Glucose Metabolism-Modifying Natural Materials for Potential Feed Additive Development
Abstract
1. Introduction
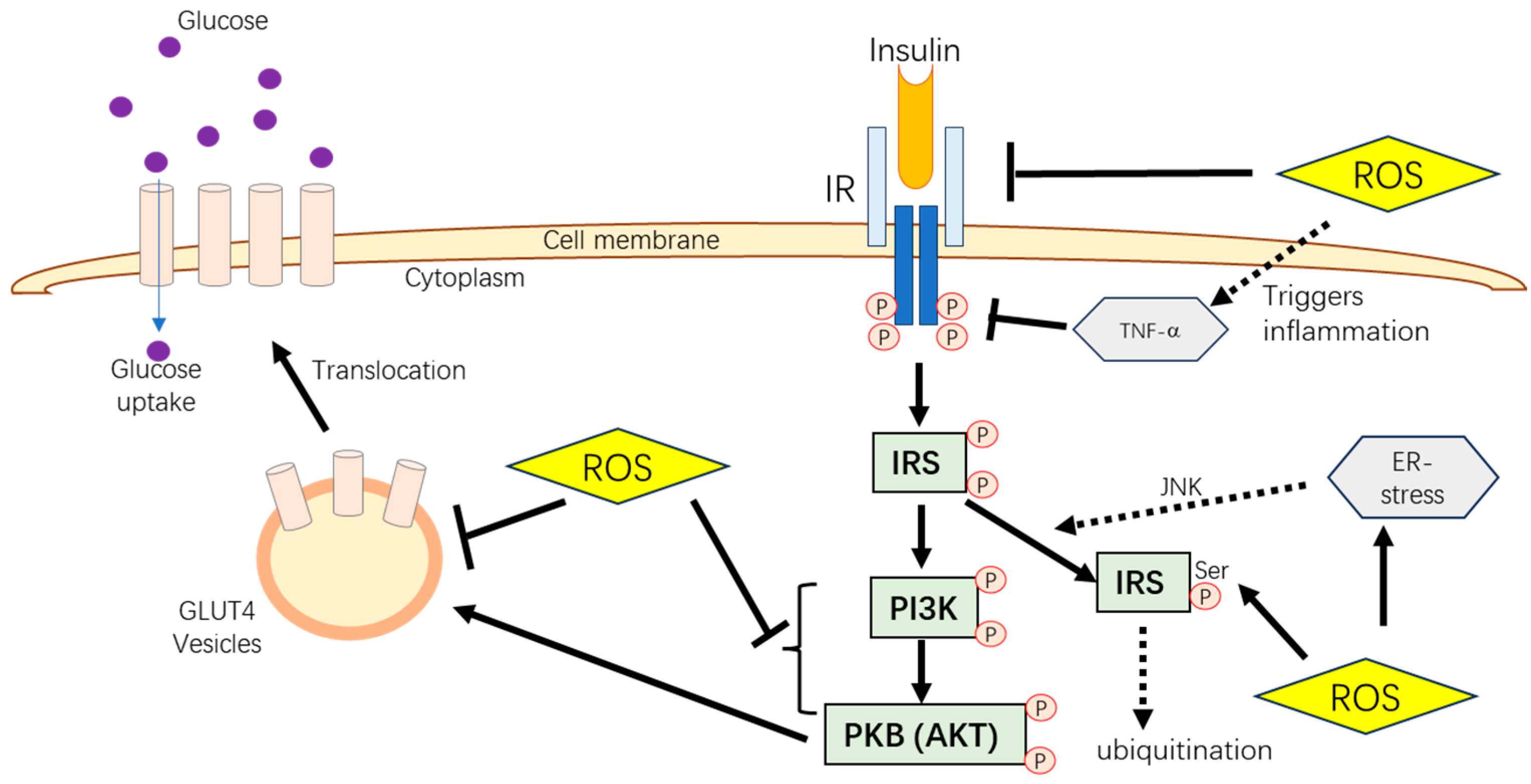
2. Insulin-Dependent Glucose Metabolism Pathways
3. Major Challenges Related to Glucose Metabolism in Animal Production
3.1. Poultry
3.2. Swine
3.3. Dairy Ruminants
3.3.1. Dairy Cattle
3.3.2. Dairy Sheep and Goats
| Farm Animals | Production and Health Issues Related to Insulin-Dependent Glucose Metabolism | References |
