Exploring Cationic Guar Gum: Innovative Hydrogels and Films for Enhanced Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cationic Guar Gum-Based Formulations
2.3. Semisolid Characterization
2.4. Films’ Characterization
2.5. Fourier Transformed Infrared (FTIR) Spectroscopy
2.6. Chemometrics Analysis—PCA Model
2.7. Bioadhesive Strength
2.8. Antioxidant Activity
2.9. Biocompatibility Evaluation
2.9.1. Hemolysis Assay
2.9.2. Cell Viability Assay
2.10. Wound-Healing Assay
2.11. Statistical Analyses
3. Results and Discussion
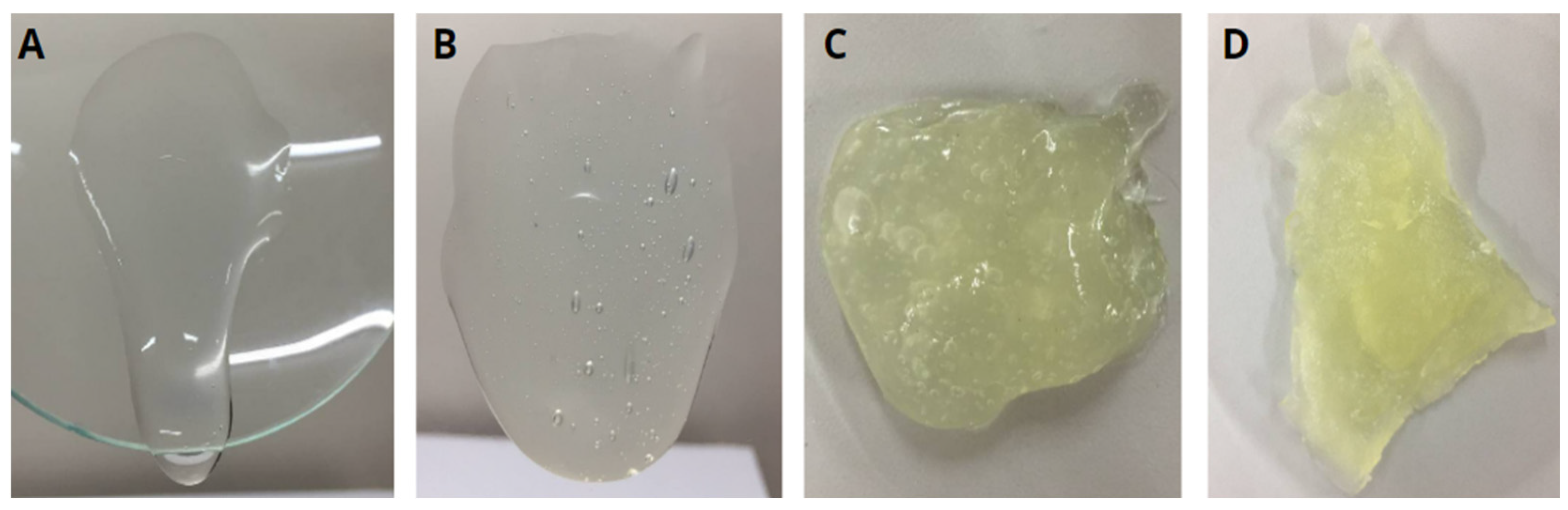
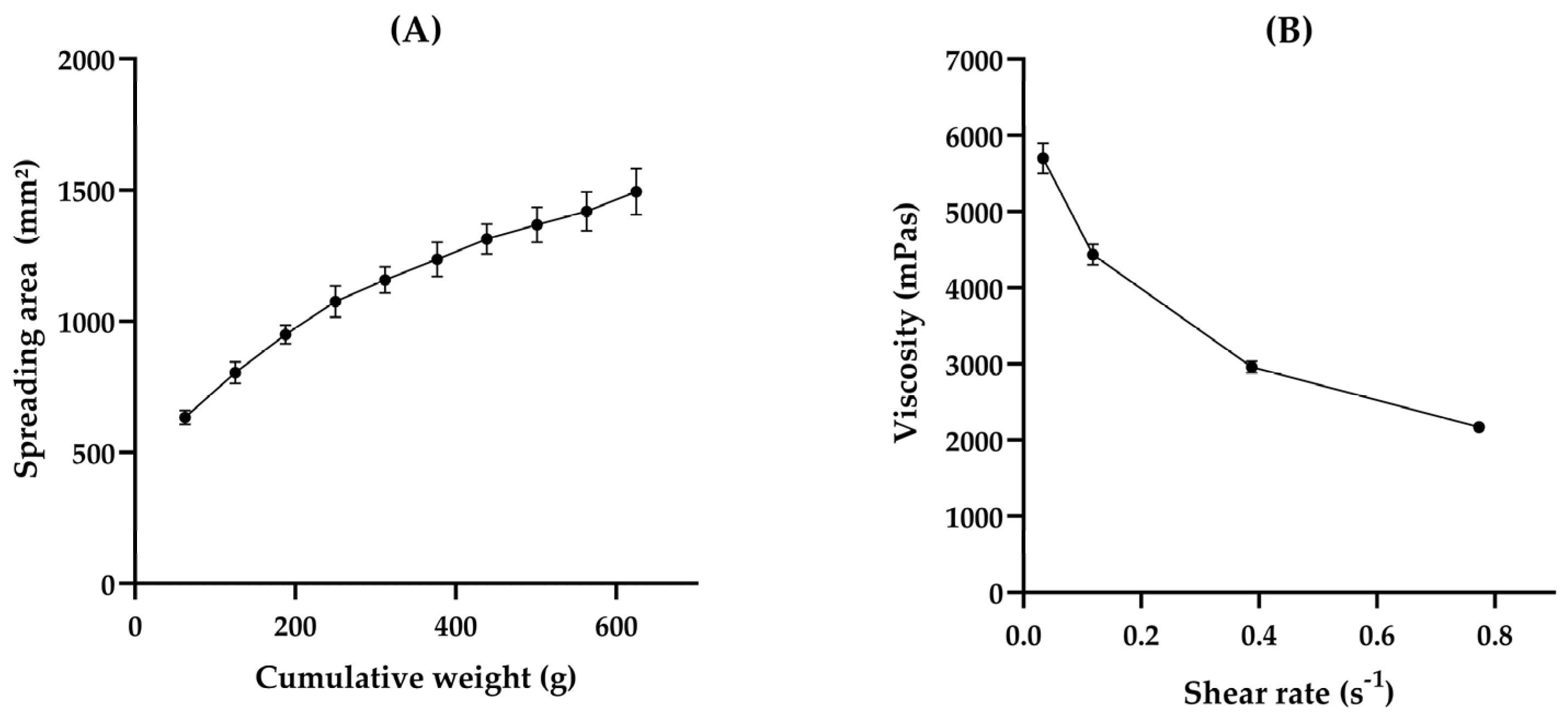
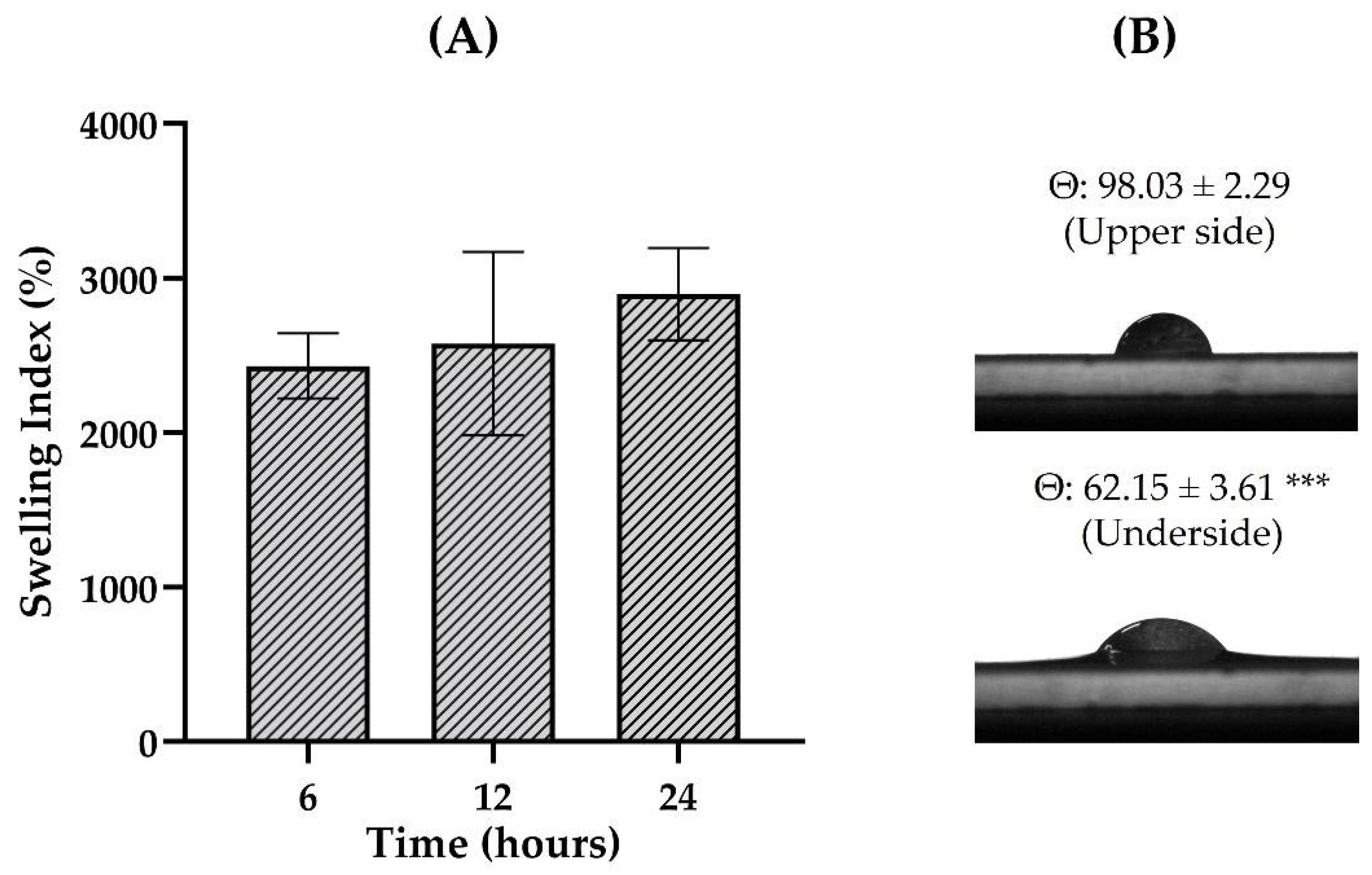
3.1. HG Preparation and Characterization
3.2. Film Preparation and Characterization
3.3. FTIR Spectroscopy
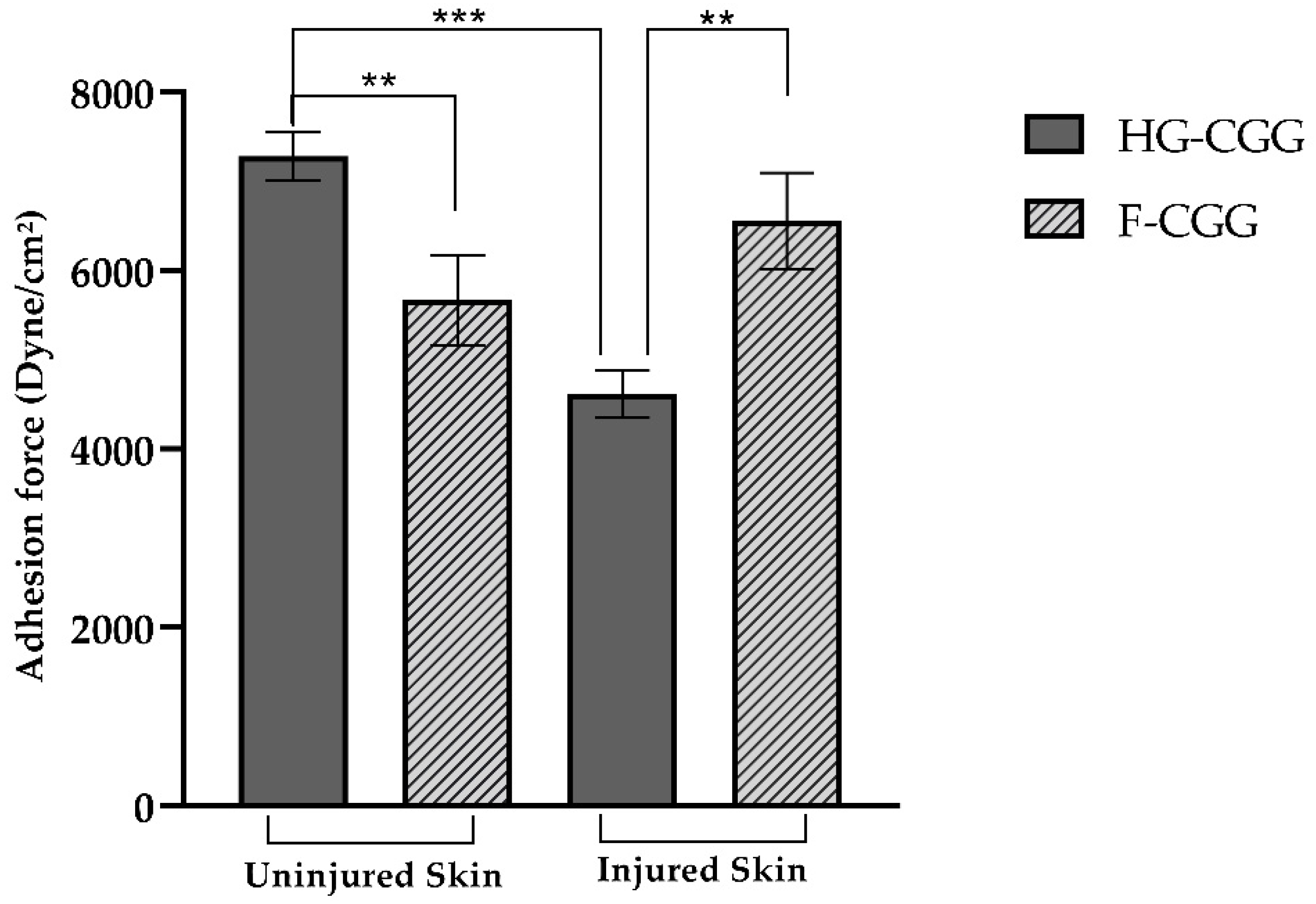
3.4. Bioadhesive Strength
3.5. Antioxidant Activity
3.6. Safety Assays
3.7. Wound-Healing Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, V.; Trivedi, P. In Vitro and In Vivo Characterization of Pharmaceutical Topical Nanocarriers Containing Anticancer Drugs for Skin Cancer Treatment. In Lipid Nanocarriers for Drug Targeting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 563–627. [Google Scholar] [CrossRef]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and Function of the Epidermis Related to Barrier Properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.H.M.; Cobre, A.d.F.; Pontarolo, R.; Ferreira, L.M. Status and Future Scope of Soft Nanoparticles-Based Hydrogel in Wound Healing. Pharmaceutics 2023, 15, 874. