Multifunctional Bioactivity Electrospinning Nanofibers Encapsulating Emodin Provides a Potential Postoperative Management Strategy for Skin Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coaxial Electrospinning Nanofibers
2.3. Characterization of Electrospinning Nanofibers
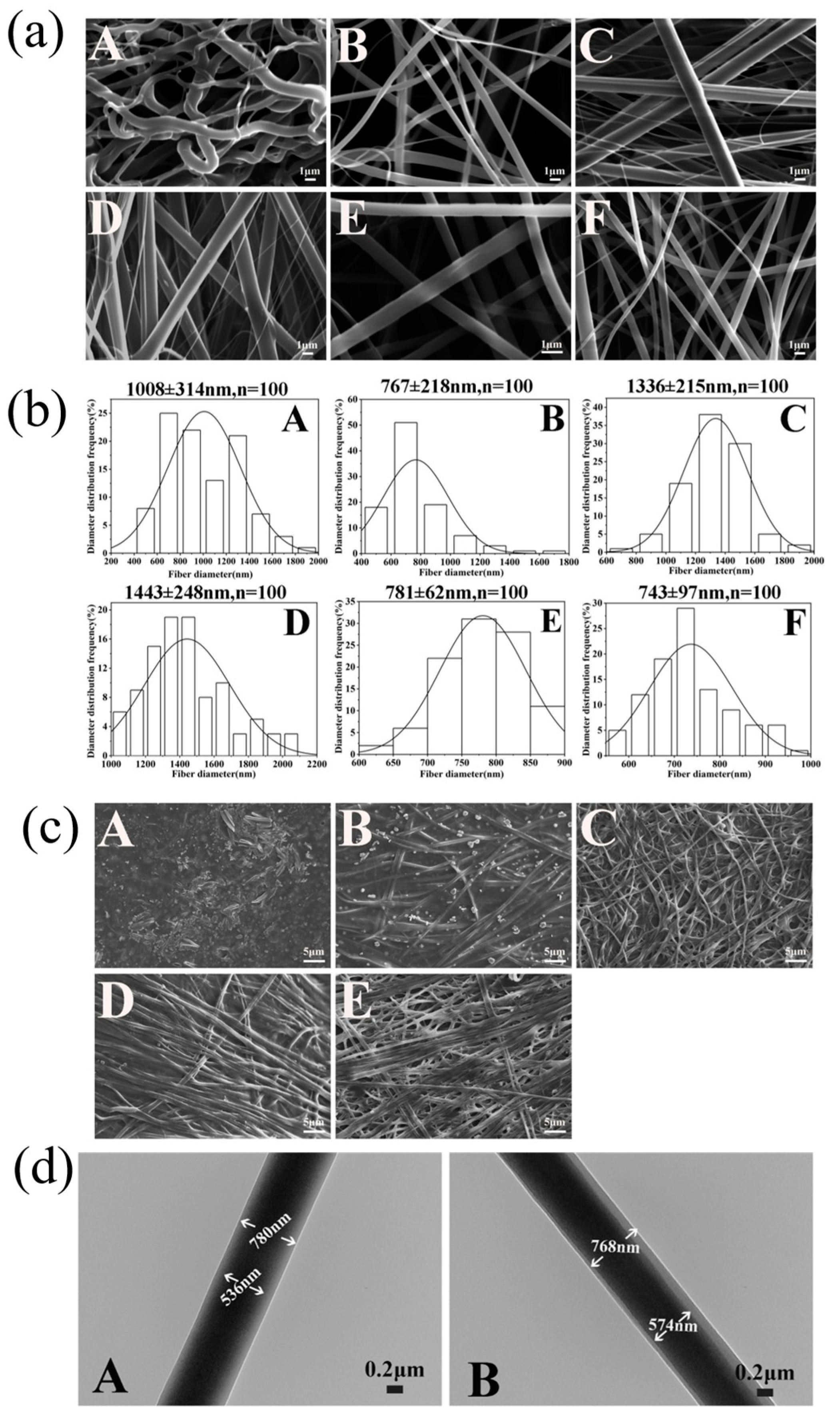
2.3.1. Morphologies
2.3.2. Attenuated Total Reflection–Fourier Transform Infrared Spectra Analysis
2.3.3. X-ray Diffraction
2.3.4. Differential Scanning Calorimetry
2.3.5. Wettability and Swelling Ratio
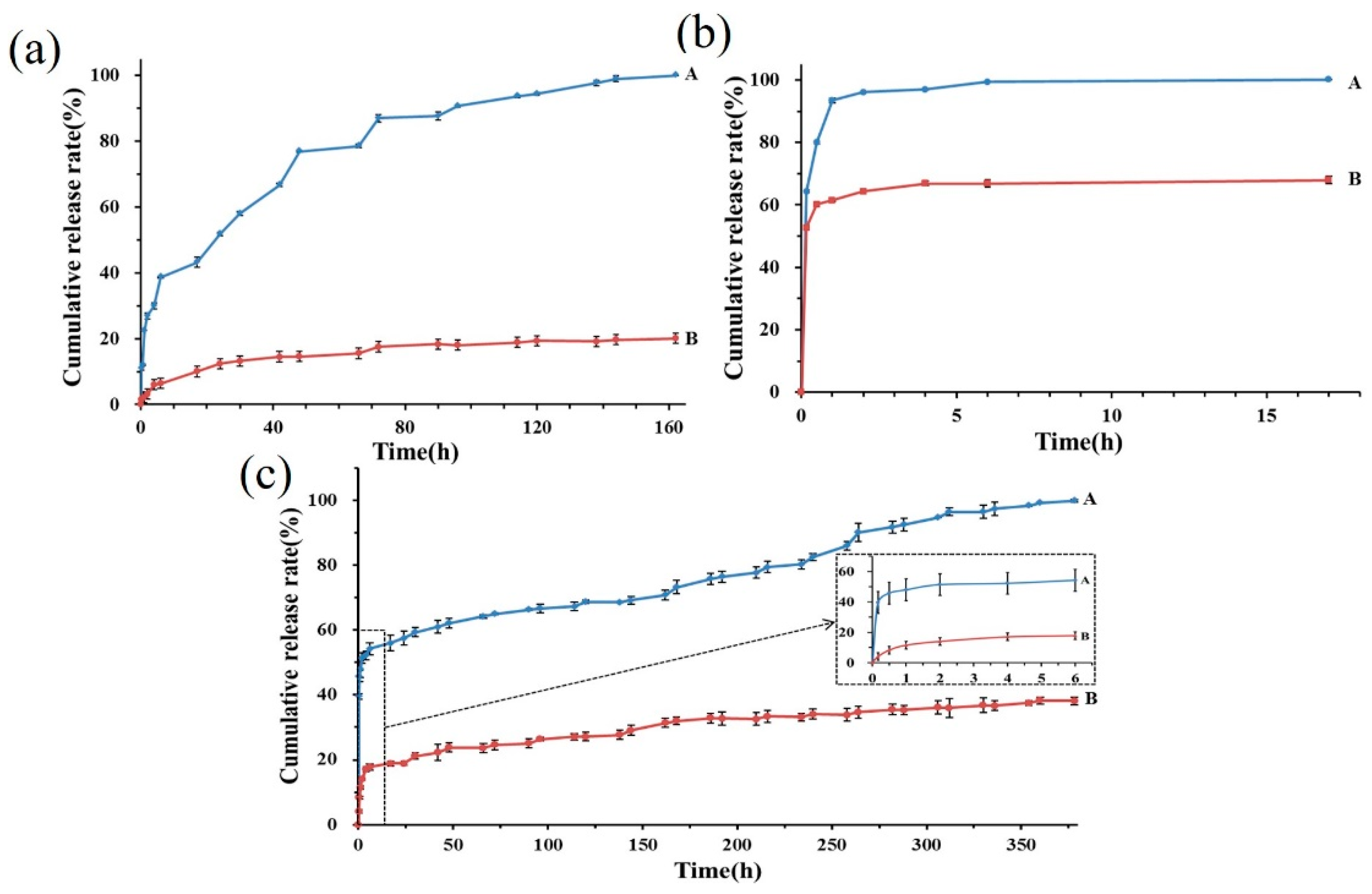
2.3.6. Encapsulation Efficiency and Drug Release Profiles
2.4. Cytocompatibility
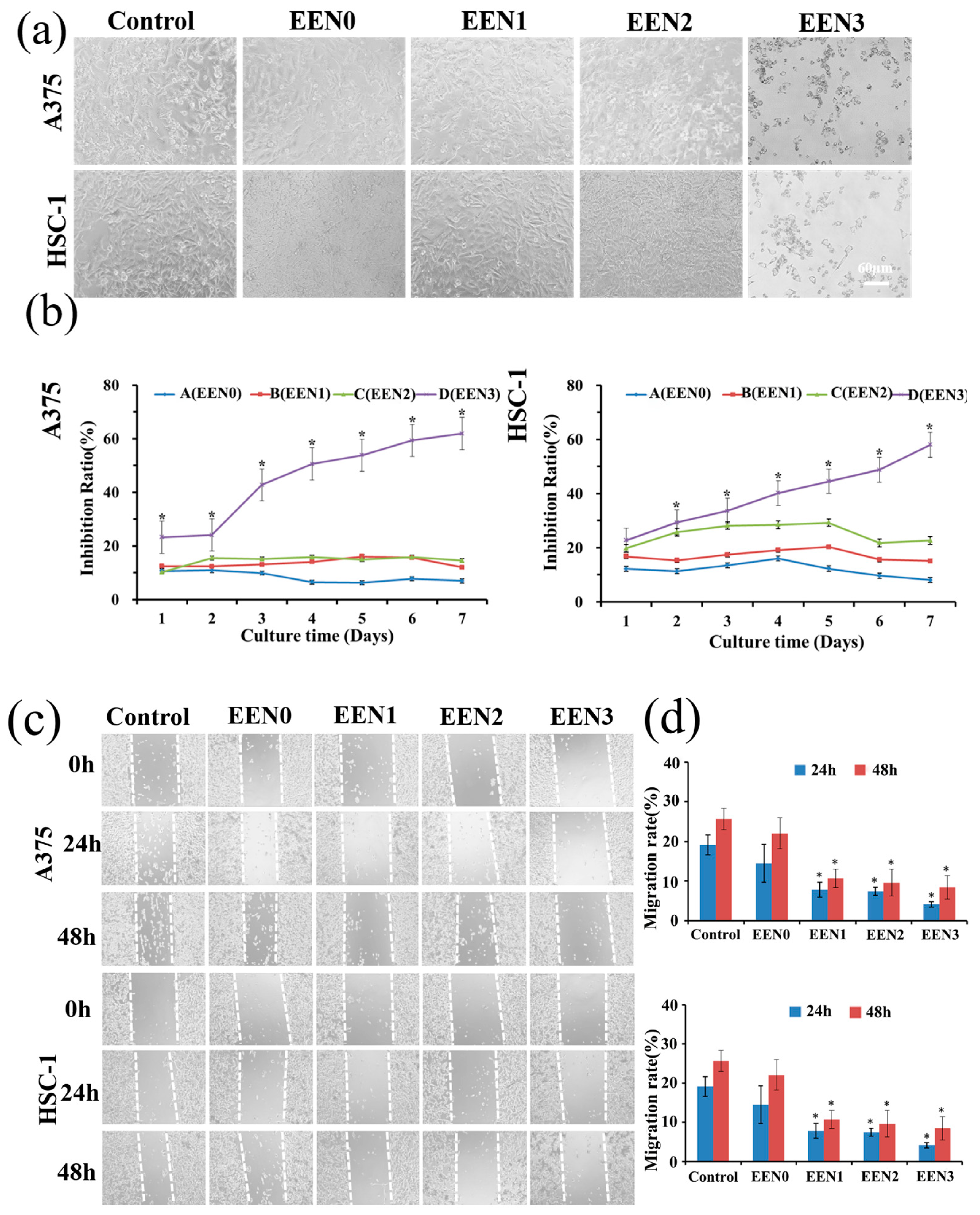
2.5. Anti-Cancer Effects
2.6. Mechanism of Anti-Cancer Effects
2.6.1. Cell Death Inhibitors
2.6.2. MDA Level
2.6.3. ROS Level
2.7. Anti-Bacterial Activities
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimized Electrospinning Conditions
3.2. Characterization
3.2.1. Morphology
3.2.2. ATR-FTIR Spectra Analysis
3.2.3. X-ray Diffraction
3.2.4. Differential Scanning Calorimetry
3.2.5. Swelling Ratio and Contact Angle
3.2.6. Encapsulation Efficiency and Release Profiles
3.3. Cytocompatibility with Normal Cells
3.4. Anti-Cancer Effects
3.5. Anti-Cancer Effect Mechanism
3.5.1. Inhibit Cancer Cell Proliferation via Necroptosis and Apoptosis Pathway
3.5.2. MDA Level in Tumor Cells
3.5.3. ROS Level in Tumor Cells
3.6. Anti-Bacterial Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, J. Screening and Prevention of Skin Cancer. JAMA-J. Am. Med. Assoc. 2023, 329, 1324. [Google Scholar] [CrossRef] [PubMed]
- Rojas, K.D.; Perez, M.E.; Marchetti, M.A.; Nichols, A.J.; Penedo, F.J.; Jaimes, N. Skin cancer: Primary, secondary, and tertiary prevention. Part II. J. Am. Acad. Dermatol. 2022, 87, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol. Cancer 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K. HIV and skin cancer risk. Nat. Med. 2022, 24, 521–530. [Google Scholar] [CrossRef]
