Herbal Formula Extract Ameliorates Anxiety and Cognitive Impairment via Regulation of the Reelin/Dab-1 Pathway in a Murine Model of Post-Traumatic Stress Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of the PTSD Mouse Model
2.2. Preparation of HFEs
2.3. HFE Administration
2.4. Open Field Test (OFT)
2.5. Y-Maze Test
2.6. Tissue Preparation
2.7. Serum Corticosterone and Serotonin Assays
2.8. Immunostaining
2.9. Western Blot Analysis
2.10. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.11. Bisulfite Modification and PCR of Genomic DNA
2.12. Quantitative DNA Methylation Assay
2.13. High Performance Liquid Chromatography (HPLC)/Photodiode Array Detector (PDA) Analysis of HFE
2.14. Statistical Analyses
3. Results
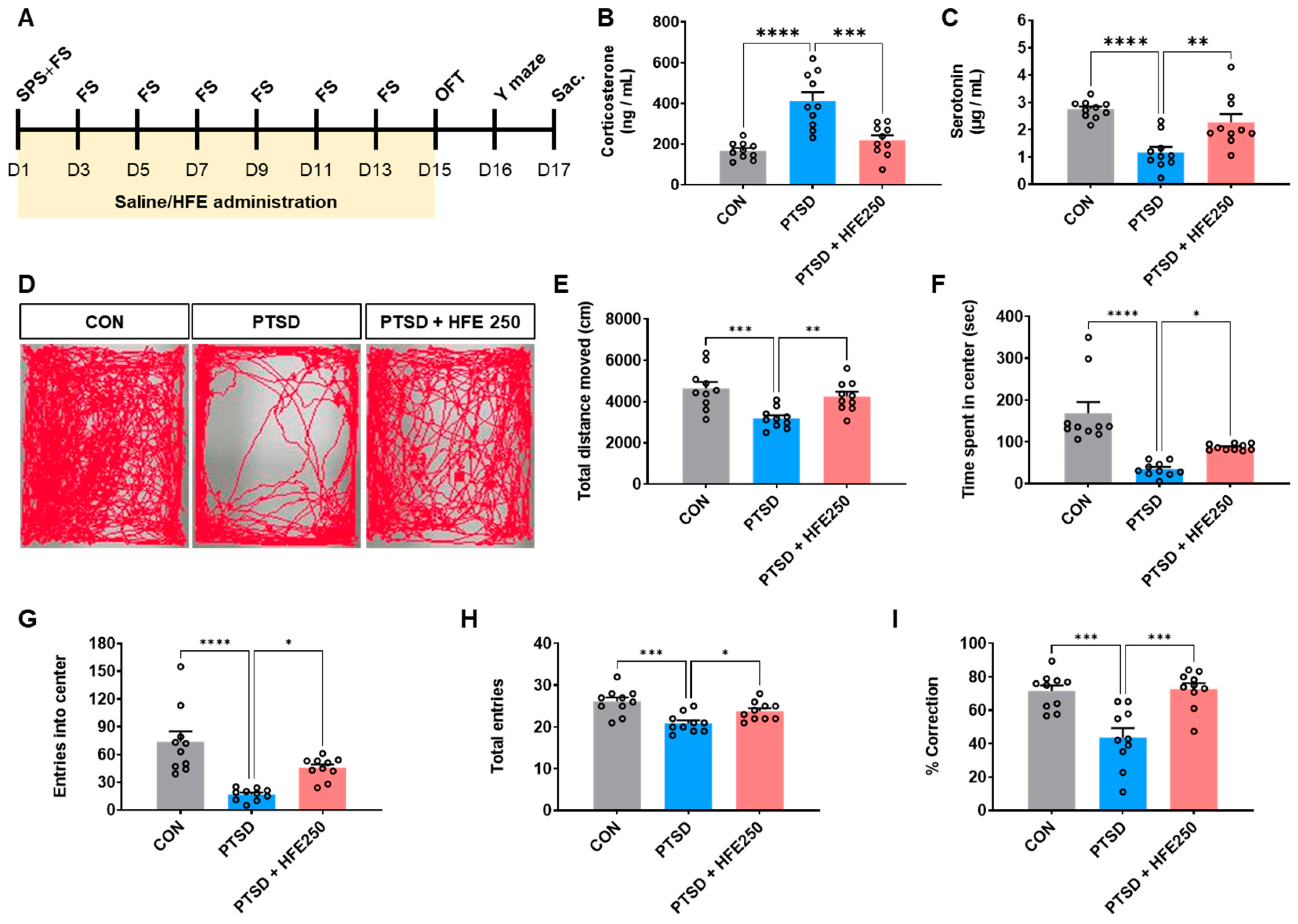
3.1. Effect of HFE Administration on Serum Corticosterone and Serotonin Levels in PTSD Mice
3.2. HFE Administration Ameliorates PTSD-like Behavioral Abnormalities
3.3. Hippocampal Dysfunctions in PTSD Mice Are Reduced by HFE Administration
3.4. HFE Administration Reverses PTSD-Induced Reduction in the Reelin/Dab1 Pathway in the Hippocampus
3.5. Treatment with HFE Restored Reelin Methylation of the Hippocampus in PTSD Mice
3.6. Treatment with HFE Increases the Expression of Synaptic Plasticity-Associated Genes in the Hippocampus of PTSD Mice
3.7. Identification of Active Compounds of HFE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blum, K.; Gondre-Lewis, M.C.; Modestino, E.J.; Lott, L.; Baron, D.; Siwicki, D.; McLaughlin, T.; Howeedy, A.; Krengel, M.H.; Oscar-Berman, M.; et al. Understanding the Scientific Basis of Post-traumatic Stress Disorder (PTSD): Precision Behavioral Management Overrides Stigmatization. Mol. Neurobiol. 2019, 56, 7836–7850. [Google Scholar] [CrossRef]
- Besnard, A.; Sahay, A. Adult Hippocampal Neurogenesis, Fear Generalization, and Stress. Neuropsychopharmacology 2016, 41, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.P.; Hayes, S.; Miller, D.R.; Lafleche, G.; Logue, M.W.; Verfaellie, M. Automated measurement of hippocampal subfields in PTSD: Evidence for smaller dentate gyrus volume. J. Psychiatr. Res. 2017, 95, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Torres-Reveron, A.; Gray, J.D.; Melton, J.T.; Punsoni, M.; Tabori, N.E.; Ward, M.J.; Frys, K.; Iadecola, C.; Milner, T.A. Early postnatal exposure to methylphenidate alters stress reactivity and increases hippocampal ectopic granule cells in adult rats. Brain Res. Bull. 2009, 78, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Winzenried, E.T.; Everett, A.C.; Saito, E.R.; Miller, R.M.; Johnson, T.; Neal, E.; Boyce, Z.; Smith, C.; Jensen, C.; Kimball, S.; et al. Effects of a True Prophylactic Treatment on Hippocampal and Amygdala Synaptic Plasticity and Gene Expression in a Rodent Chronic Stress Model of Social Defeat. Int. J. Mol. Sci. 2023, 24, 11193. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, R.N.; Quintanilla, L.J.; Song, J. Epigenetic regulation in the neurogenic niche of the adult dentate gyrus. Neurosci. Lett. 2022, 766, 136343. [Google Scholar] [CrossRef] [PubMed]
