Combinatory Effect of Pequi Oil (Caryocar brasiliense)-Based Nanoemulsions Associated to Docetaxel and Anacardic Acid (Anacardium occidentale) in Triple-Negative Breast Cancer Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of Pequi Oil Nanoformulations Containing DTX or AA
2.3. Dynamic Light Scattering (DLS) and Stability under Time
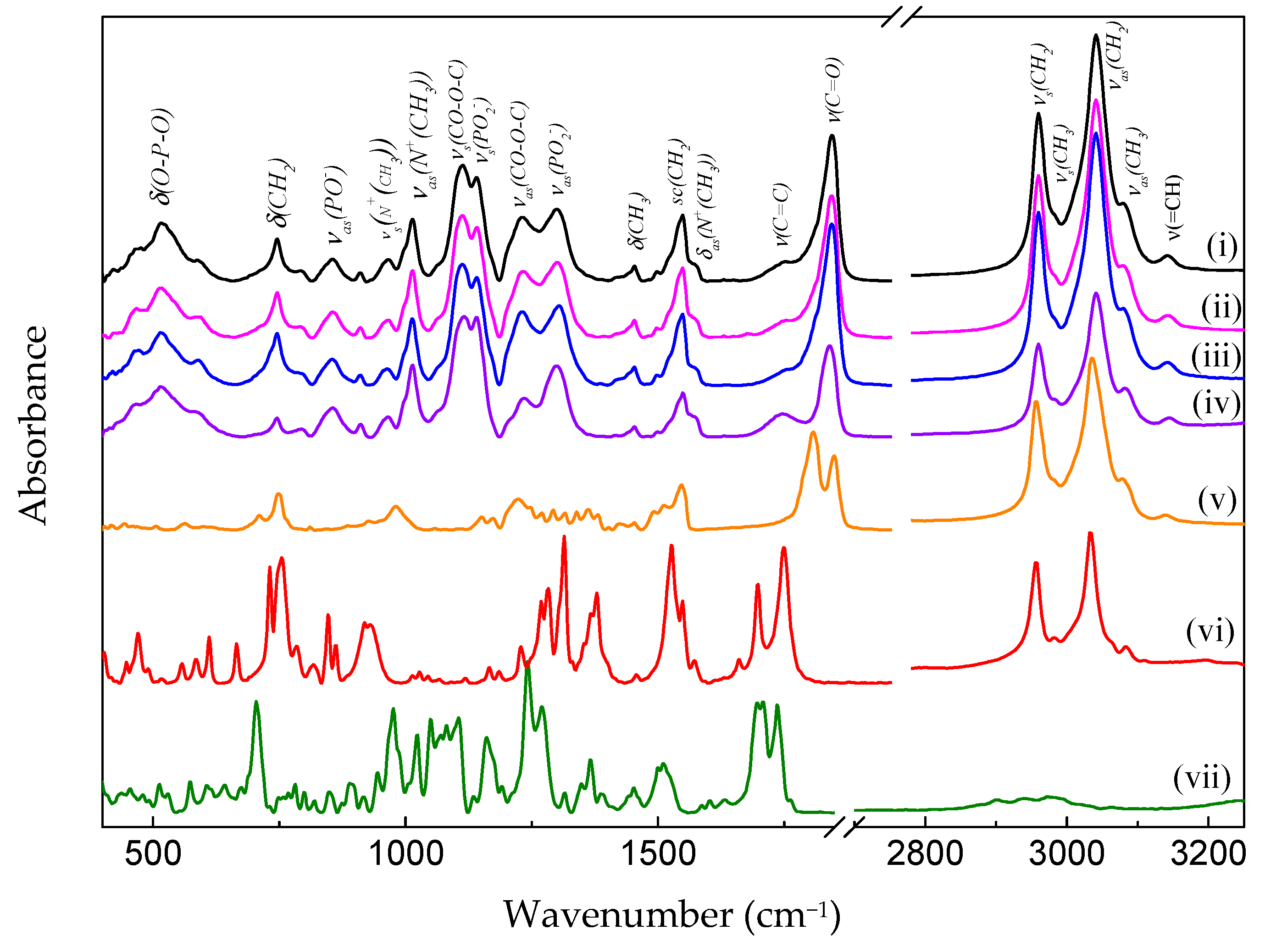
2.4. Analysis of Infrared Spectrophotometry—FTIR