- Ma, H.; Yu, Q.; Qu, Y.; Zhu, Y.; Wu, C. Manganese silicate nanospheres-incorporated hydrogels: Starvation therapy and tissue regeneration. Bioact. Mater. 2021, 6, 4558–4567. [Google Scholar]
- Xi, Y.; Ge, J.; Wang, M.; Chen, M.; Niu, W.; Cheng, W.; Xue, Y.; Lin, C.; Lei, B. Bioactive Anti-inflammatory, Antibacterial, Antioxidative Silicon-Based Nanofibrous Dressing Enables Cutaneous Tumor Photothermo-Chemo Therapy and Infection-Induced Wound Healing. ACS Nano 2020, 14, 2904–2916. [Google Scholar] [CrossRef]
- Fan, C.; Xu, Q.; Hao, R.; Wang, C.; Que, Y.; Chen, Y.; Yang, C.; Chang, J. Multi-functional wound dressings based on silicate bioactive materials. Biomaterials 2022, 287, 121652. [Google Scholar] [CrossRef]
- Nowak-Perlak, M.; Bromke, M.A.; Ziolkowski, P.; Wozniak, M. The Comparison of the Efficiency of Emodin and Aloe-Emodin in Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 6276. [Google Scholar] [CrossRef]
- Ye, P.; Wei, S.; Luo, C.; Wang, Q.; Li, A.; Wei, F. Long-Term Effect against Methicillin-Resistant Staphylococcus aureus of Emodin Released from Coaxial Electrospinning Nanofiber Membranes with a Biphasic Profile. Biomolecules 2020, 10, 362. [Google Scholar] [CrossRef]
- Hu, Q.; Yao, J.; Wu, X.; Li, J.; Li, G.; Tang, W.; Liu, J.; Wan, M. Emodin attenuates severe acute pancreatitis-associated acute lung injury by suppressing pancreatic exosome-mediated alveolar macrophage activation. Acta Pharm. Sin. B 2022, 12, 3986–4003. [Google Scholar] [CrossRef] [PubMed]
- Dumit, V.I.; Zerbes, R.M.; Kaeser-Pebernard, S.; Rackiewicz, M.; Wall, M.T.; Gretzmeier, C.; Kuttner, V.; van der Laan, M.; Braun, R.J.; Dengjel, J. Respiratory status determines the effect of emodin on cell viability. Oncotarget 2017, 8, 37478–37490. [Google Scholar] [CrossRef]
