Biological Evaluations and Computer-Aided Approaches of Janus Kinases 2 and 3 Inhibitors for Cancer Treatment: A Review
Abstract
1. Introduction
2. The JAK2 Target
3. The JAK3 Target
4. JAK2 Inhibitors
5. Natural Derived JAK2 Inhibitors
6. JAK3 Inhibitors
7. Natural Derived JAK3 Inhibitors
8. Dual JAK2/3 Inhibitors
9. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Bose, S.; Banerjee, S.; Mondal, A.; Chakraborty, U.; Pumarol, J.; Croley, C.R.; Bishayee, A. Targeting the JAK/STAT Signaling Pathway Using Phytocompounds for Cancer Prevention and Therapy. Cells 2020, 11, 1451. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Leonard, W.J.; O’Shea, J.J. Jaks and STATs: Biological implications. Annu. Rev. Immunol. 1998, 16, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Ihle, J.N.; Witthuhn, B.A.; Quelle, F.W.; Yamamoto, K.; Silvennoinen, O. Signaling through the hematopoietic cytokine receptors. Annu. Rev. Immunol. 1995, 13, 369–398. [Google Scholar] [CrossRef] [PubMed]
- Hammarén, H.M.; Virtanen, A.T.; Raivola, J.; Silvennoinen, O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine 2019, 118, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Haan, C.; Kreis, S.; Margue, C.; Behrmann, I. Jaks and cytokine receptors–an intimate relationship. Biochem. Pharmacol. 2006, 72, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Pölläniemi, A.; Virtanen, A.; Silvennoinen, O.; Haikarainen, T. Development of an enzyme-coupled activity assay for Janus kinase 2 inhibitor screening. SLAS Discov. 2023, 28, 180–187. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef]
- Li, Y.; Guo, F.; Chen, T.; Zhang, L.; Qin, Y. Anthraquinone derivative C10 inhibits proliferation and cell cycle progression in colon cancer cells via the Jak2/Stat3 signaling pathway. Toxicol. Appl. Pharmacol. 2021, 418, 115481. [Google Scholar] [CrossRef]
- Pardanani, A.; Gotlib, J.R.; Jamieson, C.; Cortes, J.E.; Talpaz, M.; Stone, R.M.; Silverman, M.H.; Gilliland, D.G.; Shorr, J.; Tefferi, A. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J. Clin. Oncol. 2011, 9, 789–796. [Google Scholar] [CrossRef]
- Harrison, C.; Kiladjian, J.J.; Al-Ali, H.K.; Gisslinger, H.; Waltzman, R.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.; Knoops, L.; et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012, 366, 787–798. [Google Scholar] [CrossRef]
- Plimack, E.R.; Lorusso, P.M.; McCoon, P.; Tang, W.; Krebs, A.D.; Curt, G.; Eckhardt, S.G. AZD1480: A phase I study of a novel JAK2 inhibitor in solid tumors. Oncol. 2013, 18, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Quintás-Cardama, A.; Vaddi, K.; Liu, P.; Manshouri, T.; Li, J.; Scherle, P.A.; Caulder, E.; Wen, X.; Li, Y.; Waeltz, P.; et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: Therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010, 115, 3109–3117. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. JAK2 inhibitors for myeloproliferative neoplasms: What is next? Blood 2017, 130, 115–125. [Google Scholar] [CrossRef]
- Telliez, J.B.; Dowty, M.E.; Wang, L.; Jussif, J.; Lin, T.; Li, L.; Moy, E.; Balbo, P.; Li, W.; Zhao, Y.; et al. Discovery of a JAK3-selective inhibitor: Functional differentiation of JAK3-selective inhibition over pan-JAK or JAK1-selective inhibition. ACS Chem. Biol. 2016, 11, 3442–3451. [Google Scholar] [CrossRef]
- Forster, M.; Liang, X.J.; Schröder, M.; Gerstenecker, S.; Chaikuad, A.; Knapp, S.; Laufer, S.; Gehringer, M. Discovery of a Novel Class of Covalent Dual Inhibitors Targeting the Protein Kinases BMX and BTK. Int. J. Mol. Sci. 2020, 21, 9269. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Marín, H.A.; Tosti, A. Evaluating the Therapeutic Potential of Ritlecitinib for the Treatment of Alopecia Areata. Drug Des. Dev. Ther. 2022, 16, 363–374. [Google Scholar] [CrossRef]