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Henrique Marcondes Sari, M.; Mota Ferreira, L.; Cruz, L. The Use of Natural Gums to Produce Nano-Based Hydrogels and Films for Topical Application. Int. J. Pharm. 2022, 626, 122166. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Alcalá-Alcalá, S.; Almarhoon, Z.M.; Turgumbayeva, A.; Gürer, E.S.; De Los Dolores Campos-Echeverria, M.; Cortés, H.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Sharifi-Rad, J.; et al. Novel Ultra-Stretchable and Self-Healing Crosslinked Poly (Ethylene Oxide)-Cationic Guar Gum Hydrogel. J. Biol. Eng. 2023, 17, 64. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Zhao, Z. Facile Fabrication of Self-Healing, Injectable and Antimicrobial Cationic Guar Gum Hydrogel Dressings Driven by Hydrogen Bonds. Carbohydr. Polym. 2023, 310, 120723. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Li, Z.; Wang, X.; Duan, C.; Zhao, W.; Xiong, C.; Nie, S.; Xu, Y.; Ni, Y. A Multifunctional Self-Crosslinked Chitosan/Cationic Guar Gum Composite Hydrogel and Its Versatile Uses in Phosphate-Containing Water Treatment and Energy Storage. Carbohydr. Polym. 2020, 244, 116472. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, J. Thin Films as an Emerging Platform for Drug Delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Leng, Q.Q.; Li, Y.; Pang, X.L.; Wang, B.Q.; Wu, Z.X.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S.Z. Curcumin Nanoparticles Incorporated in PVA/Collagen Composite Films Promote Wound Healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Parekh, K.; Mehta, T.A.; Dhas, N.; Kumar, P.; Popat, A. Emerging Nanomedicines for the Treatment of Atopic Dermatitis. AAPS PharmSciTech 2021, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, S.; Dhiman, A. Design of Antibiotic Containing Hydrogel Wound Dressings: Biomedical Properties and Histological Study of Wound Healing. Int. J. Pharm. 2013, 457, 82–91. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Wang, Y.; Lu, H.; Nie, S.; He, H.; Duan, C.; Ni, Y. Injectable All-Polysaccharide Self-Assembling Hydrogel: A Promising Scaffold for Localized Therapeutic Proteins. Cellulose 2019, 26, 6891–6901. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Du, B.; Li, P.; Li, H. Associating and Rheological Behaviors of Fluorinated Cationic Guar Gum in Aqueous Solutions. Carbohydr. Polym. 2013, 95, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tiwary, A.K.; Kaur, G. Investigations on Interpolymer Complexes of Cationic Guar Gum and Xanthan Gum for Formulation of Bioadhesive Films. Res. Pharm. Sci. 2010, 5, 79. [Google Scholar] [PubMed]