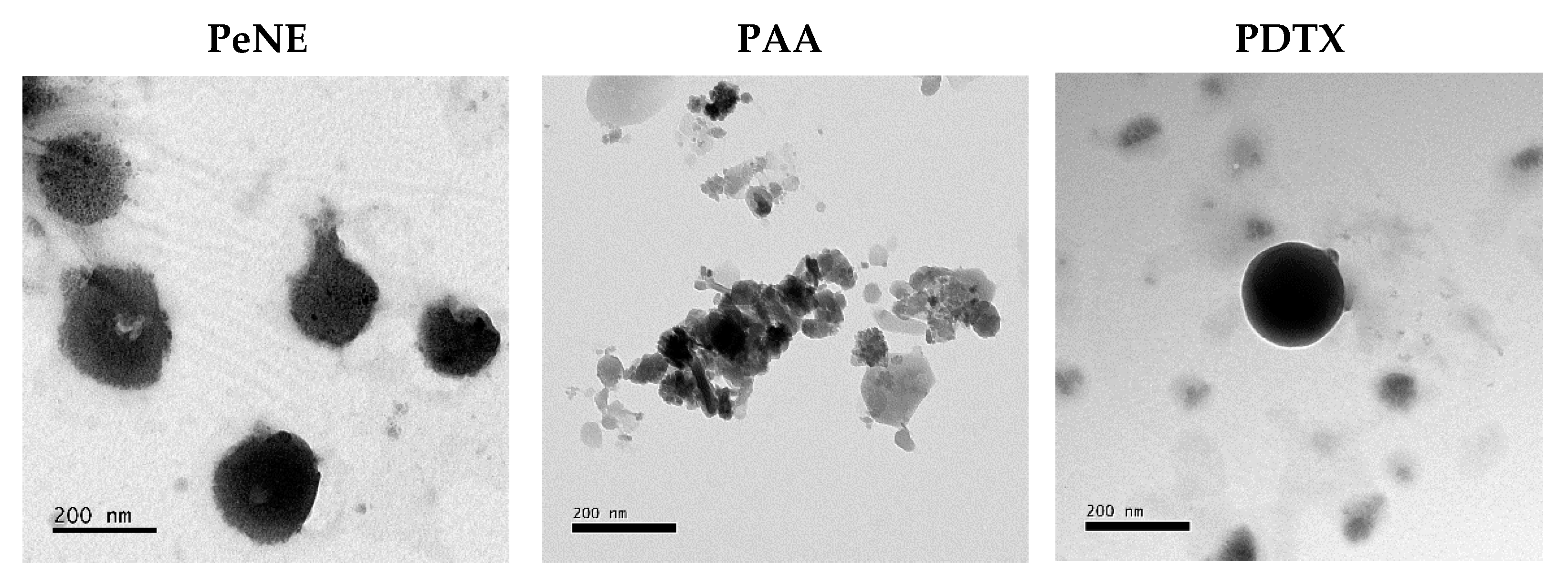
2.5. Electronic Transmission Microscopy
2.6. Cell Culture
2.7. Cell Treatment
2.8. Cell Viability Assay
2.9. Plasma Membrane Integrity and Cell Count
2.10. Flow Cytometry
2.10.1. Cells Treatment
2.10.2. Lysosomal Membrane Permeabilization
2.10.3. Mitochondrial Membrane Potential and Cells Morphologic Aspects
2.10.4. ROS Level