- Jiang, J.K.; Ghoreschi, K.; Deflorian, F.; Chen, Z.; Perreira, M.; Pesu, M.; Smith, J.; Nguyen, D.T.; Liu, E.H.; Leister, W.; et al. Examining the chirality, conformation and selective kinase inhibition of 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile (CP-690,550). J. Med. Chem. 2008, 51, 8012–8018. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Chen, C.; Huan, X.; Huang, H.; Wang, M.; Zhang, J.; Yan, Y.; Liu, J.; Zhang, T.; Zhang, D. Design, synthesis, and pharmacological evaluation of 4- or 6-phenyl-pyrimidine derivatives as novel and selective Janus kinase 3 inhibitors. Eur. J. Med. Chem. 2020, 191, 112148. [Google Scholar] [CrossRef]
- Su, W.; Chen, Z.; Liu, M.; He, R.; Liu, C.; Li, R.; Gao, M.; Zheng, M.; Tu, Z.; Zhang, Z.; et al. Design, synthesis and structure-activity relationship studies of pyrido[2,3-d]pyrimidin-7-ones as potent Janus Kinase 3 (JAK3) covalent inhibitors. Bioorg. Med. Chem. Lett. 2022, 64, 128680. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Diao, Y.; Li, W.; Luo, Y.; Yang, T.; Zhao, Y.; Qi, T.; Xu, F.; Ma, X.; Ge, H.; et al. Design, synthesis and structure-activity relationship study of aminopyridine derivatives as novel inhibitors of Janus kinase 2. Bioorg. Med. Chem. Lett. 2019, 29, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Li, L.; Ho, Y.; Lin, A.; Li, L.; Stein, A.; Forman, S.; Perrotti, D.; Jove, R.; Bhatia, R. Role of altered growth factor receptor-mediated JAK2 signaling in growth and maintenance of human acute myeloid leukemia stem cells. Blood 2014, 123, 2826–2837. [Google Scholar] [CrossRef]
- Dosil, M.; Wang, S.; Lemischka, I.R. Mitogenic signalling and substrate specificity of the Flk2/Flt3 receptor tyrosine kinase in fibroblasts and interleukin 3-dependent hematopoietic cells. Mol. Cell. Biol. 1993, 13, 6572–6585. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef]
- Alicea-Velázquez, N.L.; Boggon, T.J. The use of structural biology in Janus kinase targeted drug discovery. Curr. Drug Targets 2011, 12, 546–555. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009, 228, 273–287. [Google Scholar] [CrossRef]
- Virtanen, A.T.; Haikarainen, T.; Sampathkumar, P.; Palmroth, M.; Liukkonen, S.; Liu, J.; Nekhotiaeva, N.; Hubbard, S.R.; Silvennoinen, O. Identification of Novel Small Molecule Ligands for JAK2 Pseudokinase Domain. Pharmaceuticals 2023, 16, 75. [Google Scholar] [CrossRef]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef]
- Doheny, D.; Sirkisoon, S.; Carpenter, R.L.; Aguayo, N.R.; Regua, A.T.; Anguelov, M.; Manore, S.G.; Arrigo, A.; Jalboush, S.A.; Wong, G.L.; et al. Combined inhibition of JAK2-STAT3 and SMO-GLI1/tGLI1 pathways suppresses breast cancer stem cells, tumor growth, and metastasis. Oncogene 2020, 39, 6589–6605. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Y.; Wang, S.; Shen, Q.; Zhou, X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018, 415, 117–128. [Google Scholar] [CrossRef]
- Raivola, J.; Haikarainen, T.; Silvennoinen, O. Characterization of JAK1 Pseudokinase Domain in Cytokine Signaling. Cancers 2020, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Raivola, J.; Hammarén, H.M.; Virtanen, A.T.; Bulleeraz, V.; Ward, A.C.; Silvennoinen, O. Hyperactivation of Oncogenic JAK3 Mutants Depend on ATP Binding to the Pseudokinase Domain. Front. Oncol. 2018, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Ugo, V.; Le Couédic, J.P.; Staerk, J.; Delhommeau, F.; Lacout, C.; Garçon, L.; Raslova, H.; Berger, R.; Bennaceur-Griscelli, A.; et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005, 434, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Vassiliou, G.S.; Bench, A.J.; Boyd, E.M.; Curtin, N.; et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005, 365, 1054–1061. [Google Scholar] [CrossRef]
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 2005, 352, 1779–1790. [Google Scholar] [CrossRef]
- Zhao, R.; Xing, S.; Li, Z.; Fu, X.; Li, Q.; Krantz, S.B.; Zhao, Z.J. Identification of an acquired JAK2 mutation in polycythemia vera. J. Biol. Chem. 2005, 280, 22788–22792. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, C.J.; Yu, R.N.; Shu, L.; Zhang, T.T.; Zhang, D.Y. Discovery of novel selective Janus kinase 2 (JAK2) inhibitors bearing a 1H-pyrazolo[3,4-d]pyrimidin-4-amino scaffold. Bioorg. Chem. 2019, 27, 1562–1576. [Google Scholar] [CrossRef]
