A Descriptive Review on the Potential Use of Diatom Biosilica as a Powerful Functional Biomaterial: A Natural Drug Delivery System
Abstract
1. Introduction
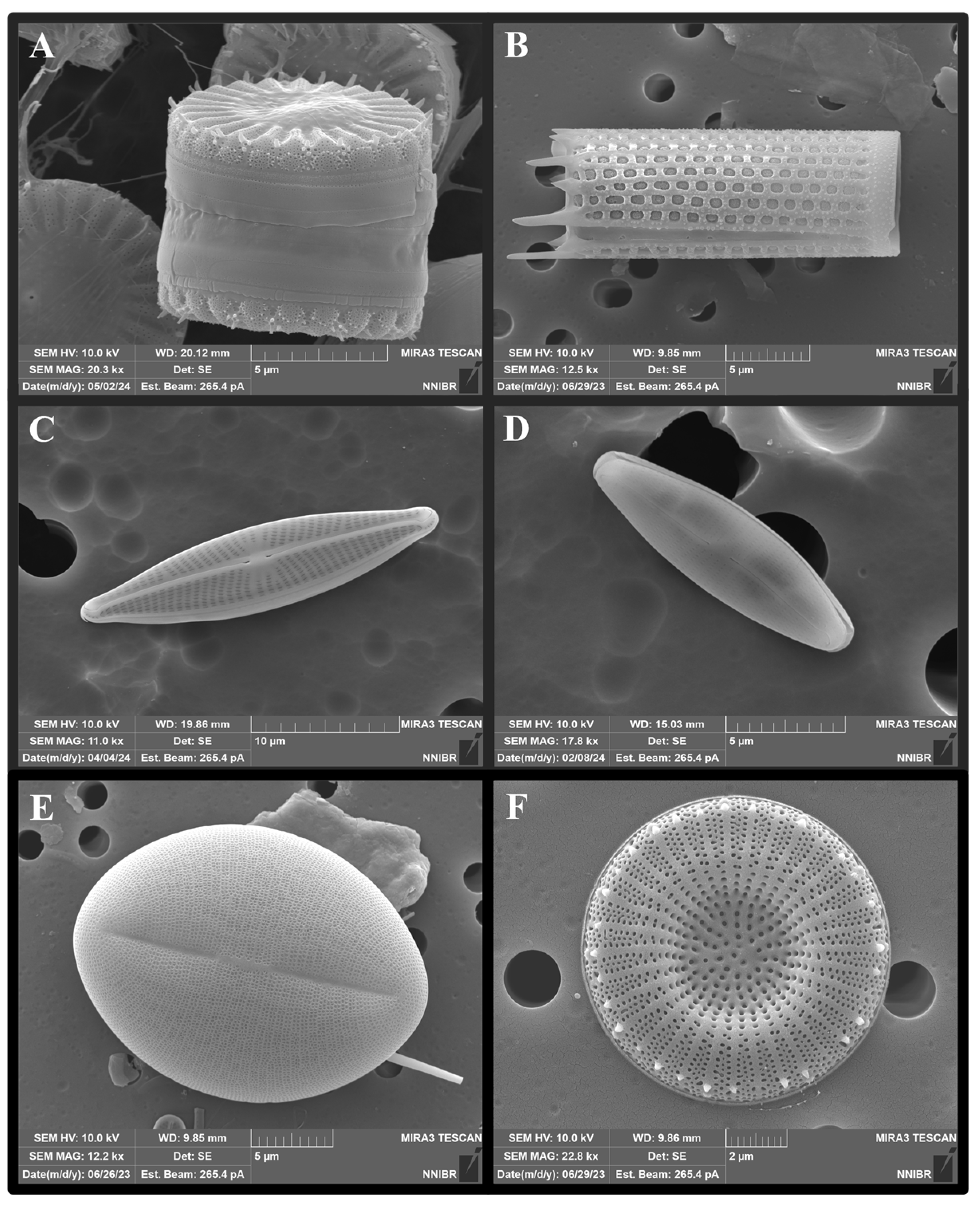
2. Characteristics of DB
2.1. The Mechanism of DB Synthesis
2.2. The DB Structure
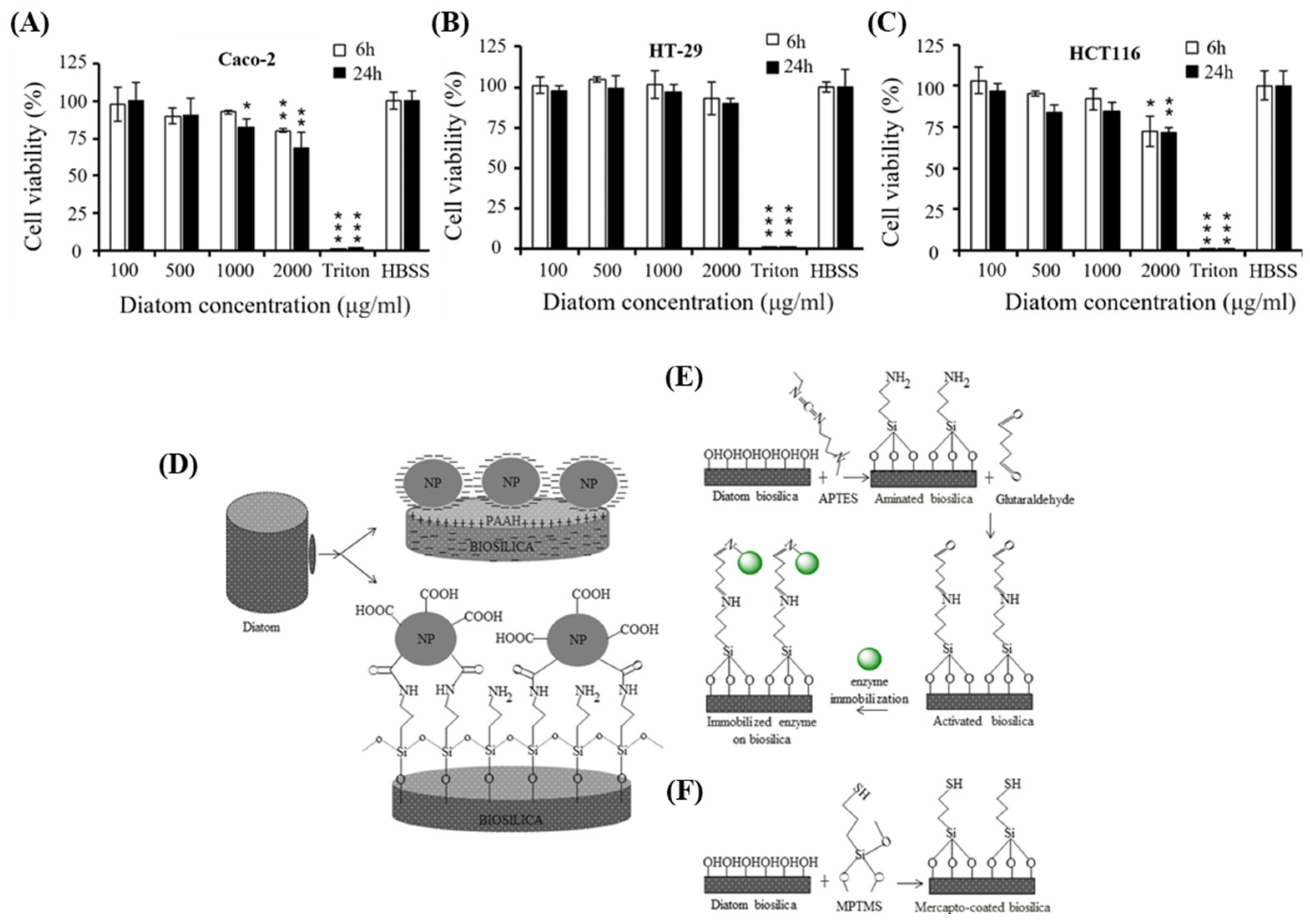
2.3. Biocompatibility of DB
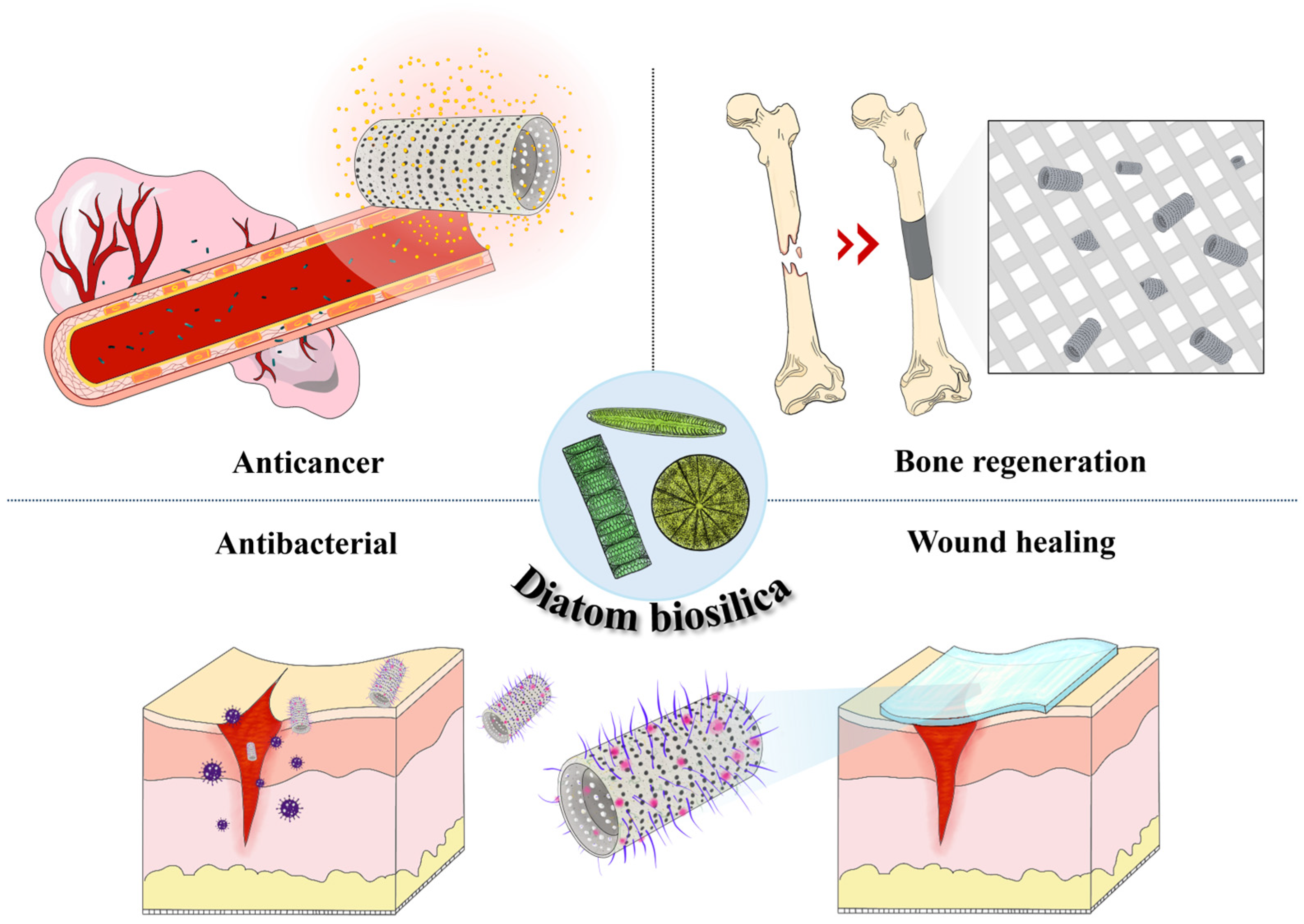
3. DB-Based Drug Delivery System
3.1. Anticancer Drug Therapy
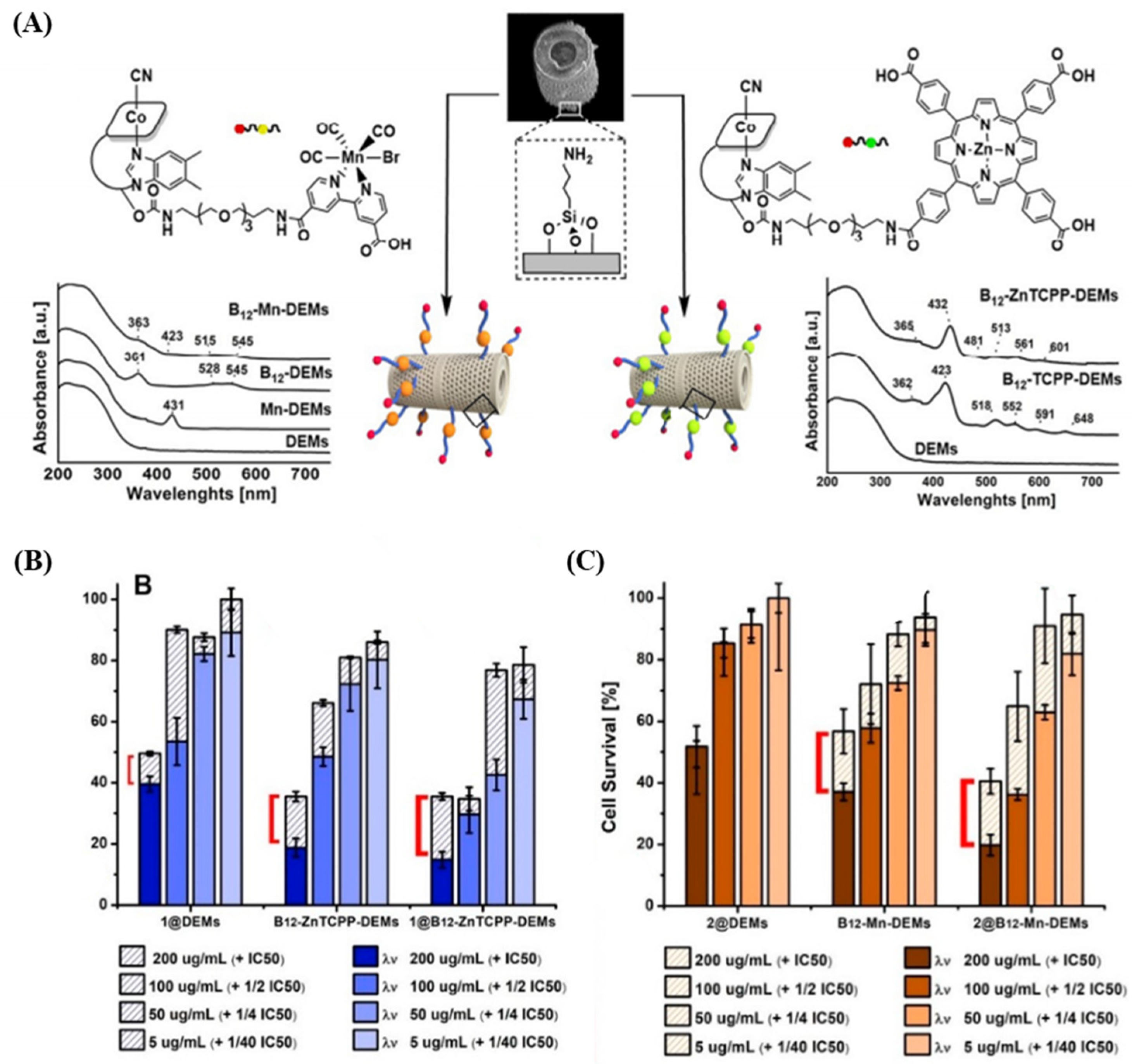
3.2. Anticancer DDS through DB Surface Functionalization
3.3. Anti-Inflammatory and Antibiotic Therapy
3.4. Bone Regeneration
3.5. Wound Healing
4. Secondary DDS: The Use of Lipid Nanoparticles (LNP) and Hydrogels
4.1. Electrostatic Interaction (e.g., Cationic LNPs and Fe3+/Dopamine Complex)
4.2. Secondary DDS (with Hydrogel)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kociolek, J.P. A worldwide listing and biogeography of freshwater diatom genera: A phylogenetic perspective. Diatom Res. 2018, 33, 509–534. [Google Scholar] [CrossRef]
- Wang, J.; Soininen, J.; Heino, J. Ecological Indicators for Aquatic Biodiversity, Ecosystem Functions, Human Activities and Climate Change. Ecol. Indic. 2021, 132, 108250. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, J. Comparative analysis of organelle genomes provides conflicting evidence between morphological similarity and phylogenetic relationship in diatoms. Front. Mar. Sci. 2024, 10, 1283893. [Google Scholar] [CrossRef]
- Li, F.; Beardall, J.; Gao, K. Diatom performance in a future ocean: Interactions between nitrogen limitation, temperature, and CO2-induced seawater acidification. ICES J. Mar. Sci. 2018, 75, 1451–1464. [Google Scholar] [CrossRef]
- Wiemer, G.; Dziadek, R.; Kopf, A. The Enigmatic Consolidation of Diatomaceous Sediment. Mar. Geol. 2017, 385, 173–184. [Google Scholar] [CrossRef]
- Zuluaga-Astudillo, D.; Ruge, J.C.; Camacho-Tauta, J.; Reyes-Ortiz, O.; Caicedo-Hormaza, B. Diatomaceous Soils and Advances in Geotechnical Engineering—Part, I. Appl. Sci. 2023, 13, 549. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms Green Nanotechnology for Biosilica-Based Drug Delivery Systems. Pharmaceutics 2018, 10, 242. [Google Scholar] [CrossRef]
- Ghobara, M.; El-Sheekh, M.; Hamed, A.F.; Abdelhamid, M.A.A.; Pack, S.P. Diatom Nanostructured Biosilica. In Value-Added Products from Algae: Phycochemical Production and Applications; Springer International Publishing: Cham, Switzerland, 2023; pp. 461–492. [Google Scholar]
- Rabiee, N.; Khatami, M.; Jamalipour Soufi, G.; Fatahi, Y.; Iravani, S.; Varma, R.S. Diatoms with Invaluable Applications in Nanotechnology, Biotechnology, and Biomedicine: Recent Advances. ACS Biomater. Sci. Eng. 2021, 7, 3053–3068. [Google Scholar] [CrossRef]
- De Tommasi, E.; De Luca, A.C. Diatom Biosilica in Plasmonics: Applications in Sensing, Diagnostics and Therapeutics. Biomed. Opt. Express 2022, 13, 3080. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent Progress in Synthesis, Functionalization, Biocompatibility, and Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898. [Google Scholar] [CrossRef]
- Panwar, V.; Dutta, T. Diatom Biogenic Silica as a Felicitous Platform for Biochemical Engineering: Expanding Frontiers. ACS Appl. Bio Mater. 2019, 2, 2295–2316. [Google Scholar] [CrossRef] [PubMed]
- Dhanker, R.; Singh, P.; Sharma, D.; Tyagi, P.; Kumar, M.; Singh, R.; Prakash, S. Diatom Silica a Potential Tool as Biosensors and for Biomedical Field. In Insights into the World of Diatoms: From Essentials to Applications; Springer Nature: Singapore, 2023; pp. 175–193. [Google Scholar]
- Lim, H.; Seo, Y.; Kwon, D.; Kang, S.; Yu, J.; Park, H.; Lee, S.D.; Lee, T. Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier. Pharmaceutics 2023, 15, 2434. [Google Scholar] [CrossRef] [PubMed]
- Gnanamoorthy, P.; Anandhan, S.; Prabu, V.A. Natural Nanoporous Silica Frustules from Marine Diatom as a Biocarrier for Drug Delivery. J. Porous Mater. 2014, 21, 789–796. [Google Scholar] [CrossRef]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A Review of Drug Delivery Systems Based on Nanotechnology and Green Chemistry: Green Nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, R.; Rathee, J.; Salunke, D.B.; Mehta, S.K. Green Nanotechnology-Driven Drug Delivery Assemblies. ACS Omega 2019, 4, 8804–8815. [Google Scholar] [CrossRef] [PubMed]
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.-Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature Engineered Diatom Biosilica as Drug Delivery Systems. J. Control. Release 2018, 281, 70–83. [Google Scholar] [CrossRef]
- Pu, Y.; Wei, M.; Witkowski, A.; Krzywda, M.; Wang, Y.; Li, W. A Hybrid Biomaterial of Biosilica and C-Phycocyanin for Enhanced Photodynamic Effect towards Tumor Cells. Biochem. Biophys. Res. Commun. 2020, 533, 573–579. [Google Scholar] [CrossRef]
