 | I | 48 | zimberelimab (AB122) | Renal cell carcinoma, non-small cell lung cancer, squamous cell carcinoma of the head and neck, breast cancer, colorectal cancer, melanoma, bladder cancer, ovarian cancer, endometrial cancer, Merkel cell carcinoma, gastroesophageal cancer, castration-resistant prostate cancer | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-Y.; Chang, Y.-C.; Yu, C.-Y.; Sung, W.-W. Targeting the Adenosine A2A Receptor as a Novel Therapeutic Approach for Renal Cell Carcinoma: Mechanisms and Clinical Trial Review. Pharmaceutics 2024, 16, 1127. https://doi.org/10.3390/pharmaceutics16091127
Chen T-Y, Chang Y-C, Yu C-Y, Sung W-W. Targeting the Adenosine A2A Receptor as a Novel Therapeutic Approach for Renal Cell Carcinoma: Mechanisms and Clinical Trial Review. Pharmaceutics. 2024; 16(9):1127. https://doi.org/10.3390/pharmaceutics16091127
Chicago/Turabian StyleChen, Ting-Yu, Ya-Chuan Chang, Chia-Ying Yu, and Wen-Wei Sung. 2024. "Targeting the Adenosine A2A Receptor as a Novel Therapeutic Approach for Renal Cell Carcinoma: Mechanisms and Clinical Trial Review" Pharmaceutics 16, no. 9: 1127. https://doi.org/10.3390/pharmaceutics16091127
APA StyleChen, T.-Y., Chang, Y.-C., Yu, C.-Y., & Sung, W.-W. (2024). Targeting the Adenosine A2A Receptor as a Novel Therapeutic Approach for Renal Cell Carcinoma: Mechanisms and Clinical Trial Review. Pharmaceutics, 16(9), 1127. https://doi.org/10.3390/pharmaceutics16091127