- Xiong, H.; Du, W.; Zhang, Y.J.; Hong, J.; Su, W.Y.; Tang, J.T.; Wang, Y.C.; Lu, R.; Fang, J.Y. Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol. Carcinog. 2012, 51, 174–184. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, F.; Li, G.; Li, G.; Yang, X.; Liu, L.; Zhang, R.; Zhang, B.; Feng, Y. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018, 18, 25. [Google Scholar] [CrossRef]
- Leroy, E.; Constantinescu, S.N. Rethinking JAK2 inhibition: Towards novel strategies of more specific and versatile janus kinase inhibition. Leukemia 2017, 31, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, P.L.; Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Gadina, M.; Schreiber, R.D. Cytokine signaling in 2002: New surprises in the Jak/Stat pathway. Cell 2002, 109, S121–S131. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Shi, W.; Zhao, D.; Wang, Q.; Chang, X.; He, X.; Wang, X.; Gao, Y.; Lu, P.; Zhang, X.; et al. Design and synthesis of boron-containing diphenylpyrimidines as potent BTK and JAK3 dual inhibitors. Bioorg. Med. Chem. 2020, 28, 115236. [Google Scholar] [CrossRef] [PubMed]
- Sanachai, K.; Mahalapbutr, P.; Hengphasatporn, K.; Shigeta, Y.; Seetaha, S.; Tabtimmai, L.; Langer, T.; Wolschann, P.; Kittikool, T.; Yotphan, S.; et al. Pharmacophore-Based Virtual Screening and Experimental Validation of Pyrazolone-Derived Inhibitors toward Janus Kinases. ACS Omega 2022, 7, 33548–33559. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Rassidakis, G.Z.; Lin, Q.; Atwell, C.; Medeiros, L.J.; Amin, H.M. Jak3 activation is significantly associated with ALK expression in anaplastic large cell lymphoma. Hum. Pathol. 2005, 36, 939–944. [Google Scholar] [CrossRef]
- Gee, K.; Kozlowski, M.; Kryworuchko, M.; Diaz-Mitoma, F.; Kumar, A. Differential effect of IL-4 and IL-13 on CD44 expression in the Burkitt’s lymphoma B cell line BL30/B95-8 and in Epstein-Barr virus (EBV) transformed human B cells: Loss of IL-13 receptors on Burkitt’s lymphoma B cells. Cell. Immunol. 2001, 211, 131–142. [Google Scholar] [CrossRef]
- Yared, M.A.; Khoury, J.D.; Medeiros, L.J.; Rassidakis, G.Z.; Lai, R. Activation status of the JAK/STAT3 pathway in mantle cell lymphoma. Arch. Pathol. Lab. Med. 2005, 129, 990–996. [Google Scholar] [CrossRef]
- Malamut, G.; El Machhour, R.; Montcuquet, N.; Martin-Lannerée, S.; Dusanter-Fourt, I.; Verkarre, V.; Mention, J.J.; Rahmi, G.; Kiyono, H.; Butz, E.A.; et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J. Clin. Investig. 2010, 120, 2131–2143. [Google Scholar] [CrossRef]
- Forster, M.; Gehringer, M.; Laufer, S.A. Recent advances in JAK3 inhibition: Isoform selectivity by covalent cysteine targeting. Bioorg. Med. Chem. Lett. 2017, 27, 4229–4237. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; El-Kady, D.S.; Abd-Rabou, A.A.; Tantawy, M.A.; AbdElhalim, M.M.; Elazabawy, S.R.; Abdallah, A.E.M.; Elmegeed, G.A. Synthesis of novel hybrid hetero-steroids: Molecular docking study augmented anti-proliferative properties against cancerous cells. Steroids 2020, 54, 108527. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, T.; Xu, L.; Dong, Y.; Luo, Y.; Wang, C.; Han, Y.; Chen, K.; Qin, M.; Liu, Y.; et al. Discovery of 4-piperazinyl-2-aminopyrimidine derivatives as dual inhibitors of JAK2 and FLT3. Eur. J. Med. Chem. 2019, 181, 111590. [Google Scholar] [CrossRef] [PubMed]
- Jyothi-Buggana, S.; Paturi, M.C.; Perka, H.; Gade, D.R.; Vvs, R.P. Novel 2,4-disubstituted quinazolines as cytotoxic agents and JAK2 inhibitors: Synthesis, in vitro evaluation and molecular dynamics studies. Comput. Biol. Chem. 2019, 79, 110–118. [Google Scholar] [CrossRef]
- Sinha, I.; Null, K.; Wolter, W.; Suckow, M.A.; King, T.; Pinto, J.T.; Sinha, R. Methylseleninic acid downregulates hypoxia-inducible factor-1α in invasive prostate cancer. Int. J. Cancer 2012, 130, 1430–1439. [Google Scholar] [CrossRef]