- Ding, Y.; Mu, Y.; Hu, Y.; Liu, J.; Su, C.; Sun, X.; Chen, X.; Jia, N.; Feng, C. Zinc-Mineralized Diatom Biosilica/Hydroxybutyl Chitosan Composite Hydrogel for Diabetic Chronic Wound Healing. J. Colloid Interface Sci. 2024, 656, 1–14. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, H.A.; Choi, J.W.; Kim, M.Y.; Go, G.M. Compositional characteristics of the Microalga Melosira nummuloides Mass-cultured using Jeju lava seawater. Korean J. Fish. Aquat. Sci. 2022, 55, 91–101. [Google Scholar]
- Saoud, H.A.A.L.; Sprynskyy, M.; Pashaei, R.; Kawalec, M.; Pomastowski, P.; Buszewski, B. Diatom Biosilica: Source, Physical-Chemical Characterization, Modification, and Application. J. Sep. Sci. 2022, 45, 3362–3376. [Google Scholar] [CrossRef]
- Delasoie, J.; Zobi, F. Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Mayzel, B.; Aram, L.; Varsano, N.; Wolf, S.G.; Gal, A. Structural evidence for extracellular silica formation by diatoms. Nat. Commun. 2021, 12, 4639. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, F.; Brunner, E. Silicic Acid Uptake and Storage by Diatoms. In The Molecular Life of Diatoms; Springer International Publishing: Cham, Switzerland, 2022; pp. 345–365. [Google Scholar]
- Hildebrand, M. Diatoms, Biomineralization Processes, and Genomics. Chem. Rev. 2008, 108, 4855–4874. [Google Scholar] [CrossRef]
- Oa, T. Silicon Uptake in Diatoms Revisited A Model for Saturable and Nonsaturable Uptake Kinetics and the Role of silicon transporters. Plant Physiol. 2008, 146, 1397–1407. [Google Scholar]
- Reichelt, T.; Bode, T.; Jordan, P.; Brunner, E. Towards the Chemical Analysis of Diatoms Silicon Storage pools: A differential centrifugation-based separation approach. Minerals 2023, 13, 653. [Google Scholar] [CrossRef]
- Kröger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-Specific Polyamines from Diatoms Control Silica Morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Clarson, S.J. Silicification and Biosilicification: Part 4. Effect of Template Size on the Formation of Silica. J. Inorg. Organomet. Polym. 2002, 12, 109–116. [Google Scholar] [CrossRef]
- Van de Meene, A.M.L.; Pickett-Heaps, J.D. Valve Morphogenesis in the Centric Diatom Proboscia Alata Sundstrom. J. Phycol. 2002, 38, 351–363. [Google Scholar] [CrossRef]
- Van De Meene, A.M.L.; Pickett-Heaps, J.D. Valve Morphogenesis in the Centric Diatom Rhizosolenia Setigera (Bacillariophyceae, Centrales) and Its Taxonomic Implications. Eur. J. Phycol. 2004, 39, 93–104. [Google Scholar] [CrossRef]
- Cvjetinovic, J.; Luchkin, S.Y.; Davidovich, N.A.; Bedoshvili, Y.D.; Salimon, A.I.; Korsunsky, A.M.; Gorin, D.A. Characterization of diatom silica exoskeletons using atomic force microscopy: Topography and mechanical properties. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, J.; Wang, X.; Cheng, J.J.; Wen, Z. Selective Adsorption of Pb (II) from Aqueous Solution Using Porous Biosilica Extracted from Marine Diatom Biomass: Properties and Mechanism. Appl. Surf. Sci. 2017, 396, 965–977. [Google Scholar] [CrossRef]
- Vona, D.; Cicco, S.R.; la Forgia, F.M.; Vacca, M.; Porrell, A.; Caggiano, G.; Angelis, M.D.; Gesualdo, L.; Farinola, G.M. All Bio-Based µ-Beads from Microalgae for Probiotics Delivery. Adv. Sustain. Syst. 2024, 2400384. [Google Scholar] [CrossRef]
- Konak, B.M.K.; Bakar, M.E.; Ahan, R.E.; Özyürek, E.U.; Dökmeci, S.; Şeker, U.Ö.Ş. A living material platform for the biomineralization of biosilica. Mater. Today Bio. 2022, 17, 100461. [Google Scholar]
- Min, K.H.; Kim, D.H.; Shin, J.W.; Ki, M.R.; Pack, S.P. Microalgae-derived peptide with dual-functionalities of silica deposition and antimicrobial activity for biosilica-based biomaterial design. Process Biochem. 2024, 146, 204–213. [Google Scholar] [CrossRef]
- Sharma, N.; Simon, D.P.; Diaz-Garza, A.M.; Fantino, E.; Messaabi, A.; Meddeb-Mouelhi, F.; Germain, H.; Desgagné-Penix, I. Diatoms Biotechnology: Various Industrial Applications for a Greener Tomorrow. Front. Mar. Sci. 2021, 8, 636613. [Google Scholar] [CrossRef]
- Mishra, M.; Arukha, A.P.; Bashir, T.; Yadav, D.; Prasad, G.B.K.S. All New Faces of Diatoms: Potential Source of Nanomaterials and Beyond. Front. Microbiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Gristina, R.; Sardella, E.; Ragni, R.; Presti, M.L.; Farinola, G.M. Biosilica from Living Diatoms: Investigations on Biocompatibility of Bare and Chemically Modified Thalassiosira Weissflogii Silica Shells. Bioengineering 2016, 3, 1500223. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Chen, F.; Cai, W. Biodegradable and Renal Clearable Inorganic Nanoparticles. Adv. Sci. 2016, 3, 1500223. [Google Scholar] [CrossRef]
- Maher, S.; Kumeria, T.; Wang, Y.; Kaur, G.; Fathalla, D.; Fetih, G.; Santos, A.; Habib, F.; Evdokiou, A.; Losic, D. From The Mine to Cancer Therapy: Natural and Biodegradable Theranostic Silicon Nanocarriers from Diatoms for Sustained Delivery of Chemotherapeutics. Adv. Healthc. Mater. 2016, 5, 2667–2678. [Google Scholar] [CrossRef]