|---|---|---|
| Poultry |
| [29,30] |
| [32,34,36] | |
| [37,38] | |
| Swine |
| [39] |
| [40,41,42] | |
| [43,45] | |
| Dairy cattle (especially for high yield) |
| [47] |
| [49] | |
| [54] | |
| [50] | |
| Dairy sheep and goat |
| [56] |
| [56] |
4. Effects of Stress on Animal Glucose Metabolism
Effects of Oxidative Damage on Animal Glucose Metabolism
5. Glucose-Modifying Materials as Potential Feed Additives
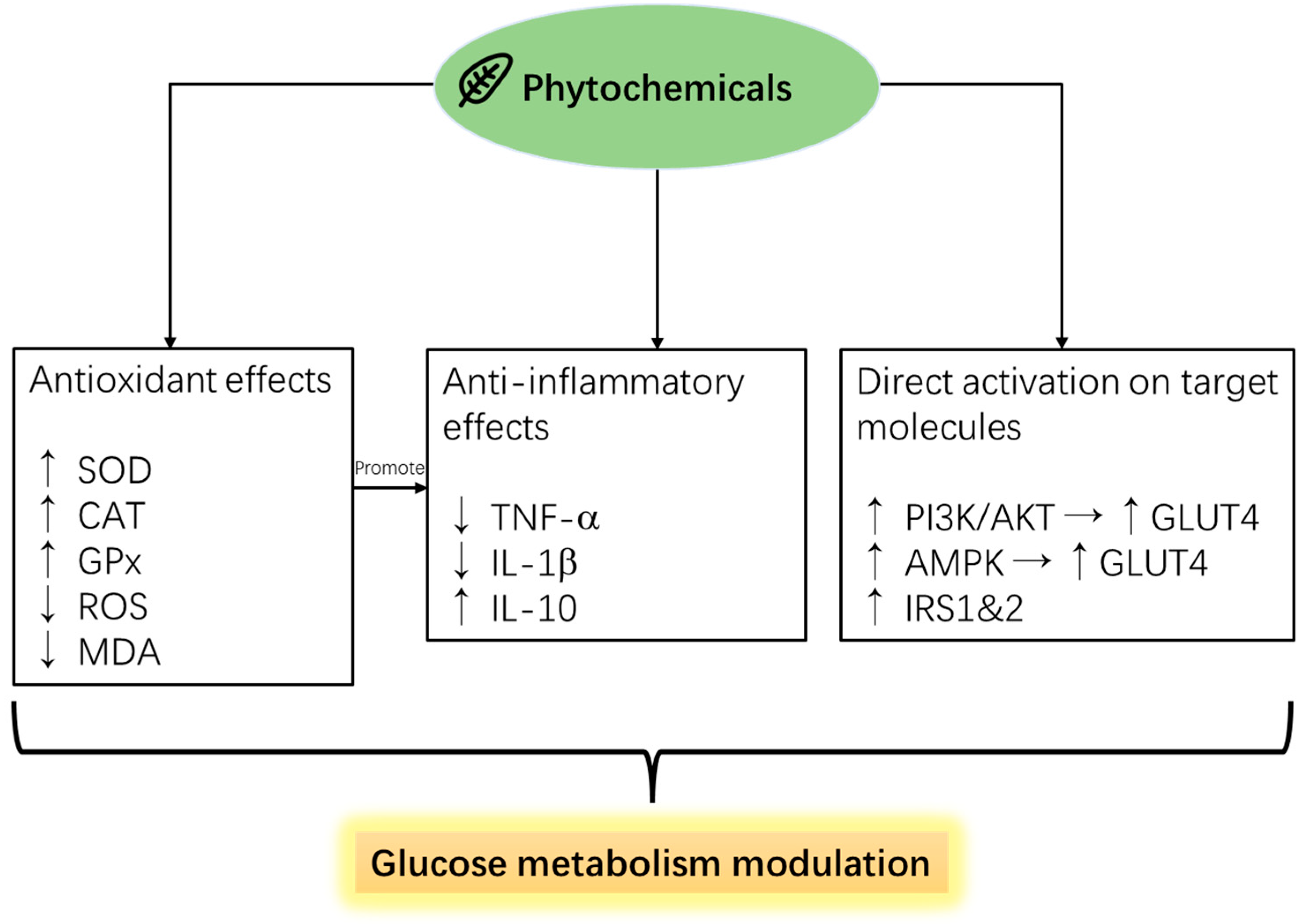
5.1. Phytochemicals
5.1.1. Polyphenols
Gallic Acid
Olive Pomace
Resveratrol
Curcumin
Rosmarinic Acid
Quercetin
5.1.2. Terpene-Based Essential Oils
5.2. Probiotics
5.2.1. Lactic Acid Bacteria
5.2.2. Clostridium butyricum
5.2.3. Bacillus sp.
5.3. Prebiotics
5.4. Symbiotics
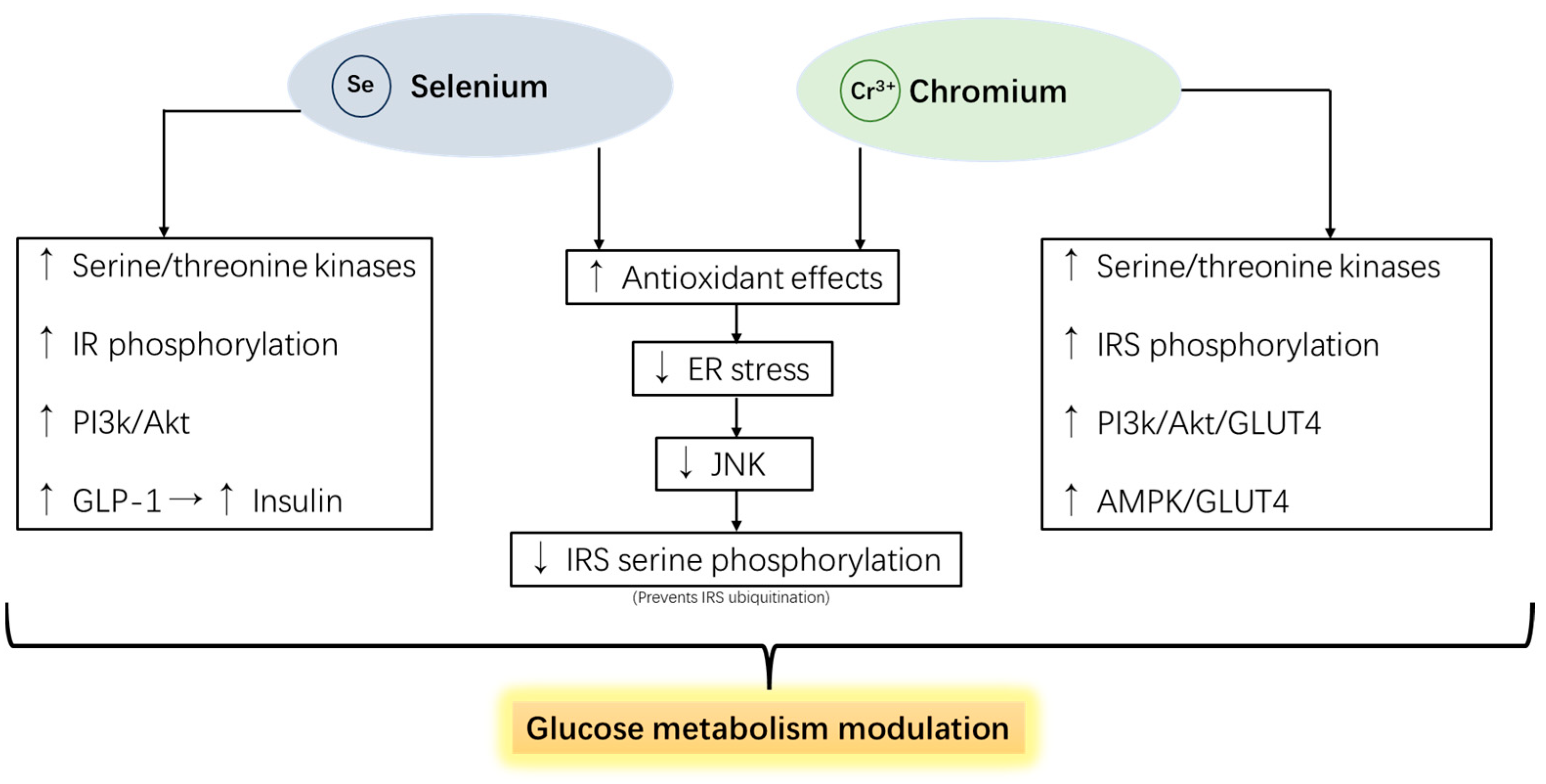
5.5. Selenium and Chromium
6. Limitations for the Feed Additives
7. Research Gaps and Future Direction
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elischer, M.F.; Sordillo, L.M.; Siegford, J.M.; Karcher, E.L. Short communication: Characterizing metabolic and oxidant status of pastured dairy cows postpartum in an automatic milking system. J. Dairy Sci. 2015, 98, 7083–7089. [Google Scholar] [CrossRef] [PubMed]
- Heiser, A.; McCarthy, A.; Wedlock, N.; Meier, S.; Kay, J.; Walker, C.; Crookenden, M.A.; Mitchell, M.D.; Morgan, S.; Watkins, K.; et al. Grazing dairy cows had decreased interferon-γ, tumor necrosis factor, and interleukin-17 and increased expression of interleukin-10 during the first week after calving. J. Dairy Sci. 2015, 98, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Farooq, M.; Lee, D.J.; Siddique, K.H.M. Sustainable agricultural practices for food security and ecosystem services. Environ. Sci. Pollut. Res. 2022, 29, 84076–84095. [Google Scholar] [CrossRef] [PubMed]
- Gundala, R.R.; Singh, A. What motivates consumers to buy organic foods? Results of an empirical study in the United States. PLoS ONE 2021, 16, e0257288. [Google Scholar] [CrossRef]
- Yue, C.; Lai, Y.; Wang, J.; Mitchell, P. Consumer Preferences for Sustainable Product Attributes and Farm Program Features. Sustainability 2020, 12, 7388. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy. Biomolecules 2023, 13, 1402. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef] [PubMed]
- Mansuy-Aubert, V.; Ravussin, Y. Short chain fatty acids: The messengers from down below. Front. Neurosci. 2023, 17, 1197759. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Stanić, Z. Curcumin, a Compound from Natural Sources, a True Scientific Challenge—A Review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2020, 25, 63. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values, and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics, and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Thorens, B.; Mueckler, M. Glucose transporters in the 21st Century. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E141–E145. [Google Scholar] [CrossRef]
- Veys, K.; Fan, Z.; Ghobrial, M.; Bouché, A.; García-Caballero, M.; Vriens, K.; Conchinha, N.V.; Seuwen, A.; Schlegel, F.; Gorski, T.; et al. Role of the GLUT1 Glucose Transporter in Postnatal CNS Angiogenesis and Blood-Brain Barrier Integrity. Circ. Res. 2020, 127, 466–482. [Google Scholar] [CrossRef]
- Ibrahim, D.; Moustafa, A.; Metwally, A.S.; Nassan, M.A.; Abdallah, K.; Eldemery, F.; Tufarelli, V.; Laudadio, V.; Kishawy, A.T.Y. Potential application of cornelian cherry extract on broiler chickens: Growth, expression of antioxidant biomarker and glucose transport genes, and oxidative stability of frozen meat. Animals 2021, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Na, L.X.; Zhang, Y.L.; Li, Y.; Liu, L.Y.; Li, R.; Kong, T.; Sun, C.H. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Kang, J.; Cao, Y.; Fan, S.; Yang, H.; An, Y.; Pan, Y.; Tie, L.; Li, X. Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic β-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways. Apoptosis 2015, 20, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis was revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Cousin, C. Oxidative stress: A biomarker for nutritional or environmental effects in farm animals. Animal 2016, 10, 972–981. [Google Scholar]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Braun, E.J.; Sweazea, K.L. Glucose regulation in birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 1–9. [Google Scholar] [CrossRef]
- Simon, J.; Rideau, N.; Taouis, M.; Dupont, J. Plasma insulin levels are rather similar in chicken and rat. Gen. Comp. Endocrinol. 2011, 171, 267–268. [Google Scholar] [CrossRef]
- Dupont, J.; Tesseraud, S.; Derouet, M.; Collin, A.; Rideau, N. Insulin immuno-neutralization in chicken: Effects on insulin signaling and gene expression in liver and muscle. J. Endocrinol. 2008, 197, 531–542. [Google Scholar] [CrossRef]
- Dupont, J.; Dagou, C.; Derouet, M.; Simon, J.; Taouis, M. Early steps of insulin receptor signaling in chicken and rat: Apparent refractoriness in chicken muscle. Domest. Anim. Endocrinol. 2004, 26, 127–142. [Google Scholar] [CrossRef]
- Zhao, J.P.; Lin, H.; Jiao, H.C.; Song, Z.G. Corticosterone suppresses insulin- and NO-stimulated muscle glucose uptake in broiler chickens (Gallus gallus domesticus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Tetrault, M.-A.; Takahashi, K.; Ouyang, J.; Pratx, G.; Normandin, M.; Fakhri, G. Dependence of fluorodeoxyglucose (FDG) uptake on cell cycle and dry mass: A single-cell study using a multi-modal radiography platform. Sci. Rep. 2020, 10, 4280. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Tao, Y.; Zhang, X.; Pan, J.; Zhu, X.; Wang, H.; Du, P.; Zhu, Y.; Huang, Y.; Chen, W. Dynamic changes of blood glucose, serum biochemical parameters and gene expression in response to exogenous insulin in Arbor Acres broilers and Silky fowls. Sci. Rep. 2020, 10, 6697. [Google Scholar] [CrossRef] [PubMed]
- Sumners, L.H.; Zhang, W.; Zhao, X.; Honaker, C.F.; Zhang, S.; Cline, M.A.; Siegel, P.B.; Gilbert, E.R. Chickens from lines artificially selected for juvenile low and high body weight differ in glucose homeostasis and pancreas physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 172, 57–65. [Google Scholar] [CrossRef]
- Meng, J.; Ma, N.; Liu, H.; Liu, J.; Liu, J.; Wang, J.; He, X.; Zhao, X. Untargeted and targeted metabolomics profiling reveals the underlying pathogenesis and abnormal arachidonic acid metabolism in laying hens with fatty liver hemorrhagic syndrome. Poult. Sci. 2021, 100, 101320. [Google Scholar] [CrossRef]