- Tarrado-Castellarnau, M.; Cortés, R.; Zanuy, M.; Tarragó-Celada, J.; Polat, I.H.; Hill, R.; Fan, T.W.; Link, W.; Cascante, M. Methylseleninic acid promotes antitumour effects via nuclear FOXO3a translocation through Akt inhibition. Pharmacol. Res. 2015, 102, 218–234. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Qiu, J.; Jiang, K.; Zhao, G.; Wu, H.; Deng, G.; Qiu, C. Correction to: Methylseleninic Acid Suppresses Breast Cancer Growth via the JAK2/STAT3 Pathway. Reprod. Sci. 2021, 28, 614. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Diao, Y.; Ge, H.; Xu, F.; Zhu, L.; Zhao, Z.; Li, H. Discovery and optimization of 2-aminopyridine derivatives as novel and selective JAK2 inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127048. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Chen, C.; Ye, T.; Han, Y.; Hou, Y.; Liu, Y.; Gong, P.; Qin, M.; Zhao, Y. Discovery and rational design of 2-aminopyrimidine-based derivatives targeting Janus kinase 2 (JAK2) and FMS-like tyrosine kinase 3 (FLT3). Bioorg. Chem. 2020, 104, 104361. [Google Scholar] [CrossRef]
- Bottiglieri, T. S-Adenosyl-L-methionine (SAMe): From the bench to the bedside—Molecular basis of a pleiotrophic molecule. Am. J. Clin. Nutr. 2002, 76, 1151S–1157S. [Google Scholar] [CrossRef]
- Mahmood, N.; Cheishvili, D.; Arakelian, A.; Tanvir, I.; Khan, H.A.; Pépin, A.S.; Szyf, M.; Rabbani, S.A. Methyl donor S-adenosylmethionine (SAM) supplementation attenuates breast cancer growth, invasion, and metastasis in vivo; therapeutic and chemopreventive applications. Oncotarget 2017, 9, 5169–5183. [Google Scholar] [CrossRef]
- Ma, D.; Shen, B.; Seewoo, V.; Tong, H.; Yang, W.; Cheng, X.; Jin, Z.; Peng, C.; Qiu, W. GADD45β induction by S-adenosylmethionine inhibits hepatocellular carcinoma cell proliferation during acute ischemia-hypoxia. Oncotarget 2016, 7, 37215–37225. [Google Scholar] [CrossRef] [PubMed]
- Parashar, S.; Cheishvili, D.; Arakelian, A.; Hussain, Z.; Tanvir, I.; Khan, H.A.; Szyf, M.; Rabbani, S.A. S-adenosylmethionine blocks osteosarcoma cells proliferation and invasion in vitro and tumor metastasis in vivo: Therapeutic and diagnostic clinical applications. Cancer Med. 2015, 4, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Li, T.W.; Zhang, Q.; Oh, P.; Xia, M.; Chen, H.; Bemanian, S.; Lastra, N.; Circ, M.; Moyer, M.P.; Mato, J.M.; et al. S-Adenosylmethionine and methylthioadenosine inhibit cellular FLICE inhibitory protein expression and induce apoptosis in colon cancer cells. Mol. Pharmacol. 2009, 76, 192–200. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, T.; Yuan, F.; Gao, X.; Jia, G.; Tian, Z. S-adenosylmethionine induces apoptosis and cycle arrest of gallbladder carcinoma cells by suppression of JAK2/STAT3 pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2507–2515. [Google Scholar] [CrossRef]
- Speich, B.; Ame, S.M.; Ali, S.M.; Alles, R.; Hattendorf, J.; Utzinger, J.; Albonico, M.; Keiser, J. Efficacy and safety of nitazoxanide, albendazole, and nitazoxanide-albendazole against Trichuris trichiura infection: A randomized controlled trial. PLoS Negl. Trop. Dis. 2012, 6, e1685. [Google Scholar] [CrossRef]
- Stockis, A.; Allemon, A.M.; De Bruyn, S.; Gengler, C. Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses. Int. J. Clin. Pharmacol. Ther. 2002, 40, 213–220. [Google Scholar] [CrossRef]
- Di Santo, N.; Ehrisman, J. Research perspective: Potential role of nitazoxanide in ovarian cancer treatment. Old drug, new purpose? Cancers 2013, 5, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.A.; El-Sherbeeny, N.A.; Helmi, N.; Alazragi, R.; Salem, N.; Elaidy, S.M. Synthetic antiprotozoal thiazolide drug induced apoptosis in colorectal cancer cells: Implications of IL-6/JAK2/STAT3 and p53/caspases-dependent signaling pathways based on molecular docking and in vitro study. Mol. Cell Biochem. 2020, 469, 143–157. [Google Scholar] [CrossRef]
- Nafie, M.S.; Mahgoub, S.; Amer, A.M. Antimicrobial and antiproliferative activities of novel synthesized 6-(quinolin-2-ylthio) pyridine derivatives with molecular docking study as multi-targeted JAK2/STAT3 inhibitors. Chem. Biol. Drug Des. 2021, 97, 553–564. [Google Scholar] [CrossRef]
