Glioblastoma Multiforme: Sensitivity to Antimicrobial Peptides LL-37 and PG-1, and Their Combination with Chemotherapy for Predicting the Overall Survival of Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Characteristics of Patients
2.2. Cell Culture
2.3. MTT Assay
2.4. Determination of IC50 Dose, Combination Index and Combination Effects
2.5. Wistar Rat Intracerebral C6 Glioma Model
2.6. Reagents
2.7. Statistical Analysis
3. Results
3.1. Personalized In Vitro Cytotoxic Effects of Chemotherapy Drugs, LL-37, PG-1, and Their Combinations on Patients’ GBM Cells Using the MTT Assay
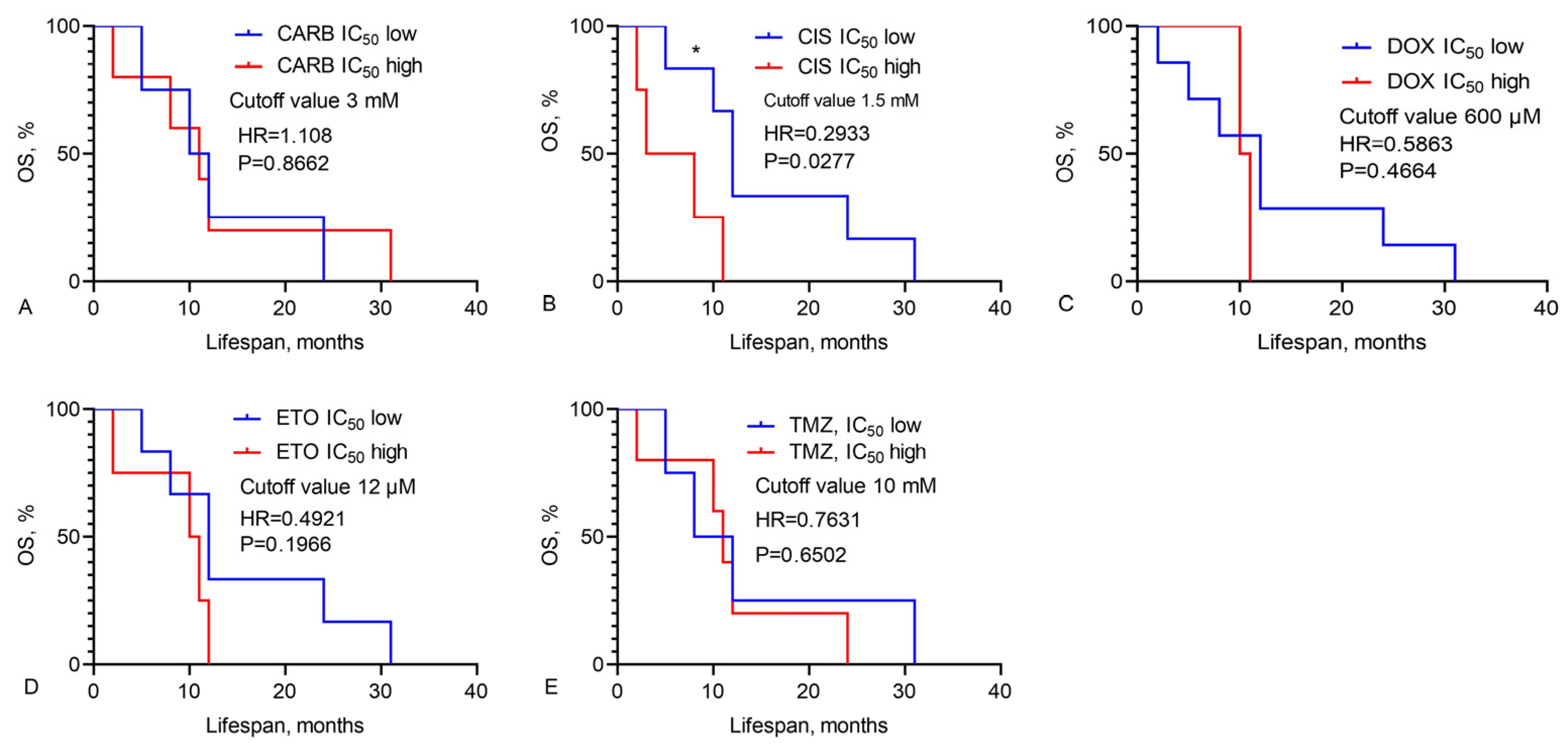
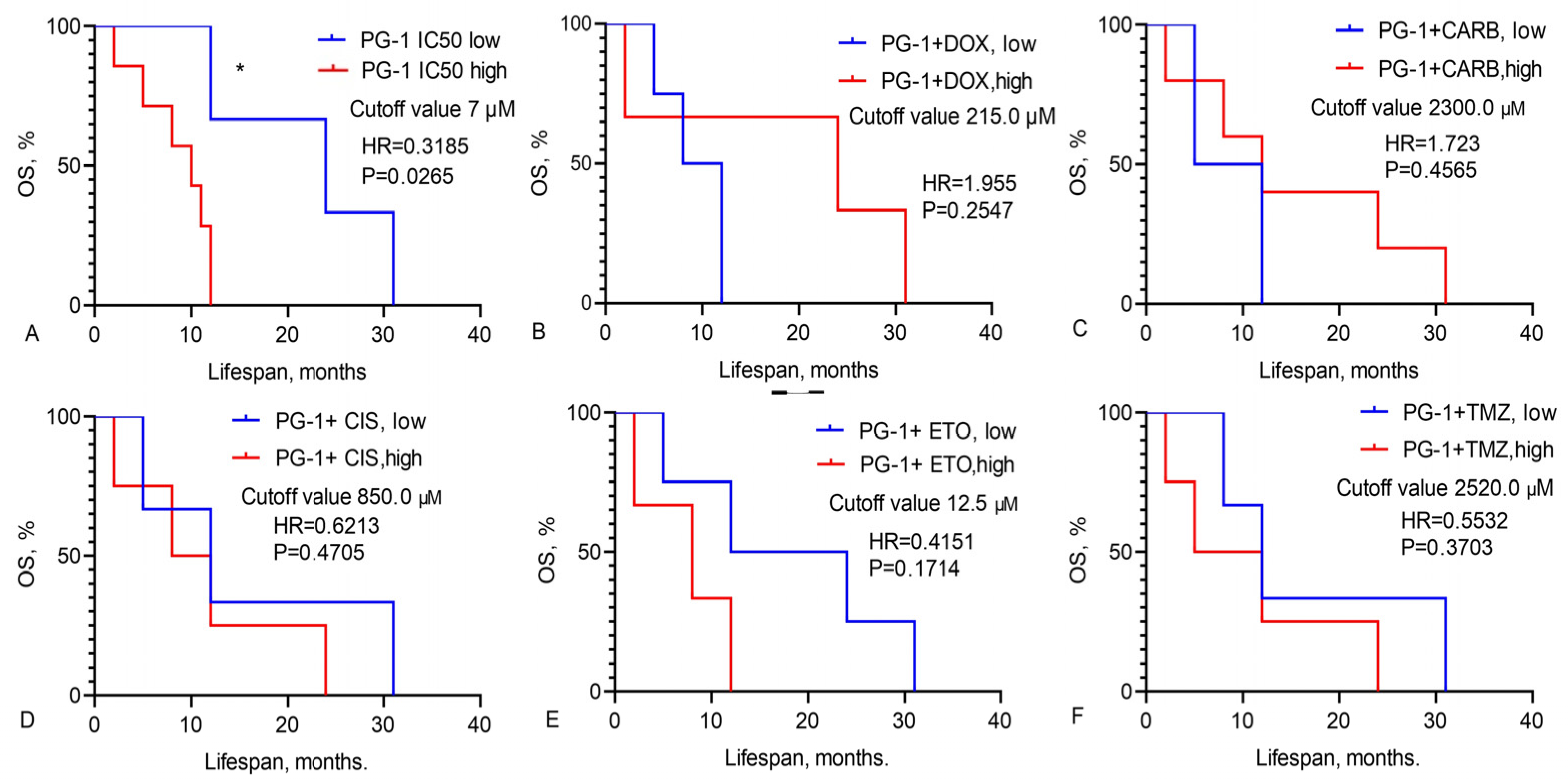
3.2. Prediction of GBM Patients’ Overall Survival Based on IC50 Values of Chemotherapy Drugs, LL-37, PG-1, and Their Combinations
3.3. Study of Survival Rates in Wistar Rats with a C6 Glioma Following Intranasal Administration of LL-37 and PG-1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holecki, T.; Węgrzyn, M.; Frączkiewicz-Wronka, A.; Sobczyk, K. Oncological Diseases and Social Costs Considerations on Undertaken Health Policy Interventions. Int. J. Environ. Res. Public Health 2020, 17, 2837. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- The International Agency for Research on Cancer (GLOBOCAN). Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 24 July 2024).