- Persaud, N.S.; Cates, H.M. The Epigenetics of Anxiety Pathophysiology: A DNA Methylation and Histone Modification Focused Review. eNeuro 2023, 10, 1–20. [Google Scholar]
- Guo, J.; Yang, Y.; Jiang, X.; Guo, M.; Li, X.; Huang, P.; Liu, Z. Differential promoter methylation and G-712A polymorphism of brain-derived neurotrophic factor in post-traumatic stress disorder patients of Li and Han populations in Hainan province. Gene 2021, 769, 145192. [Google Scholar] [CrossRef]
- Raza, Z.; Hussain, S.F.; Foster, V.S.; Wall, J.; Coffey, P.J.; Martin, J.F.; Gomes, R.S.M. Exposure to war and conflict: The individual and inherited epigenetic effects on health, with a focus on post-traumatic stress disorder. Front. Epidemiol. 2023, 3, 1066158. [Google Scholar] [CrossRef]
- Chou, P.C.; Huang, Y.C.; Yu, S. Mechanisms of Epigenetic Inheritance in Post-Traumatic Stress Disorder. Life 2024, 14, 98. [Google Scholar] [CrossRef]
- Bock, H.H.; May, P. Canonical and Non-canonical Reelin Signaling. Front. Cell Neurosci. 2016, 10, 166. [Google Scholar] [CrossRef]
- Ishii, K.; Kohno, T.; Sakai, K.; Hattori, M. Reelin regulates the migration of late-born hippocampal CA1 neurons via cofilin phosphorylation. Mol. Cell. Neurosci. 2023, 124, 103794. [Google Scholar] [CrossRef] [PubMed]
- Lossi, L.; Castagna, C.; Granato, A.; Merighi, A. The Reeler Mouse: A Translational Model of Human Neurological Conditions, or Simply a Good Tool for Better Understanding Neurodevelopment? J. Clin. Med. 2019, 8, 2088. [Google Scholar] [CrossRef] [PubMed]
- Badea, A.; Nicholls, P.J.; Johnson, G.A.; Wetsel, W.C. Neuroanatomical phenotypes in the reeler mouse. Neuroimage 2007, 34, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Sawahata, M.; Mori, D.; Arioka, Y.; Kubo, H.; Kushima, I.; Kitagawa, K.; Sobue, A.; Shishido, E.; Sekiguchi, M.; Kodama, A.; et al. Generation and analysis of novel Reln-deleted mouse model corresponding to exonic Reln deletion in schizophrenia. Psychiatry Clin. Neurosci. 2020, 74, 318–327. [Google Scholar] [CrossRef]
- Liao, J.; Dong, G.; Wulaer, B.; Sawahata, M.; Mizoguchi, H.; Mori, D.; Ozaki, N.; Nabeshima, T.; Nagai, T.; Yamada, K. Mice with exonic RELN deletion identified from a patient with schizophrenia have impaired visual discrimination learning and reversal learning in touchscreen operant tasks. Behav. Brain Res. 2022, 416, 113569. [Google Scholar] [CrossRef]
- Johnston, J.N.; Allen, J.; Shkolnikov, I.; Sanchez-Lafuente, C.L.; Reive, B.S.; Scheil, K.; Liang, S.; Christie, B.R.; Kalynchuk, L.E.; Caruncho, H.J. Reelin Rescues Behavioral, Electrophysiological, and Molecular Metrics of a Chronic Stress Phenotype in a Similar Manner to Ketamine. eNeuro 2023, 10, 1–13. [Google Scholar] [CrossRef]
- Sowndharya, S.; Rajan, K.E. Environmental enrichment improves social isolation-induced memory impairment: The possible role of ITSN1-Reelin-AMPA receptor signaling pathway. PLoS ONE 2024, 19, e0294354. [Google Scholar] [CrossRef]
- Sawahata, M.; Asano, H.; Nagai, T.; Ito, N.; Kohno, T.; Nabeshima, T.; Hattori, M.; Yamada, K. Microinjection of Reelin into the mPFC prevents MK-801-induced recognition memory impairment in mice. Pharmacol. Res. 2021, 173, 105832. [Google Scholar] [CrossRef]