- Li, W.; Yuan, B.; Zhao, Y.; Lu, T.; Zhang, S.; Ding, Z.; Wang, D.; Zhong, S.; Gao, G.; Yan, M. Transcriptome profiling reveals target in primary myelofibrosis together with structural biology study on novel natural inhibitors regarding JAK2. Aging 2021, 13, 8248–8275. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.F.; Li, Z.C.; Chen, P.P.; Zhou, X. Bufothionine induced the mitochondria-mediated apoptosis in H22 liver tumor and acute liver injury. Chin. Med. 2015, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.S.; Shen, F.X.; Xie, R.F.; Zhou, G.; Feng, Y.M.; Zhou, X. Bufothionine induces autophagy in H22 hepatoma-bearing mice by inhibiting JAK2/STAT3 pathway, a possible anti-cancer mechanism of cinobufacini. J. Ethnopharmacol. 2021, 270, 113848. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Shen, P.; Wang, H.; Qin, L.; Ren, J.; Sun, Q.; Ge, R.; Bian, J.; Zhong, Y.; Li, Z.; et al. Discovery of imidazopyrrolopyridines derivatives as novel and selective inhibitors of JAK2. Eur. J. Med. Chem. 2021, 218, 113394. [Google Scholar] [CrossRef] [PubMed]
- Sanachai, K.; Aiebchun, T.; Mahalapbutr, P.; Seetaha, S.; Tabtimmai, L.; Maitarad, P.; Xenikakis, I.; Geronikaki, A.; Choowongkomon, K.; Rungrotmongkol, T. Discovery of novel JAK2 and EGFR inhibitors from a series of thiazole-based chalcone derivatives. RSC Med. Chem. 2021, 12, 430–438. [Google Scholar] [CrossRef]
- Newton, A.S.; Liosi, M.E.; Henry, S.P.; Deiana, L.; Faver, J.C.; Krimmer, S.G.; Puleo, D.E.; Schlessinger, J.; Jorgensen, W.L. Indoloxytriazines as binding molecules for the JAK2 JH2 pseudokinase domain and its V617F variant. Tetrahedron Lett. 2021, 77, 153248. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Shaheen, S.; Kattan, S.W.; Alelwani, W.; Barnawi, I.O.; Elmgeed, G.A.; Nafie, M.S. Cytotoxicity, in silico predictions and molecular studies for androstane heterocycle compounds revealed potential antitumor agent against lung cancer cells. J. Biomol. Struct. Dyn. 2022, 40, 4352–4365. [Google Scholar] [CrossRef]
- Singh, A.; Mishra, A. Molecular modelling study to discover novel JAK2 signaling pathway inhibitor. J. Biomol. Struct. Dyn. 2023, 41, 5827–5838. [Google Scholar] [CrossRef]
- He, L.; Liu, J.; Zhao, H.L.; Zhang, L.C.; Yu, R.L.; Kang, C.M. De novo design of dual-target JAK2, SMO inhibitors based on deep reinforcement learning, molecular docking and molecular dynamics simulations. Biochem. Biophys. Res. Commun. 2023, 638, 23–27. [Google Scholar] [CrossRef]
- Guo, Y.; Zou, Y.; Chen, Y.; Deng, D.; Zhang, Z.; Liu, K.; Tang, M.; Yang, T.; Fu, S.; Zhang, C.; et al. Design, synthesis and biological evaluation of purine-based derivatives as novel JAK2/BRD4(BD2) dual target inhibitors. Bioorg. Chem. 2023, 132, 106386. [Google Scholar] [CrossRef]
- Diao, Y.; Liu, D.; Ge, H.; Zhang, R.; Jiang, K.; Bao, R.; Zhu, X.; Bi, H.; Liao, W.; Chen, Z.; et al. Macrocyclization of linear molecules by deep learning to facilitate macrocyclic drug candidates discovery. Nat. Commun. 2023, 14, 4552. [Google Scholar] [CrossRef] [PubMed]
- Suriya, U.; Mahalapbutr, P.; Geronikaki, A.; Kartsev, V.; Zubenko, A.; Divaeva, L.; Chekrisheva, V.; Petrou, A.; Oopkaew, L.; Somngam, P.; et al. Discovery of furopyridine-based compounds as novel inhibitors of Janus kinase 2: In silico and in vitro studies. Int. J. Biol. Macromol. 2024, 260 Pt 2, 129308. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Park, J.; Han, E.T.; Park, W.S.; Han, J.H.; Chun, W. Drug Repositioning via Graph Neural Networks: Identifying Novel JAK2 Inhibitors from FDA-Approved Drugs through Molecular Docking and Biological Validation. Molecules 2024, 29, 1363. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Himejima, M. Combination effects of antifungal nagilactones against Candida albicans and two other fungi with phenylpropanoids. J. Nat. Prod. 1993, 56, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Yao, S.; Ye, Y.; Yu, Q. 3-Deoxy-2β,16-dihydroxynagilactone E, a natural compound from Podocarpus nagi, preferentially inhibits JAK2/STAT3 signaling by allosterically interacting with the regulatory domain of JAK2 and induces apoptosis of cancer cells. Acta Pharmacol. Sin. 2019, 40, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Niu, J.; Wang, F.; Hu, L.; Yu, Q. A natural compound derivative P-13 inhibits STAT3 signaling by covalently inhibiting Janus kinase 2. Investig. New Drugs 2019, 37, 452–460. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, J.; Liu, S.; Nasser, M.I.; Wei, W.; Mao, T.; Liu, X.; Zou, X.; Li, J.; Li, X. Rosmanol induces breast cancer cells apoptosis by regulating PI3K/AKT and STAT3/JAK2 signaling pathways. Oncol. Lett. 2021, 22, 631. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; Chen, H.P.; Tan, B.Z.; Yu, H.H.; Huang, X.S. Triptolide avoids cisplatin resistance and induces apoptosis via the reactive oxygen species/nuclear factor-κB pathway in SKOV3PT platinum-resistant human ovarian cancer cells. Oncol. Lett. 2013, 6, 1084–1092. [Google Scholar] [CrossRef]