- Sreedevi Madhavikutty, A.; Singh Chandel, A.K.; Tsai, C.C.; Inagaki, N.F.; Ohta, S.; Ito, T. PH Responsive Cationic Guar Gum-Borate Self-Healing Hydrogels for Muco-Adhesion. Sci. Technol. Adv. Mater. 2023, 24, 2175586. [Google Scholar] [CrossRef]
- Da Silva, M.J.F.; Rodrigues, A.M.; Vieira, I.R.S.; Neves, G.D.A.; Menezes, R.R.; Gonçalves, E.D.G.D.R.; Pires, M.C.C. Development and Characterization of a Babassu Nut Oil-Based Moisturizing Cosmetic Emulsion with a High Sun Protection Factor. RSC Adv. 2020, 10, 26268–26276. [Google Scholar] [CrossRef]
- Rigo, L.A.; Weber, J.; Silva, C.B.; Beck, R.C. Evaluation of the Spreadability of Pharmaceutical or Cosmetic Semisolid Formulations Using Scanned Images. Lat. Am. J. Pharm. 2012, 31, 1387–1391. [Google Scholar]
- Gehrcke, M.; de Bastos Brum, T.; da Rosa, L.S.; Ilha, B.D.; Soares, F.Z.M.; Cruz, L. Incorporation of Nanocapsules into Gellan Gum Films: A Strategy to Improve the Stability and Prolong the Cutaneous Release of Silibinin. Mater. Sci. Eng. C 2021, 119, 111624. [Google Scholar] [CrossRef]
- ASTM D882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2024. Available online: https://www.astm.org/d0882-02.html (accessed on 31 July 2024).