- Telese, F.; Ma, Q.; Perez, P.M.; Notani, D.; Oh, S.; Li, W.; Comoletti, D.; Ohgi, K.A.; Taylor, H.; Rosenfeld, M.G. LRP8-Reelin-Regulated Neuronal Enhancer Signature Underlying Learning and Memory Formation. Neuron 2015, 86, 696–710. [Google Scholar] [CrossRef]
- Williams, J.W., Jr.; Gierisch, J.M.; McDuffie, J.; Strauss, J.L.; Nagi, A. An Overview of Complementary and Alternative Medicine Therapies for Anxiety and Depressive Disorders: Supplement to Efficacy of Complementary and Alternative Medicine Therapies for Posttraumatic Stress Disorder; Department of Veterans Affairs: Washington, DC, USA, 2011. [Google Scholar]
- Wu, Y.Y.; Xu, Y.M.; Lau, A.T.Y. Epigenetic effects of herbal medicine. Clin. Epigenetics 2023, 15, 85. [Google Scholar] [CrossRef]
- Duan, X.; Wen, J.; Zhang, M.; Wang, C.; Xiang, Y.; Wang, L.; Yu, C.; Deng, G.; Yan, M.; Zhang, B.; et al. Glycyrrhiza uralensis Fisch. and its active components mitigate Semen Strychni-induced neurotoxicity through regulating high mobility group box 1 (HMGB1) translocation. Biomed. Pharmacother. 2022, 149, 112884. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.X.; Xiang, Q.; Wen, Z.; He, D.; Wu, X.M.; Hu, G.Z. Neuroprotective effect of Atractylodes macrocephalaon polysaccharides in vitro on neuronal apoptosis induced by hypoxia. Mol. Med. Rep. 2014, 9, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xie, S.; Wang, H.; Li, Y.; Lai, Z.; Sun, S.; Pan, R.; Huang, Y.; Cai, J. The combination of Astragalus membranaceus and ligustrazine mitigates cerebral ischemia-reperfusion injury via regulating NR2B-ERK/CREB signaling. Brain Behav. 2023, 13, e2867. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, M.; Xiao, H.; Ye, J.; Cao, L.; Li, W.; Sun, G. Advancements in research on the effects of panax notoginseng saponin constituents in ameliorating learning and memory disorders. Heliyon 2024, 10, e28581. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Morinobu, S.; Takei, S.; Fuchikami, M.; Matsuki, A.; Yamawaki, S.; Liberzon, I. Single prolonged stress: Toward an animal model of posttraumatic stress disorder. Depress. Anxiety 2009, 26, 1110–1117. [Google Scholar] [CrossRef]
- Park, H.R.; Cai, M.; Yang, E.J. Novel Psychopharmacological Herbs Relieve Behavioral Abnormalities and Hippocampal Dysfunctions in an Animal Model of Post-Traumatic Stress Disorder. Nutrients 2023, 15, 3815. [Google Scholar] [CrossRef]
- da Silva, V.A.; Dantas Mde, S.; Silva, L.A.; Carneiro, J.G.; Schamber-Reis, B.L. Testosterone Depletion Induces Demethylation of Murine Reelin Promoter CpG Dinucleotides: A Preliminary Study. Biomed. Res. Int. 2015, 2015, 286369. [Google Scholar] [CrossRef]
- Pine, D.S.; Wise, S.P.; Murray, E.A. Evolution, Emotion, and Episodic Engagement. Am. J. Psychiatry 2021, 178, 701–714. [Google Scholar] [CrossRef]
- Plumpe, T.; Ehninger, D.; Steiner, B.; Klempin, F.; Jessberger, S.; Brandt, M.; Romer, B.; Rodriguez, G.R.; Kronenberg, G.; Kempermann, G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006, 7, 77. [Google Scholar] [CrossRef]