- Liu, Q.; Hodge, J.; Wang, J.; Wang, Y.; Wang, L.; Singh, U.; Li, Y.; Yao, Y.; Wang, D.; Ai, W.; et al. Emodin reduces Breast Cancer Lung Metastasis by suppressing Macrophage-induced Breast Cancer Cell Epithelial-mesenchymal transition and Cancer Stem Cell formation. Theranostics 2020, 10, 8365–8381. [Google Scholar] [CrossRef] [PubMed]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul, M.Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, S.; Bramanti, A.; Nicoletti, F.; Fagone, P.; Kaluderovic, G.N.; Maksimovic-Ivanic, D. Naturally occurring compounds in differentiation based therapy of cancer. Biotechnol. Adv. 2018, 36, 1622–1632. [Google Scholar] [CrossRef]
- Way, T.D.; Huang, J.T.; Chou, C.H.; Huang, C.H.; Yang, M.H.; Ho, C.T. Emodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the beta-catenin and Akt pathways. Eur. J. Cancer 2014, 50, 366–378. [Google Scholar] [CrossRef]
- Shia, C.S.; Hou, Y.C.; Tsai, S.Y.; Huieh, P.H.; Leu, Y.L.; Chao, P.D. Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. J. Pharm. Sci. 2010, 99, 2185–2195. [Google Scholar] [CrossRef]
- Liu, W.; Tang, L.; Ye, L.; Cai, Z.; Xia, B.; Zhang, J.; Hu, M.; Liu, Z. Species and Gender Differences Affect the Metabolism of Emodin via Glucuronidation. AAPS J. 2010, 12, 424–436. [Google Scholar] [CrossRef]
- Ban, E.; Park, M.; Jeong, S.; Kwon, T.; Kim, E.H.; Jung, K.; Kim, A. Poloxamer-Based Thermoreversible Gel for Topical Delivery of Emodin: Influence of P407 and P188 on Solubility of Emodin and Its Application in Cellular Activity Screening. Molecules 2017, 22, 246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Dong, D.; Lu, D.; Wang, S.; Wu, B. Cremophor EL-based nanoemulsion enhances transcellular permeation of emodin through glucuronidation reduction in UGT1A1-overexpressing MDCKII cells. Int. J. Pharmaceut. 2016, 501, 190–198. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Z.; Sun, R.; Mo, Z.; Jin, G.; Wei, F.; Hu, J.; Guan, W.; Zhong, N. Preparation of lung-targeting, emodin-loaded polylactic acid microspheres and their properties. Int. J. Mol. Sci. 2014, 15, 6241–6251. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Nie, W.; Wang, Y.C.; Shen, Y.; Li, Y.; Gan, S.J. Electrospun emodin polyvinylpyrrolidone blended nanofibrous membrane: A novel medicated biomaterial for drug delivery and accelerated wound healing. J. Mater. Sci.-Mater. Med. 2012, 23, 2709–2716. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, M.S.; Akhavan-Mahdavi, S.; Hasan, A.; Kharazmi, M.S.; Jafari, S.M. Chitosan-based electrospun nanofibers for diabetic foot ulcer management; recent advances. Carbohyd. Polym. 2023, 313, 120512. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.D.; Powell, J.D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef]
- Quinones, E.D.; Lu, T.Y.; Liu, K.T.; Fan, Y.J.; Chuang, E.Y.; Yu, J. Glycol chitosan/iron oxide/polypyrrole nanoclusters for precise chemodynamic/photothermal synergistic therapy. Int. J. Biol. Macromol. 2022, 203, 268–279. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef]
- Liang, X.; Mu, M.; Fan, R.; Zou, B.; Guo, G. Functionalized chitosan as a promising platform for cancer immunotherapy: A review. Carbohyd. Polym. 2022, 290, 119452. [Google Scholar] [CrossRef]
- Zaiki, Y.; Iskandar, A.; Wong, T.W. Functionalized chitosan for cancer nano drug delivery. Biotechnol. Adv. 2023, 67, 108200. [Google Scholar] [CrossRef]
- Jie, X.; Shiu, B.C.; Zhang, Y.; Wu, H.; Ye, Y.; Fang, R. Chitosan-Urushiol nanofiber membrane with enhanced acid resistance and broad-spectrum antibacterial activity. Carbohyd. Polym. 2023, 312, 120792. [Google Scholar] [CrossRef]
- Salas, C.; Thompson, Z.; Bhattarai, N. 15—Electrospun chitosan fibers. In Electrospun Nanofibers; Afshari, M., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 371–398. [Google Scholar]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Gao, J.; Yang, R.; Yuan, C.; Wang, R.; Zou, Q.; Zuo, Y.; Zhu, M.; Li, Y.; Man, Y.; et al. A baicalin-loaded coaxial nanofiber scaffold regulated inflammation and osteoclast differentiation for vascularized bone regeneration. Bioact. Mater. 