- Reolon, J.B.; Saccol, C.P.; Osmari, B.F.; Oliveira, D.B.d.; Prado, V.C.; Cabral, F.L.; da Rosa, L.S.; Rechia, G.C.; Leal, D.B.R.; Cruz, L. Karaya/Gellan-Gum-Based Bilayer Films Containing 3,3′-Diindolylmethane-Loaded Nanocapsules: A Promising Alternative to Melanoma Topical Treatment. Pharmaceutics 2023, 15, 2234. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, T.; Rothmaier, M.; Zander, H.; Ring, J.; Gutermuth, J.; Anliker, M.D. Acid-Coated Textiles (PH 5.5–6.5)—A New Therapeutic Strategy for Atopic Eczema? Acta Derm. Venereol. 2015, 95, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.M.S.; El Zein, A.K.; Cobre, A.d.F.; Lazo, R.E.L.; Reolon, J.B.; Marchiori, C.; Costa, J.S.d.; Pontarolo, R.; Fajardo, A.R.; Sari, M.H.M.; et al. Aloe Vera Miller Extract as a Plasticizer Agent to Polymeric Films: A Structural and Functional Component. J. Drug Deliv. Sci. Technol. 2024, 99, 105982. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal Component Analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Mishra, P.; Rutledge, D.N.; Roger, J.M.; Wali, K.; Khan, H.A. Chemometric Pre-Processing Can Negatively Affect the Performance of near-Infrared Spectroscopy Models for Fruit Quality Prediction. Talanta 2021, 229, 122303. [Google Scholar] [CrossRef]
- Osmari, B.F.; Giuliani, L.M.; Reolon, J.B.; Rigo, G.V.; Tasca, T.; Cruz, L. Gellan Gum-Based Hydrogel Containing Nanocapsules for Vaginal Indole-3-Carbinol Delivery in Trichomoniasis Treatment. Eur. J. Pharm. Sci. 2020, 151, 105379. [Google Scholar] [CrossRef]
- Barbalho, G.N.; Matos, B.N.; Espirito Santo, M.E.L.; Silva, V.R.C.; Chaves, S.B.; Gelfuso, G.M.; Cunha-Filho, M.; Gratieri, T. In Vitro Skin Model for the Evaluation of Burn Healing Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2021, 62, 102330. [Google Scholar] [CrossRef]
- Sari, M.H.M.; Saccol, C.P.; Custódio, V.N.; da Rosa, L.S.; da Costa, J.S.; Fajardo, A.R.; Ferreira, L.M.; Cruz, L. Carrageenan-Xanthan Nanocomposite Film with Improved Bioadhesion and Permeation Profile in Human Skin: A Cutaneous-Friendly Platform for Ketoprofen Local Delivery. Int. J. Biol. Macromol. 2024, 265, 130864. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.H.M.; Fulco, B.d.C.W.; Ferreira, L.M.; Pegoraro, N.S.; Brum, E.d.S.; Casola, K.K.; Marchiori, M.C.L.; de Oliveira, S.M.; Nogueira, C.W.; Cruz, L. Nanoencapsulation Potentiates the Cutaneous Anti-Inflammatory Effect of p,P′-Methoxyl-Diphenyl Diselenide: Design, Permeation, and in Vivo Studies of a Nanotechnological-Based Carrageenan Gum Hydrogel. Eur. J. Pharm. Sci. 2020, 153, 105500. [Google Scholar] [CrossRef]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-Based Biomaterials for Pharmaceutical and Biomedical Applications: A Focus on Topical Drug Administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Salehi, M.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Ebrahimi-Barough, S. Accelerating Healing of Excisional Wound with Alginate Hydrogel Containing Naringenin in Rat Model. Drug Deliv. Transl. Res. 2021, 11, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Tong, M.Q.; Wang, L.F.; Chen, R.; Li, X.Z.; Sohawon, Y.; Yao, Q.; Xiao, J.; Zhao, Y.Z. Thiolated γ-Polyglutamic Acid as a Bioadhesive Hydrogel-Forming Material: Evaluation of Gelation, Bioadhesive Properties and Sustained Release of KGF in the Repair of Injured Corneas. Biomater. Sci. 2019, 7, 2582–2599. [Google Scholar] [CrossRef]
- Merg, C.D.; Reolon, J.B.; Rechia, G.C.; Cruz, L. Locust Bean Gum Hydrogels Are Bioadhesive and Improve Indole-3-Carbinol Cutaneous Permeation: Influence of the Polysaccharide Concentration. Braz. J. Pharm. Sci. 2023, 59, e21770. [Google Scholar] [CrossRef]