- Zhuang, Y.; Xing, C.; Cao, H.; Zhang, C.; Luo, J.; Guo, X.; Hu, G. Insulin resistance and metabonomics analysis of fatty liver haemorrhagic syndrome in laying hens induced by a high-energy low-protein diet. Sci. Rep. 2019, 9, 10141. [Google Scholar] [CrossRef]
- Nematbakhsh, S.; Pei Pei, C.; Selamat, J.; Nordin, N.; Idris, L.H.; Abdull Razis, A.F. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes 2021, 12, 414. [Google Scholar] [CrossRef]
- Alvarenga, R.R.; Zangeronimo, M.G.; Pereira, L.J.; Rodrigues, P.B.; Gomide, E.M. Lipoprotein Metabolism in Poultry. World’s Poult. Sci. J. 2011, 67, 431–440. [Google Scholar] [CrossRef]
- Kemp, B.; Soede, N.M.; Vesseur, P.; Helmond, F.A.; Spoorenberg, J.; Frankena, K. Glucose Tolerance of Pregnant Sows Is Related to Postnatal Pig Mortality. J. Anim. Sci. 1996, 74, 879–885. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar]
- Watson, C.J. Alveolar cells in the mammary gland: Lineage commitment and cell death. Biochem. J. 2022, 479, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, E.; Le Floc’h, N.; Etienne, M.; Ramaekers, P.; Sève, B.; Père, M.C. Reduced feed intake of lactating primiparous sows is associated with increased insulin resistance during the peripartum period and is not modified through supplementation with dietary tryptophan. J. Anim. Sci. 2010, 88, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.A.; Briskey, E.J.; Hoekstra, W.G.; Bray, R.W. Glucose Tolerance in Swine as Related to Post-Mortem Muscle Characteristics. J. Anim. Sci. 1962, 21, 543–548. [Google Scholar] [CrossRef]
- Apaoblaza, A.; Gerrard, S.D.; Matarneh, S.K.; Wicks, J.C.; Kirkpatrick, L.; England, E.M.; Scheffler, T.L.; Duckett, S.K.; Shi, H.; Silva, S.L.; et al. Muscle from grass- and grain-fed cattle differs energetically. Meat Sci. 2020, 161, 107996. [Google Scholar] [CrossRef]
- Edwards, L.N.; Engle, T.E.; Correa, J.A.; Paradis, M.A.; Grandin, T.; Anderson, D.B. The relationship between exsanguination blood lactate concentration and carcass quality in slaughter pigs. Meat Sci. 2010, 85, 435–440. [Google Scholar] [CrossRef]
- Bellinger, D.A.; Merricks, E.P.; Nichols, T.C. Swine Models of Type 2 Diabetes Mellitus: Insulin Resistance, Glucose Tolerance, and Cardiovascular Complications. ILAR J. 2006, 47, 243–258. [Google Scholar] [CrossRef]
- Qiao, K.; Jiang, R.; Contreras, G.A.; Xie, L.; Pascottini, O.B.; Opsomer, G.; Dong, Q. The Complex Interplay of Insulin Resistance and Metabolic Inflammation in Transition Dairy Cows. Animals 2024, 14, 832. [Google Scholar] [CrossRef]
- Herdt, T.H. Variability characteristics and test selection in herd-level nutritional and metabolic profile testing. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 387–403. [Google Scholar] [CrossRef]
- Holtenius, P.; Holtenius, K. New aspects of ketone bodies in energy metabolism of dairy cows: A review. Transbound. Emerg. Dis. 1996, 43, 579–587. [Google Scholar] [CrossRef]
- Andersson, L. Subclinical ketosis in dairy cows. Metabolic diseases of ruminant livestock. Vet. Clin. N. Am. Food Anim. Pract. 1988, 4, 233–251. [Google Scholar] [CrossRef]
- De Koster, J.D.; Opsomer, G. Insulin resistance in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. Association of oxidative status and insulin sensitivity in periparturient dairy cattle: An observational study. J. Anim. Physiol. Anim. Nutr. 2016, 100, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Block, E. Transition cow research—What makes sense today? In Proceedings of the High Plains Dairy Conference, Amarillo, TX, USA, 10–12 March 2010; pp. 75–98. [Google Scholar]
- Ingvartsen, K.L. Feeding and management-related diseases in the transition cow: Physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim. Feed Sci. Technol. 2006, 126, 175–213. [Google Scholar] [CrossRef]
- Joy, A.; Dunshea, F.R.; Leury, B.J.; Clarke, I.J.; DiGiacomo, K.; Chauhan, S.S. Resilience of small ruminants to climate change and increased environmental temperature: A review. Animals 2020, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Marteniuk, J.V.; Herdt, T.H. Pregnancy toxemia and ketosis of ewes and does. Vet. Clin. N. Am. Food Anim. Pract. 1988, 4, 307–315. [Google Scholar] [CrossRef]
- Ji, X.; Liu, N.; Wang, Y.; Ding, K.; Huang, S.; Zhang, C. Pregnancy toxemia in ewes: A review of molecular metabolic mechanisms and management strategies. Metabolites 2023, 13, 149. [Google Scholar] [CrossRef]
- Huang, Y.; Kong, Y.; Li, B.; Zhao, C.; Loor, J.J.; Tan, P.; Yuan, Y.; Zeng, F.; Zhu, X.; Qi, S.; et al. Effects of perinatal stress on the metabolites and lipids in plasma of dairy goats. Stress Biol. 2023, 3, 11. [Google Scholar] [CrossRef]
- Cabiddu, A.; Dattena, M.; Decandia, M.; Molle, G.; Lopreiato, V.; Minuti, A.; Trevisi, E. The effect of parity number on the metabolism, inflammation, and oxidative status of dairy sheep during the transition period. J. Dairy Sci. 2020, 103, 8564–8575. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Butler, A.E.; Jamialahmadi, T.; Sahebkar, A. Molecular mechanisms linking stress and insulin resistance. EXCLI J. 2022, 21, 317–334. [Google Scholar]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Ruzzin, J.; Wagman, A.S.; Jensen, J. Glucocorticoid-induced insulin resistance in skeletal muscles: Defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia 2005, 48, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Derouet, M.; Simon, J.; Taouis, M. Corticosterone alters insulin signaling in chicken muscle and liver at different steps. J. Endocrinol. 1999, 162, 67–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Müssig, K.; Fiedler, H.; Staiger, H.; Weigert, C.; Lehmann, R.; Schleicher, E.D.; Häring, H.U. Insulin-induced stimulation of JNK and the PI 3-kinase/mTOR pathway leads to phosphorylation of serine 318 of IRS-1 in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2005, 335, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Contreras-Ferrat, A.; Lavandero, S.; Jaimovich, E.; Klip, A. Calcium signaling in insulin action on striated muscle. Cell Calcium 2014, 56, 390–396. [Google Scholar] [CrossRef]
- Xu, C.; Shu, S.; Xia, C.; Wang, B.; Zhang, H.Y. Investigation on the Relationship of Insulin Resistance and Ketosis in Dairy Cows. J. Vet. Sci. Technol. 2014, 5, 162. [Google Scholar]