- Zhai, W.; He, C.; Wu, L.; Zhou, Y.; Chen, H.; Chang, J.; Zhang, H. Degradation of Hollow Mesoporous Silica Nanoparticles in Human Umbilical Vein Endothelial Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 B, 1397–1403. [Google Scholar] [CrossRef]
- Anglin, E.J.; Cheng, L.; Freeman, W.R.; Sailor, M.J. Porous Silicon in Drug Delivery Devices and Materials. Adv. Drug Deliv. Rev. 2008, 60, 1266–1277. [Google Scholar] [CrossRef]
- Maher, S.; Kumeria, T.; Aw, M.S.; Losic, D. Diatom Silica for Biomedical Applications: Recent Progress and Advances. Adv. Healthc. Mater. 2018, 7, e1800552. [Google Scholar] [CrossRef]
- Chianese, C.T.G.; Terracciano, M.; de Stefano, L.; Rea, I. Nanostructured Biosilica of Diatoms: From Water World to Biomedical Applications. Appl. Sci. 2020, 10, 6811. [Google Scholar] [CrossRef]
- Zhang, H.; Shahbazi, M.-A.; Mäkilä, E.M.; da Silva, T.H.; Reis, R.L.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Diatom Silica Microparticles for Sustained Release and Permeation Enhancement Following Oral Delivery of Prednisone and Mesalamine. Biomaterials 2013, 34, 9210–9219. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, e1705328. [Google Scholar] [CrossRef] [PubMed]
- Irrechukwu, O.; Yeager, R.; David, R.; Ekert, J.; Saravanakumar, A.; Choi, C.K. Applications of Microphysiological Systems to Disease Models in the Biopharmaceutical Industry: Opportunities and Challenges. ALTEX-Altern. Anim. Exp. 2023, 40, 485–518. [Google Scholar] [CrossRef]
- Sahlgren, C.; Meinander, A.; Zhang, H.; Cheng, F.; Preis, M.; Xu, C.; Salminen, T.A.; Toivola, D.; Abankwa, D.; Rosling, A.; et al. Tailored Approaches in Drug Development and Diagnostics: From Molecular Design to Biological Model Systems. Adv. Healthc. Mater. 2017, 6, 1700258. [Google Scholar] [CrossRef] [PubMed]
- Zitter, R.; Chugh, R.M.; Saha, S. Patient Derived Ex-Vivo Cancer Models in Drug Development, Personalized Medicine, and Radiotherapy. Cancers 2022, 14, 3006. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, C.; Miranda, B.; Chianese, G.; De Stefano, L.; Forestiere, C.; Pirozzi, M.; Rea, I. Design of gelatin-capped plasmonic-diatomite nanoparticles with enhanced galunisertib loading capacity for drug delivery applications. Int. J. Mol. Sci. 2021, 22, 10755. [Google Scholar] [CrossRef]
- Thabet, Y.; Klingmann, V.; Breitkreutz, J. Drug Formulations: Standards and Novel Strategies for Drug Administration in Pediatrics. J. Clin. Pharmacol. 2018, 58, 26–35. [Google Scholar] [CrossRef]
- Glassman, P.M.; Muzykantov, V.R. Pharmacokinetic and Pharmacodynamic Properties of Drug Delivery Systems. J. Pharmacol. Exp. Ther. 2019, 370, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, R.; Musumeci, T.; Carbone, C.; Pignatello, R. Nanotechnologies for Intranasal Drug Delivery: An Update of Literature. Pharm. Dev. Technol. 2021, 26, 824–845. [Google Scholar] [CrossRef] [PubMed]
- Adeosun, S.O.; Ilomuanya, M.O.; Gbenebor, O.P.; Dada, M.O.; Odili, C.C. Biomaterials for Drug Delivery: Sources, Classification, Synthesis, Processing, and Applications. In Advanced Functional Materials; IntechOpen: London, UK, 2020; pp. 141–167. [Google Scholar]
- Hakim, L.K.; Yazdanian, M.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Khayatan, D.; Seifalian, A.; Ranjbar, R.; Yazdanian, A. Biocompatible and Biomaterials Application in Drug Delivery System in Oral Cavity. Evid. Based Complement. Altern. Med. 2021, 2021, 9011226. [Google Scholar] [CrossRef] [PubMed]
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Khan, M.I.; Almasry, W.A.; Kang, I.-K. Biocompatible Polymers and Their Potential Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef]
- Phogat, S.; Saxena, A.; Kapoor, N.; Aggarwal, C.; Tiwari, A. Diatom Mediated Smart Drug Delivery System. J. Drug Deliv. Sci. Technol. 2021, 63, 102433. [Google Scholar] [CrossRef]
- Le, T.D.H. Hydrophobic and hydrophilic drug loading capacity of micro diatom frustule from diatomite. J. Tech. Educ. Sci. 2021, 16, 35–40. [Google Scholar]
- Li, M.; Wu, J.; Lin, D.; Yang, J.; Jiao, N.; Wang, Y.; Liu, L. A Diatom-Based Biohybrid Microrobot with a High Drug-Loading Capacity and PH-Sensitive Drug Release for Target Therapy. Acta Biomater. 2022, 154, 443–453. [Google Scholar] [CrossRef]
- Delalat, B.; Sheppard, V.C.; Rasi Ghaemi, S.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted Drug Delivery Using Genetically Engineered Diatom Biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef]
- Delasoie, J.; Schiel, P.; Vojnovic, S.; Nikodinovic-Runic, J.; Zobi, F. Photoactivatable Surface-Functionalized Diatom Microalgae for Colorectal Cancer Targeted Delivery and Enhanced Cytotoxicity of Anticancer Complexes. Pharmaceutics 2020, 12, 480. [Google Scholar] [CrossRef]
- Kumeria, T.; Bariana, M.; Altalhi, T.; Kurkuri, M. Graphene Oxide Decorated Diatom Silica Particles as New Nano-Hybrids: Towards Smart Natural Drug Microcarriers. J. Mater. Chem. B. 2013, 1, 6302–6311. [Google Scholar] [CrossRef]