- Giuliani, L.M.; Pegoraro, N.S.; Camponogara, C.; Osmari, B.F.; de Bastos Brum, T.; Reolon, J.B.; Rechia, G.C.; Oliveira, S.M.; Cruz, L. Locust Bean Gum-Based Hydrogel Containing Nanocapsules for 3,3′-Diindolylmethane Delivery in Skin Inflammatory Conditions. J. Drug Deliv. Sci. Technol. 2022, 78, 103960. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Azambuja, J.H.; da Silveira, E.F.; Cervi, V.F.; Marchiori, M.C.L.; Maria-Engler, S.S.; Wink, M.R.; Azevedo, J.G.; Nogueira, C.W.; et al. Xanthan Gum-Based Hydrogel Containing Nanocapsules for Cutaneous Diphenyl Diselenide Delivery in Melanoma Therapy. Investig. New Drugs 2020, 38, 662–674. [Google Scholar] [CrossRef]
- Sharadha, M.; Gowda, D.V.; Vishal Gupta, N.; Akhila, A.R. View of An Overview on Topical Drug Delivery System—Updated Review. Int. J. Res. Pharm. Sci. 2020, 11, 368–385. [Google Scholar] [CrossRef]
- Chin, J.S.; Madden, L.; Chew, S.Y.; Becker, D.L. Drug Therapies and Delivery Mechanisms to Treat Perturbed Skin Wound Healing. Adv. Drug Deliv. Rev. 2019, 149–150, 2–18. [Google Scholar] [CrossRef]
- Debebe, D.; Gabriel, T.; Brhane, Y.; Temesgen, A.; Nigatu, M.; Marew, T. Comparative In Vitro Evaluation of Brands of Clotrimazole Cream Formulations Marketed in Ethiopia. J. Drug Deliv. Ther. 2018, 8, 17–22. [Google Scholar] [CrossRef]
- Chyzy, A.; Plonska-Brzezinska, M.E. Hydrogel Properties and Their Impact on Regenerative Medicine and Tissue Engineering. Molecules 2020, 25, 5795. [Google Scholar] [CrossRef]
- Prado, V.C.; Moenke, K.; Osmari, B.F.; Pegoraro, N.S.; Oliveira, S.M.; Cruz, L. Development of Guar Gum Hydrogel Containing Sesamol-Loaded Nanocapsules Designed for Irritant Contact Dermatitis Treatment Induced by Croton Oil Application. Pharmaceutics 2023, 15, 285. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Sharma, D.; Xia, Y.; Saini, A.; You, X.; Su, Y.; Chen, L.; Yadav, C.; Li, X. Layer-by-Layer Assembly of Cationic Guar Gum, Cellulose Nanocrystals and Hydroxypropyl Methylcellulose Based Multilayered Composite Films. Cellulose 2021, 28, 8445–8457. [Google Scholar] [CrossRef]
- Dai, L.; Long, Z.; Chen, J.; An, X.; Cheng, D.; Khan, A.; Ni, Y. Robust Guar Gum/Cellulose Nanofibrils Multilayer Films with Good Barrier Properties. ACS Appl. Mater. Interfaces 2017, 9, 5477–5485. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Ceccarini, M.R.; Calarco, P.; Scuota, S.; Conte, C.; Primavilla, S.; Ricci, M.; Perioli, L. Bioadhesive Polymeric Films Based on Usnic Acid for Burn Wound Treatment: Antibacterial and Cytotoxicity Studies. Colloids Surf. B Biointerfaces 2019, 178, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.E.; Zulfakar, M.H.; Ng, S.F. Alginate Based Bilayer Hydrocolloid Films as Potential Slow-Release Modern Wound Dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef]
- Bombaldi de Souza, R.F.; Bombaldi de Souza, F.C.; Bierhalz, A.C.K.; Pires, A.L.R.; Moraes, Â.M. Biopolymer-Based Films and Membranes as Wound Dressings. In Biopolymer Membranes and Films: Health, Food, Environment, and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–194. [Google Scholar] [CrossRef]
- Contardi, M.; Russo, D.; Suarato, G.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Penna, I.; Margaroli, N.; Summa, M.; Spanò, R.; Tassistro, G.; et al. Polyvinylpyrrolidone/Hyaluronic Acid-Based Bilayer Constructs for Sequential Delivery of Cutaneous Antiseptic and Antibiotic. Chem. Eng. J. 2019, 358, 912–923. [Google Scholar] [CrossRef]
- Chu, M.; Feng, N.; An, H.; You, G.; Mo, C.; Zhong, H.; Pan, L.; Hu, D. Design and Validation of Antibacterial and PH Response of Cationic Guar Gum Film by Combining Hydroxyethyl Cellulose and Red Cabbage Pigment. Int. J. Biol. Macromol. 2020, 162, 1311–1322. [Google Scholar] [CrossRef]