- Zhou, J.; Huang, K.; Lei, X.G. Selenium and diabetes—Evidence from animal studies. Free Radic. Biol. Med. 2013, 65, 1548–1556. [Google Scholar] [CrossRef]
- Victor, P.; Umapathy, D.; George, L.; Juttada, U.; Ganesh, G.V.; Amin, K.N.; Viswanathan, V.; Ramkumar, K.M. Crosstalk between endoplasmic reticulum stress and oxidative stress in the progression of diabetic nephropathy. Cell Stress Chaperones 2021, 26, 311–321. [Google Scholar] [CrossRef]
- Nakatani, Y.; Kaneto, H.; Kawamori, D.; Yoshiuchi, K.; Hatazaki, M.; Matsuoka, T.A.; Ozawa, K.; Ogawa, S.; Hori, M.; Yamasaki, Y.; et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J. Biol. Chem. 2005, 280, 847–851. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 2005, 54, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Gorgun, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Lee, T.T. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals—A review. Asian-Australas. J. Anim. Sci. 2019, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, C.; Fernández-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Farina, M.; Aschner, M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay. Biochim. Biophys. Acta 2019, 1863, 129258. [Google Scholar] [CrossRef]
- Willemsen, H.; Swennen, Q.; Everaet, N.; Geraert, P.A.; Mercier, Y.; Stinckens, A.; Decuypere, E.; Buyse, J. Effects of dietary supplementation of methionine and its hydroxyl analog DL-2-hydroxy-4-methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperature. Poult. Sci. 2011, 90, 2311–2320. [Google Scholar] [CrossRef]
- Bae, H.; Jeong, C.H.; Cheng, W.N.; Hong, K.; Seo, H.G.; Han, S.G. Oxidative stress-induced inflammatory responses and effects of N-acetylcysteine in bovine mammary alveolar cells. J. Dairy Res. 2017, 84, 418–425. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, Q.; Chen, Y.; Pan, R.; Kuang, S.; Liu, G.; Sun, G.; Sun, X. Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci. Rep. 2017, 7, 44239. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Best, L.; Elliott, A.C.; Brown, P.D. Curcumin induces electrical activity in rat pancreatic beta-cells by activating the volume-regulated anion channel. Biochem. Pharmacol. 2007, 73, 1768–1775. [Google Scholar] [CrossRef]
- Kord, M.T.; Pourrajab, F.; Hekmatimoghaddam, S. Ginger extract increases GLUT-4 expression preferentially through AMPK than PI3K signalling pathways in C2C12 muscle cells. Diabetes Metab. Syndr. Obes. 2020, 13, 3231–3238. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union 2003, L268, 29–43. [Google Scholar]
- Thanner, S.; Drissner, D.; Walsh, F. Antimicrobial resistance in agriculture. mBio 2016, 7, e02227-15. [Google Scholar] [CrossRef]
- Wei, C.; Yang, K.; Zhao, G.; Lin, S.; Xu, Z. Effect of dietary supplementation of gallic acid on nitrogen balance, nitrogen excretion pattern and urinary nitrogenous constituents in beef cattle. Arch. Anim. Nutr. 2016, 70, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Samuel, K.G.; Wang, J.; Yue, H.Y.; Wu, S.G.; Zhang, H.J.; Duan, Z.Y.; Qi, G.H. Effects of dietary gallic acid supplementation on performance, antioxidant status, and jejunum intestinal morphology in broiler chicks. Poult. Sci. 2017, 96, 2768–2775. [Google Scholar] [CrossRef]
- Mektrirat, R.; Chuammitri, P.; Navathong, D.; Khumma, T.; Srithanasuwan, A.; Suriyasathaporn, W. Exploring the potential immunomodulatory effects of gallic acid on milk phagocytes in bovine mastitis caused by Staphylococcus aureus. Front. Vet. Sci. 2023, 10, 1255058. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Gao, G.; Bontempo, V.; Chen, C.; Schroyen, M.; Li, X.; Jiang, X. The influence of dietary gallic acid on growth performance and plasma antioxidant status of high and low weaning weight piglets. Animals 2021, 11, 3323. [Google Scholar] [CrossRef]
- Prasad, C.N.; Anjana, T.; Banerji, A.; Gopalakrishnapillai, A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010, 584, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Doan, K.V.; Ko, C.M.; Kinyua, A.W.; Yang, D.J.; Choi, Y.H.; Oh, I.Y.; Ko, A.; Choi, J.W.; Jeong, Y.; Jung, M.H.; et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology 2015, 156, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods: A review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Dunne, G. Transforming olive waste into animal feed. Int. J. Clin. Nutr. Diet. 2019, 5, 142. [Google Scholar] [CrossRef]
- De Oliveira, C.O.; Roll, A.A.P.; Gonçalves, F.M.M.; Lopes, D.C.N.; Xavier, E.G. Olive pomace for the feeding of commercial poultry: Effects on performance, meat and eggs quality, haematological parameters, microbiota and immunity. World’s Poult. Sci. J. 2021, 77, 363–376. [Google Scholar] [CrossRef]
- Chaves, B.W.; Valles, G.A.F.; Scheibler, R.B.; Schafhauser Junior, J.; Nornberg, J.L. Milk yield of cows submitted to different levels of olive pomace in the diet. Acta Sci. Anim. Sci. 2020, 43, e51158. [Google Scholar] [CrossRef]
- Tzamaloukas, O.; Neofytou, M.C.; Simitzis, P.E. Application of olive by-products in livestock with emphasis on small ruminants: Implications on rumen function, growth performance, milk and meat quality. Animals 2021, 11, 531. [Google Scholar] [CrossRef]
- Claro-Cala, C.M.; Quintela, J.C.; Pérez-Montero, M.; Miñano, J.; de Sotomayor, M.A.; Herrera, M.D.; Rodríguez-Rodríguez, A.R. Pomace olive oil concentrated in triterpenic acids restores vascular function, glucose tolerance, and obesity progression in mice. Nutrients 2020, 12, 323. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products: Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cells. Food Res. Int. 2018, 113, 392–400. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Nie, X.; He, Z.; Xiong, T.; Li, Y.; Bai, Y.; Zhang, H. Research Note: Dietary resveratrol supplementation improves the hepatic antioxidant capacity and attenuates lipopolysaccharide-induced inflammation in yellow-feathered broilers. Poult. Sci. 2023, 102, 102370. [Google Scholar] [CrossRef] [PubMed]
- Alba, D.F.; Campigotto, G.; Cazarotto, C.J.; Dos Santos, D.S.; Gebert, R.R.; Reis, J.H.; Souza, C.F.; Baldissera, M.D.; Gindri, A.L.; Kempka, A.P.; et al. Use of grape residue flour in lactating dairy sheep in heat stress: Effects on health, milk production and quality. J. Therm. Biol. 2019, 82, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Burgess, T.A.; Robich, M.P.; Chu, L.M.; Bianchi, C.; Sellke, F.W. Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle. Arch. Surg. 2011, 146, 556–564. [Google Scholar] [CrossRef]