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Mohammed, S.; Dinesan, M.; Ajayakumar, T. Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: A retrospective study. Rep. Pract. Oncol. Radiother. 2022, 27, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Aravantinou-Fatorou, A.; Georgakopoulou, V.E.; Mathioudakis, N.; Papalexis, P.; Tarantinos., K.; Trakas, I.; Trakas, N.; Spandidos, D.A.; Fotakopoulos, G. Comparison of the outcomes following bevacizumab and/or temozolamide/radiosurgery treatment in patients with glioblastoma. Mol. Clin. Oncol. 2023, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Lee, S.; Kim, H.; Kang, H.; Youn, H.; Jo, S.; Youn, B.; Kim, H.Y. Revisiting Platinum-Based Anticancer Drugs to Overcome Gliomas. Int. J. Mol. Sci. 2021, 22, 5111. [Google Scholar] [CrossRef]
- Leonard, A.; Wolff, J.E. Etoposide improves survival in high-grade glioma: A meta-analysis. Anticancer Res. 2013, 33, 3307–3315. [Google Scholar]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef]
- Abou-Antoun, T.J.; Hale, J.S.; Lathia, J.D.; Dombrowski, S.M. Brain Cancer Stem Cells in Adults and Children: Cell Biology and Therapeutic Implications. Neurotherapeutics 2017, 14, 372–384. [Google Scholar] [CrossRef]
- Olivier, C.; Oliver, L.; Lalier, L.; Vallette, F.M. Drug Resistance in Glioblastoma: The Two Faces of Oxidative Stress. Front. Mol. Biosci. 2021, 7, 620677. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Wu, H.; Huang, J.; Wang, W.; Ge, K.; Li, G.; Zhong, J.; Huang, Q. LAMP2: A major update of the database linking antimicrobial peptides. Database 2020, 2020, baaa061. [Google Scholar] [CrossRef] [PubMed]
- Büyükkiraz, M.E.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef]
- Burdukiewicz, M.; Sidorczuk, K.; Rafacz, D.; Pietluch, F.; Bąkała, M.; Słowik, J.; Gagat, P. CancerGram: An Effective Classifier for Differentiating Anticancer from Antimicrobial Peptides. Pharmaceutics 2020, 12, 1045. [Google Scholar] [CrossRef]
- Hemmati, S.; Kazerooni, H.R. Polypharmacological Cell-Penetrating Peptides from Venomous Marine Animals Based on Immunomodulating, Antimicrobial, and Anticancer Properties. Mar. Drugs 2022, 20, 763. [Google Scholar] [CrossRef]
- Umnyakova, E.S.; Zharkova, M.S.; Berlov, M.N.; Shamova, O.V.; Kokryakov, V.N. Human antimicrobial peptides in autoimmunity. Autoimmunity 2020, 53, 137–147. [Google Scholar] [CrossRef]
- Kuroda, K.; Okumura, K.; Isogai, H.; Isogai, E. The Human Cathelicidin Antimicrobial Peptide LL-37 and Mimics are Potential Anticancer Drugs. Front. Oncol. 2015, 5, 144. [Google Scholar] [CrossRef]
- Chen, X.; Zou, X.; Qi, G.; Tang, Y.; Guo, Y.; Si, J.; Liang, L. Roles and Mechanisms of Human Cathelicidin LL-37 in Cancer. Cell. Physiol. Biochem. 2018, 47, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.-Q.; Zhang, S.-Q.; Bai, J.-X.; Lau, C.-L.; Sze, S.C.-W.; Yung, K.K.-L.; Ko, J.K.-S. The human cathelicidin peptide LL-37 inhibits pancreatic cancer growth by suppressing autophagy and reprogramming of the tumor immune microenvironment. Front. Pharmacol. 2022, 13, 906625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, W.; Gong, W.; Yoshimura, T.; Chen, K.; Wang, J.M. The Critical Role of the Antimicrobial Peptide LL-37/CRAMP in Protection of Colon Microbiota Balance, Mucosal Homeostasis, Anti-Inflammatory Responses, and Resistance to Carcinogenesis. Crit. Rev. Immunol. 2019, 39, 83–92. [Google Scholar] [CrossRef]