- Tomoda, B.T.; Yassue-Cordeiro, P.H.; Ernesto, J.V.; Lopes, P.S.; Péres, L.O.; da Silva, C.F.; de Moraes, M.A. Characterization of Biopolymer Membranes and Films: Physicochemical, Mechanical, Barrier, and Biological Properties. In Biopolymer Membranes and Films: Health, Food, Environment, and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–95. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of Advances in Polymeric Wound Dressing Films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally Disintegrating Films: A Modern Expansion in Drug Delivery System. Saudi Pharm. J. 2016, 24, 537–546. [Google Scholar] [CrossRef]
- Gehrcke, M.; Martins, C.C.; de Bastos Brum, T.; da Rosa, L.S.; Luchese, C.; Wilhelm, E.A.; Soares, F.Z.M.; Cruz, L. Novel Pullulan/Gellan Gum Bilayer Film as a Vehicle for Silibinin-Loaded Nanocapsules in the Topical Treatment of Atopic Dermatitis. Pharmaceutics 2022, 14, 2352. [Google Scholar] [CrossRef]
- Evans, N.D.; Oreffo, R.O.C.; Healy, E.; Thurner, P.J.; Man, Y.H. Epithelial Mechanobiology, Skin Wound Healing, and the Stem Cell Niche. J. Mech. Behav. Biomed. Mater. 2013, 28, 397–409. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. How to Assess Orodispersible Film Quality? A Review of Applied Methods and Their Modifications. Acta Pharm. 2019, 69, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Wang, H.J.; Hou, X.S.; Raza, A.; Koyama, Y.; Ito, T.; Wang, J.Y. Preparation of Stretchable Composite Film and Its Application in Skin Burn Repair. J. Mech. Behav. Biomed. Mater. 2021, 113, 104114. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Feng, J.; Shi, J.; He, L.; Guo, P.; Guan, S.; Fu, H.; Ao, Y. Ultra-Stretchable, Self-Recovering, Self-Healing Cationic Guar Gum/Poly(Stearyl Methacrylate-Co-Acrylic Acid) Hydrogels. Carbohydr. Polym. 2021, 256, 117563. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Ayyoub, A.M.d.M.d.; Summa, M.; Kossyvaki, D.; Fadda, M.; Liessi, N.; Armirotti, A.; Fragouli, D.; Bertorelli, R.; Athanassiou, A. Self-Adhesive and Antioxidant Poly(Vinylpyrrolidone)/Alginate-Based Bilayer Films Loaded with Malva Sylvestris Extracts as Potential Skin Dressings. ACS Appl. Bio Mater. 2022, 5, 2880–2893. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, I.; Lopreiato, M.; D’abusco, A.S.; Di Lisio, V.; Martinelli, A.; Piozzi, A.; Francolini, I. Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings. Int. J. Mol. Sci. 2020, 21, 2070. [Google Scholar] [CrossRef]
- Johnson, W.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Galactomannans as Used in Cosmetics. Int. J. Toxicol. 2015, 34, 35S–65S. [Google Scholar] [CrossRef]
- Shaikh, R.; Raj Singh, T.; Garland, M.; Woolfson, A.; Donnelly, R. Mucoadhesive Drug Delivery Systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef]
- Trindade, G.A.D.M.; Alves, L.A.; Dallabrida, K.G.; Sari, M.H.M.; Reolon, J.B.; Pontarolo, R.; Ferreira, L.M. Formulação de Gel-Creme a Base de Óleo de Camomila (Matricaria chamomilla L.) Com Potencial Bioadesivo, Oclusivo e Antioxidante Para Aplicação Cutânea. Rev. Bras. Multidiscip. 2024, 27, 54–69. [Google Scholar] [CrossRef]
- Dodi, G.; Sabau, R.E.; Crețu, B.E.B.; Gardikiotis, I. Exploring the Antioxidant Potential of Gellan and Guar Gums in Wound Healing. Pharmaceutics 2023, 15, 2152. [Google Scholar] [CrossRef]
- Baawad, A.; Rice, C.; Hamil, T.; Murphy, K.; Park, J.; Kim, D.S. Molecular Weight Effects of Low Acyl Gellan Gum on Antioxidant Capacity and Rheological Properties. J. Food Sci. 2021, 86, 4275–4287. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tong, X.; You, S.; Mao, R.; Cai, E.; Pan, W.; Zhang, C.; Hu, R.; Shen, J. Mild Hyperthermia-Assisted ROS Scavenging Hydrogels Achieve Diabetic Wound Healing. ACS Macro Lett. 2022, 11, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Hwang, J.; Lee, H.; Hammond, P.T.; Choi, J.; Hong, J. In Vitro Blood Cell Viability Profiling of Polymers Used in Molecular Assembly. Sci. Rep. 2017, 7, 9481. [Google Scholar] [CrossRef]