- Son, M.J.; Minakawa, M.; Miura, Y.; Yagasaki, K. Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice. Eur. J. Nutr. 2013, 52, 1607–1619. [Google Scholar] [CrossRef]
- Castro, A.J.; Frederico, M.J.; Cazarolli, L.H.; Bretanha, L.C.; Tavares, L.C.; Buss, Z.S.; Dutra, M.F.; de Souza, A.Z.; Pizzolatti, M.G.; Silva, F.R. Betulinic acid and 1,25(OH)₂ vitamin D₃ share intracellular signal transduction in glucose homeostasis. Arch. Surg. 2014, 48, 18–27. [Google Scholar]
- Deng, Y.T.; Chang, T.W.; Lee, M.S.; Lin, J.K. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.M.; Kim, E.K.; Lee, J.O.; Lee, S.K.; Jung, J.H.; You, G.Y.; Park, S.H.; Suh, P.G.; Kim, H.S. Curcumin stimulates glucose uptake through AMPK-p38 MAPK pathways in L6 myotube cells. J. Cell. Physiol. 2010, 223, 771–778. [Google Scholar] [CrossRef]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Alshehri, M.A.; Sayed, S.; Shukry, M.; El-Sayed, Y.S. The Impact of Curcumin on Growth Performance, Growth-Related Gene Expression, Oxidative Stress, and Immunological Biomarkers in Broiler Chickens at Different Stocking Densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Yu, L.; Li, S.; Zhu, N.; You, J. Curcumin Supplementation Improves Growth Performance and Anticoccidial Index by Improving the Antioxidant Capacity, Inhibiting Inflammatory Responses, and Maintaining Intestinal Barrier Function in Eimeria tenella-Infected Broilers. Animals 2024, 14, 1223. [Google Scholar] [CrossRef]
- Novakoski, P.V.; de Vitt, M.G.; Molosse, V.L.; Xavier, A.C.H.; Wagner, R.; Klein, B.; Milarch, C.F.; Leonardi, L.E.; Kozloski, G.V.; Vedovatto, M.; et al. The addition of curcumin to the diet of post-weaning dairy calves: Effects on ruminal fermentation, immunological, and oxidative responses. Trop. Anim. Health Prod. 2024, 56, 142. [Google Scholar] [CrossRef] [PubMed]
- Fafoula, O.; Alkhayyat, H.; Hussain, K. Prolonged hyperinsulinaemic hypoglycaemia in newborns with intrauterine growth retardation. Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F467. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Thirone, A.; Huang, X.; Klip, A. Differential contribution of insulin receptor substrates 1 versus 2 to insulin signaling and glucose uptake in L6 myotubes. J. Biol. Chem. 2005, 280, 19426–19435. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; He, J.; Zhao, Y.; Shen, M.; Zhang, L.; Zhong, X.; Wang, C.; Wang, T. Effect of Curcumin on Growth Performance, Inflammation, Insulin level, and Lipid Metabolism in Weaned Piglets with IUGR. Animals 2019, 9, 1098. [Google Scholar] [CrossRef]
- Chiofalo, V.; Luigi, L.; Fiumanò, R.; Riolo, E.; Chiofalo, B. Influence of dietary supplementation of Rosmarinus officinalis L. on performances of dairy ewes organically managed. Small Rumin. Res. 2012, 104, 122–128. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Dai, D.; Cao, Z.; Wang, Y.; Li, S.; Wang, W. Preliminary Investigation of the Effects of Rosemary Extract Supplementation on Milk Production and Rumen Fermentation in High-Producing Dairy Cows. Antioxidants 2022, 11, 1715. [Google Scholar] [CrossRef]
- Yang, M.; Yin, Y.; Wang, F.; Bao, X.; Long, L.; Tan, B.; Yin, Y.; Chen, J. Effects of dietary rosemary extract supplementation on growth performance, nutrient digestibility, antioxidant capacity, intestinal morphology, and microbiota of weaning pigs. J. Anim. Sci. 2021, 99, 2311–2320. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Huang, X.; Zhang, X.; Deng, P.; Jiang, G.; Dai, Q. Dietary rosemary extract modulated gut microbiota and influenced the growth, meat quality, serum biochemistry, antioxidant, and immune capacities of broilers. Front. Microbiol. 2022, 13, 1024682. [Google Scholar] [CrossRef]
- Runtuwene, J.; Cheng, K.C.; Asakawa, A.; Amitani, H.; Amitani, M.; Morinaga, A.; Inui, A. Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des. Dev. Ther. 2016, 10, 2193–2202. [Google Scholar]
- Vlavcheski, F.; Naimi, M.; Murphy, B.; Hudlicky, T.; Tsiani, E. Rosmarinic acid, a rosemary extract polyphenol, increases skeletal muscle cell glucose uptake and activates AMPK. Molecules 2017, 22, 1669. [Google Scholar] [CrossRef]
- Serra, C.A.; Dos Reis, A.F.; Calsa, B.; Bueno, C.S.; Helaehil, J.V.; de Souza, S.A.R.; de Oliveira, C.A.; Vanzella, E.C.; do Amaral, M.E.C. Quercetin prevents insulin dysfunction in hypertensive animals. J. Diabetes Metab. Disord. 2022, 21, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, B.; Zhou, S.; Liu, J.; Lu, H.; Wu, H.; Ding, M.; Li, Y. Quercetin ameliorates chicken quality by activating the PI3K/PKB/AMPK signaling pathway in broilers. Front. Vet. Sci. 2022, 9, 951512. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Chaudhry, M.T.; Xiao, F.; Mao, Y.; Wang, M.; Wang, B.; Wang, S.; Li, Y. The effects and mechanism of quercetin dietary supplementation in streptozotocin-induced hyperglycemic Arbor Acre broilers. Oxid. Med. Cell. Longev. 2020, 2020, 9585047. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.A.; Elbestawy, A.R.; El-Far, A.H.; Noreldin, A.E.; Emam, M.; Baty, R.S.; Albadrani, G.M.; Abdel-Daim, M.M.; Abd El-Hamid, H.S. Quercetin dietary supplementation advances growth performance, gut microbiota, and intestinal mRNA expression genes in broiler chickens. Animals 2021, 11, 2302. [Google Scholar] [CrossRef] [PubMed]
- Stoldt, A.-K.; Mielenz, M.; Nürnberg, G.; Sauerwein, H.; Esatbeyoglu, T.; Wagner, A.E.; Rimbach, G.; Starke, A.; Wolffram, S.; Metges, C.C. Effects of a six-week intraduodenal supplementation with quercetin on liver lipid metabolism and oxidative stress in peripartal dairy cows. J. Anim. Sci. 2016, 94, 1913–1923. [Google Scholar] [CrossRef]
- Park, J.H.; Sureshkumar, S.; Kim, I.H. Influences of dietary flavonoid (quercetin) supplementation on growth performance and immune response of growing pigs challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. Technol. 2020, 62, 605–613. [Google Scholar] [CrossRef]
- Casalino, G.; Dinardo, F.R.; D’Amico, F.; Bozzo, G.; Bove, A.; Camarda, A.; Lombardi, R.; Dimuccio, M.M.; Circella, E. Antimicrobial efficacy of cinnamon essential oil against avian pathogenic Escherichia coli from poultry. Animals 2023, 13, 2639. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano essential oil induces SOD1 and GSH expression through Nrf2 activation and alleviates hydrogen peroxide-induced oxidative damage in IPEC-J2 Cells. Oxid. Med. Cell. Longev. 2016, 2016, 5987183. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Sun, H.; Ao, J.; Long, G.; Jiang, S. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. BioMed Res. Int. 2015, 2015, 525218. [Google Scholar] [CrossRef]