2.10.5. DNA Fragmentation Assay and Cell-Cycle
2.10.6. Annexin-V FITC/Propidium Iodide (PI) Staining
2.10.7. Multicaspase Assay
2.11. Clonogenic Assay
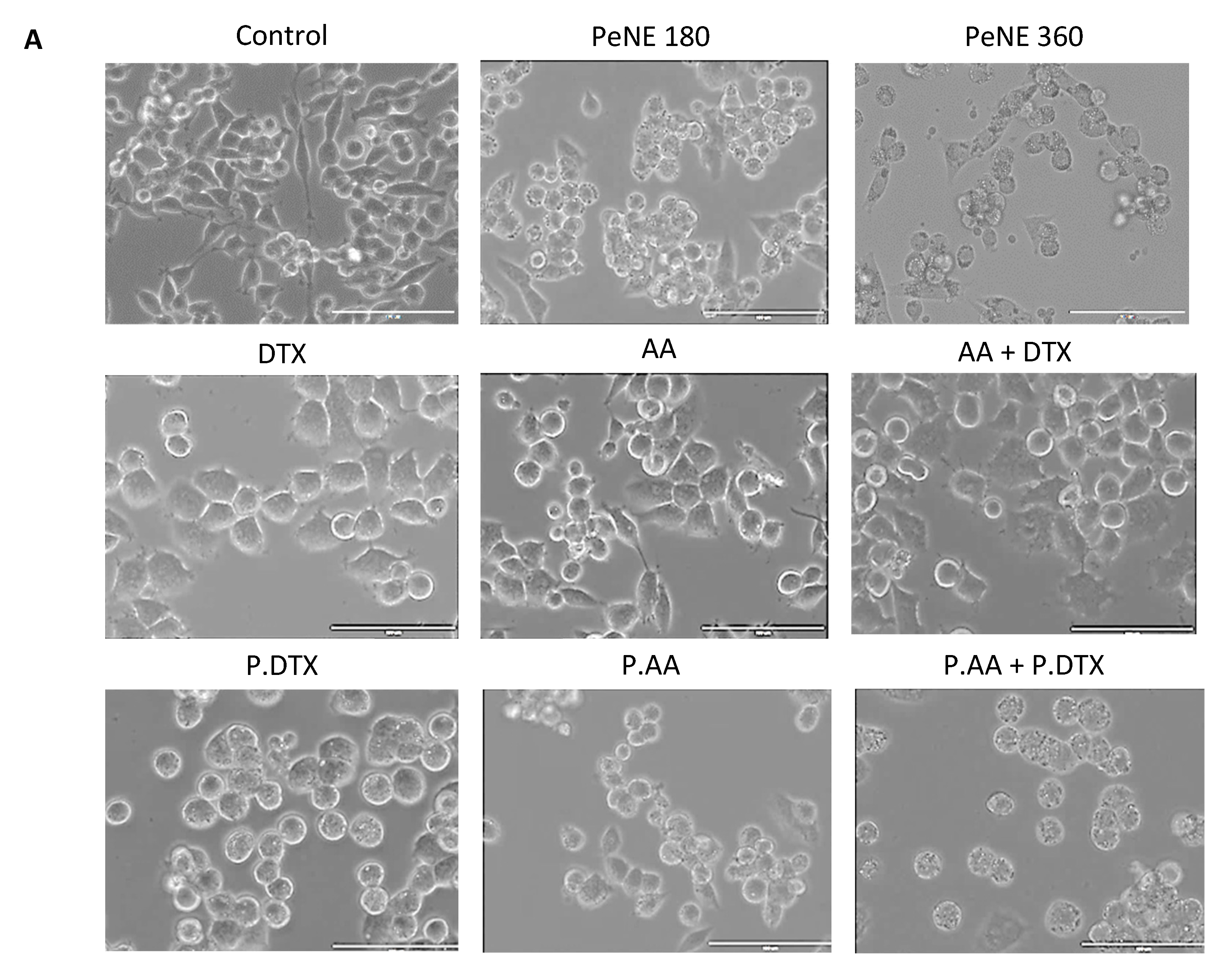
2.12. Cell Morphology Analysis by Optical Microscopy
2.13. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Nanoemulsions
3.2. Combined Effect of PDTX and PAA on Triple Negative Breast Cancer Cells (4T1)