- Wei, Y.M.; Wang, Y.H.; Xue, H.Q.; Luan, Z.H.; Liu, B.W.; Ren, J.H. Triptolide, A potential autophagy modulator. Chin. J. Integr. Med. 2019, 25, 233–240. [Google Scholar] [CrossRef]
- Zhong, Y.; Le, F.; Cheng, J.; Luo, C.; Zhang, X.; Wu, X.; Xu, F.; Zuo, Q.; Tan, B. Triptolide inhibits JAK2/STAT3 signaling and induces lethal autophagy through ROS generation in cisplatin-resistant SKOV3/DDP ovarian cancer cells. Oncol. Rep. 2021, 45, 69. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Niu, Y.; Xia, J.; Fan, L.; Wu, Y.; Gao, W. Procyanidins mediates antineoplastic effects against non-small cell lung cancer via the JAK2/STAT3 pathway. Transl. Cancer Res. 2021, 10, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Joo, S.H.; Seo, J.H.; Kim, J.; Yoon, G.; Jeon, Y.J.; Lee, M.H.; Chae, J.I.; Kim, W.K.; Shim, J.H. Licochalcone H Induces Cell Cycle Arrest and Apoptosis in Human Skin Cancer Cells by Modulating JAK2/STAT3 Signaling. Biomol. Ther. 2022, 30, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A. Computational modeling and in vitro evaluation identified natural product-Z218 as a novel Janus kinase 2 (JAK2) inhibitor to combat β-thalassemia. Biotechnol. Appl. Biochem. 2023, 70, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Upreti, S.; Muduli, K.; Pradhan, J.; Elangovan, S.; Samant, M. Identification of novel inhibitors from Urtica spp. against TNBC targeting JAK2 receptor for breast cancer therapy. Med. Oncol. 2023, 40, 326. [Google Scholar] [CrossRef] [PubMed]
- Vaziri-Amjad, S.; Rahgosha, R.; Taherkhani, A. Potential JAK2 Inhibitors from Selected Natural Compounds: A Promising Approach for Complementary Therapy in Cancer Patients. Evid. Based Complement. Alternat. Med. 2024, 2024, 1114928. [Google Scholar] [CrossRef]
- Yu, R.N.; Chen, C.J.; Shu, L.; Yin, Y.; Wang, Z.J.; Zhang, T.T.; Zhang, D.Y. Structure-based design and synthesis of pyrimidine-4,6-diamine derivatives as Janus kinase 3 inhibitors. Bioorg. Med. Chem. 2019, 27, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.G.; Wang, J.A.; Meng, L.; Pei, X.; Zhang, L.; An, L.; Li, C.L.; Miao, Y.L. Design, synthesis, biological activity evaluation of 3-(4-phenyl-1H-imidazol-2-yl)-1H-pyrazole derivatives as potent JAK 2/3 and aurora A/B kinases multi-targeted inhibitors. Eur. J. Med. Chem. 2021, 209, 112934. [Google Scholar] [CrossRef]
- Medvedeva, S.M.; Shikhaliev, K.S. Synthesis of 4,5-Dihydro-1H-[1,2]dithiolo[3,4-c]quinoline-1-thione Derivatives and Their Application as Protein Kinase Inhibitors. Molecules 2022, 27, 4033. [Google Scholar] [CrossRef]
- Wei, J.; Pan, Y.; Shen, Z.; Shen, L.; Xu, L.; Yu, W.; Huang, W. A hybrid energy-based and AI-based screening approach for the discovery of novel inhibitors of JAK3. Front. Med. 2023, 10, 1182227. [Google Scholar] [CrossRef]
- Faris, A.; Cacciatore, I.; Ibrahim, I.M.; Al-Mughram, M.H.; Hadni, H.; Tabti, K.; Elhallaoui, M. In silico computational drug discovery: A Monte Carlo approach for developing a novel JAK3 inhibitors. J. Biomol. Struct. Dyn. 2023, 20, 1–23. [Google Scholar] [CrossRef]