- Zhao, B.C.; Wang, T.H.; Chen, J.; Qiu, B.H.; Xu, Y.R.; Zhang, Q.; Li, J.J.; Wang, C.J.; Nie, Q.F.; Li, J.L. Effects of dietary supplementation with a carvacrol–cinnamaldehyde–thymol blend on growth performance and intestinal health of nursery pigs. Porc. Health Manag. 2023, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Mai, Y.; Qiu, X.; Yuan, W.; Li, Y.; Luo, C.; Liu, Y.; Zhang, G.; Zhao, G.; Luo, J.D. Carvacrol attenuates diabetic cardiomyopathy by modulating the PI3K/AKT/GLUT4 pathway in diabetic mice. Front. Pharmacol. 2019, 10, 998. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Murali, K.Y.; Tandon, V.; Murthy, P.S.; Chandra, R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.E.; Ort, S.B.; Aragona, K.M.; Cabral, R.G.; Erickson, P.S. Effect of cinnamaldehyde on feed intake, rumen fermentation, and nutrient digestibility in lactating dairy cows. J. Anim. Sci. 2019, 97, 1819–1827. [Google Scholar] [CrossRef]
- Benchaar, C. Feeding oregano oil and its main component carvacrol does not affect ruminal fermentation, nutrient utilization, methane emissions, milk production, or milk fatty acid composition of dairy cows. J. Dairy Sci. 2020, 103, 1516–1527. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Terzić-Vidojević, A.; Veljović, K.; Tolinački, M.; Živković, M.; Lukić, J.; Lozo, J.; Fira, Đ.; Jovčić, B.; Strahinić, I.; Begović, J.; et al. Diversity of non-starter lactic acid bacteria in autochthonous dairy products from Western Balkan Countries—Technological and probiotic properties. Food Res. Int. 2020, 136, 109494. [Google Scholar] [CrossRef]
- Zhang, W.H.; Jiang, Y.; Zhu, Q.F.; Gao, F.; Dai, S.F.; Chen, J.; Zhou, G.H. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 2011, 52, 292–301. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Fernández-Rubio, C.; Ordóñez, C.; Abad-González, J.; Garcia-Gallego, A.; Honrubia, M.P.; Mallo, J.J.; Balaña-Fouce, R. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult. Sci. 2009, 88, 943–948. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, G.; Meng, X.; Wang, X.; Zhao, Z.; Zhou, S.; Li, G.; Zhang, Q.; Wei, X. Effects of Lactobacillus plantarum and Pediococcus acidilactici co-fermented feed on growth performance and gut microbiota of nursery pigs. Front. Vet. Sci. 2022, 9, 1076906. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Galle, S.; Yang, Y.; Landero, J.L.; Beltranena, E.; Gänzle, M.G.; Zijlstra, R.T. Effects of feeding fermented wheat with Lactobacillus reuteri on gut morphology, intestinal fermentation, nutrient digestibility, and growth performance in weaned pigs. J. Anim. Sci. 2016, 94, 4677–4687. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.H.; Chen, Y.P.; Chen, M.J. Selecting probiotics with the abilities of enhancing GLP-1 to mitigate the progression of type 1 diabetes in vitro and in vivo. J. Funct. Foods 2015, 18, 473–486. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Wang, J.; Qiu, X.; Qi, R.; Huang, J. Identification of differentially expressed miRNAs after Lactobacillus reuteri treatment in the ileum mucosa of piglets. Genes. Genom. 2020, 42, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Cassir, N.; Benamar, S.; Scola, B.L. Clostridium butyricum: From beneficial to a new emerging pathogen. Clin. Microbiol. Infect. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Ariyoshi, T.; Hagihara, M.; Takahashi, M.; Mikamo, H. Effect of Clostridium butyricum on gastrointestinal infections. Biomedicines 2022, 10, 483. [Google Scholar] [CrossRef]
- Casas, G.A.; Blavi, L.; Cross, T.W.L.; Lee, A.H.; Swanson, K.S.; Stein, H.H. Inclusion of the direct-fed microbial Clostridium butyricum in diets for weanling pigs increases growth performance and tends to increase villus height and crypt depth, but does not change intestinal microbial abundance. J. Anim. Sci. 2020, 98, skz372. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Zhan, X.; Zeng, X.; Zhou, L.; Cao, G.; Chen, A.; Yang, C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Huang, L.; Xia, Z. A discovery of relevant hepatoprotective effects and underlying mechanisms of dietary Clostridium butyricum against corticosterone-induced liver injury in Pekin ducks. Microorganisms 2019, 7, 358. [Google Scholar] [CrossRef]
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci. Rep. 2017, 7, 7046. [Google Scholar]
- Sun, R.; Li, D.; Sun, M.; Miao, X.; Jin, X.; Xu, X.; Su, Y.; Xu, H.; Wang, J.; Niu, H. Bacillus natto ameliorates obesity by regulating PI3K/AKT pathways in rats. Biochem. Biophys. Res. Commun. 2022, 603, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Y.; Cui, Y.; Gao, B.; Zhang, H.; Jiang, Q.; Loor, J.J.; Deng, Z.; Xu, C. Bacillus subtilis produces amino acids to stimulate protein synthesis in ruminal tissue explants via the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta–serine/threonine kinase–mammalian target of rapamycin complex 1 pathway. Front. Vet. Sci. 2022, 9, 852321. [Google Scholar]
- Chen, X.; Zhao, H.; Meng, F.; Zhou, L.; Pang, X.; Lu, Z.; Lu, Y. Ameliorated effects of a lipopeptide surfactin on insulin resistance in vitro and in vivo. Food Sci. Nutr. 2022, 10, 2455–2469. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Afzal, N.U.; Wann, S.B.; Kalita, J.; Manna, P. A ~24 kDa protein isolated from protein isolates of Hawaijar, popular fermented soy food of North-East India exhibited promising antidiabetic potential via stimulating PI3K/AKT/GLUT4 signaling pathway of muscle glucose metabolism. Int. J. Biol. Macromol. 2023, 224, 1025–1039. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. Multi-strain probiotics: Synergy among isolates enhances biological activities. Biology 2021, 10, 322. [Google Scholar] [CrossRef]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Moroti, C.; Souza Magri, L.F.; de Rezende, C.M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef]
- Ryan, W.R.; DeSocio, E.S.; Youngers, M.E.; Lockard, C.G.; Richards, C.J.; Trojan, S.J.; Hergenreder, J.E.; Wilson, B.K. Effects of feeding CLOSTAT (Bacillus subtilis PB6) on the clinical health, performance, and carcass characteristics of feedlot steers. Transl. Anim. Sci. 2023, 7, txad047. [Google Scholar] [CrossRef]