- Bariana, M.; Aw, M.S.; Kurkuri, M.; Losic, D. Tuning Drug Loading and Release Properties of Diatom Silica Microparticles by Surface Modifications. Int. J. Pharm. 2013, 443, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Vona, D.; Leone, G.; Ragni, R.; Palumbo, F.; Evidente, A.; Vurro, M.; Farinola, G.M.; Cicco, S.R. Diatoms Biosilica as Efficient Drug-Delivery System. MRS Adv. 2016, 1, 3825–3830. [Google Scholar] [CrossRef]
- Kabir, A.; Nazeer, N.; Bissessur, R.; Ahmed, M. Diatoms Embedded, Self-Assembled Carriers for Dual Delivery of Chemotherapeutics in Cancer Cell Lines. Int. J. Pharm. 2020, 573, 118887. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Rossier, J.; Haeni, L.; Rothen-Rutishauser, B.; Zobi, F. Slow-Targeted Release of a Ruthenium Anticancer Agent from Vitamin B12 Functionalized Marine Diatom Microalgae. Dalton Trans. 2018, 48, 17221–17232. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-Mensah, J.; Losic, D. Porous Silica Microshells from Diatoms as Biocarrier for Drug Delivery Applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
- Saxena, A.; Dutta, A.; Kapoor, N.; Kumar, A.; Tiwari, A. Envisaging Marine Diatom Thalassiosira Weissflogii as a SMART Drug Delivery System for Insoluble Drugs. J. Drug Deliv. Sci. Technol. 2022, 68, 102983. [Google Scholar] [CrossRef]
- Vona, D.; Flemma, A.; Piccapane, F.; Cotugno, P.; Cicco, S.R.; Armenise, V.; Vicente-Garcia, C.; Giangregorio, M.M.; Procino, G.; Ragni, R. Drug Delivery through Epidermal Tissue Cells by Functionalized Biosilica from Diatom Microalgae. Mar. Drugs 2023, 21, 438. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Addai-Mensah, J.; Losic, D. Silica Microcapsules from Diatoms as New Carrier for Delivery of Therapeutics. Nanomedicine 2011, 6, 1159–1173. [Google Scholar] [CrossRef]
- Mancera-Andrade, E.I.; Parsaeimehr, A.; Ruiz-Ruiz, F.; Rorrer, G.L.; González-Valdez, J.; Iqbal, H.M.N.; Parra-Saldivar, R. Isorhamnetin Encapsulation into Biogenic Silica from Cyclotella sp. Using a Microfluidic Device for Drug Delivery Applications. Biocatal. Agric. Biotechnol. 2019, 19, 101175. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Bin Jumah, M.N.; Othman, S.I.; Alruhaimi, R.S.; Al-Khalawi, N.; Salama, Y.F.; Allam, A.A.; Abukhadra, M.R. Synthesis of Chitosan/Diatomite Composite as an Advanced Delivery System for Ibuprofen Drug; Equilibrium Studies and the Release Profile. Acs Omega 2021, 6, 13406–13416. [Google Scholar] [CrossRef]
- Le, T.D.H.; Bonani, W.; Speranza, G.; Sglavo, V.; Ceccato, R.; Maniglio, D.; Motta, A.; Migliaresi, C. Processing and Characterization of Diatom Nanoparticles and Microparticles as Potential Source of Silicon for Bone Tissue Engineering. Mater. Sci. Eng. C 2016, 59, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, S.; Shen, K.; Song, Y.; Zhang, J.; Su, W.; Yang, X. Study on the Hemostasis Characteristics of Biomaterial Frustules Obtained from Diatom Navicula australoshetlandica sp. Materials 2021, 14, 3752. [Google Scholar] [CrossRef]
- Losic, D.; Yu, Y.; Aw, M.S.; Simovic, S.; Thierry, B.; Addai-Mensah, J. Surface functionalisation of diatoms with dopamine modified iron-oxide nanoparticles: Toward magnetically guided drug microcarriers with biologically derived morphologies. Chem. Commun. 2010, 46, 6323–6325. [Google Scholar] [CrossRef]
- Bariana, M.; Aw, M.S.; Losic, D. Tailoring Morphological and Interfacial Properties of Diatom Silica Microparticles for Drug Delivery Applications. Adv. Powder Technol. 2013, 24, 757–763. [Google Scholar] [CrossRef]
- Milović, M.; Simović, S.; Lošić, D.; Dashevskiy, A.; Ibrić, S. Solid Self-Emulsifying Phospholipid Suspension (SSEPS) with Diatom as a Drug Carrier. Eur. J. Pharm. Sci. 2014, 63, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Uthappa, U.T.; Sriram, G.; Brahmkhatri, V.; Kigga, M. Xerogel Modified Diatomaceous Earth Microparticles for Controlled Drug Release Studies. New J. Chem. 2018, 14, 11964–11971. [Google Scholar] [CrossRef]
- Serhan, M.; Jackemeyer, D.; Long, M.; Sprowls, M.; Perez, I.D.; Maret, W.; Chen, F.; Tao, N.; Forzani, E. Total iron measurement in human serum with a novel smartphone-based assay. J. Transl. Eng. Health Med. 2020, 8, 2800309. [Google Scholar] [CrossRef]
- Javalkote, V.S.; Pandey, A.P.; Puranik, P.R.; Deshmukh, P.K. Magnetically Responsive Siliceous Frustules for Efficient Chemotherapy. Mater. Sci. Eng. C 2015, 50, 107–116. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Leone, G.; De Giglio, E.; Bonifacio, M.A.; Cometa, S.; Fiore, S.; Palumbo, F.; Ragni, R.; Farinola, G.M. In Vivo Functionalization of Diatom Biosilica with Sodium Alendronate as Osteoactive Material. Mater. Sci. Eng. C 2019, 104, 109897. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Giglio, E.D.; Cometa, S.; Mattioli-, M.; Palumbo, F.; Ragni, R.; Farinola, G.M. Chemically Modified Diatoms Biosilica for Bone Cell Growth with Combined Drug-Delivery and Antioxidant Properties. Chempluschem 2015, 80, 1104–1112. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Radakovic, N.; Pavic, A.; Zobi, F. Neovascularization effects of carbon monoxide releasing drugs chemisorbed on coscinodiscus diatoms carriers characterized by spectromicroscopy imaging. Appl. Sci. 2020, 10, 7380. [Google Scholar] [CrossRef]