- Faris, A.; Ibrahim, I.M.; Alnajjar, R.; Hadni, H.; Bhat, M.A.; Yaseen, M.; Chakraborty, S.; Alsakhen, N.; Shamkh, I.M.; Mabood, F.M.; et al. QSAR-driven screening uncovers and designs novel pyrimidine-4,6-diamine derivatives as potent JAK3 inhibitors. J. Biomol. Struct. Dyn. 2023, 7, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Gao, Y.Q.; Deng, Y.J.; Zhang, H.H.; Wu, Y.R.; Hu, Y.; Mei, Q.X. Identification of Chinese Herbal Compounds with Potential as JAK3 Inhibitors. Evid. Based Complement. Alternat. Med. 2019, 2019, 4982062. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.M.; Xu, T.; Tu, Z.C.; Zhu, H.J.; Cheng, Y.X. Sulfur and nitrogen-containing compounds from the whole bodies of Blaps japanensis. Bioorg. Chem. 2020, 102, 104086. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Yi, E.H.; Jee, J.G.; Jeong, A.J.; Sandoval, C.; Park, I.C.; Baeg, G.H.; Ye, S.K. Tubulosine selectively inhibits JAK3 signalling by binding to the ATP-binding site of the kinase of JAK3. J. Cell Mol. Med. 2020, 24, 7427–7438. [Google Scholar] [CrossRef]
- Sanachai, K.; Mahalapbutr, P.; Tabtimmai, L.; Seetaha, S.; Kittikool, T.; Yotphan, S.; Choowongkomon, K.; Rungrotmongkol, T. Discovery of JAK2/3 Inhibitors from Quinoxalinone-Containing Compounds. ACS Omega 2022, 7, 33587–33598. [Google Scholar] [CrossRef] [PubMed]
- Sanachai, K.; Mahalapbutr, P.; Tabtimmai, L.; Seetaha, S.; Kaekratoke, N.; Chamni, S.; Azam, S.S.; Choowongkomon, K.; Rungrotmongkol, T. In Silico and In Vitro Study of Janus Kinases Inhibitors from Naphthoquinones. Molecules 2023, 28, 597. [Google Scholar] [CrossRef]



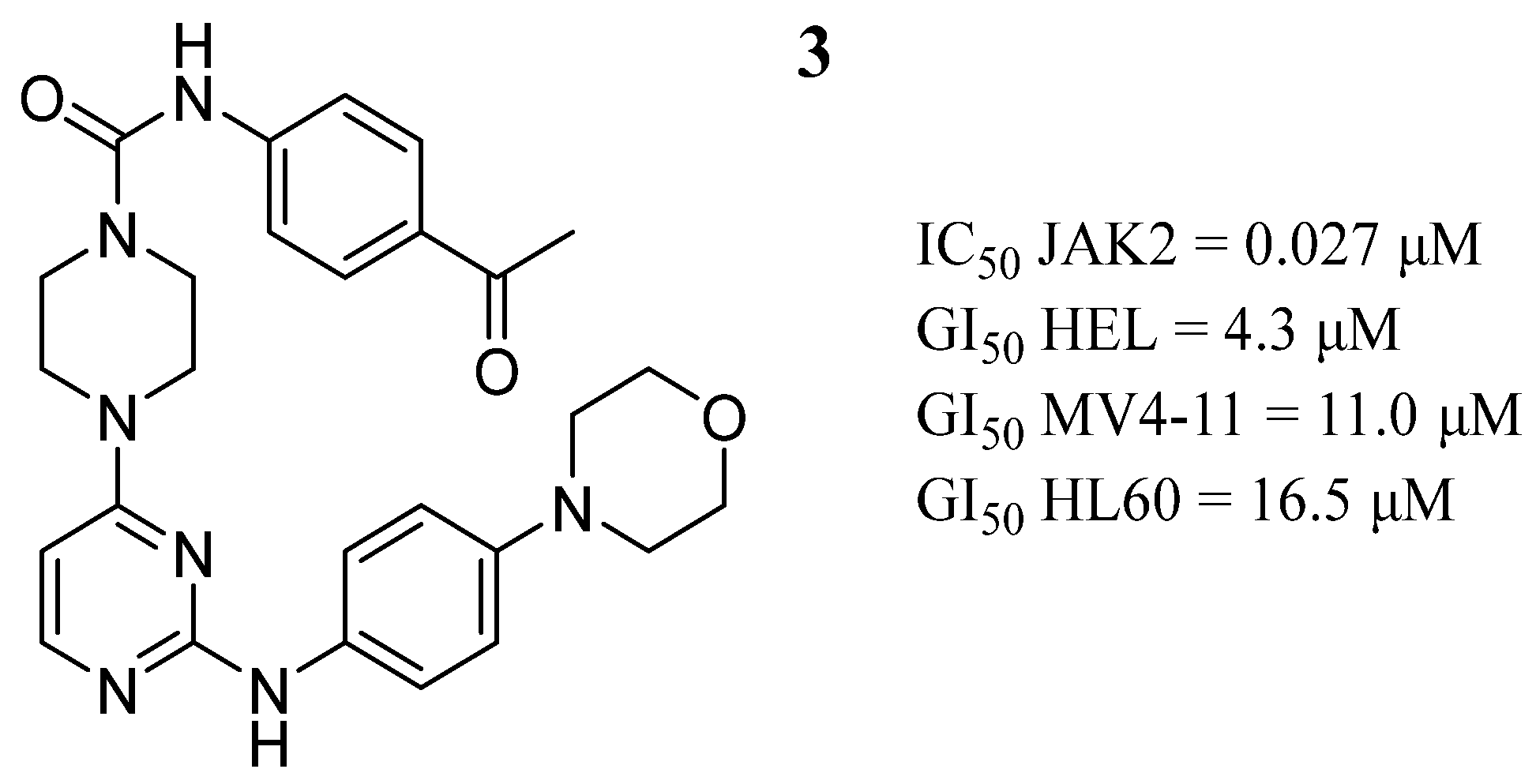




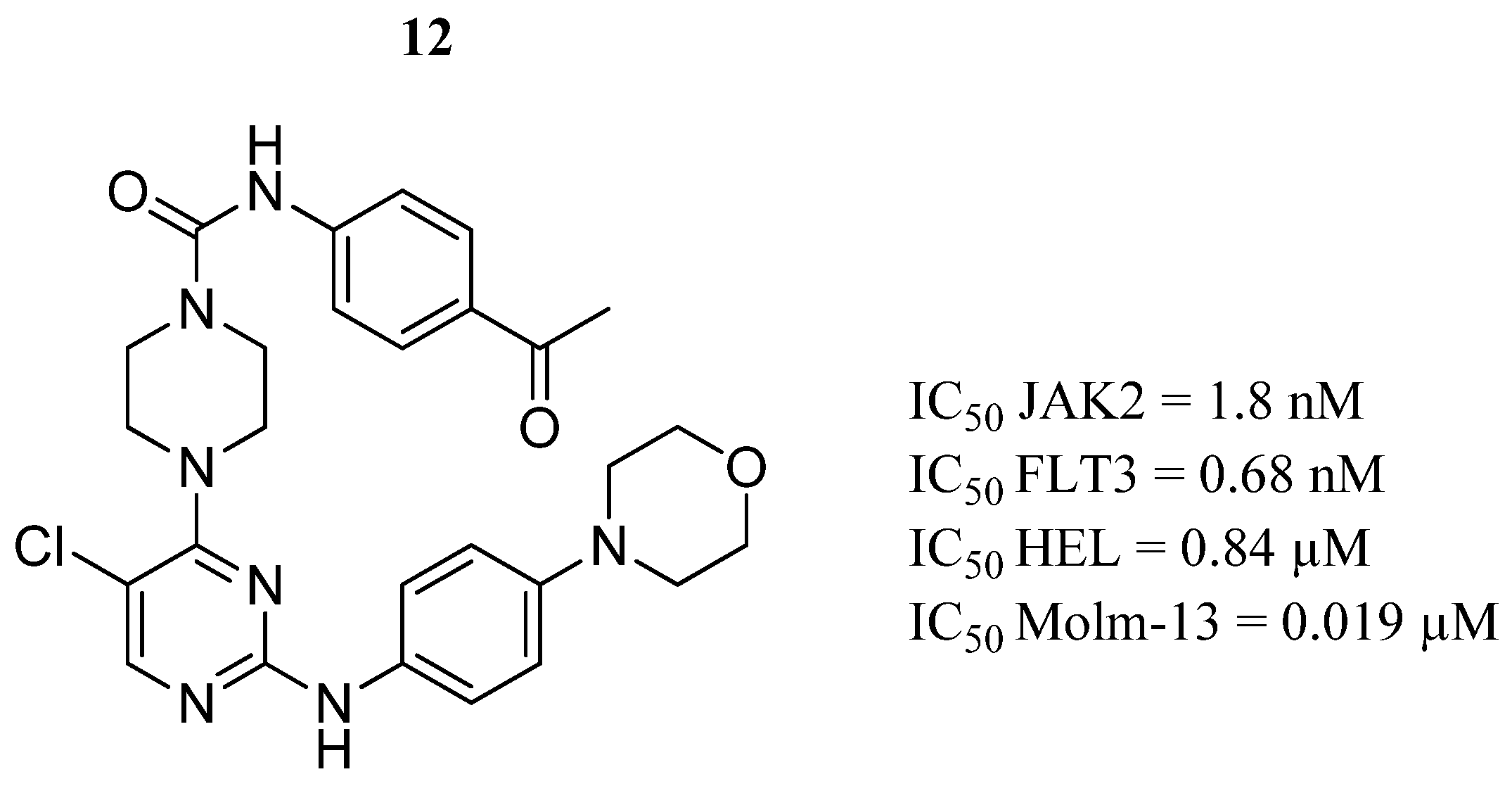




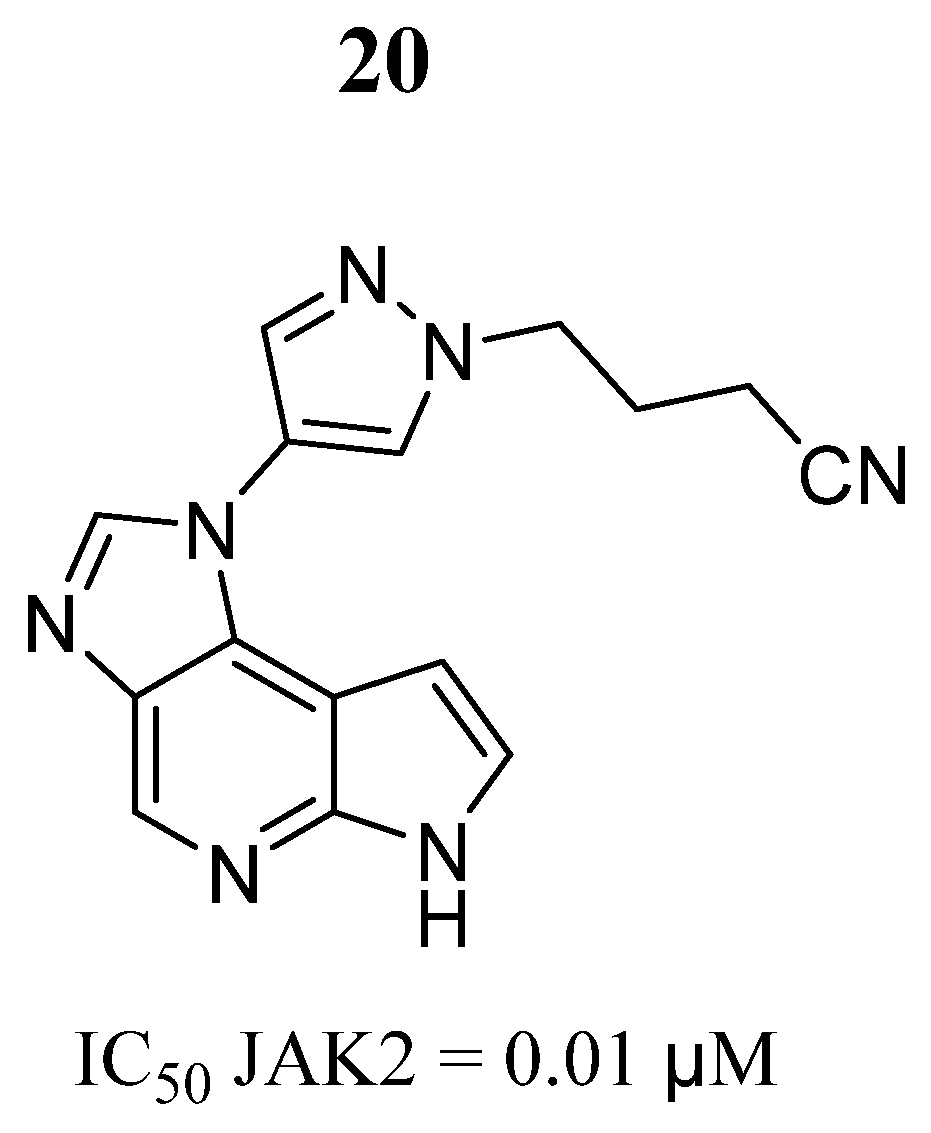
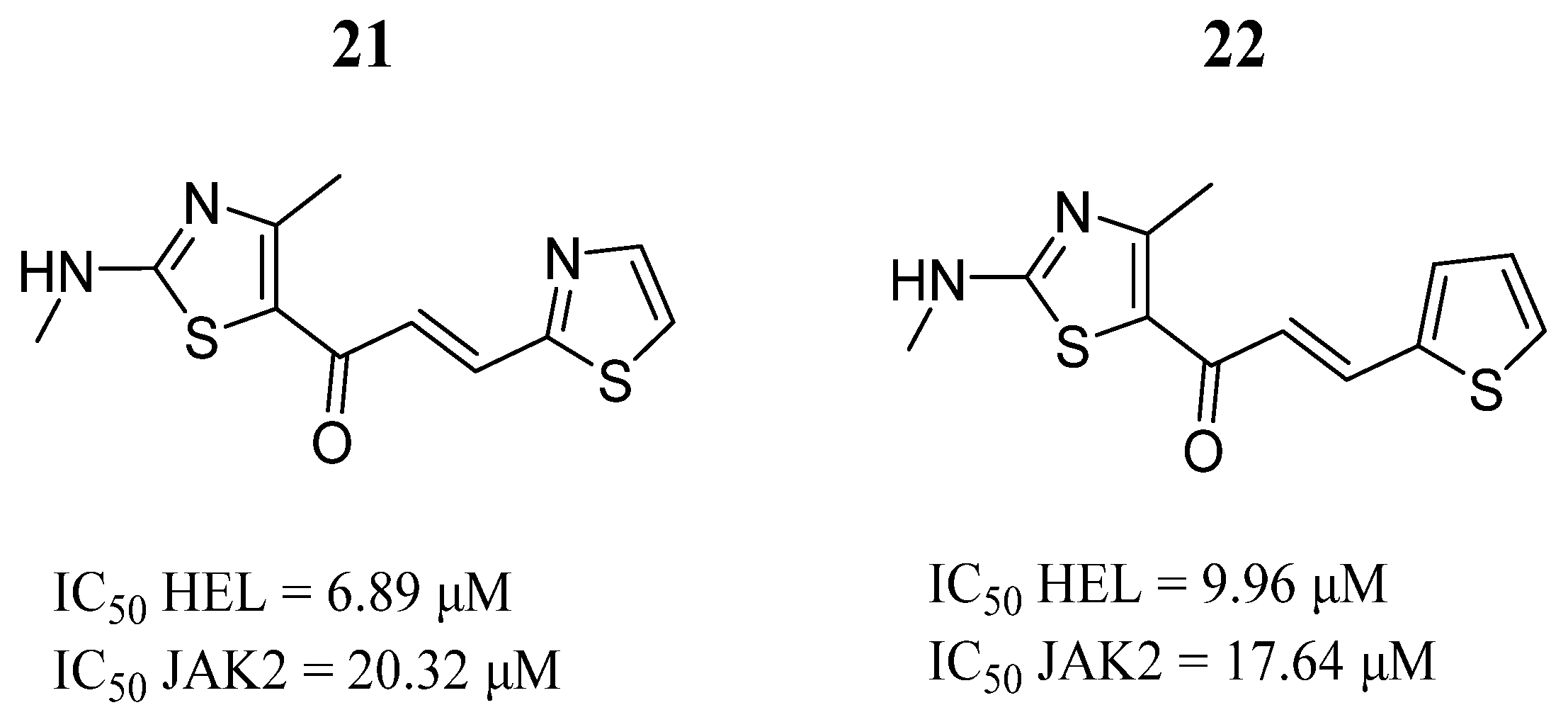


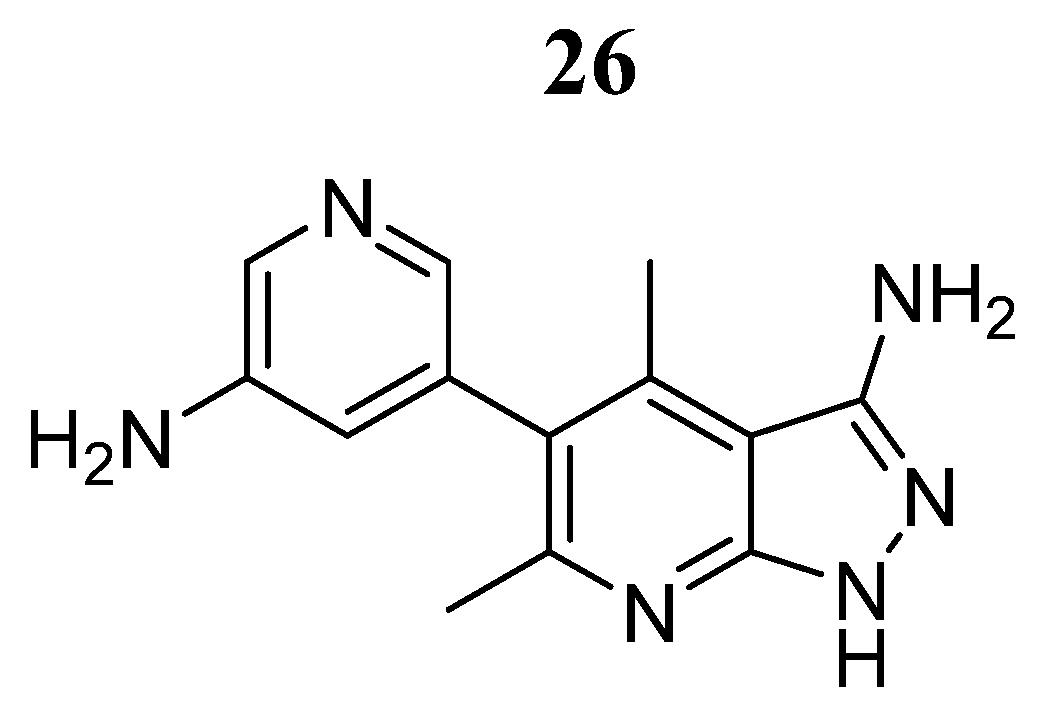


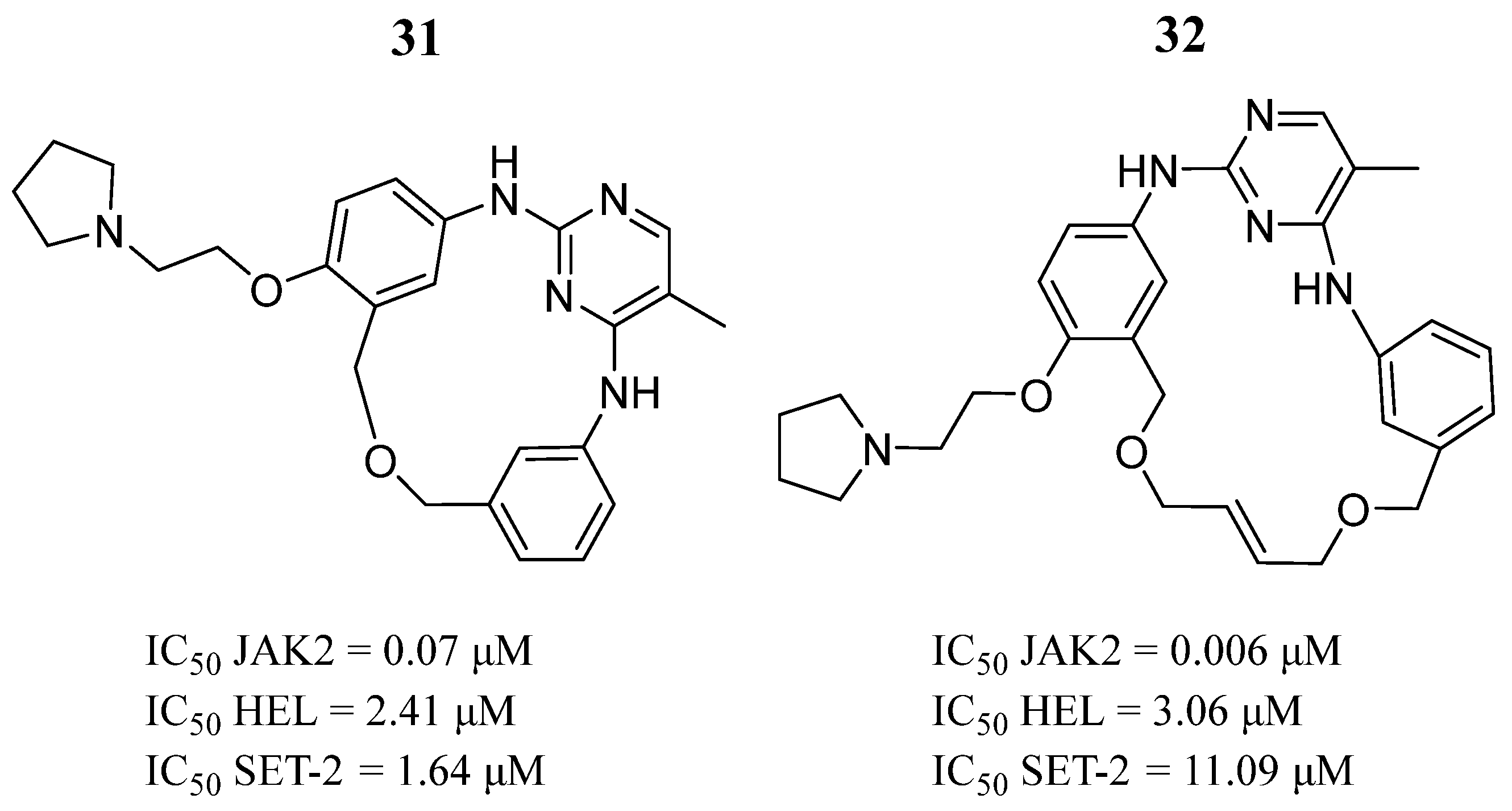
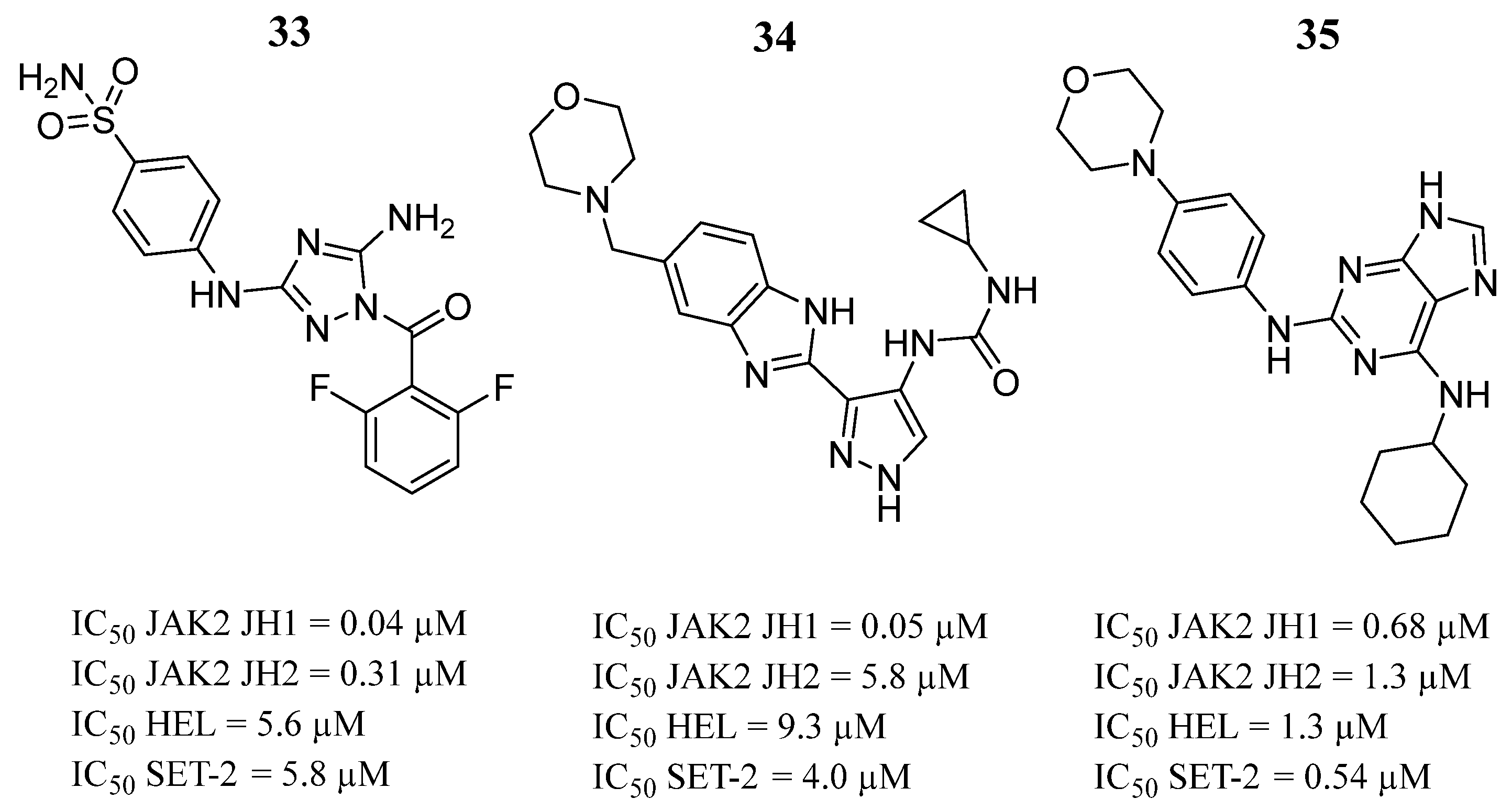
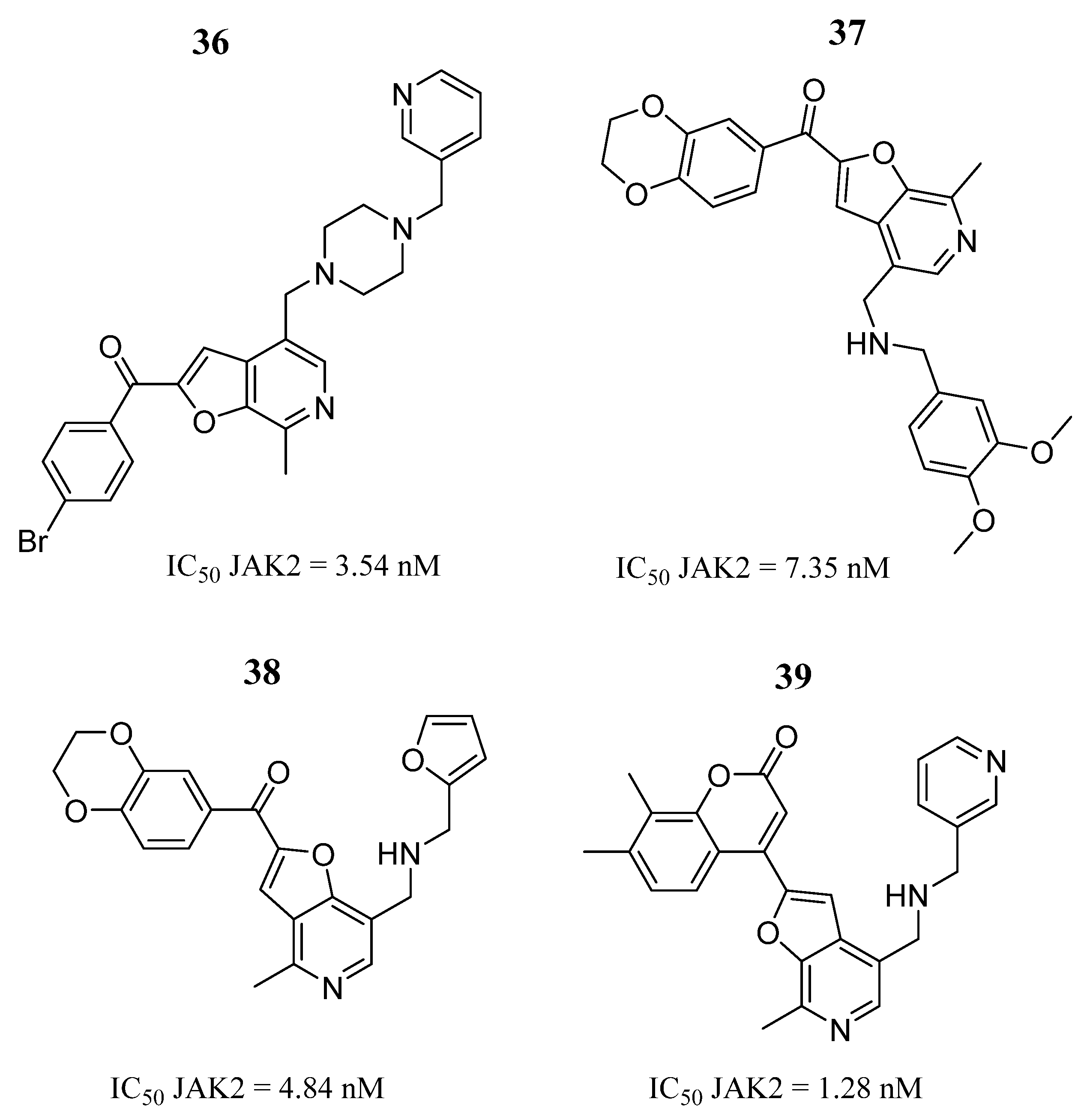
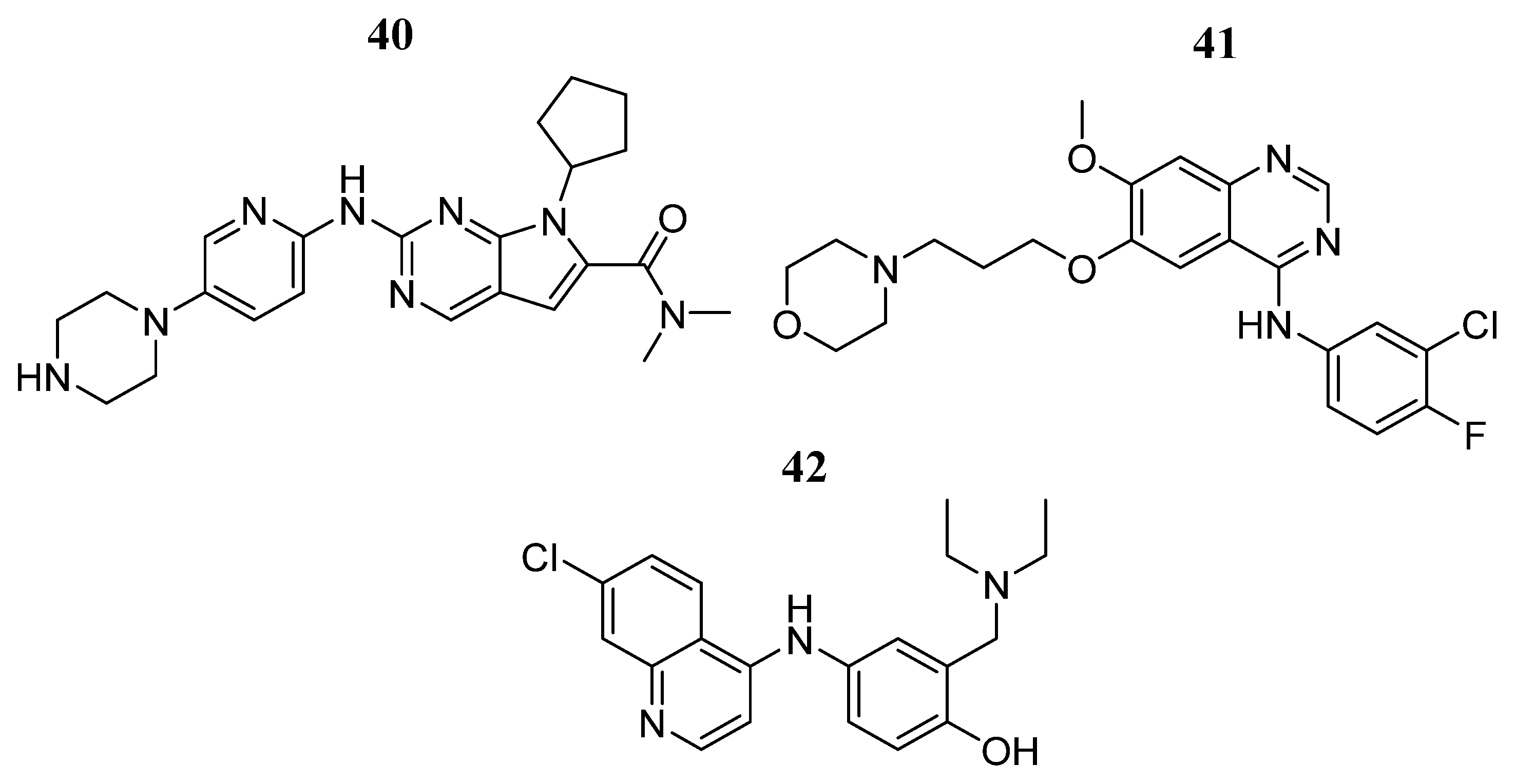
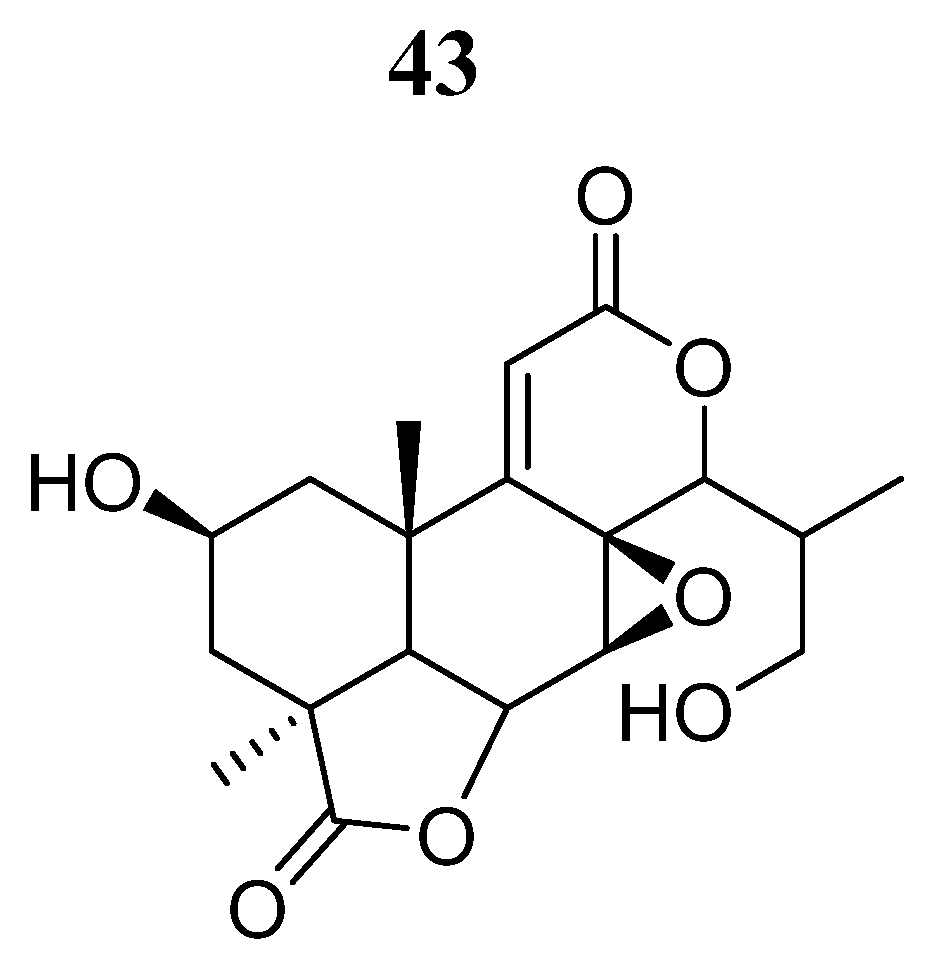
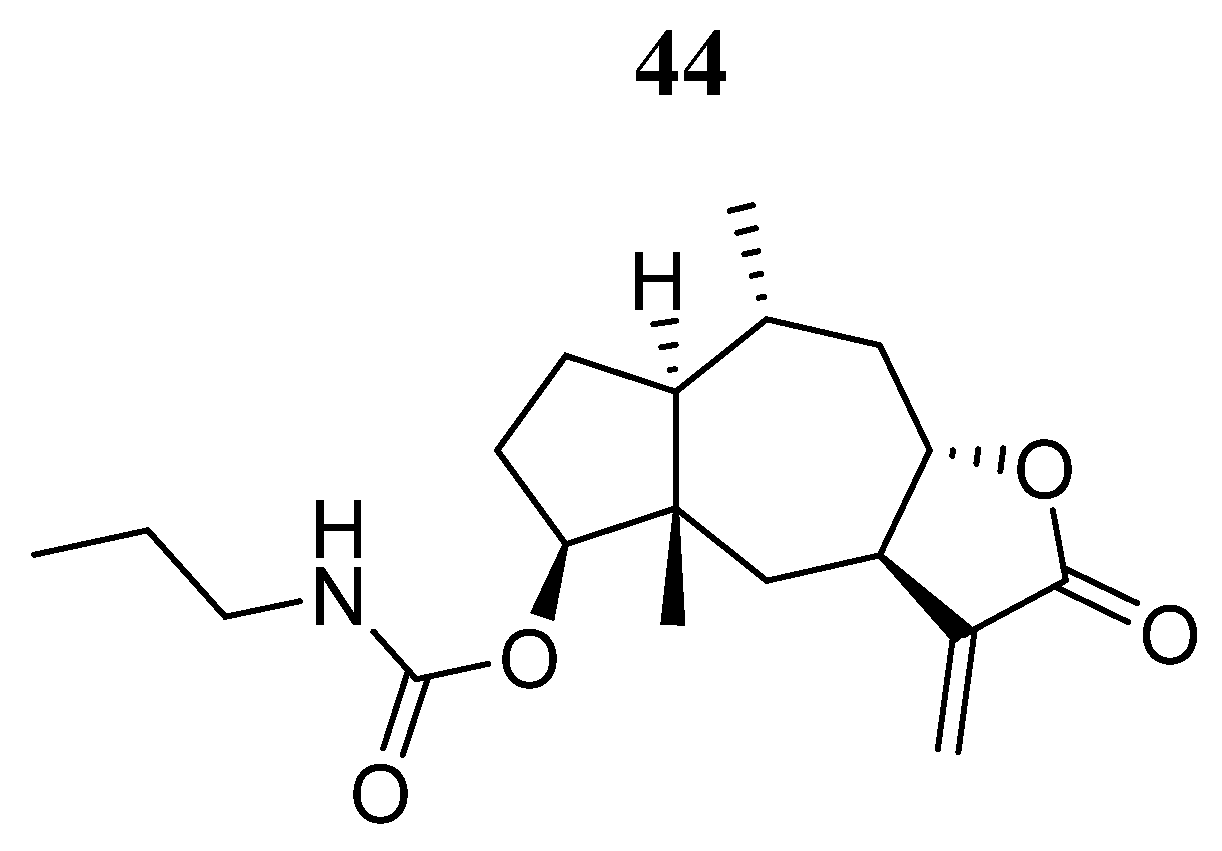

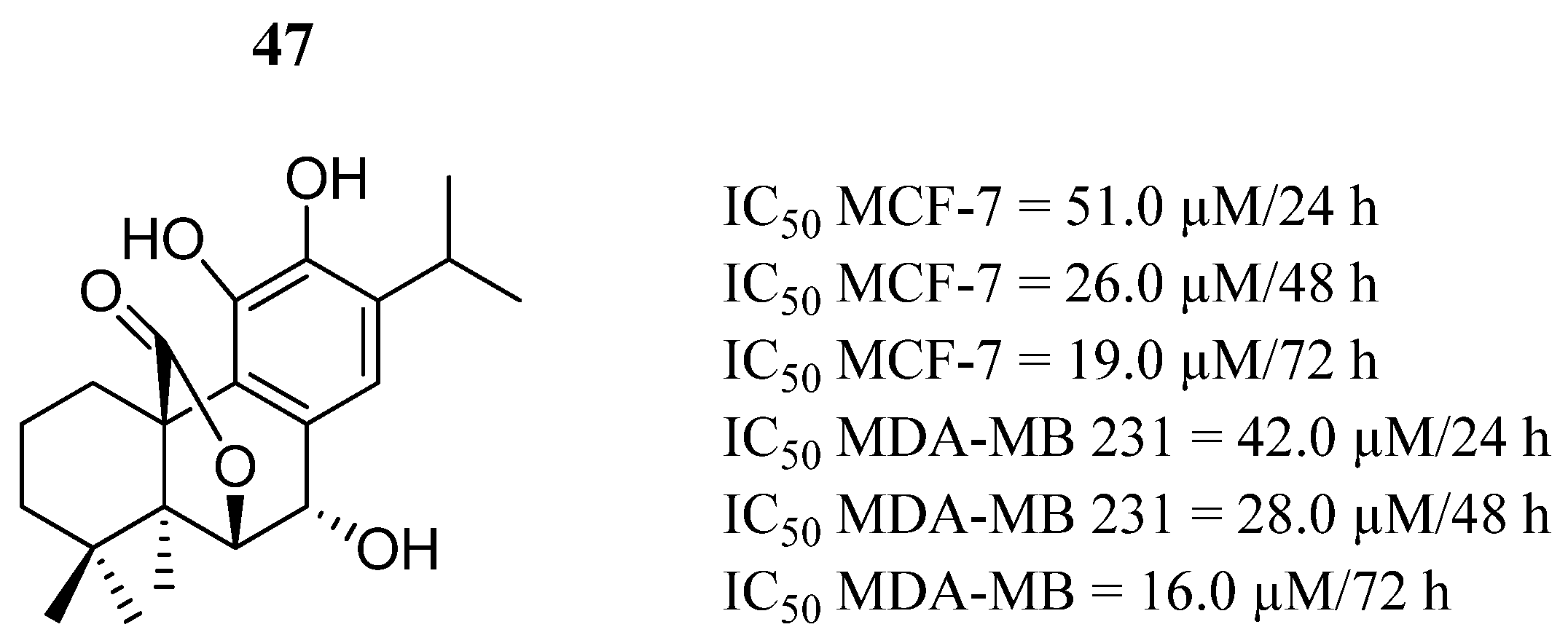



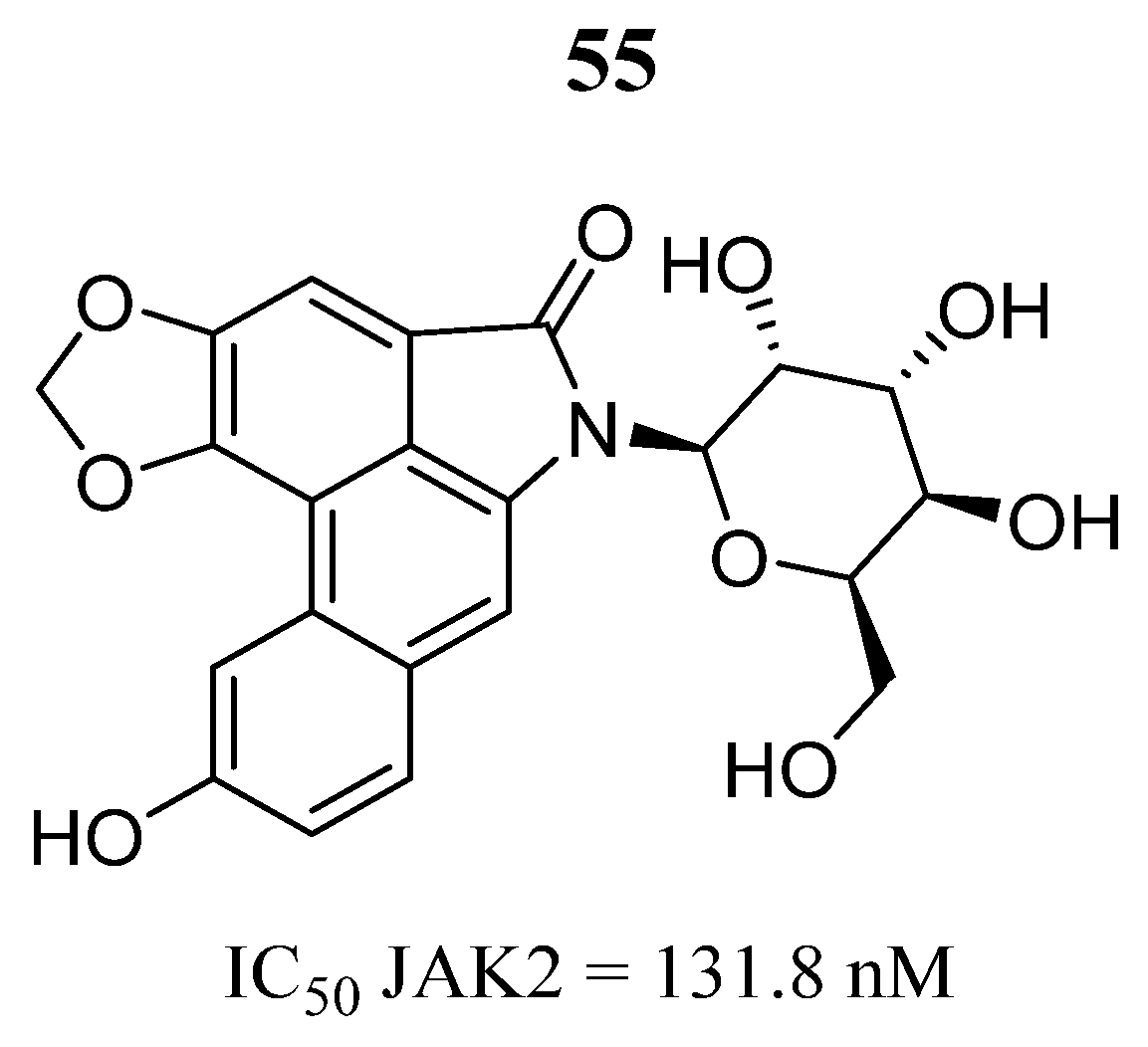

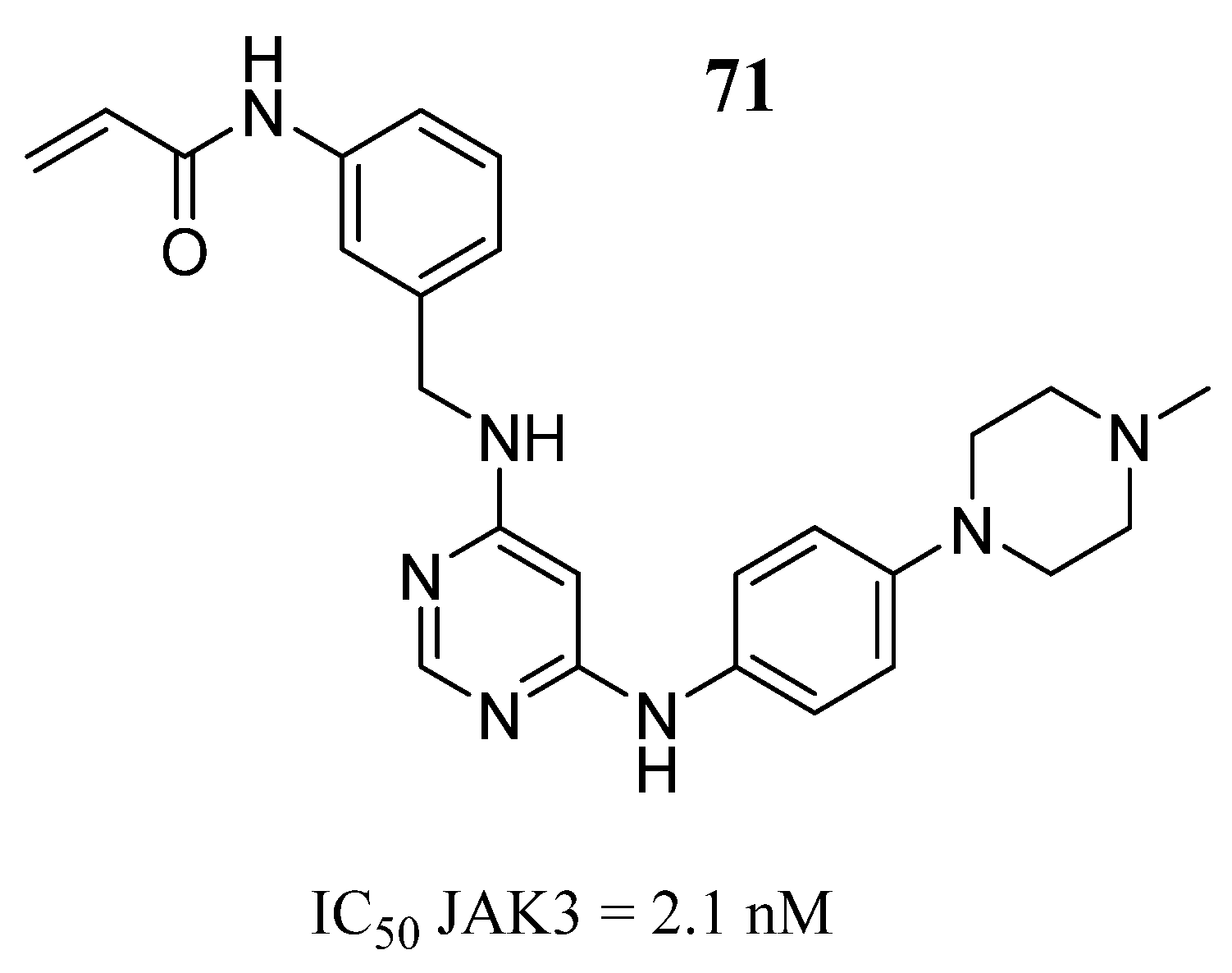

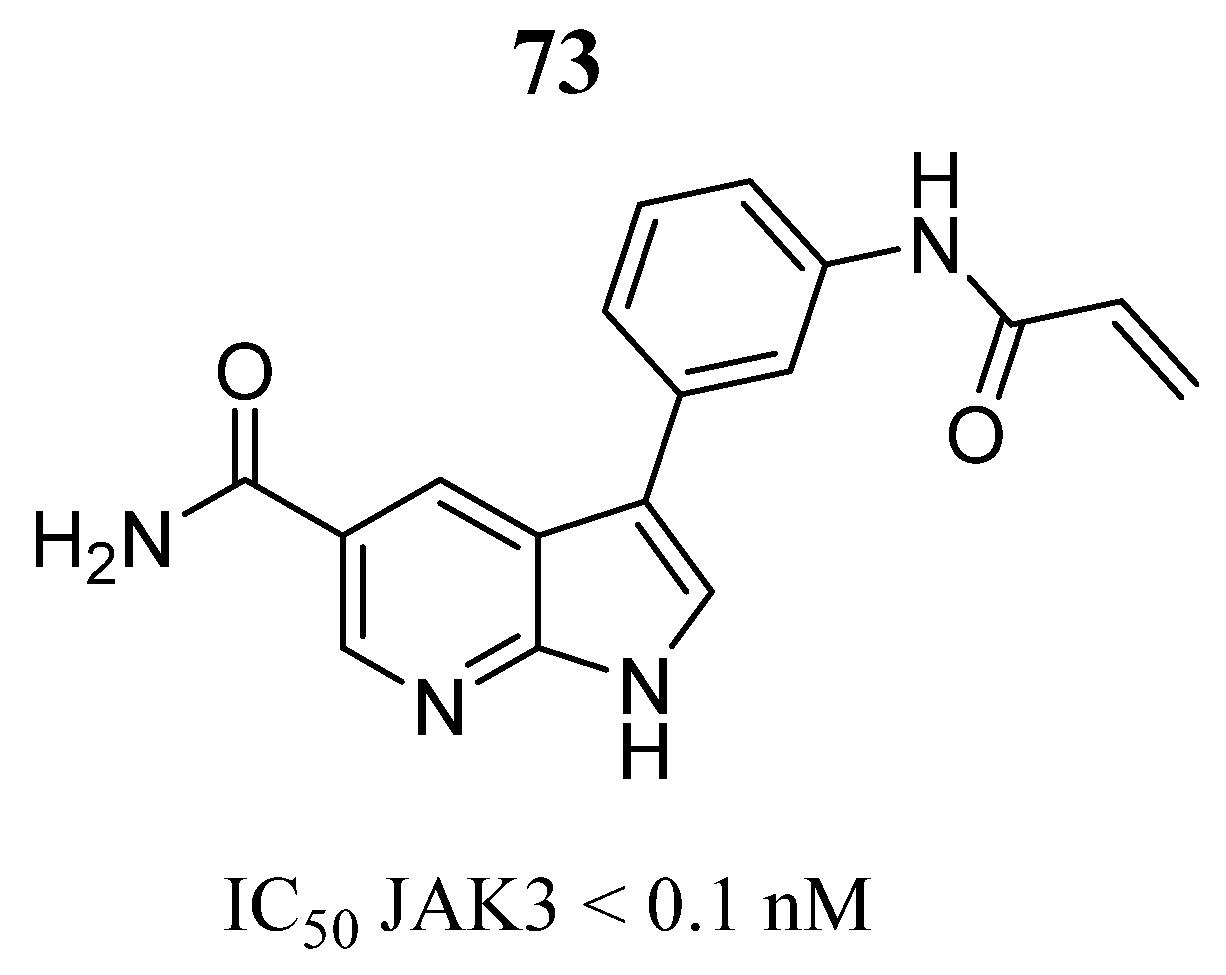

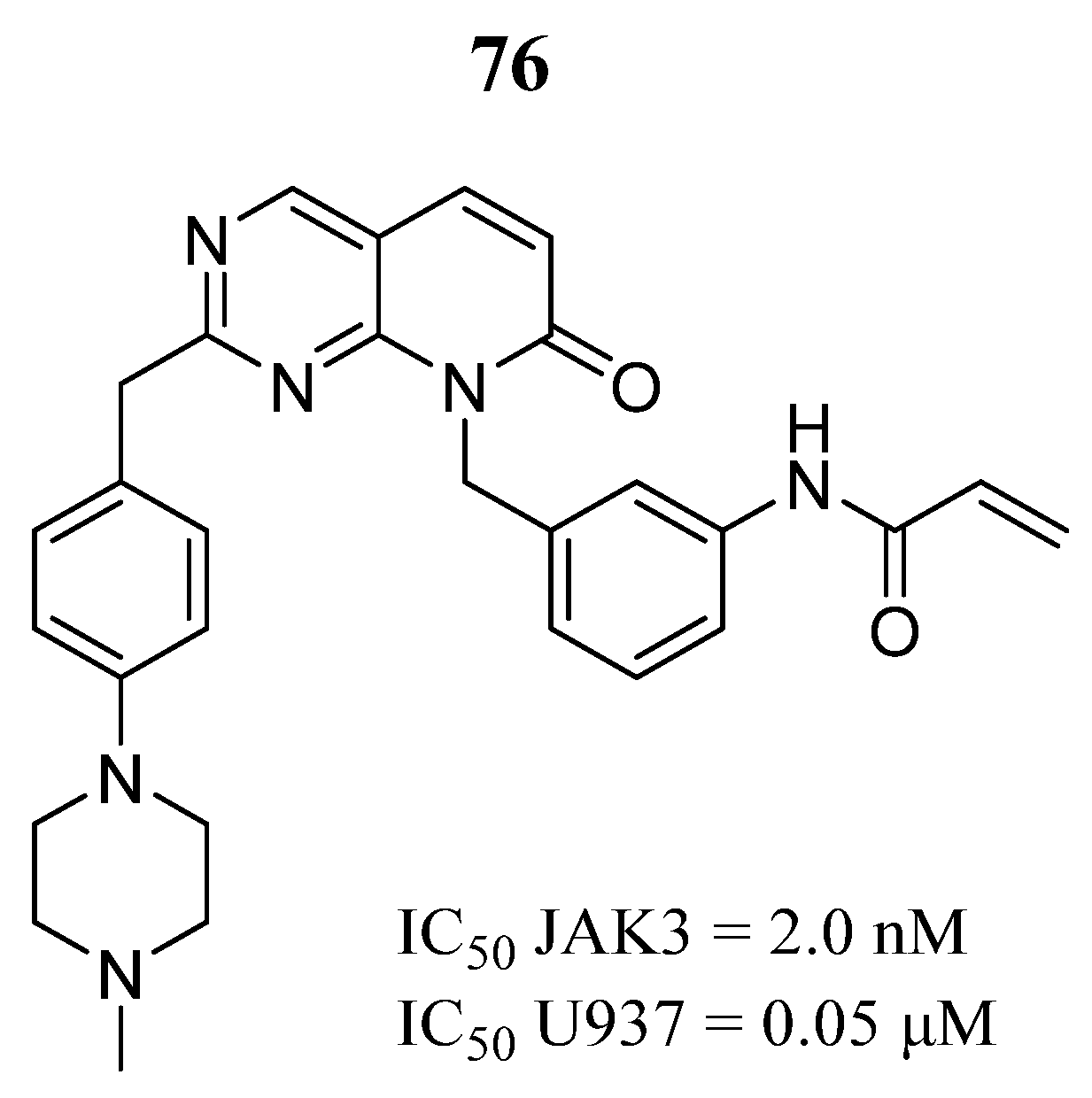





| Compound | Activity | Disease | Toxic Effects |
|---|---|---|---|
 Ruxolitinib | IC50 = 2.8 nM | Polycythemia, Myelofibrosis, Various cancers | Diarrhea, abdominal pain, anemia, thrombocytopenia |
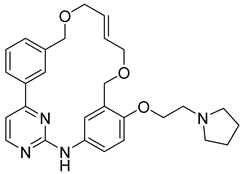 Pacritinib | IC50 = 23 nM | Myeloid leukemias, Myelofibrosis | Cardiovascular and hemorrhagic events |
 AZD1480 | Ki = 0.26 nM | Myeloproliferative diseases, Solid tumors | Dizziness, anxiety, memory loss, ataxia, hallucinations, behavior changes |
| Compound | Activity | Disease | Toxic Effects |
|---|---|---|---|
 Ritlecitinib | IC50 = 33.1 nM | Alopecia areata, Vitiligo, Ulcerative colitis, Rheumatoid arthritis, Crohn’s disease | Hepatotoxicity, Pruritus, Influenza |
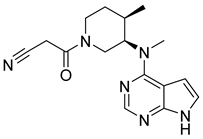 Tofacitinib | IC50 = 1 nM | Transplant patients, Autoimmune disease, Rheumatoid arthritis | Infection, Cytopenias |
| Compound | Anticancer Activity | Enzyme Activity | In Silico Analysis | References |
|---|---|---|---|---|
| JAK2 | ||||
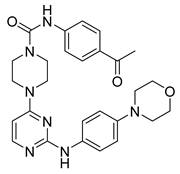 3 | GI50 HEL = 4.3 μM GI50 MV4-11 = 11.0 μM GI50 HL60 = 16.5 μM | IC50 = 0.027 μM | Binds to the ATP-binding site, two hydrogen bonds with Leu932, a network-like alkyl-π interaction with the adjacent Ala880, Leu855, a hydrogen bond with Lys943 and another with Leu855. | [53] |
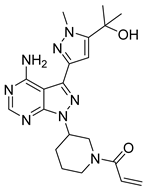 5 | IC50 HEL = 6.46 μM | IC50 = 0.0065 μM | Two hydrogen bonds with Leu932 and two π-π interactions with Tyr931, a hydrogen bond with Lys857, two hydrogen bonds with residue Tyr931, and hydrogen bonding with Ser936 and Asp939 via a water molecule, near the ATP-binding site. | [39] |
 15 | IC50 MCF-7 = 6.39 µM IC50 A549 = 6.9 µM | - | Hydrogen bond with the residue Leu932, lipophilic interactions with the nonpolar amino acid residues Leu83, Leu855, Val863, Pro933, Met929, Ala880 and Leu932 within the receptor pocket. | [70] |
 20 | IC50 = 0.01 μM | Hydrogen bonds with Glu930 and Leu932, hydrogen bonds with Lys882. | [74] | |
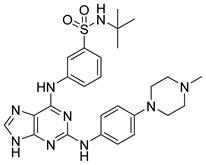 30 | - | IC50 = 0.022 μM | Hydrogen bonds with Pro375, Lys378, Asp381, Tyr390, and Asn433 | [80] |
 34 | IC50 HEL = 5.6 µM IC50 SET-2 = 5.8 µM | IC50 = 0.04 µM | Two hydrogen bonds with Val629 and Glu627, hydrogen bond with the Lys640 side chain of the αD helix. | [28] |