- Dowd, S.E.; Callaway, T.R.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Hagevoort, R.G.; Edrington, T.S. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008, 8, 125. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Martin, R.; Chamignon, C.; Mhedbi-Hajri, N.; Chain, F.; Derrien, M.; Escribano-Vázquez, U.; Garault, P.; Cotillard, A.; Pham, H.-P.; Chervaux, C.; et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 2019, 9, 5398. [Google Scholar] [CrossRef]
- Jana, U.K.; Suryawanshi, R.K.; Prajapati, B.P.; Kango, N. Prebiotic Mannooligosaccharides: Synthesis, Characterization and Bioactive Properties. Food Chem. 2021, 342, 128328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, L.; Tan, W.; Li, Y.; Sun, L.; Zhu, X.; Wang, S.; Gao, P.; Zhu, C.; Shu, G.; et al. Mannan Oligosaccharides Promoted Skeletal Muscle Hypertrophy through the Gut Microbiome and Microbial Metabolites in Mice. Foods 2023, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, J.; Li, Y.; Wang, N.; Yan, Q.; Jiang, Z. Manno-oligosaccharides from cassia seed gum ameliorate inflammation and improve glucose metabolism in diabetic rats. Food Funct. 2022, 13, 6674–6687. [Google Scholar] [CrossRef]
- Saeed, M.; Ahmad, F.; Arain, M.; Abd El-Hack, M.; Emam, M.; Bhutto, Z.; Moshaveri, A. Use of Mannan-Oligosaccharides (MOS) As a Feed Additive in Poultry Nutrition. J. Anim. Sci. 2017, 7, 94–103. [Google Scholar]
- Bagheri, M.; Ghorbani, G.R.; Rahmani, H.R.; Khorvash, M.; Nili, N.; Südekum, K.-H. Effect of Live Yeast and Mannan-oligosaccharides on Performance of Early-lactation Holstein Dairy Cows. Asian-Australas. J. Anim. Sci. 2009, 22, 938–944. [Google Scholar] [CrossRef]
- Li, T.; Xu, L.; Yan, Q.; Liu, J.; Jiang, Z. Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet-induced inflammation, glucose, and lipid metabolism in the liver of mice. Food Sci. Hum. Wellness 2022, 11, 1064–1075. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rajeswari, D. Biomedical aspects of beta-glucan on glucose metabolism and its role on primary gene PIK3R1. J. Funct. Foods 2022, 99, 105296. [Google Scholar] [CrossRef]
- Erdem, N.; Montero, E.; Roep, B.O. Breaking and restoring immune tolerance to pancreatic beta-cells in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 397–403. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Miao, M.; Dai, Y.; Rui, C.; Fan, Y.; Wang, X.; Fan, C.; Mu, J.; Hou, W.; Dong, Z.; Li, P.; et al. Dietary supplementation of inulin alleviates metabolism disorders in gestational diabetes mellitus mice via RENT/AKT/IRS/GLUT4 pathway. Diabetol. Metab. Syndr. 2021, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, P.; Thiyagarajan, G.; Arun Babu, R.; Lakshmi, B.S. Raffinose from Costus speciosus attenuates lipid synthesis through modulation of PPARs/SREBP1c and improves insulin sensitivity through PI3K/AKT. Chem. Biol. Interact. 2018, 284, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wang, X.; Cho, S.B.; Wu, Y.L.; Wei, C.; Han, S.; Bao, L.; Wu, Q.; Ao, W.; Nan, J.X. Agriophyllum oligosaccharides ameliorate diabetic insulin resistance through INS-R/IRS/GLUT4-mediated insulin pathway in db/db mice and MIN6 cells. Front. Pharmacol. 2021, 12, 656220. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Guo, X.; Zhou, Y.; Cao, G. The effects of probiotics/synbiotics on glucose and lipid metabolism in women with gestational diabetes mellitus: A meta-analysis of randomized controlled trials. Nutrients 2023, 15, 1375. [Google Scholar] [CrossRef]
- Horvath, A.; Leber, B.; Feldbacher, N.; Tripolt, N.; Rainer, F.; Blesl, A.; Trieb, M.; Marsche, G.; Sourij, H.; Stadlbauer, V. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: A randomized, double-blind, placebo-controlled pilot study. Eur. J. Nutr. 2020, 59, 2969–2983. [Google Scholar] [CrossRef]
- Khan, A.; Sarkar, E.; Afrin, G.; Misra, A.; Chandra, A. Role of symbiotics in the treatment of diabetes mellitus via modification of the immune system. In Biochemical Immunology of Diabetes and Associated Complications; Tripathi, P., Tripathi, R.P., Kaushik, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 289–303. ISBN 9780443131950. [Google Scholar]
- Sun, Z.; Shao, Y.; Yan, K.; Yao, T.; Liu, L.; Sun, F.; Wu, J.; Huang, Y. The link between trace metal elements and glucose metabolism: Evidence from zinc, copper, iron, and manganese-mediated metabolic regulation. Metabolites 2023, 13, 1048. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, M.; Zhou, J.; Yang, Z.; Xiao, M.; Du, L.; Bao, M.; Ju, J.; Dong, C.; Zheng, Y.; et al. Effects of organic trace minerals chelated with oligosaccharides on growth performance, blood parameters, slaughter performance, and meat quality in sheep. Front. Vet. Sci. 2024, 11, 1366314. [Google Scholar] [CrossRef]
- Harvey, K.M.; Cooke, R.F.; Marques, R.D.S. Supplementing Trace Minerals to Beef Cows during Gestation to Enhance Productive and Health Responses of the Offspring. Animals 2021, 11, 1159. [Google Scholar] [CrossRef]
- Mohammadi, F.; Soltani, A.; Ghahremanloo, A.; Javid, H.; Hashemy, S.I. The thioredoxin system and cancer therapy: A review. Cancer Chemother. Pharmacol. 2019, 84, 925–935. [Google Scholar] [CrossRef]
- Fontenelle, L.C.; Feitosa, M.M.; Morais, J.B.S.; Severo, J.S.; Freitas, T.E.C.D.; Beserra, J.B.; Henriques, G.S.; Marreiro, D.D.N. The role of selenium in insulin resistance. Braz. J. Pharm. Sci. 2018, 54, e00139. [Google Scholar] [CrossRef]
- Torres, D.J.; Alfulaij, N.; Berry, M.J. Stress and the Brain: An Emerging Role for Selenium. Front. Neurosci. 2021, 15, 666601. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, B.M.; Leal, D.F.; Frias-De-Diego, A.; Browning, M.; Odle, J.; Crisci, E. The health benefits of selenium in food animals: A review. J. Anim. Sci. Biotechnol. 2022, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.; Samóra, T.; Libera, A.; Netto, A.; Junior, L.; Blagitz, M.; El Faro, L.; Souza, F.; Batista, C.; Salles, F.; et al. Selenium and vitamin E supplementation ameliorates the oxidative stress of lactating cows. Livest. Sci. 2021, 255, 104807. [Google Scholar] [CrossRef]
- Krejpcio, Z. Chromium and Insulin Signaling. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Wang, Z.Q.; Zhang, X.H.; Russell, J.C.; Hulver, M.; Cefalu, W.T. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR rats. J. Nutr. 2006, 136, 415–420. [Google Scholar] [CrossRef]