- Huang, L.-H.; Rau, C.-S.; Liu, Y.-W.; Lin, H.-P.; Wu, Y.-C.; Tsai, C.-W.; Chien, P.-C.; Wu, C.-J.; Huang, C.-Y.; Hsieh, T.-M.; et al. Cathelicidin Antimicrobial Peptide Acts as a Tumor Suppressor in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 15652. [Google Scholar] [CrossRef] [PubMed]
- Tokajuk, J.; Deptuła, P.; Piktel, E.; Daniluk, T.; Chmielewska, S.; Wollny, T.; Wolak, P.; Fiedoruk, K.; Bucki, R. Cathelicidin LL-37 in Health and Diseases of the Oral Cavity. Biomedicines 2022, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.; Sung, J.J.; To, K.F.; Yu, L.; Li, H.T.; Li, Z.J.; Chu, K.M.; Yu, J.; Cho, C.H. The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J. Cell. Physiol. 2010, 223, 178–186. [Google Scholar] [CrossRef]
- Ren, S.X.; Cheng, A.S.L.; To, K.F.; Tong, J.H.M.; Li, M.S.; Shen, J.; Wong, C.C.; Zhang, L.; Chan, R.L.; Wang, X.J.; et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012, 72, 6512–6523. [Google Scholar] [CrossRef] [PubMed]
- Podaza, E.; Palacios, F.; Croci, D.O.; Risnik, D.; Yan, X.J.; Almejún, M.B.; Colado, A.; Elías, E.E.; Borge, M.; Morande, P.E.; et al. Expression and function of cathelicidin hCAP18/LL-37 in chronic lymphocytic leukemia. Haematologica 2020, 105, e465–e469. [Google Scholar] [CrossRef]
- Guo, C.; Rosoha, E.; Lowry, M.B.; Borregaard, N.; Gombart, A.F. Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. J. Nutr. Biochem. 2013, 24, 754–759. [Google Scholar] [CrossRef]
- Lee, M.; Shi, X.; Barron, A.E.; McGeer, E.; McGeer, P.L. Human antimicrobial peptide LL-37 induces glial-mediated neuroinflammation. Biochem. Pharmacol. 2015, 94, 130–141. [Google Scholar] [CrossRef]
- Chernov, A.N.; Tsapieva, A.N.; Alaverdian, D.A.; Filatenkova, T.A.; Galimova, E.S.; Suvorova, M.; Shamova, O.V.; Suvorov, A.N. In vitro evaluation of cytotoxic effect of Streptococcus pyogenes strains, Protegrin PG-1, Cathelicidin LL-37, Nerve Growth Factor and chemotherapy on C6 glioma cell line. Molecules 2022, 27, 569. [Google Scholar] [CrossRef]
- Chernov, A.; Filatenkova, T.; Alaverdian, D.; Tsapieva, A.; Kim, A.; Fedorov, E.; Scliar, S.S.; Matsko, M.V.; Galimova, E.S.; Shamova, O.V. Anticancer Effect of Cathelicidin LL-37, Protegrin PG-1, Nerve Growth Factor NGF, and Temozolomide: Impact on the Mitochondrial Metabolism, Clonogenic Potential, and Migration of Human U251 Glioma Cells. Molecules 2022, 27, 4988. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Freshney, R.I.; Griffiths, B.; Hay, R.J.; Reid, Y.A.; Carmiol, S.; Kunz-Schugart, L. Animal Cell Culture: A Practical Approach, 3rd ed.; Masters, J.R.W., Ed.; Oxford University Press: London, UK, 2000. [Google Scholar]
- Amini, S.; White, M.K. (Eds.) Neuronal Cell Culture. Methods and Protocols; Humana: Totowa, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Assay Guidance Manual. Cell Viability Assays; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013. [Google Scholar]
- Chernov, A.; Kudryavtsev, I.; Komlev, A.; Alaverdian, D.; Tsapieva, A.; Galimova, E.; Shamova, O. Nerve Growth Factor, Antimicrobial Peptides and Chemotherapy: Glioblastoma Combination Therapy to Improve Their Efficacy. Biomedicines 2023, 11, 3009. [Google Scholar] [CrossRef] [PubMed]