- Sasirekha, R.; Sheena, T.S.; Deepika, M.S.; Santhanam, P.; Townley, H.E.; Jeganathan, K.; Kumar, S.D.; Premkumar, K. Surface Engineered Amphora Subtropica Frustules Using Chitosan as a Drug Delivery Platform for Anticancer Therapy. Mater. Sci. Eng. C 2019, 94, 56–64. [Google Scholar] [CrossRef]
- Wang, L.; Pan, K.; Li, J.; Li, Y.; Zhu, B.; Wang, Y.; Feng, C.; Han, J. Influence of the Physicochemical Characteristics of Diatom Frustules on Hemorrhage Control. Biomater. Sci. 2019, 7, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Cao, Z.; Liu, J.; Sun, X.; Qiu, K.; Mu, Y.; Cong, X.; Wang, X.; Chen, X.; Jia, N. The Hierarchical Porous Structures of Diatom Biosilica-Based Hemostat: From Selective Adsorption to Rapid Hemostasis. J. Colloid Interface Sci. 2023, 651, 544–557. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; de la Lastra, J.M.P. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Pandya, P.; Giram, P.; Bhole, R.P.; Chang, H.I.; Raut, S.Y. Nanocarriers based oral lymphatic drug targeting: Strategic bioavailability enhancement approaches. J. Drug Deliv. Sci. Technol. 2021, 64, 102585. [Google Scholar] [CrossRef]
- Johnson, L.T.; Zhang, D.; Zhou, K.; Lee, S.M.; Liu, S.; Dilliard, S.A.; Farbiak, L.; Chatterjee, S.; Lin, Y.H.; Siegwart, D.J. Lipid Nanoparticle (LNP) Chemistry Can Endow Unique in Vivo RNA Delivery Fates within the Liver That Alter Therapeutic Outcomes in a Cancer Model. Mol. Pharm. 2022, 19, 3973–3986. [Google Scholar] [CrossRef]
- Duan, H.; Liu, Y.; Gao, Z.; Huang, W. Recent Advances in Drug Delivery Systems for Targeting Cancer Stem Cells. Acta Pharm. Sin. B 2021, 11, 55–70. [Google Scholar] [CrossRef]
- Li, Y.; Lv, W.; Wang, L.; Zhang, Y.; Yang, L.; Wang, T.; Zhu, L.; Wang, Y.; Wang, W. Photo-Triggered Nucleus Targeting for Cancer Drug Delivery. Nano Res. 2021, 14, 2630–2636. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Kigga, M.; Sriram, G.; Ajeya, K.V.; Jung, H.-Y.; Neelgund, G.M.; Kurkuri, M.D. Facile Green Synthetic Approach of Bio Inspired Polydopamine Coated Diatoms as a Drug Vehicle for Controlled Drug Release and Active Catalyst for Dye Degradation. Microporous Mesoporous Mater. 2019, 288, 109572. [Google Scholar] [CrossRef]
- Senapati, S.; Shukla, R.; Tripathi, Y.B.; Mahanta, A.K.; Rana, D.; Maiti, P. Engineered Cellular Uptake and Controlled Drug Delivery Using Two Dimensional Nanoparticle and Polymer for Cancer Treatment. Mol. Pharm. 2018, 15, 679–694. [Google Scholar] [CrossRef]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous Silica Materials: From Physico-Chemical Properties to Enhanced Dissolution of Poorly Water-Soluble Drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Khan, R.U.; Iqbal, M.S.; Khan, H.A.; Khalid, I.; Yousaf, A.M.; Khalid, S.H.; Asghar, S.; et al. In Vitro and in Vivo Evaluation of Velpatasvir-Loaded Mesoporous Silica Scaffolds. A Prospective Carrier for Drug Bioavailability Enhancement. Pharmaceutics 2020, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Tesson, B.; Genet, M.J.; Fernandez, V.; Degand, S.; Rouxhet, P.G.; Martin-Jézéquel, V. Surface Chemical Composition of Diatoms. ChemBioChem 2009, 10, 2011–2024. [Google Scholar] [CrossRef] [PubMed]
- Rogato, A.; De Tommasi, E. Physical, chemical, and genetic techniques for diatom frustule modification: Applications in nanotechnology. Appl. Sci. 2020, 10, 8738. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Chronic inflammatory diseases, anti-inflammatory agents and their delivery nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M.; Kayagaki, N. Dying Cells Fan the Flames of Inflammation. Science 2021, 374, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Review Anti-Inflammatory Agents Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Luo, X.; Matranga, C.; Tan, S.; Alba, N.; Cui, X.T. Carbon Nanotube Nanoreservior for Controlled Release of Anti-Inflammatory Dexamethasone. Biomaterials 2011, 32, 6316–6323. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Friedman, M. Sustained-Release Drug Delivery of Antimicrobials in Controlling of Supragingival Oral Biofilms. Expert Opin. Drug Deliv. 2017, 14, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, X.; Yang, Y.; Qiao, H.; Yu, Z.; Liu, Y.; Wang, J.; Tang, T. Bacteria-targeting nanoparticles with microenvironment-responsive antibiotic release to eliminate intracellular Staphylococcus aureus and associated infection. ACS Appl. Mater. Interfaces 2018, 10, 14299–14311. [Google Scholar] [CrossRef]