- Mackowiak, P.; Krejpcio, Z.; Sassek, M.; Kaczmarek, P.; Hertig, I.; Chmielewska, J.; Wojciechowicz, T.; Szczepankiewicz, D.; Wieczorek, D.; Szymusiak, H.; et al. Evaluation of insulin binding and signaling activity of newly synthesized chromium(III) complexes in vitro. Mol. Med. Rep. 2010, 3, 347–353. [Google Scholar]
- Zhao, P.; Wang, J.; Ma, H.; Xiao, Y.; He, L.; Tong, C.; Wang, Z.; Zheng, Q.; Dolence, E.K.; Nair, S.; et al. A newly synthetic chromium complex-chromium (D-phenylalanine)3 activates AMP-activated protein kinase and stimulates glucose transport. Biochem. Pharmacol. 2009, 77, 1002–1010. [Google Scholar] [CrossRef]
- Hua, Y.; Clark, S.; Ren, J.; Sreejayan, N. Molecular mechanisms of chromium in alleviating insulin resistance. J. Nutr. Biochem. 2012, 23, 313–319. [Google Scholar] [CrossRef]
- Hayirli, A.; Bremmer, D.R.; Bertics, S.J.; Socha, M.T.; Grummer, R.R. Effect of chromium supplementation on production and metabolic parameters in periparturient dairy cows. J. Dairy Sci. 2001, 84, 1218–1230. [Google Scholar] [CrossRef]
- Yasui, T.; McArt, J.A.; Ryan, C.M.; Gilbert, R.O.; Nydam, D.V.; Valdez, F.; Griswold, K.E.; Overton, T.R. Effects of chromium propionate supplementation during the periparturient period and early lactation on metabolism, performance, and cytological endometritis in dairy cows. J. Dairy Sci. 2014, 97, 6400–6410. [Google Scholar] [CrossRef]
- Sumner, J.; Valdez, F.; McNamara, J. Effects of chromium propionate on response to an intravenous glucose tolerance test in growing Holstein heifers. J. Dairy Sci. 2007, 90, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cottrell, J.J.; Wijesiriwardana, U.; Kelly, F.W.; Chauhan, S.S.; Pustovit, R.V.; Gonzales-Rivas, P.A.; DiGiacomo, K.; Leury, B.J.; Celi, P.; et al. Effects of chromium supplementation on physiology, feed intake, and insulin related metabolism in growing pigs subjected to heat stress. Transl. Anim. Sci. 2017, 1, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sahin, N.; Hayirli, A.; Orhan, C.; Tuzcu, M.; Akdemir, F.; Komorowski, J.R.; Sahin, K. Effects of the supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress. Poult. Sci. 2017, 96, 4317–4324. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.N.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; Oz, F. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.C.; Gray, G.C. Pro-oxidant activity of flavonoids: Effects on glutathione and glutathione S-transferase in isolated rat liver nuclei. Cancer Lett. 1996, 104, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Joyner, P.M. Protein Adducts and Protein Oxidation as Molecular Mechanisms of Flavonoid Bioactivity. Molecules 2021, 26, 5102. [Google Scholar] [CrossRef]
- Janeiro, P.; Oliveira Brett, A.M. Catechin Electrochemical Oxidation Mechanisms. Anal. Chim. Acta 2004, 518, 109–115. [Google Scholar] [CrossRef]
- Brett, A.M.O.; Ghica, M.-E. Electrochemical Oxidation of Quercetin. Electroanalysis 2003, 15, 1745–1750. [Google Scholar] [CrossRef]
- Taras, D.; Vahjen, W.; Macha, M.; Simon, O. Performance, diarrhea incidence, and occurrence of Escherichia coli virulence genes during long-term administration of a probiotic Enterococcus faecium strain to sows and piglets. J. Anim. Sci. 2006, 84, 608–617. [Google Scholar] [CrossRef]
- Hamid, N.H.; Daud, H.M.; Kayansamruaj, P.; Hassim, H.A.; Mohd Yusoff, M.S.; Abu Bakar, S.N.; Srisapoome, P. Short- and long-term probiotic effects of Enterococcus hirae isolated from fermented vegetable wastes on the growth, immune responses, and disease resistance of hybrid catfish (Clarias gariepinus × Clarias macrocephalus). Fish. Shellfish. Immunol. 2021, 114, 1–19. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Durand, H. Probiotics in animal nutrition and health. Benef. Microbes 2010, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- FDA 21 CFR Part 573.304. Federal Register Vol. 81, No. 107. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=573.304 (accessed on 12 September 2024).
- Lloyd, K.E.; Fellner, V.; McLeod, S.J.; Fry, R.S.; Krafka, K.; Lamptey, A.; Spears, J.W. Effects of supplementing dairy cows with chromium propionate on milk and tissue chromium concentrations. J. Dairy Sci. 2010, 93, 4774–4780. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.W.; Lloyd, K.E.; Pickworth, C.A.; Huang, Y.L.; Krafka, K.; Hyda, J.; Grimes, J.L. Chromium propionate in broilers: Human food and broiler safety. Poult. Sci. 2019, 98, 6579–6585. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.B. Chromium: Celebrating 50 years as an essential element? Dalton Trans. 2010, 39, 3787–3794. [Google Scholar] [CrossRef]
- Stout, M.D.; Nyska, A.; Collins, B.J.; Witt, K.L.; Kissling, G.E.; Malarkey, D.E.; Hooth, M.J. Chronic toxicity and carcinogenicity studies of chromium picolinate monohydrate administered in feed to F344/N rats and B6C3F1 mice for 2 years. Food Chem. Toxicol. 2009, 47, 729–733. [Google Scholar] [CrossRef]
- Semo, D.; Reinecke, H.; Godfrey, R. Gut microbiome regulates inflammation and insulin resistance: A novel therapeutic target to improve insulin sensitivity. Signal Transduct. Target. Ther. 2024, 9, 35. [Google Scholar] [CrossRef]
- Youssef, M.A.; El-Ashker, M.R.; Younis, M.S. The effect of subclinical ketosis on indices of insulin sensitivity and selected metabolic variables in transition dairy cattle. Comp. Clin. Pathol. 2017, 26, 329–334. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-C.; Hoe, B.-C.; Li, X.; Lian, D.; Zeng, X. Glucose Metabolism-Modifying Natural Materials for Potential Feed Additive Development. Pharmaceutics 2024, 16, 1208. https://doi.org/10.3390/pharmaceutics16091208
Lin W-C, Hoe B-C, Li X, Lian D, Zeng X. Glucose Metabolism-Modifying Natural Materials for Potential Feed Additive Development. Pharmaceutics. 2024; 16(9):1208. https://doi.org/10.3390/pharmaceutics16091208
Chicago/Turabian StyleLin, Wei-Chih, Boon-Chin Hoe, Xianming Li, Daizheng Lian, and Xiaowei Zeng. 2024. "Glucose Metabolism-Modifying Natural Materials for Potential Feed Additive Development" Pharmaceutics 16, no. 9: 1208. https://doi.org/10.3390/pharmaceutics16091208
APA StyleLin, W.-C., Hoe, B.-C., Li, X., Lian, D., & Zeng, X. (2024). Glucose Metabolism-Modifying Natural Materials for Potential Feed Additive Development. Pharmaceutics, 16(9), 1208. https://doi.org/10.3390/pharmaceutics16091208