3.3. PDTX + PAA Modified the Morphology of Breast Cancer Cells
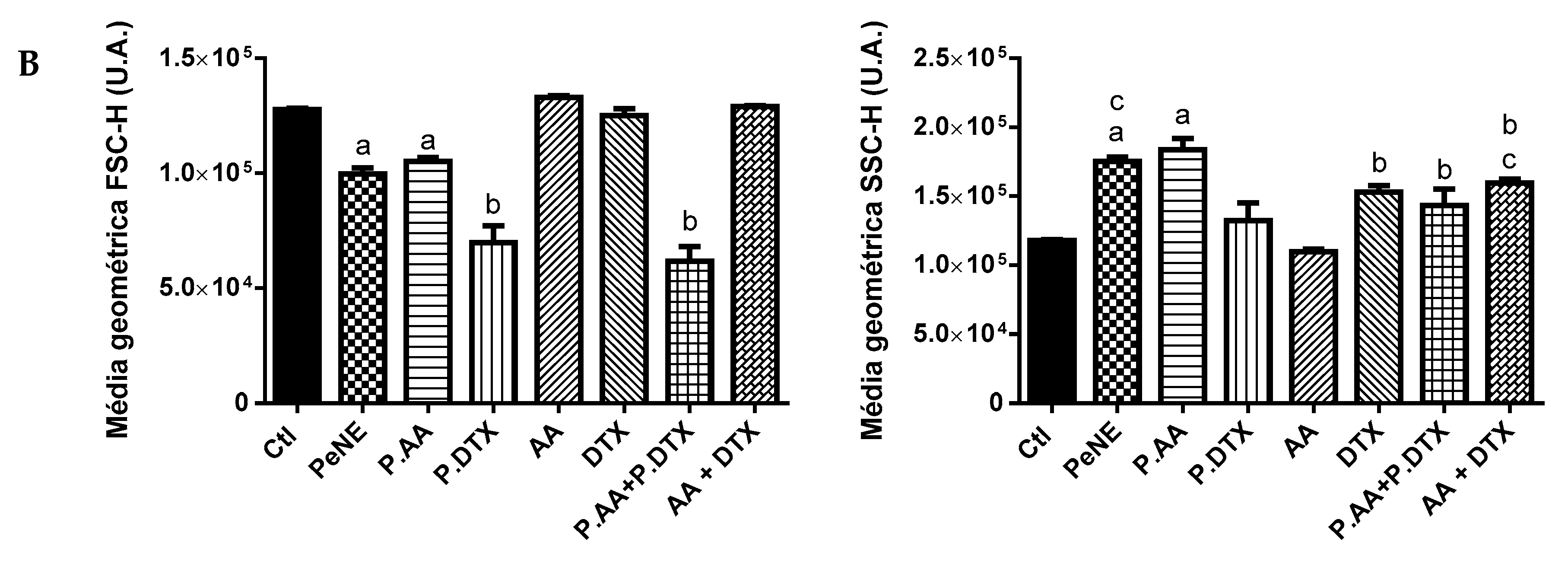
3.4. PAA + PDTX Significantly Impacted 4T1 Key Organelles
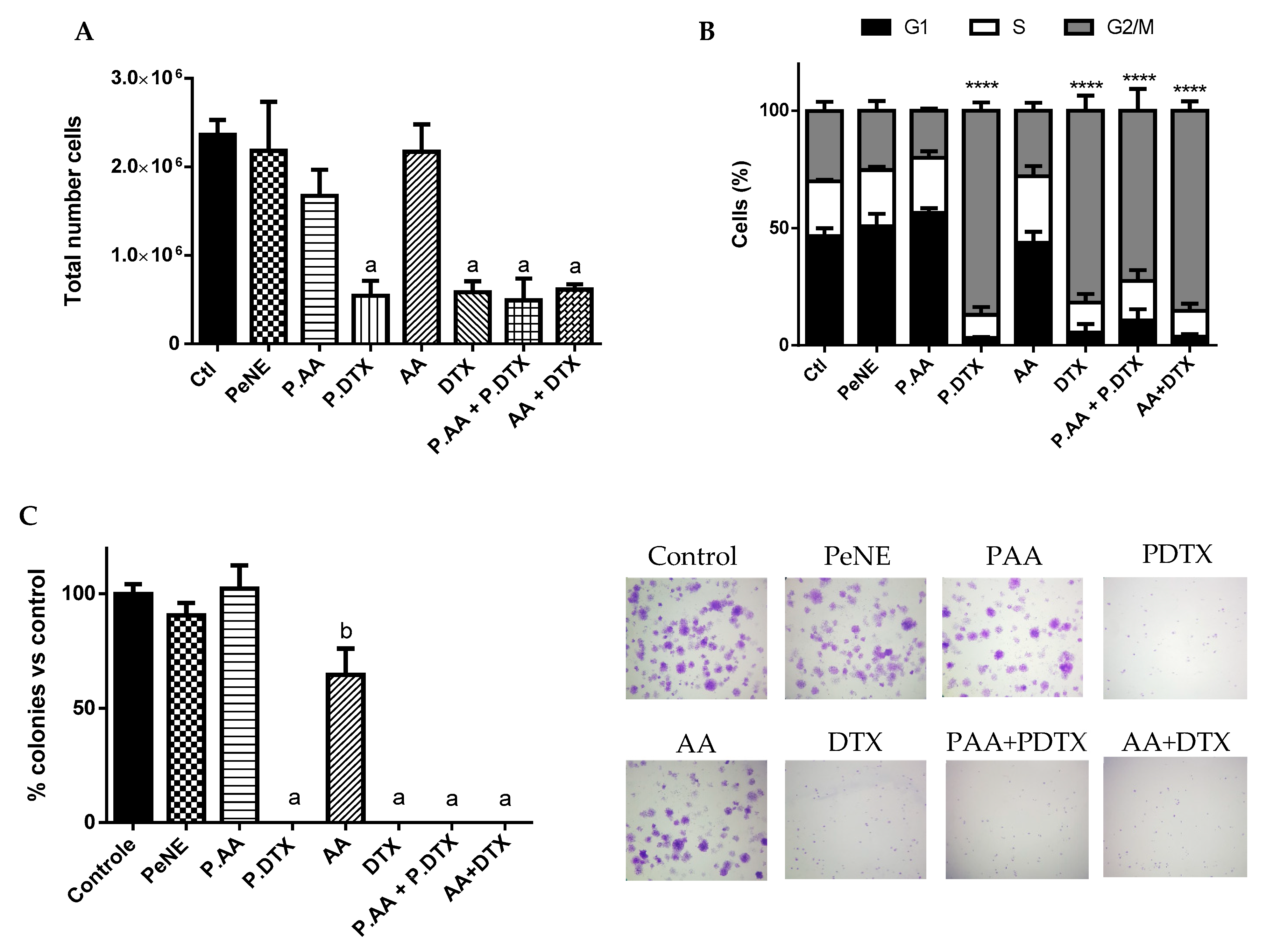
3.5. PAA + PDTX Reduced Cell Proliferation
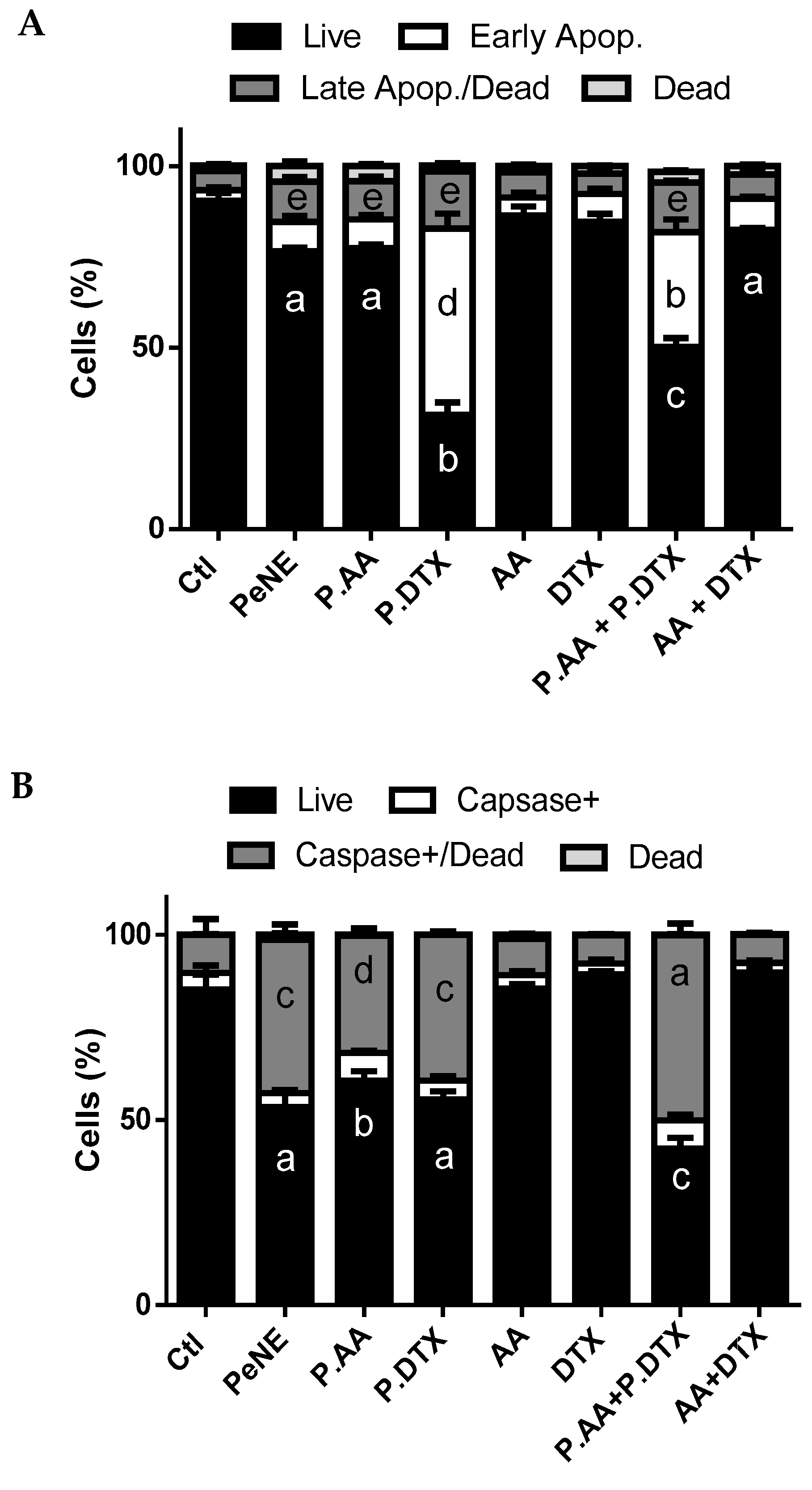
3.6. PAA + PDTX Promoted Phosphatidylserine Exposure and Multicaspase Activation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 13 August 2024).