- Jin, K.; Zhang, S.; Jiang, C.; Liu, R.; Chen, B.; Zhao, H.; Zhang, Q.; Shen, Z.; Xu, P.; Hu, X.; et al. The role of reelin in the pathological mechanism of depression from clinical to rodents. Psychiatry Res. 2022, 317, 114838. [Google Scholar] [CrossRef] [PubMed]
- Wasser, C.R.; Herz, J. Reelin: Neurodevelopmental Architect and Homeostatic Regulator of Excitatory Synapses. J. Biol. Chem. 2017, 292, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Kheirbek, M.A.; Klemenhagen, K.C.; Sahay, A.; Hen, R. Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nat. Neurosci. 2012, 15, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.K.; Liu, Z.T.; Pang, J.L.; Wu, H.B.; Liu, X.; Yang, Z.M.; Li, X.; Chen, J.S. A network pharmacology study with molecular docking to investigate the possibility of licorice against posttraumatic stress disorder. Metab. Brain Dis. 2021, 36, 1763–1777. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, S.; Wu, F.; Zheng, Q.; Zhang, F.; Luo, Y.; Jian, X. Atractylenolide III reduces depressive- and anxiogenic-like behaviors in rat depression models. Neurosci. Lett. 2021, 759, 136050. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkader, H.A.E.; Abdou, H.M.; Khamiss, O.A.; Essawy, A.E. Anti-anxiety and antidepressant-like effects of astragaloside IV and saponins extracted from Astragalus spinosus against the bisphenol A-induced motor and cognitive impairments in a postnatal rat model of schizophrenia. Environ. Sci. Pollut. Res. Int. 2021, 28, 35171–35187. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Cho, S.G.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Ginsenoside Rb1 rescues anxiety-like responses in a rat model of post-traumatic stress disorder. J. Nat. Med. 2016, 70, 133–144. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Pinna, G. Animal Models of PTSD: The Socially Isolated Mouse and the Biomarker Role of Allopregnanolone. Front. Behav. Neurosci. 2019, 13, 114. [Google Scholar] [CrossRef]
- Tortora, F.; Hadipour, A.L.; Battaglia, S.; Falzone, A.; Avenanti, A.; Vicario, C.M. The Role of Serotonin in Fear Learning and Memory: A Systematic Review of Human Studies. Brain Sci. 2023, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, P.; Rodriguez-Moreno, A. The Impact of Studying Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Chenani, A.; Weston, G.; Ulivi, A.F.; Castello-Waldow, T.P.; Huettl, R.E.; Chen, A.; Attardo, A. Repeated stress exposure leads to structural synaptic instability prior to disorganization of hippocampal coding and impairments in learning. Transl. Psychiatry 2022, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Kalisch, R.; Russo, S.J.; Muller, M.B. Neurobiology and systems biology of stress resilience. Physiol. Rev. 2024, 104, 1205–1263. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Garcia, A.; Knafo, S.; Pereda-Perez, I.; Esteban, J.A.; Venero, C.; Armario, A. Administration of the TrkB receptor agonist 7,8-dihydroxyflavone prevents traumatic stress-induced spatial memory deficits and changes in synaptic plasticity. Hippocampus 2016, 26, 1179–1188. [Google Scholar] [CrossRef]