- Weiss, A.M.; Lopez, M.A.; Rawe, B.W.; Manna, S.; Chen, Q.; Mulder, E.J.; Rowan, S.J.; Esser-Kahn, A.P. Understanding How Cationic Polymers’ Properties Inform Toxic or Immunogenic Responses via Parametric Analysis. Macromolecules 2023, 56, 7286–7299. [Google Scholar] [CrossRef]
- Correia, J.S.; Mirón-Barroso, S.; Hutchings, C.; Ottaviani, S.; Somuncuoğlu, B.; Castellano, L.; Porter, A.E.; Krell, J.; Georgiou, T.K. How Does the Polymer Architecture and Position of Cationic Charges Affect Cell Viability? Polym. Chem. 2022, 14, 303–317. [Google Scholar] [CrossRef]
- Vihar, B.; Rožanc, J.; Krajnc, B.; Gradišnik, L.; Milojević, M.; Činč Ćurić, L.; Maver, U. Investigating the Viability of Epithelial Cells on Polymer Based Thin-Films. Polymers 2021, 13, 2311. [Google Scholar] [CrossRef] [PubMed]
- Lopéz-Martínez, E.E.; Claudio-Rizo, J.A.; Caldera-Villalobos, M.; Becerra-Rodríguez, J.J.; Cabrera-Munguía, D.A.; Cano-Salazar, L.F.; Betancourt-Galindo, R. Hydrogels for Biomedicine Based on Semi-Interpenetrating Polymeric Networks of Collagen/Guar Gum: Applications in Biomedical Field and Biocompatibility. Macromol. Res. 2022, 30, 384–390. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Ghorbani, M.; Abdyazdani, N. Reinforcement of Hydrogel Scaffold Using Oxidized-Guar Gum Incorporated with Curcumin-Loaded Zein Nanoparticles to Improve Biological Performance. Int. J. Biol. Macromol. 2021, 167, 59–65. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Yang, Z.; Jiao, J.; Zhao, Z.; Liu, Y. “All-in-One” Self-Healing and Injectable Cationic Guar Gum Hydrogel Dressing Functionalized by Bioactive Complexes Composed of Natural Small Molecules. Int. J. Biol. Macromol. 2024, 275, 133517. [Google Scholar] [CrossRef]

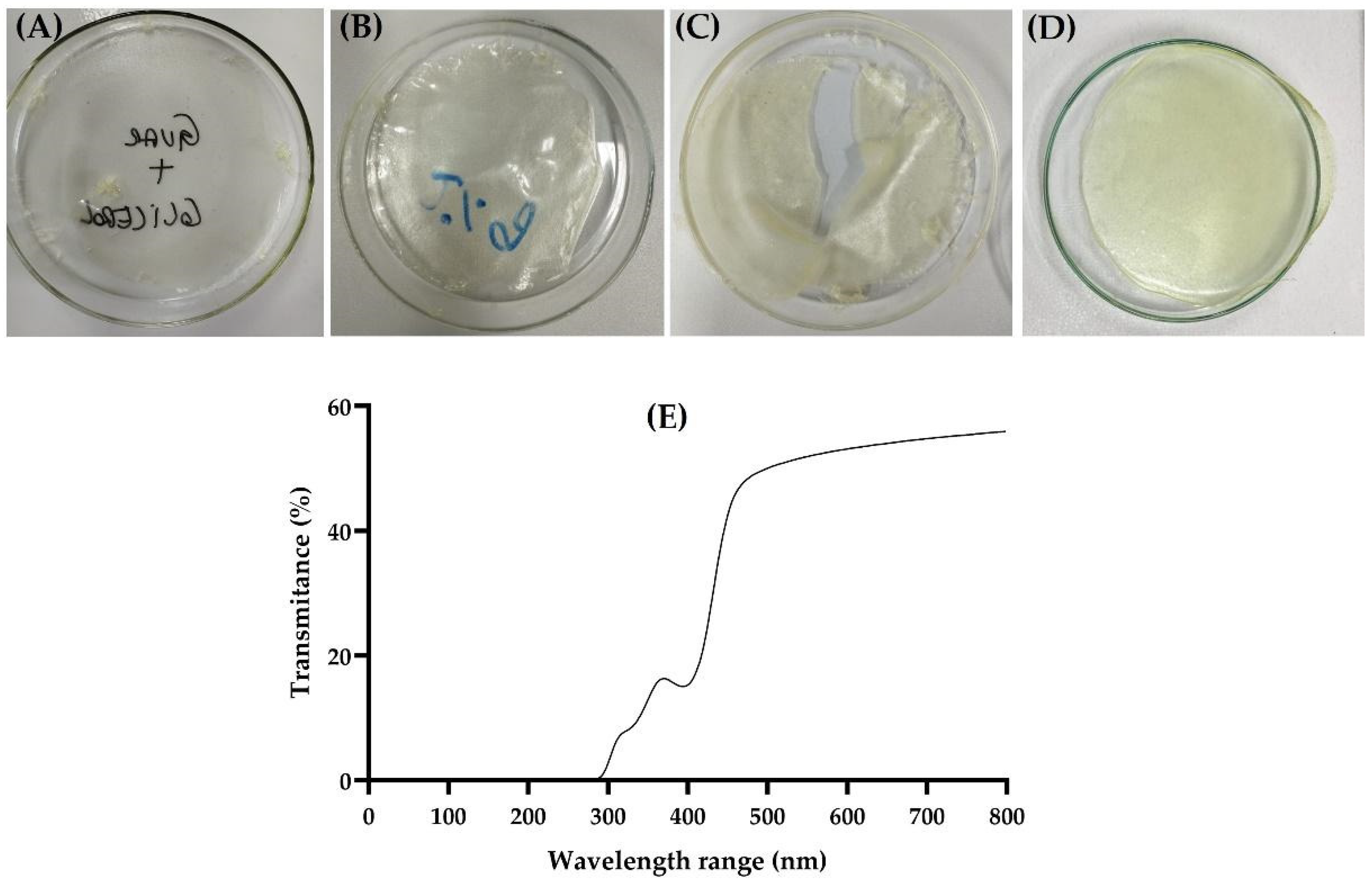






| Parameter | Value |
|---|---|
| Thickness (mm) | 0.63 ± 0.07 |
| Transparency (%) | 52.46 ± 3.29 |
| Weight uniformity (mg/cm2) | 82.03 ± 1.91 |
| Folding endurance | >300 |
| Tensile strength (MPa) | 1.16 ± 0.24 |
| Elongation (%) | 40.38 ± 5.00 |
| Young’s modulus (MPa) | 2.96 ± 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallabrida, K.G.; Braz, W.C.; Marchiori, C.; Alves, T.M.; Cruz, L.S.; Trindade, G.A.d.M.; Machado, P.; da Rosa, L.S.; Khalil, N.M.; Rego, F.G.d.M.; et al. Exploring Cationic Guar Gum: Innovative Hydrogels and Films for Enhanced Wound Healing. Pharmaceutics 2024, 16, 1233. https://doi.org/10.3390/pharmaceutics16091233
Dallabrida KG, Braz WC, Marchiori C, Alves TM, Cruz LS, Trindade GAdM, Machado P, da Rosa LS, Khalil NM, Rego FGdM, et al. Exploring Cationic Guar Gum: Innovative Hydrogels and Films for Enhanced Wound Healing. Pharmaceutics. 2024; 16(9):1233. https://doi.org/10.3390/pharmaceutics16091233
Chicago/Turabian StyleDallabrida, Kamila Gabrieli, Willer Cezar Braz, Crisleine Marchiori, Thainá Mayer Alves, Luiza Stolz Cruz, Giovanna Araujo de Morais Trindade, Patrícia Machado, Lucas Saldanha da Rosa, Najeh Maissar Khalil, Fabiane Gomes de Moraes Rego, and et al. 2024. "Exploring Cationic Guar Gum: Innovative Hydrogels and Films for Enhanced Wound Healing" Pharmaceutics 16, no. 9: 1233. https://doi.org/10.3390/pharmaceutics16091233
APA StyleDallabrida, K. G., Braz, W. C., Marchiori, C., Alves, T. M., Cruz, L. S., Trindade, G. A. d. M., Machado, P., da Rosa, L. S., Khalil, N. M., Rego, F. G. d. M., Fajardo, A. R., Ferreira, L. M., Sari, M. H. M., & Reolon, J. B. (2024). Exploring Cationic Guar Gum: Innovative Hydrogels and Films for Enhanced Wound Healing. Pharmaceutics, 16(9), 1233. https://doi.org/10.3390/pharmaceutics16091233