- Zhou, Z.; Edil, B.H.; Li, M. Combination therapies for cancer: Challenges and opportunities. BMC Med. 2023, 21, 171. [Google Scholar] [CrossRef]
- Xiong, N.; Wu, H.; Yu, Z. Advancements and challenges in triple-negative breast cancer: A comprehensive review of therapeutic and diagnostic strategies. Front. Oncol. 2024, 14, 1405491. [Google Scholar] [CrossRef]
- Onyinyechi, O.; Gantumur, B.; Akala, O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Rinad, M.; Ordóñez-Morán, P.; Allegrucci, C. Challenges for Triple Negative Breast Cancer Treatment: Defeating Heterogeneity and Cancer Stemness. Cancers 2022, 14, 4280. [Google Scholar] [CrossRef]
- Jadia, R.; Scandore, C.; Rai, P. Nanoparticles for Effective Combination Therapy of Cancer. Int. J. Nanotechnol. Nanomed. 2016, 1, 1–27. [Google Scholar]
- Kushwah, V.; Katiyar, S.S.; Dora, C.P.; Agrawal, A.K.; Lamprou, D.A.; Gupta, R.C.; Jain, S. Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater. 2018, 73, 424–436. [Google Scholar] [CrossRef]
- Farha, N.G.; Kasi, A. Docetaxel. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Qin, A.; Wells, L.; Malhotra, B.; Gadgeel, S.; Schneider, B.J.; Ramnath, N.; Rice, J.D.; Kalemkerian, G.P. A Phase II Trial of Pevonedistat and Docetaxel in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2024, 25, 128–134. [Google Scholar] [CrossRef]
- Schultz, D.J.; Wickramasinghe, N.S.; Ivanova, M.M.; Isaacs, S.M.; Dougherty, S.M.; Imbert-Fernandez, Y.; Cunningham, A.R.; Chen, C.; Klinge, C.M. Anacardic acid inhibits estrogen receptor alpha-DNA binding and reduces target gene transcription and breast cancer cell proliferation. Mol. Cancer Ther. 2010, 9, 0978. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, X.; Cai, H.; Zhang, P.; Kong, D.; Ge, X.; Du, M.; Liang, R.; Dong, W. Anticancer Effects of Plant Derived Anacardic Acid on Human Breast Cancer MDA-MB-231 Cells. Am. J. Transl. Res. 2018, 10, 2424–2434. [Google Scholar]
- Tomasiak, P.; Janisiak, J.; Rogińska, D.; Perużyńska, M.; Machaliński, B.; Tarnowski, M. Garcinol and Anacardic Acid, Natural Inhibitors of Histone Acetyltransferases, Inhibit Rhabdomyosarcoma Growth and Proliferation. Molecules 2023, 28, 5292. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Santhosh, M.S.; Kemparaju, K.; Girish, K.S. Emerging Roles of Anacardic Acid and Its Derivatives: A Pharmacological Overview. Basic. Clin. Pharmacol. Toxicol. 2011, 110, 122–132. [Google Scholar] [CrossRef]
- Kushwah, V.; Jain, D.K.; Agrawal, A.K.; Jain, S. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Colloids Surf. B Biointerfaces 2018, 172, 213–223. [Google Scholar] [CrossRef]
- Singh, K.S.; Singh, S.; Wlillard, J.; Singh, R. Drug Delivery Approaches for Breast Cancer. Int. J. Nanomed. 2017, 12, 6205–6218. [Google Scholar] [CrossRef]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed.Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Rocha, M.; Chaves, N.; Bao, S. Nanobiotechnology for Breast Cancer Treatment. Breast Cancer—From Biology to Medicine. In Breast Cancer—From Biology to Medicine; IntechOpen: London, UK, 2017. [Google Scholar]
- Kumar, G.P.; Divya, A. Nanoemulsion Based Targeting in Cancer Therapeutics. Med. Chem. 2015, 5, 272–284. [Google Scholar]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter. 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Preeti, S.; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Sehrawat, R. Nanoemulsion: An emerging novel technology for improving the bioavailability of drugs. Scientific 2023, 25, 1–25. [Google Scholar] [CrossRef]
- Ombredane, A.S.; Araujo, V.H.S.; Borges, C.O.; Costa, P.L.; Landim, M.G.; Pinheiro, A.C.; Szlachetka, I.O.; Benedito, L.E.C.; Espindola, L.S.; Dias, D.J.S.; et al. Nanoemulsion-Based Systems as a Promising Approach for Enhancing the Antitumoral Activity of Pequi Oil (Caryocar Brasilense Cambess.) in Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2020, 58, 101819. [Google Scholar] [CrossRef]