- Ardi, Z.; Ritov, G.; Lucas, M.; Richter-Levin, G. The effects of a reminder of underwater trauma on behaviour and memory-related mechanisms in the rat dentate gyrus. Int. J. Neuropsychopharmacol. 2014, 17, 571–580. [Google Scholar] [CrossRef]
- Hester, M.S.; Tulina, N.; Brown, A.; Barila, G.; Elovitz, M.A. Intrauterine inflammation reduces postnatal neurogenesis in the hippocampal subgranular zone and leads to accumulation of hilar ectopic granule cells. Brain Res. 2018, 1685, 51–59. [Google Scholar] [CrossRef]
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef]
- Joly-Amado, A.; Kulkarni, N.; Nash, K.R. Reelin Signaling in Neurodevelopmental Disorders and Neurodegenerative Diseases. Brain Sci. 2023, 13, 1479. [Google Scholar] [CrossRef]
- Imai, H.; Shoji, H.; Ogata, M.; Kagawa, Y.; Owada, Y.; Miyakawa, T.; Sakimura, K.; Terashima, T.; Katsuyama, Y. Dorsal Forebrain-Specific Deficiency of Reelin-Dab1 Signal Causes Behavioral Abnormalities Related to Psychiatric Disorders. Cereb. Cortex. 2017, 27, 3485–3501. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.N.; Thacker, J.S.; Desjardins, C.; Kulyk, B.D.; Romay-Tallon, R.; Kalynchuk, L.E.; Caruncho, H.J. Ketamine Rescues Hippocampal Reelin Expression and Synaptic Markers in the Repeated-Corticosterone Chronic Stress Paradigm. Front. Pharmacol. 2020, 11, 559627. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, W.; Wang, Z.; Zhang, R.; Xie, Y.; Guo, S.; Jiao, L.; Hong, Y.; Di, Z.; Wang, G.; et al. Reduced Neuronal cAMP in the Nucleus Accumbens Damages Blood-Brain Barrier Integrity and Promotes Stress Vulnerability. Biol. Psychiatry 2020, 87, 526–537. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Dai, X.; Xiao, N.; Ye, Q.; Chen, X. A Moderate Duration of Stress Promotes Behavioral Adaptation and Spatial Memory in Young C57BL/6J Mice. Brain Sci. 2022, 12, 1081. [Google Scholar] [CrossRef] [PubMed]
- Brymer, K.J.; Johnston, J.; Botterill, J.J.; Romay-Tallon, R.; Mitchell, M.A.; Allen, J.; Pinna, G.; Caruncho, H.J.; Kalynchuk, L.E. Fast-acting antidepressant-like effects of Reelin evaluated in the repeated-corticosterone chronic stress paradigm. Neuropsychopharmacology 2020, 45, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Maag, J.L.; Kaczorowski, D.C.; Panja, D.; Peters, T.J.; Bramham, C.R.; Wibrand, K.; Dinger, M.E. Widespread promoter methylation of synaptic plasticity genes in long-term potentiation in the adult brain in vivo. BMC Genom. 2017, 18, 250. [Google Scholar] [CrossRef]
- Sui, L.; Wang, Y.; Ju, L.H.; Chen, M. Epigenetic regulation of reelin and brain-derived neurotrophic factor genes in long-term potentiation in rat medial prefrontal cortex. Neurobiol. Learn. Mem. 2012, 97, 425–440. [Google Scholar] [CrossRef]