- Peng, G.; Cai, J.; Wang, Z.; Zhang, W.; Xu, J.; Zhang, D.; Gong, D. Facile Fabrication of Diatomite Biosilica-Based Nasal Drug Delivery Vehicle for Enhanced Treatment of Allergic Rhinitis. Colloids Surf. B Biointerfaces 2024, 234, 113715. [Google Scholar] [CrossRef]
- Kushioka, J.; Kwoon, S.; Chow, H.; Toya, M.; Tsubosaka, M.; Shen, H. Bone Regeneration in Inflammation with Aging and Cell—Based Immunomodulatory Therapy. Inflamm. Regen. 2023, 43, 29. [Google Scholar] [CrossRef]
- Jang, J.-W.; Min, K.-E.; Kim, C.; Shin, J.; Lee, J.; Yi, S. Scaffold Characteristics, Fabrication Methods, and Biomaterials for the Bone Tissue Engineering. Int. J. Precis. Eng. Manuf. 2023, 24, 511–529. [Google Scholar] [CrossRef]
- Abbas, M.; Alqahtani, M.S.; Alhifzi, R. Recent developments in polymer nanocomposites for bone regeneration. Int. J. Mol. Sci. 2023, 24, 3312. [Google Scholar] [CrossRef]
- Gu, W.; Wu, C.; Chen, J.; Xiao, Y. Bone Diseases and Bone Regeneration Nanotechnology in the Targeted Drug Delivery for Bone Diseases and Bone Regeneration. Int. J. Nanomed. 2013, 8, 2305–2317. [Google Scholar] [CrossRef]
- Newman, M.R.; Benoit, D.S.W. ScienceDirect Local and Targeted Drug Delivery for Bone Regeneration. Curr. Opin. Biotechnol. 2016, 40, 125–132. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Zewail, M.B.; Noshy, M.; Abdelfatah, A.M.; Doghish, A.S. Smart/Stimuli-Responsive Hydrogels: State-of-the-Art Platforms for Bone Tissue Engineering. Appl. Mater. Today 2022, 29, 101560. [Google Scholar] [CrossRef]
- Municoy, S.; Alvare Echazu, M.I.; Antezana, P.E.; Galdoporpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem 2018, 6, 499. [Google Scholar] [CrossRef]
- Dalgic, A.D.; Atila, D.; Tezcaner, A.; Gürses, S.; Keskin, D. Diatom Silica Frustules-Doped Fibers for Controlled Release of Melatonin for Bone Regeneration. Eur. Polym. J. 2023, 186, 111858. [Google Scholar] [CrossRef]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The Synergistic Effects of Sr and Si Bioactive Ions on Osteogenesis, Osteoclastogenesis and Angiogenesis for Osteoporotic Bone Regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef]
- Lu, X.; Yu, S.; Chen, G.; Zheng, W.; Peng, J.; Huang, X.; Chen, L. Insight into the Roles of Melatonin in Bone Tissue and Bone-Related Diseases. Int. J. Mol. Med. 2021, 47, 82. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Lin, J.; Chen, R.; Huang, Y.; Liu, Y.; Bai, J.; Ge, G.; Shi, X.; Chen, Y.; Shi, J.; et al. Sustained Release of Melatonin from GelMA Liposomes Reduced Osteoblast Apoptosis and Improved Implant Osseointegration in Osteoporosis. Oxidative Med. Cell. Longev. 2020, 2020, 6797154. [Google Scholar] [CrossRef]
- Mohammadi, M.; Abbaszadeh, S.; Nosrati-siahmazgi, V. Heliyon Diatom-Guided Bone Healing via a Hybrid Natural Scaffold. Heliyon 2024, 10, e25878. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M.; Hall, R.P. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Leaper, D.J.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Extending the TIME concept: What have we learned in the past 10 years? Int. Wound J. 2012, 9, 1–19. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound Repair at a Glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.; Mahnoud, N.N.; Sharifi, S.; Gould, L.J.; Mahmoudi, M. Chronic wound healing models. ACS Pharmacol. Transl. Sci. 2023, 6, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Han, H.; Li, M.; Li, N.; Wang, Q.; Qin, X.; Wang, X.; Yu, J.; Li, Y.; Li, F.; et al. Nanofibers Reinforced Injectable Hydrogel with Self-Healing, Antibacterial, and Hemostatic Properties for Chronic Wound Healing. J. Colloid Interface Sci. 2021, 596, 312–323. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Wang, Y.; Kong, Q. Inflammation-responsive drug-loaded hydrogels with sequential hemostasis, antibacterial, and anti-inflammatory behavior for chronically infected diabetic wound treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef]
- Liu, L.; Hu, E.; Yu, K.; Xie, R.; Lu, F.; Lu, B.; Bao, R.; Li, Q.; Dai, F.; Lan, G. Recent Advances in Materials for Hemostatic Management. Biomater. Sci. 2021, 9, 7343–7378. [Google Scholar] [CrossRef]
- Wang, C.; Niu, H.; Ma, X.; Hong, H.; Yuan, Y.; Liu, C. Bioinspired, injectable, quaternized hydroxyethyl cellulose composite hydrogel coordinated by mesocellular silica foam for rapid, noncompressible hemostasis and wound healing. ACS Appl. Mater. Interfaces 2019, 11, 34595–34608. [Google Scholar] [CrossRef]
- Sun, X.; Li, N.; Su, C.; Mu, Y.; Cong, X.; Cao, Z.; Wang, H.; Yang, X.; Chen, X.; Feng, C. Diatom-inspired bionic hydrophilic polysaccharide adhesive for rapid sealing hemostasis. ACS Nano. 2023, 17, 19121–19135. [Google Scholar] [CrossRef] [PubMed]
- Khraisheh, M.A.M.; Al-Ghouti, M.A.; Allen, S.J.; Ahmad, M.N. Effect of OH and silanol groups in the removal of dyes from aqueous solution using diatomite. Water Res. 2005, 39, 922–932. [Google Scholar] [CrossRef]