- Ombredane, A.S.; Silva, L.R.A.; Araujo, V.H.; Costa, P.L.; Silva, L.C.; Sampaio, M.C.; Lima, M.C.F.; Veiga Junior, V.F.; Vieira, I.J.C.; Azevedo, R.B.; et al. Pequi oil (Caryocar brasilense Cambess.) nanoemulsion alters cell proliferation and damages key organelles in triple-negative breast cancer cells in vitro. Biomed. Pharmacother. 2022, 153, 113348. [Google Scholar] [CrossRef]
- Miranda-Vilela, A.L.; Pereira, L.C.S.; Gonçalves, C.A.; Grisolia, C.K. Pequi Fruit (Caryocar Brasiliense Camb.) Pulp Oil Reduces Exercise-Induced Inflammatory Markers and Blood Pressure of Male and Female Runners. Nutr. Res. 2009, 29, 850–858. [Google Scholar] [CrossRef]
- Colombo, N.B.R.; Rangel, M.P.; Martins, V.; Hage, M.; Gelain, D.P.; Barbeiro, D.F.; Grisolia, C.K.; Parra, E.R.; Capelozzi, V.L. Caryocar Brasiliense Camb Protects against Genomic and Oxidative Damage in Urethane-Induced Lung Carcinogenesis. Braz. J. Med. Biol. Res. 2015, 48, 852–862. [Google Scholar] [CrossRef]
- Palmeira, S.M.; Silva, P.R.P.; Ferrão, J.S.P.; Ladd, A.A.B.L.; Dagli, M.L.Z.; Grisolia, C.K.; Hernandez-Blazquez, F.J. Chemopreventive Effects of Pequi Oil (Caryocar Brasiliense Camb.) on Preneoplastic Lesions in a Mouse Model of Hepatocarcinogenesis. Eur. J. Cancer Prev. 2016, 25, 299–305. [Google Scholar] [CrossRef]
- Guedes, A.M.M.; de Faria-Machado, A.F. Pequi: A Brazilian fruit with potential uses for the fat industry. OCL 2017, 24, D507. [Google Scholar] [CrossRef]
- Carneiro, C.R.; Alhaji, A.M.; da Silva, C.A.S.; de Sousa, R.C.S.; Monteiro, S.; dos Reis Coimbra, J.S. Potential Challenges of the Extraction of Carotenoids and Fatty Acids from Pequi (Caryocar brasiliense) Oil. Foods 2023, 12, 1907. [Google Scholar] [CrossRef]
- Miranda-Vilela, A.L.; Portilho, F.L.; De Araujo, V.G.B.; Mezzomo, B.P.; De Almeida Santos, M.F.M.; Lacava, Z.G.M. The Protective Effects of Nutritional Antioxidant Therapy on Ehrlich Solid Tumor-Bearing Mice Depend on the Type of Antioxidant Therapy Chosen: Histology, Genotoxicity and Hematology Evaluations. J. Nutr. Biochem. 2011, 22, 1091–1098. [Google Scholar] [CrossRef]
- Miranda-Vilela, A.L.; Peixoto, R.C.; Longo, J.P.; Silva, C.D.O.; Portilho, F.A.; Miranda, K.L.; Sartoratto, P.P.; Báo, S.N.; Azevedo, R.B.; Lacava, Z.G. Dextran-functionalized magnetic fluid mediating magnetohyperthermia combined with preventive antioxidant pequi-oil supplementation: Potential use against cancer. J. Biomed. Nanotechnol. 2013, 9, 1261–1271. [Google Scholar] [CrossRef]
- Miranda-Vilela, A.L.; Grisolia, C.K.; Longo, J.P.F.; Peixoto, R.C.A.; Almeida, M.C.; Barbosa, L.C.P.; Roll, M.M.; Portilho, F.A.; Estevanato, L.L.C.; Bocca, A.L.; et al. Oil Rich in Carotenoids Instead of Vitamins C and E as a Better Option to Reduce Doxorubicin-Induced Damage to Normal Cells of Ehrlich Tumor-Bearing Mice: Hematological, Toxicological and Histopathological Evaluations. J. Nutr. Biochem. 2014, 25, 1161–1176. [Google Scholar] [CrossRef]
- Ombredane, A.S. Investigação do Efeito de Nanoemulsão à Base de óleo de Pequi e de sua Associação com Docetaxel e ácido Anacárdico em células de Câncer de Mama, in vitro e in vivo. Ph.D. Thesis, University of Brasilia, Brasília, Brazil, 2021. [Google Scholar]
- Sahin, C.; Magomedova, L.; Ferreira, T.A.M.; Liu, J.; Tiefenbach, J.; Alves, P.S.; Queiroz, F.J.G.; de Oliveira, A.S.; Bhattacharyya, M.; Grouleff, J.; et al. Phenolic Lipids Derived from Cashew Nut Shell Liquid to Treat Metabolic Diseases. J. Med. Chem. 2022, 65, 1961–1978. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Analysis of combined drug effects: A new look at a very old problem. Trends Pharmacol. Sci. 1983, 4, 450–454. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Kroemer, G. Lysosomal Membrane Permeabilization in Cell Death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef]
- Pasciu, V.; Nieddu, M.; Sotgiu, F.D.; Baralla, E.; Berlinguer, F. An Overview on Assay Methods to Quantify ROS and Enzymatic Antioxidants in Erythrocytes and Spermatozoa of Small Domestic Ruminants. Animals 2023, 13, 2300. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Guerra, M.J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Deshmukh, R.; Rahman, A. Nanoemulsion: Promising Nanocarrier System for Delivery of Herbal Bioactives. J. Drug Deliv. Sci. Technol. 2019, 51, 224–233. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh, D.F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 18, 57. [Google Scholar] [CrossRef]