 46 | - | - | Hydrogen bonding interactions with Lys882, Arg980, Ser936, Asp939, and Lys943, carbon hydrogen bonding interactions with Leu932 and Leu855, and a sulfur bonding interaction with Lue855 | [71] |
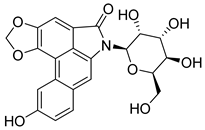 55 | - | IC50 = 131.8 nM | Interacts with the amino acid residues Ala880, Val863, Tyr931, Leu855 Asp994, Leu983, Asn981 and Arg980 | [93] |
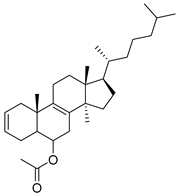 56–67 | IC50 TNBC = 90.09 to 172.16 μg/mL IC50 HEK293T = 732.52 to 1367.25 μg/mL | - | Interaction with residues of catalytic sites such as Leu983, Leu855, Val863, Arg980, Val863, Ala880, Leu855 and Leu932, in the binding pocket | [94] |
| JAK3 | ||||
 71 | - | IC50 = 2.1 nM | Bidentate hydrogen bonds with Leu905, and the Cys909, van der Waals contact with Leu956 and Leu828 in the ATP-binding pocket, a hydrogen bond with Arg953 | [96] |
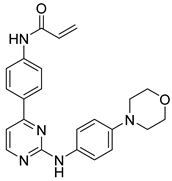 72 | - | IC50 = 1.7 nM | Bidentate hinge hydrogen bonds with Leu905, covalent bonds with Cys909, two σ-π interactions and one σ-π interaction with amino acid residues Leu828 and Gly908, a hydrogen bond with Asp912 | [20] |
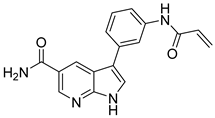 73 | - | IC50 < 0.1 nM | Hinge interaction pattern and the covalent binding of Cys909, two hydrogen bonds with Lys905, a hydrogen bond with Glu903. | [17] |
 74 | IC50 K562 = 6.72 µM | IC50 = 0.057 µM | Interaction at the binding site with Glu930, Tyr931, Leu932, Ser936 and Gly993. | [97] |
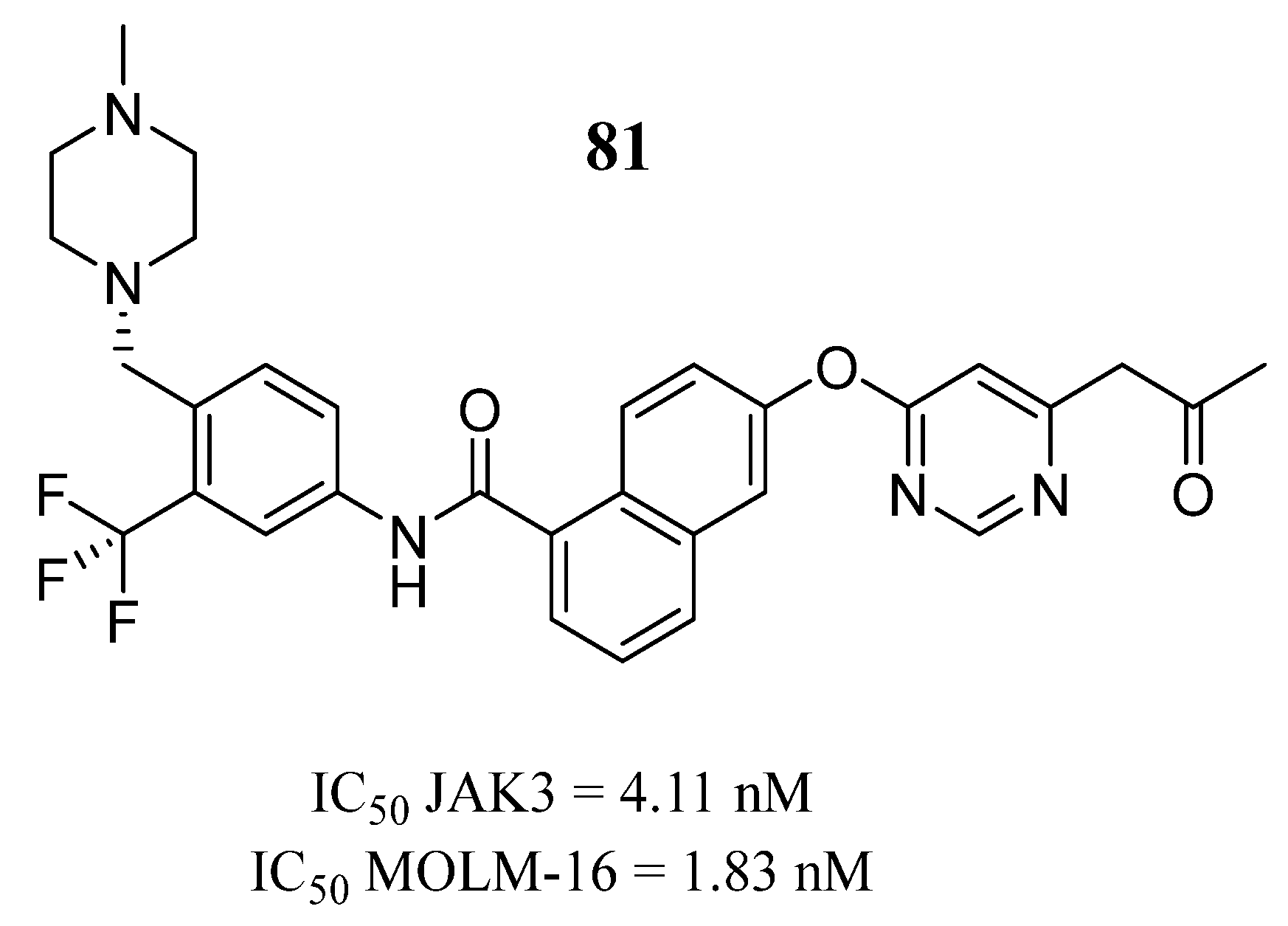
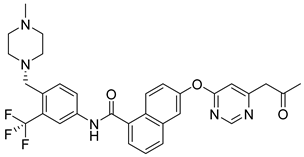 81 | IC50 MOLM-16 = 1.83 nM | IC50 = 4.11 nM | Hydrogen bond interaction with Lys855, hydrogen bond interaction with Leu905, hydrophobic interactions with Leu905, Gly906, and Arg953 | [99] |
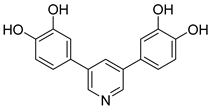 92 | IC50 A549 = 1.68 µM IC50 Huh-7 = 4.88 µM IC50 K562 = 2.13 µM | IC50 = 1.72 µM | - | [103] |
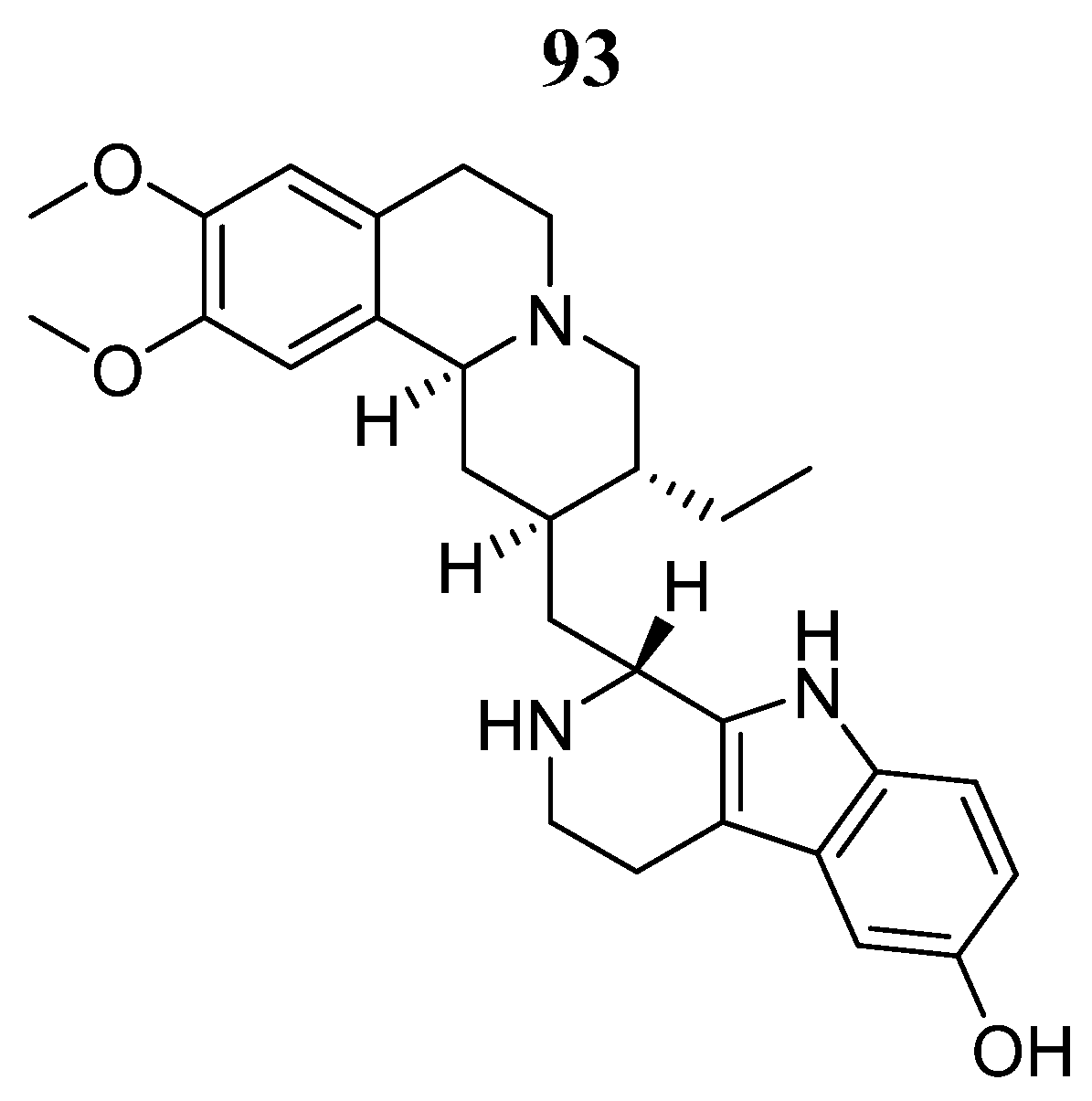
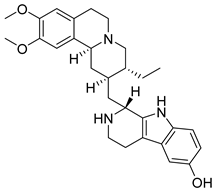 93 | - | EC50 = 1.4 μmol/L | Interaction with Val812, Ala829, Glu847, Met878, Leu881, Leu932 y Asp943. | [104] |
| JAK2/3 | ||||
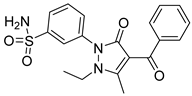 95 | - | IC50 JAK2 = 12.61 nM IC50 JAK3 = 15.80 nM | Hydrogen bonds in the hinge region with residues Glu930 and Lys932 of JAK2 and Glu903 and Lys905. | [49] |
 97 | IC50 TF1 = 15.53 μM IC50 HEL = 17.90 μM | IC50 JAK2 = 13.00 nM IC50 JAK3 = 14.86 nM | Hydrogen bonds in the hinge region with residues Ser936 and Arg938. | [105] |
 99 | IC50 TF1 = 18.10 μM IC50 HEL = 6.65 μM | IC50 JAK2 = 11.11 nM IC50 JAK3 = 10.24 nM | Hydrogen bonds with residues Y931 and L932 and hydrophobic contact with the hinge region, the G loop and the catalytic loop. | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Jiménez, L.K.; Rivera, G.; Juárez-Saldivar, A.; Ortega-Balleza, J.L.; Ortiz-Pérez, E.; Jaime-Sánchez, E.; Paz-González, A.; Lara-Ramírez, E.E. Biological Evaluations and Computer-Aided Approaches of Janus Kinases 2 and 3 Inhibitors for Cancer Treatment: A Review. Pharmaceutics 2024, 16, 1165. https://doi.org/10.3390/pharmaceutics16091165
Vázquez-Jiménez LK, Rivera G, Juárez-Saldivar A, Ortega-Balleza JL, Ortiz-Pérez E, Jaime-Sánchez E, Paz-González A, Lara-Ramírez EE. Biological Evaluations and Computer-Aided Approaches of Janus Kinases 2 and 3 Inhibitors for Cancer Treatment: A Review. Pharmaceutics. 2024; 16(9):1165. https://doi.org/10.3390/pharmaceutics16091165
Chicago/Turabian StyleVázquez-Jiménez, Lenci K., Gildardo Rivera, Alfredo Juárez-Saldivar, Jessica L. Ortega-Balleza, Eyra Ortiz-Pérez, Elena Jaime-Sánchez, Alma Paz-González, and Edgar E. Lara-Ramírez. 2024. "Biological Evaluations and Computer-Aided Approaches of Janus Kinases 2 and 3 Inhibitors for Cancer Treatment: A Review" Pharmaceutics 16, no. 9: 1165. https://doi.org/10.3390/pharmaceutics16091165
APA StyleVázquez-Jiménez, L. K., Rivera, G., Juárez-Saldivar, A., Ortega-Balleza, J. L., Ortiz-Pérez, E., Jaime-Sánchez, E., Paz-González, A., & Lara-Ramírez, E. E. (2024). Biological Evaluations and Computer-Aided Approaches of Janus Kinases 2 and 3 Inhibitors for Cancer Treatment: A Review. Pharmaceutics, 16(9), 1165. https://doi.org/10.3390/pharmaceutics16091165