- Lv, J.; Xin, Y.; Zhou, W.; Qiu, Z. The epigenetic switches for neural development and psychiatric disorders. J. Genet. Genom. 2013, 40, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.H.; Aiello, A.E.; Kim, G.S.; Xue, F.; Martin, C.L.; Ratanatharathorn, A.; Qu, A.; Koenen, K.; Galea, S.; Wildman, D.E.; et al. The impact of psychopathology, social adversity and stress-relevant DNA methylation on prospective risk for post-traumatic stress: A machine learning approach. J. Affect. Disord. 2021, 282, 894–905. [Google Scholar] [CrossRef]
- Stroud, L.R.; Jao, N.C.; Ward, L.G.; Lee, S.Y.; Marsit, C.J. Differential impact of prenatal PTSD symptoms and preconception trauma exposure on placental NR3C1 and FKBP5 methylation. Stress 2024, 27, 2321595. [Google Scholar] [CrossRef]
- Katrinli, S.; Zheng, Y.; Gautam, A.; Hammamieh, R.; Yang, R.; Venkateswaran, S.; Kilaru, V.; Lori, A.; Hinrichs, R.; Powers, A.; et al. PTSD is associated with increased DNA methylation across regions of HLA-DPB1 and SPATC1L. Brain Behav. Immun. 2021, 91, 429–436. [Google Scholar] [CrossRef]
- Logue, M.W.; Miller, M.W.; Wolf, E.J.; Huber, B.R.; Morrison, F.G.; Zhou, Z.; Zheng, Y.; Smith, A.K.; Daskalakis, N.P.; Ratanatharathorn, A.; et al. An epigenome-wide association study of posttraumatic stress disorder in US veterans implicates several new DNA methylation loci. Clin. Epigenetics 2020, 12, 46. [Google Scholar] [CrossRef]
- Sipahi, L.; Wildman, D.E.; Aiello, A.E.; Koenen, K.C.; Galea, S.; Abbas, A.; Uddin, M. Longitudinal epigenetic variation of DNA methyltransferase genes is associated with vulnerability to post-traumatic stress disorder. Psychol. Med. 2014, 44, 3165–3179. [Google Scholar] [CrossRef]
- Cosentino, L.; Witt, S.H.; Dukal, H.; Zidda, F.; Siehl, S.; Flor, H.; De Filippis, B. Methyl-CpG binding protein 2 expression is associated with symptom severity in patients with PTSD in a sex-dependent manner. Transl. Psychiatry 2023, 13, 249. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, W.; Zhu, Z.; Zhao, M.; Li, H.; Liu, D.; Pan, F. Involvement of brain-derived neurotrophic factor methylation in the prefrontal cortex and hippocampus induced by chronic unpredictable mild stress in male mice. J. Neurochem. 2023, 164, 624–642. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, W.; Jia, X.; Bade, R.; Liu, X.; Xie, Y.; Xie, W.; Jiang, S.; Shao, G. Hypoxic Preconditioning Modulates BDNF and Its Signaling through DNA Methylation to Promote Learning and Memory in Mice. ACS Chem. Neurosci. 2023, 14, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Xu, W.; Jiang, S.; Liu, X.; Zhu, H.; Wang, P.; Gao, B.; Gong, K.; Guo, G.; Sun, K.; et al. Effects of 5-Aza on neurogenesis contribute to learning and memory in the mouse hippocampus. Biomed. Pharmacother. 2022, 154, 113623. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Yamamoto, F.; Arai, T.; Yang, J.; Sakai, Y.; Itoh, M.; Mamada, N.; Sekiguchi, M.; Yamada, D.; Saitoh, A.; et al. Tyrosol Reduces Amyloid-beta Oligomer Neurotoxicity and Alleviates Synaptic, Oxidative, and Cognitive Disturbances in Alzheimer’s Disease Model Mice. J. Alzheimers Dis. 2019, 70, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Prakashkumar, N.; Sivamaruthi, B.S.; Chaiyasut, C.; Suganthy, N. Decoding the Neuroprotective Potential of Methyl Gallate-Loaded Starch Nanoparticles against Beta Amyloid-Induced Oxidative Stress-Mediated Apoptosis: An In Vitro Study. Pharmaceutics 2021, 13, 299. [Google Scholar] [CrossRef]