- Yadav, S.; Gupta, S. Development and in vitro characterization of docetaxel-loaded ligand appended solid fat nanoemulsions for potential use in breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2015, 43, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Jaromin, A.; Piwoni, A.; Mahmud, M.; Sarisozen, C.; Torchilin, V.; Gubernator, J. A Triple Co-Delivery Liposomal Carrier That Enhances Apoptosis via an Intrinsic Pathway in Melanoma Cells. Cancers 2019, 11, 1982. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. General Aspects of Nanoemulsions and Their Formulation. In Nanoemulsions: Formulation, Applications, and Characterization; Elsevier: Amsterdam, The Netherlands, 2018; Volume 43, pp. 3–20. [Google Scholar]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Wong, P.T.T. Raman Spectroscopy of Thermotropic and High-Pressure Phases of Aqueous Phospholipid Dispersions. Annu. Rev. Biophys. Bioeng. 1984, 13, 1–24. [Google Scholar] [CrossRef]
- Genova, J.; Petrov, M.; Bivas, I.; Rafailov, P.; Naradikian, H.; Katranchev, B. Fourier-Transform Infrared and Raman Characterization of Bilayer Membranes of the Phospholipid SOPC and Its Mixtures with Cholesterol. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 85–93. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Ducey, M.W.; Pemberton, J.E. Quantitative Correlation of Raman Spectral Indicators in Determining Conformational Order in Alkyl Chains. J. Phys. Chem. A 2002, 106, 6991–6998. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Hejazi, E.; Nasrollahzadeh, J.; Fatemi, R.; Mohamadi, L.B.Y.; Saliminejad, K.; Amiri, Z.; Kimiagar, M.; Houshyari, M.; Tavakoli, M.; Idali, F. Effects of Combined Soy Isoflavone Extract and Docetaxel Treatment on Murine 4T1 Breast Tumor Model. Avicenna J. Med. Biotechnol. 2015, 7, 16–21. [Google Scholar]
- Fennelly, C.; Amaravadi, R.K. Lysosomal Biology in Cancer. Methods Mol. Biol. 2017, 1594, 293–308. [Google Scholar] [PubMed]
- Towers, C.G.; Wodetzki, D.; Thorburn, A. Autophagy and cancer: Modulation of cell death pathways and cancer cell adaptations. J. Cell Biol. 2020, 219, e201909033. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Wong, I.K.; Sun, X.; Chen, Y.; Wang, L.; Yang, L.; Lu, L.; Shen, H.; Huang, D. Docetaxel Enhances Lysosomal Function through TFEB Activation. Cell Death Dis. 2018, 9, 6. [Google Scholar] [CrossRef]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomes as Mediators of Drug Resistance in Cancer. Drug Resist. Updates 2016, 24, 23–33. [Google Scholar] [CrossRef]
- Repnik, U.; Česen, M.H.; Turk, B. Lysosomal membrane permeabilization in cell death: Concepts and challenges. Mitochondrion 2014, 19, 49–57. [Google Scholar] [CrossRef]
- Imran, M.; Saleem, S.; Chaudhuri, A.; Ali, J.; Baboota, S. Docetaxel: An update on its molecular mechanisms, therapeutic trajectory and nanotechnology in the treatment of breast, lung and prostate cancer. J. Drug Deliv. Sci. Technol. 2020, 20, 101959. [Google Scholar] [CrossRef]
- Maroufi, N.F.; Vahedian, V.; Mazrakhondi, S.A.M.; Kooti, W.; Khiavy, H.A.; Bazzaz, R.; Ramezani, F.; Pirouzpanah, S.M.; Ghorbani, S.; Akbarzadeh, M.; et al. Sensitization of MDA-MBA231 breast cancer cell to docetaxel by myricetin loaded into biocompatible lipid nanoparticles via sub-G1 cell cycle arrest mechanism. Naunyn. Schmiedebergs Arch. Pharmacol. 2020, 393, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, J.R.; López-Bañuelos, L.; Vega, L. Anacardic 6-pentadecyl salicylic acid induces apoptosis in breast cancer tumor cells, immunostimulation in the host and decreases blood toxic effects of taxol in an animal model. Toxicol. Appl. Pharmacol. 2021, 410, 115359. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Diao, L.; Chen, F.; Shen, A.; Wang, S.; Jin, H.; Hu, Y. pH-sensitive nanoparticles codelivering docetaxel and dihydroartemisinin effectively treat breast cancer by enhancing reactive oxidative species-mediated mitochondrial apoptosis. Mol. Pharm. 2020, 18, 74–86. [Google Scholar] [CrossRef] [PubMed]