2022, 8, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalik, S.; Gallo, J.; Nip, J.; Katebifar, S.; Arul, M.; Lebaschi, A.; Munch, L.N.; Bartly, J.M.; Choudhary, S.; Kalajzic, I.; et al. Nanofiber matrix formulations for the delivery of Exendin-4 for tendon regeneration: In vitro and in vivo assessment. Bioact. Mater. 2023, 25, 42–60. [Google Scholar] [CrossRef]
- Ailincai, D.; Cibotaru, S.; Anisiei, A.; Coman, C.G.; Pasca, A.S.; Rosca, I.; Sandu, A.I.; Mititelu-Tartau, L.; Marin, L. Mesoporous chitosan nanofibers loaded with norfloxacin and coated with phenylboronic acid perform as bioabsorbable active dressings to accelerate the healing of burn wounds. Carbohyd. Polym. 2023, 318, 121135. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Y.; Song, Y.; He, X.; Xu, T.; Zhang, X. Hydrogel-Functionalized Bandages with Janus Wettability for Efficient Unidirectional Drug Delivery and Wound Care. ACS Nano 2024, 18, 3468–3479. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Yang, S.; Liu, P.; Zhang, B. Electrospun PCL/mupirocin and chitosan/lidocaine hydrochloride multifunctional double layer nanofibrous scaffolds for wound dressing applications. Int. J. Nanomed. 2018, 13, 5287–5299. [Google Scholar] [CrossRef]
- Yun, J.; Im, J.S.; Lee, Y.; Kim, H. Electro-responsive transdermal drug delivery behavior of PVA/PAA/MWCNT nanofibers. Eur. Polym. J. 2011, 47, 1893–1902. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Abid, S.; Hussain, T.; Nazir, A.; Zahir, A.; Ramakrishna, S.; Hameed, M.; Khenoussi, N. Enhanced antibacterial activity of PEO-chitosan nanofibers with potential application in burn infection management. Int. J. Biol. Macromol. 2019, 135, 1222–1236. [Google Scholar] [CrossRef]
- Meng, Y.; Li, X.J.; Li, Y.; Zhang, T.Y.; Liu, D.; Wu, Y.Q.; Hou, F.F.; Ye, L.; Wu, C.J.; Feng, X.D.; et al. Novel Double-Layer Dissolving Microneedles for Transmucosal Sequential Delivery of Multiple Drugs in the Treatment of Oral Mucosa Diseases. ACS Appl. Mater. Interfaces 2023, 15, 13892–13906. [Google Scholar] [CrossRef]