- Ma, S.; Chong, Y.; Zhang, R.; Quan, W.; Gui, J.; Li, L.; Wang, J.; Miao, S.; Shi, X.; Zhao, M.; et al. Glycyrrhizic acid treatment ameliorates anxiety-like behaviour via GLT1 and Per1/2-dependent pathways. J. Ethnopharmacol. 2024, 328, 118013. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, D.; Zhang, C.; Tao, Y.; Meng, Y.; Jin, M.; Song, W.; Wang, B.; Wei, L. Liquiritin Alleviates Depression-Like Behavior in CUMS Mice by Inhibiting Oxidative Stress and NLRP3 Inflammasome in Hippocampus. Evid. Based Complement. Altern. Med. 2022, 2022, 7558825. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Lu, J.; Shi, H.; Gong, S.; Wang, Y.; Hamdy, R.C.; Chua, B.H.L.; Yang, L.; Xu, X. Fisetin provides antidepressant effects by activating the tropomyosin receptor kinase B signal pathway in mice. J. Neurochem. 2017, 143, 561–568. [Google Scholar] [CrossRef] [PubMed]







| Name | Primer and Sequence (5′-3′) |
|---|---|
| Reelin | Forward-TCACTGTGTCATACGCCAAGAAC Reverse-GAGGTACAGGATGTGGATGACTGT |
| Dab-1 | Forward-CTAGGCAGAGCTCTCCATCC Reverse-GACTTATATTATCACCACTGGGCTC |
| MeCP2 | Forward-CAGCAGCATCTGCAAAGAAG Reverse-TCCACAGGCTCCTCTCTGTT |
| DNMT1 | Forward-GAGTCTTCGACGTCACACCA Reverse-AGCTACCTGCTCTGGCTCTG |
| BDNF | Forward-TTACTCTCCTGGGTTCCTGA Reverse-ACGTCCACTTCTGTTTCCTT |
| PSD-95 | Forward-GCTCCCTGGAGAATGTGCTA Reverse-TGAGAAGCACTCCGTGAACT |
| Synaptophysin | Forward-GCCTACCTTCTCCACCCTTT Reverse-GCACTACCAACGTCACAGAC |
| Cholecystokinin | Forward-ATACATCCAGCAGGTCCGCAA Reverse-CAGACATTAGAGGCGAGGGGT |
| Calbindin 1 | Forward-TCTGGCTTCATTTCGACGCTG Reverse-ACAAAGGATTTCATTTCCGGTGA |
| Syntaxin 1a | Forward-CCGAACCCCGATGAGAAGAC Reverse-TGCTCTTTAGCTTGGAGCGA |
| NeuroD6 | Forward-ATGCGACACTCAGCCTGAAA Reverse-CTGGGATTCGGGCATTACGA |
| NeuroD2 | Forward-AAGCCAGTGTCTCTTCGTGG Reverse-TTGGACAGCTTCTGCGTCTT |
| GAPDH | Forward-CCTCGTCCCGTAGACAAA Reverse-AATGAAGGGGTCGTTGATG |
| Reelin (unmethylated) | Forward-TTTTTAGTAATGTGTAAATATAGAGTTTGG Reverse-AAATAATACAAAACCAAATCATCAAA |
| Reelin (methylated) | Forward-TTAGTAACGCGTAAATATAGAGTTCG Reverse-ATAATACGAAACCAAATCGTCGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.R.; Cai, M.; Yang, E.J. Herbal Formula Extract Ameliorates Anxiety and Cognitive Impairment via Regulation of the Reelin/Dab-1 Pathway in a Murine Model of Post-Traumatic Stress Disorder. Pharmaceutics 2024, 16, 1150. https://doi.org/10.3390/pharmaceutics16091150
Park HR, Cai M, Yang EJ. Herbal Formula Extract Ameliorates Anxiety and Cognitive Impairment via Regulation of the Reelin/Dab-1 Pathway in a Murine Model of Post-Traumatic Stress Disorder. Pharmaceutics. 2024; 16(9):1150. https://doi.org/10.3390/pharmaceutics16091150
Chicago/Turabian StylePark, Hee Ra, Mudan Cai, and Eun Jin Yang. 2024. "Herbal Formula Extract Ameliorates Anxiety and Cognitive Impairment via Regulation of the Reelin/Dab-1 Pathway in a Murine Model of Post-Traumatic Stress Disorder" Pharmaceutics 16, no. 9: 1150. https://doi.org/10.3390/pharmaceutics16091150