| HD (nm) | PdI | Zeta Potential (mv) | pH | |
|---|---|---|---|---|
| PeNE | 164.8 ± 6.1 | 0.269 ± 0.015 | −19.2 ± 0.9 | 7 |
| PAA | 164.8 ± 0.7 | 0.272 ± 0.019 | −23.7 ± 0.4 | 7 |
| PDTX | 146.6 ± 3.4 | 0.257 ± 0.030 | −20.3 ± 1.6 | 7 |
| 4T1 | NIH3T3 | ||||||
|---|---|---|---|---|---|---|---|
| [Pequi Oil] µg/mL | [AA] µg/mL | [DTX] µg/mL | 24 h | 48 h | 24 h | 48 h | |
| Control | - | - | - | 100.0 ± 5.2 | 100.0 ± 19.5 | 100.0 ± 7.5 | 100.0 ± 12.6 |
| PeNE | 135 | - | - | 90.2 ± 12.8 | 91.9 ± 17.1 | 97.4 ± 7.2 | 105.8 ± 15.1 |
| 180 | - | - | 75.1 ± 10.1a | 68.7 ± 10.5 a | 99.1 ± 10.0 | 77.7 ± 9.4 | |
| PAA | 135 | 7 | - | 79.4 ± 9.3 a,b | 62.4 ± 12.2 a | 97.8 ± 8.3 | 97.4 ± 9.4 |
| 180 | 10 | - | 66.8 ± 5.9 b,d | 40.0 ± 8.3 b | 103.5 ± 13.8 | 158.2 ± 21.3 a | |
| PDTX | 135 | - | 12 | 57.1 ± 9.6 c,d | 40.7 ± 6.2 b | 73.6 ± 9.9 a | 28.7 ± 5.5 b |
| 180 | - | 16 | 50.7 ± 6.0 c,d | 36.8 ± 5.4 b | 67.7 ± 13.0 a | 16.6 ± 5.6 b | |
| AA | - | 7 | - | 100.5 ± 11.4 | 115.7 ± 21.2 | 100.4 ± 11.5 | 80.4 ± 18.7 |
| - | 10 | - | 107.7 ± 7.2 | 187.5 ± 23.0 d | 94.5 ± 10.5 | 84.9 ± 16.4 | |
| DTX | - | - | 12 | 74.2 ± 6.0 a,b | 73.6 ± 6.8 a | 68.1 ± 6.9 a | 32.3 ± 5.1 b |
| - | - | 16 | 72.9 ± 6.8 a,b | 69.0 ± 10.3 a | 64.3 ± 8.2 a | 42.9 ± 14.2 b | |
| PAA + PDTX | 135 | 7 | 12 | 45.8 ± 6.6 c | 30.3 ± 8.1 b,c | 66.5 ± 4.8 a | 18.7 ± 4.2 b |
| 135 | 7 | 12 | 39.3 ± 6.6 c | 25.9 ± 5.7 b,c | 59.2 ± 15.9 a | 14.6 ± 2.8 b | |
| 180 | 10 | 16 | 40.2 ± 5.9 c,e | 17.9 ± 3.8 c | 75.8 ± 18.9 a | 16.7 ± 3.1 b | |
| 180 | 10 | 16 | 31.4 ± 6.0 e | 12.7 ± 2.6 c | 41.7 ± 6.9 b | 14.4 ± 3.5 b | |
| AA + DTX | - | 7 | 12 | 76.6 ± 6.8 a,b | 81.8 ± 8.7 | 57.5 ± 7.5 a | 45.3 ± 23.1 b,c |
| - | 7 | 12 | 69.8 ± 10.3 b | 72.1 ± 8.0 a | 59.3 ± 6.8 a | 59.4 ± 24.2 c | |
| - | 10 | 16 | 76.3 ± 7.3 a,b | 77.7 ± 9.4 a | 59.0 ± 13.4 a | 42.9 ± 21.7 b,c | |
| - | 10 | 16 | 65.4 ± 8.9 b | 61.0 ± 4.3 a | 54.8 ± 4.7 a | 48.5 ± 20.0 b,c | |
| Fa * | DRI ** PeNE | DRI AA | DRI DTX | DRI PAA | DRI PDTX | CI *** Values | Effect | |
|---|---|---|---|---|---|---|---|---|
| P.AA | 0.52 | 1.82 | 2.71 | - | - | - | 0.91361 | Synergism |
| PDTX | 0.67 | 2.62 | - | 3.84 | - | - | 0.64067 | Synergism |
| PAA + PDTX | 0.68 | - | - | - | 2.13 | 1.90 | 0.99141 | Additive |
| AA + DTX | 0.34 | - | 1.17 | 0.73 | - | - | 2.57225 | Antagonism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ombredane, A.S.; Martins, N.O.; de Souza, G.M.V.; Araujo, V.H.S.; Szlachetka, Í.O.; da Silva, S.W.; da Rocha, M.C.O.; Oliveira, A.S.d.; Holanda, C.A.; Romeiro, L.A.S.; et al. Combinatory Effect of Pequi Oil (Caryocar brasiliense)-Based Nanoemulsions Associated to Docetaxel and Anacardic Acid (Anacardium occidentale) in Triple-Negative Breast Cancer Cells In Vitro. Pharmaceutics 2024, 16, 1170. https://doi.org/10.3390/pharmaceutics16091170
Ombredane AS, Martins NO, de Souza GMV, Araujo VHS, Szlachetka ÍO, da Silva SW, da Rocha MCO, Oliveira ASd, Holanda CA, Romeiro LAS, et al. Combinatory Effect of Pequi Oil (Caryocar brasiliense)-Based Nanoemulsions Associated to Docetaxel and Anacardic Acid (Anacardium occidentale) in Triple-Negative Breast Cancer Cells In Vitro. Pharmaceutics. 2024; 16(9):1170. https://doi.org/10.3390/pharmaceutics16091170
Chicago/Turabian StyleOmbredane, Alicia Simalie, Natália Ornelas Martins, Gabriela Mara Vieira de Souza, Victor Hugo Sousa Araujo, Ísis O. Szlachetka, Sebastião William da Silva, Márcia Cristina Oliveira da Rocha, Andressa Souza de Oliveira, Cleonice Andrade Holanda, Luiz Antonio Soares Romeiro, and et al. 2024. "Combinatory Effect of Pequi Oil (Caryocar brasiliense)-Based Nanoemulsions Associated to Docetaxel and Anacardic Acid (Anacardium occidentale) in Triple-Negative Breast Cancer Cells In Vitro" Pharmaceutics 16, no. 9: 1170. https://doi.org/10.3390/pharmaceutics16091170