- van Belle, G.; Fisher, L.D.; Heagerty, P.J.; Lumley, T. Biostatistics: A Methodology for the Health Sciences; Fisher, L.D., van Belle, G., Eds.; Jonh Wiley and Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Chernov, A.N.; Kim, A.V.; Skliar, S.S.; Fedorov, E.V.; Tsapieva, A.N.; Chutko, A.L.; Matsko, M.V.; Galimova, E.S.; Shamova, O.V. Expression of molecular markers and synergistic anticancer effects of chemotherapy with antimicrobial peptides on glioblastoma cells. Cancer Chemother. Pharmacol. 2024, 93, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Fukuda, T.; Yoneyama, H.; Katayama, M.; Isogai, H.; Okumura, K.; Isogai, E. Anti-proliferative effect of an analogue of the LL-37 peptide in the colon cancer derived cell line HCT116 p53+/+ and p53-/-. Oncol. Rep. 2012, 28, 829–834. [Google Scholar] [CrossRef]
- Zhang, Z.; Cherryholmes, G.; Chang, F.; Rose, D.M.; Schraufstatter, I.; Shively, J.E. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur. J. Immunol. 2009, 39, 3181–3194. [Google Scholar] [CrossRef]
- Tomasinsig, L.; Pizzirani, C.; Skerlavaj, B.; Pellegatti, P.; Gulinelli, S.; Tossi, A.; Di Virgilio, F.; Zanetti, M. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J. Biol. Chem. 2008, 283, 30471–30481. [Google Scholar] [CrossRef]
- Murakami, T.; Suzuki, K.; Niyonsaba, F.; Tada, H.; Reich, J.; Tamura, H.; Nagaoka, I. MrgX2-mediated internalization of LL-37 and degranulation of human LAD2 mast cells. Mol. Med. Rep. 2018, 18, 4951–4959. [Google Scholar] [CrossRef]
- Miura, S.; Garcet, S.; Li, X.; Cueto, I.; Salud-Gnilo, C.; Kunjravia, N.; Yamamura, K.; Gonzalez, J.; Murai-Yamamura, M.; Rambhia, D.; et al. Cathelicidin Antimicrobial Peptide LL37 Induces Toll-Like Receptor 8 and Amplifies IL-36γ and IL-17C in Human Keratinocytes. J. Investig. Dermatol. 2023, 143, 832–841. [Google Scholar] [CrossRef]
- Piktel, E.; Niemirowicz, K.; Wnorowska, U.; Wątek, M.; Wollny, T.; Głuszek, K.; Góźdź, S.; Levental, I.; Bucki, R. The Role of Cathelicidin LL-37 in Cancer Development. Arch. Immunol. Ther. Exp. 2016, 64, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Zharkova, M.S.; Artamonov, A.Y.; Grinchuk, T.M.; Butskina, E.A.; Pazina, T.Y.; Orlov, D.S.; Shamova, O.V. Peptides of the innate immune system modulate the cytotoxic effect of antitumor antibiotics. Ross. Immunol. J. 2016, 10, 548–550. [Google Scholar]
- Shamova, O.V.; Orlov, D.S.; Zharkova, M.S.; Balandin, S.V.; Yamschikova, E.V.; Knappe, D.; Hoffmann, R.; Kokryakov, V.N.; Ovchinnikova, T.V. Minibactenecins ChBac7.Nα and ChBac7. Nβ-Antimicrobial Peptides from Leukocytes of the Goat Capra hircus. Acta Naturae 2016, 8, 136–146. [Google Scholar] [CrossRef]
- Soundrarajan, N.; Park, S.; Le Van Chanh, Q.; Cho, H.-S.; Raghunathan, G.; Ahn, B.; Song, H.; Kim, J.-H.; Park, C. Protegrin-1 cytotoxicity towards mammalian cells positively correlates with the magnitude of conformational changes of the unfolded form upon cell interaction. Sci. Rep. 2019, 9, 11569. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Mohamed, Z.; Sasikumar, P.G.; Reddy, K.A.; Rahman, N.A.; Yusof, R. In Vitro Characterization of Novel Protegrin-1 Analogues Against Neoplastic Cells. Int. J. Pept. Res. Ther. 2014, 20, 259–267. [Google Scholar] [CrossRef]