- Townley, H.E.; Parker, A.R.; White-cooper, H. Exploitation of diatom frustules for nanotechnology: Tethering active biomolecules. Adv. Funct. Mater. 2008, 18, 369–374. [Google Scholar] [CrossRef]
- Kobayashi, M.; Juillerat, F.; Galletto, P.; Bowen, P.; Borkovec, M. Aggregation and charging of colloidal silica particles: Effect of particle size. Langmuir 2005, 21, 5761–5769. [Google Scholar] [CrossRef]
- Hassanali, A.A.; Zhang, H.; Knight, C.; Shin, Y.K.; Singer, S.J. The dissociated amorphous silica surface: Model development and evaluation. J. Chem. Theory Comput. 2010, 6, 3456–3471. [Google Scholar] [CrossRef]
- Aw, M.S.; Bariana, M.; Yu, Y.; Addai-Mensah, J.; Losic, D. Surface-functionalized diatom microcapsules for drug delivery of water-insoluble drugs. J. Biomater. Appl. 2013, 28, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, J.; Shojaedin-givi, B.; Hashemzadeh, H.; Mozafari-nia, M. Photodiagnosis and Photodynamic Therapy Capture and Detection of Rare Cancer Cells in Blood by Intrinsic Fl Uorescence of a Novel Functionalized Diatom. Photodiagnosis Photodyn. Ther. 2020, 30, 101753. [Google Scholar] [CrossRef] [PubMed]
- Rozan, H.E.; Wu, G.; Zhou, Z.; Li, Q.; Sharaf, M.; Chen, X. Colloids and Surfaces B Biointerfaces The Complex Hydrogel Based on Diatom Biosilica and Hydroxybutyl Chitosan for Wound Healing. Colloids Surf. B Biointerfaces 2022, 216, 112523. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, X.; Jiang, C.; Wang, H.; Mu, Y.; Sun, X.; Chen, X.; Feng, C. Thermo-Sensitive Hydroxybutyl Chitosan/Diatom Biosilica Hydrogel with Immune Microenvironment Regulatory for Chronic Wound Healing. Int. J. Biol. Macromol. 2024, 262, 130189. [Google Scholar] [CrossRef]
- Lee, J.; Park, E.; Fujisawa, A.; Lee, H. Diatom silica/polysaccharide elastomeric hydrogels: Adhesion and interlocking synergy. ACS Appl. Mater. Interfaces 2021, 13, 21703–21713. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Su, C.; Sun, X.; Shao, K.; Wang, X.; Mu, Y.; Chen, X.; Feng, C. Enhanced Mechanical Properties of Hydroxybutyl Chitosan Hydrogel through Anchoring Interface Effects of Diatom Biosilica. Carbohydr. Polym. 2022, 296, 119975. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, Y.; Li, J.; Mu, Y.; Zhang, X.; Zhang, K.; Liang, M.; Feng, C.; Chen, X. Multifunctional Chitosan/Dopamine/Diatom-Biosilica Composite Beads for Rapid Blood Coagulation. Carbohydr. Polym. 2018, 200, 6–14. [Google Scholar] [CrossRef]
- Hate, S.S.; Reutzel-Edens, S.M.; Taylor, L.S. Influence of drug–silica electrostatic interactions on drug release from mesoporous silica-based oral delivery systems. Mol. Pharm. 2020, 17, 3435–3446. [Google Scholar] [CrossRef]
- Todd, T.; Zhen, Z.; Tang, W.; Chen, H.; Wang, G.; Chuang, Y.J.; Xie, J. Iron oxide nanoparticle encapsulated diatoms for magnetic delivery of small molecules to tumors. Nanoscale 2014, 6, 2073–2076. [Google Scholar] [CrossRef]
- Ooi, Y.J.; Wen, Y.; Zhu, J.; Song, X.; Li, J. Surface charge switchable polymer/DNA nanoparticles responsive to tumor extracellular ph for tumor-triggered enhanced gene delivery. Biomacromolecules 2020, 21, 1136–1148. [Google Scholar] [CrossRef]
- Wu, W.; Wang, J.; Lin, Z.; Li, X.; Li, J. Tumor-Acidity Activated Surface Charge-Conversion of Polymeric Nanocarriers for Enhanced Cell Adhesion and Targeted Drug Release. Macromol. Rapid Commun. 2014, 35, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X.; Liu, Y.; Wang, G.; Falconer, R.G.; Zhao, C.X. Lipid nanoparticles for drug delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Sharma, V.; Anandhakumar, S.; Sasidharan, M. Self-Degrading Niosomes for Encapsulation of Hydrophilic and Hydrophobic Drugs: An Efficient Carrier for Cancer Multi-Drug Delivery. Mater. Sci. Eng. C 2015, 56, 393–400. [Google Scholar] [CrossRef]
- Mirchandani, Y.; Patravale, V.B.; Brijesh, S. Solid Lipid Nanoparticles for Hydrophilic Drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-based nanoparticles of targeted drug delivery system in breast cancer treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Tezgel, Ö.; Szarpark-Jankowska, A.; Arnould, A.; Auzely-Velty, R.; Texier, I. Chitosan-lipid nanoparticles (CS-LNPs): Application to siRNA delivery. J. Colloid Interface Sci. 2018, 510, 45–56. [Google Scholar] [CrossRef]
- Lv, J.; Sun, B.; Jin, J.; Jiang, W. Mechanical and Slow-Released Property of Poly (Acrylamide) Hydrogel Reinforced by Diatomite. Mater. Sci. Eng. C 2019, 99, 315–321. [Google Scholar] [CrossRef]
- Nie, J.; Pei, B.; Wang, Z.; Hu, Q. Construction of Ordered Structure in Polysaccharide Hydrogel A Review. Carbohydr. Polym. 2019, 205, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Jonker, A.M.; Löwik, D.W.; Van Hest, C.M.J. Peptide-and protein-based hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and Characterization of Hyaluronic Acid Hydrogels Crosslinked Using a Solvent-Free Process for Potential Biomedical Applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. In The Road from Nanomedicine to Precision Medicine; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2020; pp. 1117–1150. [Google Scholar]