- Shrimali, D.; Shanmugam, M.K.; Kumar, A.P.; Zhang, J.; Tan, B.K.H.; Ahn, K.S.; Sethi, G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013, 341, 139–149. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Kamran, M.Z.; Ranjan, A.; Nimesh, H.; Singh, M.; Tandon, V. Akt1/NFkappaB signaling pathway activation by a small molecule DMA confers radioprotection to intestinal epithelium in xenograft model. Free Radic. Bio Med. 2017, 108, 564–574. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, Z.; Li, M.; Yang, Y.; Zheng, B.; Wang, P.; Huang, C.; Zhou, S. The crosstalk between reactive oxygen species and noncoding RNAs: From cancer code to drug role. Mol. Cancer 2022, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, R.; Zhang, X.; Cheng, G.; Yu, P.; Chang, J.; Chen, X. Beyond Photo: Xdynamic Therapies in Fighting Cancer. Adv. Mater. 2021, 33, e2007488. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, P.; Yusufu, R.; Guan, Z.; Chen, T.; Li, S.; Feng, Y.; Zeng, X.; Lu, J.; Luo, M.; Wei, F. Multifunctional Bioactivity Electrospinning Nanofibers Encapsulating Emodin Provides a Potential Postoperative Management Strategy for Skin Cancer. Pharmaceutics 2024, 16, 1131. https://doi.org/10.3390/pharmaceutics16091131
Ye P, Yusufu R, Guan Z, Chen T, Li S, Feng Y, Zeng X, Lu J, Luo M, Wei F. Multifunctional Bioactivity Electrospinning Nanofibers Encapsulating Emodin Provides a Potential Postoperative Management Strategy for Skin Cancer. Pharmaceutics. 2024; 16(9):1131. https://doi.org/10.3390/pharmaceutics16091131
Chicago/Turabian StyleYe, Peiwen, Reyisha Yusufu, Zhenfeng Guan, Tiantian Chen, Siyi Li, Yanping Feng, Xiaoyan Zeng, Jingya Lu, Muxiang Luo, and Fenghuan Wei. 2024. "Multifunctional Bioactivity Electrospinning Nanofibers Encapsulating Emodin Provides a Potential Postoperative Management Strategy for Skin Cancer" Pharmaceutics 16, no. 9: 1131. https://doi.org/10.3390/pharmaceutics16091131