- Qin, P.; Tang, J.; Sun, D.; Yang, Y.; Liu, N.; Li, Y.; Fu, Z.; Wang, Y.; Li, C.; Li, X.; et al. Zn2+ cross-linked alginate carrying hollow silica nanoparticles loaded with RL-QN15 peptides provides promising treatment for chronic skin wounds. ACS Appl. Mater. Interfaces 2022, 14, 29491–29505. [Google Scholar] [CrossRef]






| Diatom Species | Drugs | Surface Modification | Effect | References |
|---|---|---|---|---|
| Aulacoseira sp. | Indomethacin | Dopamine-iron oxide | Anti-inflammatory | [77] |
| Aulacoseira sp. | Gentamicin and indomethacin | Hydrophobic/Hydrophilic silane | Anti-inflammatory | [65,78] |
| Aulacoseira sp. | Indomethacin | Graphene oxide | Anti-inflammatory | [64] |
| Diatomite | Prednisone and mesalamine | - | Anti-inflammatory | [47] |
| Diatomite | Carbamazepine | SEEPS (Solid self-emulsifying phospholipid suspension) | Psychomotor seizures and trigeminal neuralgia | [79] |
| Diatomite | siRNA | - | Anti-cancer | [80] |
| Aulacoseira sp. | Levofloxacin | oligo(ethylene glycol)methacrylate | Anti-inflammatory | [81] |
| Nitzschia sp. | Curcumin | CMDM- F and CMDM-I | Antibiotic | [82] |
| Thalassiosira weissfloggi sp. | Ciprofloxacin | APTES-TEMPO (2,6,6,tetramethylpiperidine N-oxy) | Bone growth | [83,84] |
| Diatomite | Doxorubicin | Magnesium thermal reduction | Anti-cancer | [43] |
| Aulacoseira sp. | Diclofenac sodium (DS) | Xerogel | Antibiotic | [68] |
| Diatomite, Coscinodiscus | Anticancer drugs | cobalamin (vitamin B12) | Anti-cancer | [63,85,86] |
| Amphora subtropica | Doxorubicin | Chitosan | Anti-cancer | [87] |
| Thalassiosira weissflogii, Thalassiosira sp., Cyclotella cryptica | - | - | Hemostasis | [88] |
| C. cryptica | - | Ca2+ | Hemostasis | [89] |
| Composite | Type | Target | Effect | References |
|---|---|---|---|---|
| Diatom/Hydroxybutyl chitosan + Zn2+ | Hydrogel dressing + Drug delivery | Diabetic chronic wound healing | Sustained release, hemostasis, wound healing, biocompatibility | [21] |
| Diatom/Hydroxybutyl chitosan + Doxycyclin | Hydrogel dressing + Drug delivery | Wound closure | Sustained release, antimicrobial, hemostasis, biocompatibility, nonadherent | [138] |
| Diatom/Hydroxybutyl chitosan + Si | Hydrogel dressing + Drug delivery | Chronic wound healing | Sustained release, reduced inflammation, angiogenesis, biocompatibility, collagen deposition | [139] |
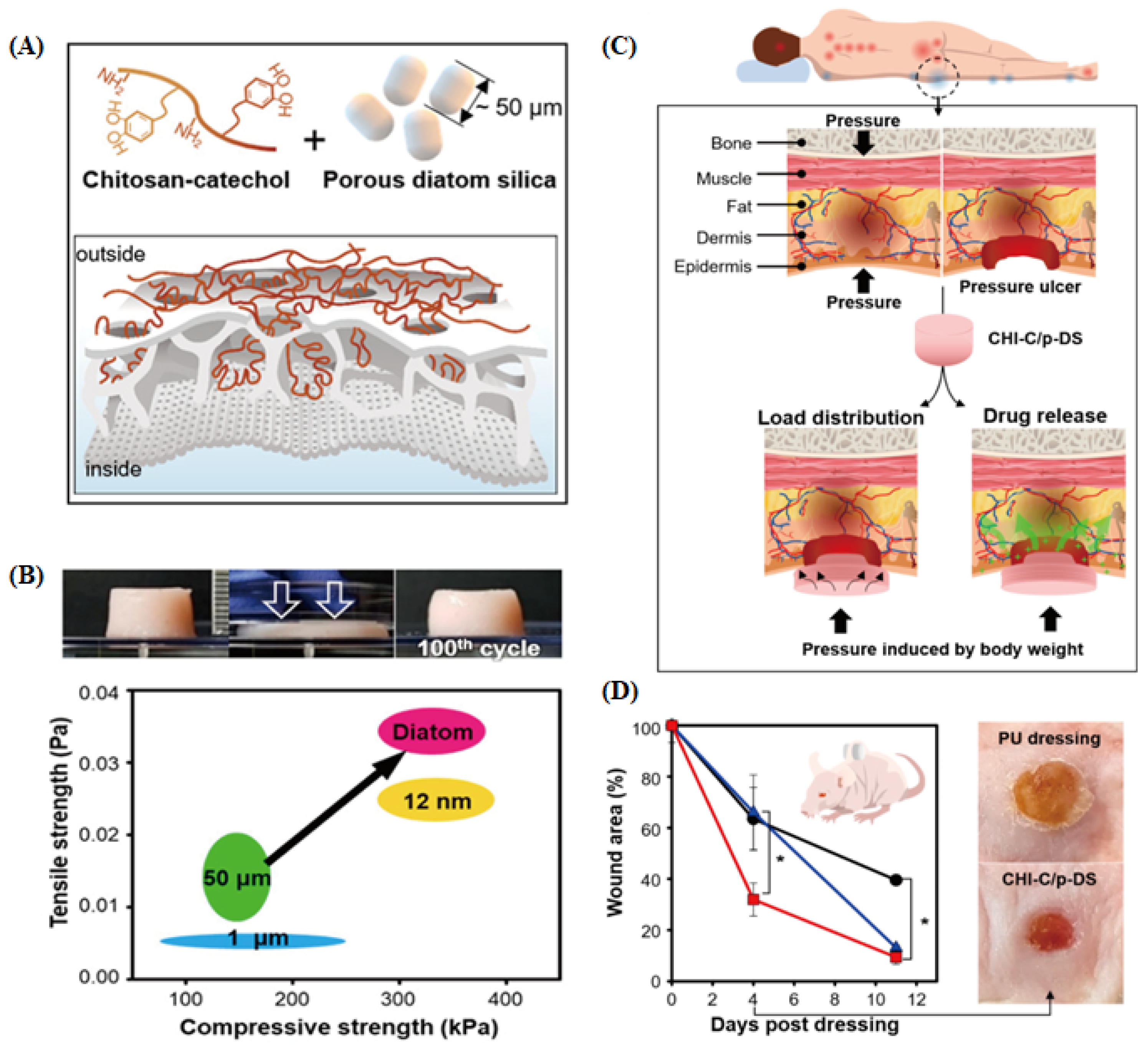
| Diatom/Catechol conjugated chitosan | Hydrogel dressing | Wound dressing for pressure ulcers | Sustained release, reinforced mechanical properties, wound healing | [140] |
| Diatom/Hydroxybutyl chitosan | Hydrogel dressing | Wound dressing | Reinforced mechanical properties, blood compatibility, thermal stability, biodegradability, biocompatibility | [141] |
| Diatom/Chitosan/Dopamine | Beads | Hemostasis | Biocompatibility, non-cytotoxicity, rapid hemostasis, enhanced absorption | [142] |
| Diatom/Polymer coating + Melatonin Diatom/Gelatin/Chitosan/Hyaluronic acid/-sitosterol Diatom/antibody/Liposome | Scaffold Scaffold Targeted drug delivery | Bone regeneration Bone regeneration Anticancer | Controlled release, increased ALP activity, enhanced osteoblast-like cell viability, enhanced porosity, biodegradability, biomineralization, anti-inflammatory, angiogenic effect natural drug carriers, biocompatible, biodegradable, surface modification, high drug loading (%) | [63,118,122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Woo, Y.; Seo, Y.; Yoo, D.; Kwon, D.; Park, H.; Lee, S.D.; Yoo, H.Y.; Lee, T. A Descriptive Review on the Potential Use of Diatom Biosilica as a Powerful Functional Biomaterial: A Natural Drug Delivery System. Pharmaceutics 2024, 16, 1171. https://doi.org/10.3390/pharmaceutics16091171
Kang S, Woo Y, Seo Y, Yoo D, Kwon D, Park H, Lee SD, Yoo HY, Lee T. A Descriptive Review on the Potential Use of Diatom Biosilica as a Powerful Functional Biomaterial: A Natural Drug Delivery System. Pharmaceutics. 2024; 16(9):1171. https://doi.org/10.3390/pharmaceutics16091171
Chicago/Turabian StyleKang, Sunggu, Yeeun Woo, Yoseph Seo, Daehyeon Yoo, Daeryul Kwon, Hyunjun Park, Sang Deuk Lee, Hah Young Yoo, and Taek Lee. 2024. "A Descriptive Review on the Potential Use of Diatom Biosilica as a Powerful Functional Biomaterial: A Natural Drug Delivery System" Pharmaceutics 16, no. 9: 1171. https://doi.org/10.3390/pharmaceutics16091171
APA StyleKang, S., Woo, Y., Seo, Y., Yoo, D., Kwon, D., Park, H., Lee, S. D., Yoo, H. Y., & Lee, T. (2024). A Descriptive Review on the Potential Use of Diatom Biosilica as a Powerful Functional Biomaterial: A Natural Drug Delivery System. Pharmaceutics, 16(9), 1171. https://doi.org/10.3390/pharmaceutics16091171