- Can, G.; Akpinar, B.; Baran, Y.; Zhivotovsky, B.; Olsson, M. 5-Fluorouracil signaling through a calcium–calmodulin-dependent pathway is required for p53 activation and apoptosis in colon carcinoma cells. Oncogene 2013, 32, 4529–4538. [Google Scholar] [CrossRef]
- Yan, H.X.; Wu, H.P.; Zhang, H.L.; Ashton, C.; Tong, C.; Wu, J. DNA damage-induced sustained p53 activation contributes to inflammation-associated hepatocarcinogenesis in rats. Oncogene 2013, 32, 4565–4571. [Google Scholar] [CrossRef]
- Gupta, K.; Kotian, A.; Subramanian, H.; Daniell, H.; Ali, H. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget 2015, 6, 28573–28587. [Google Scholar] [CrossRef] [PubMed]
- Shamova, O.V.; Orlov, D.S.; Pazina, T.Y.; Yamshchikova, E.V.; Orlov, S.B.; Zharkova, M.S. Study of the molecular and cellular bases of the cytotoxic effect of antimicrobial peptides on tumor cells. Fundamental. Res. 2012, 5, 207–212. [Google Scholar]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell. Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Nie, M.; Long, W. The role of B cells in cancer development. Front. Oncol. 2022, 12, 958756. [Google Scholar] [CrossRef]
- Kravtsov, D.S.; Erbe, A.K.; Sondel, P.M.; Rakhmilevich, A.L. Roles of CD4+ T cells as mediators of antitumor immunity. Front. Immunol. 2022, 13, 972021. [Google Scholar] [CrossRef]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neuro-Oncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Chertov, O.; Oppenheim, J.J. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 2000, 68, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, Y.L.; Jeong, Y.; Jang, Y.-S. Application of Antimicrobial Peptide LL-37 as an Adjuvant for Middle East Respiratory Syndrome-Coronavirus Antigen Induces an Efficient Protective Immune Response Against Viral Infection After Intranasal Immunization. Immune Netw. 2022, 22, e41. [Google Scholar] [CrossRef] [PubMed]


| Drugs | Dose, μM |
|---|---|
| Doxorubicin | 920.0, 460.0, 230.0, 115.0, 73.6, 36.8, 18.4 |
| Carboplatin | 26,900.0, 2690.0, 1350.0, 673.0, 269.0, 134.0 |
| Cisplatin | 1660.0, 830.0, 332.0, 166.0, 83.0, 33.2, 16.1 |
| Temozolomide | 15,500.0, 5150.0, 1550.0, 773.0, 386.0, 155.0 |
| Etoposide | 27.0, 13.5, 6.7, 3.3, 1.6, 0.8 |
| LL-37 | 32.0, 16.0, 8.0, 4.0, 2.0, 1.0 |
| PG-1 | 64.0, 32.0, 16.0, 8.0, 4.0, 2.0 |
| ID Patient | IC50, μM | ||||||
|---|---|---|---|---|---|---|---|
| DOX | CARB | TMZ | CIS | ETO | LL-37 | PG-1 | |
| 11081 | 290.4 | 29,431.0 | 16,179.5 | 2448.4 | 27.0 | 10.3 | 16.0 |
| 11961 | 3350.3 | 39,792.9 | 43,539.3 | 11,919.7 | 86.5 | 32.2 | 123.6 |
| 6770 | 850.0 | 4000.0 | 14,000.0 | 1090.0 | 26.3 | 9.5 | 8.7 |
| 7934 | 50.9 | 2000.0 | 7491.0 | 200.0 | 7.5 | 2.0 | 1.2 |
| 49142 | 548.3 | 2708.4 | 11,056.0 | 776.0 | 11.4 | 6.6 | 7.4 |
| 25873 | 560.0 | 888.8 | 8619.2 | 300.0 | 8.9 | 24.1 | 30.1 |
| 57595 | 16.9 | 3093.6 | 194.5 | 1682.3 | 7.5 | 8.3 | 8.6 |
| 55068 | 546.5 | 27,574.5 | 4789.5 | 1104.8 | 11.8 | 6.4 | 3.9 |
| 15159 | 179.2 | 116.4 | 436.8 | 698.1 | 11.4 | 32.1 | 15.8 |
| 62642 | 20.3 | 42,495.1 | 24,015.7 | 1158.5 | 32.3 | 28.1 | 34.3 |
| 60886 | 278.8 | 4498.0 | 2174.3 | >1660.0 | 6.3 | 1.1 | 1.2 |
| 18871 | 2682.8 | 24,031.9 | 11,976.9 | 1776.4 | 30.9 | 24.3 | 23.8 |
| 114495 | 3350.3 | 39,792.9 | 43,539.3 | 965.8 | 86.5 | 26.8 | 35.4 |
| 10677 | 1180.1 | 20,471.8 | 1309.1 | 2448.4 | 3.4 | 3.5 | 7.4 |
| 1401 | 920.0 | 5136.5 | 611.8 | 261.2 | 10.3 | 4.0 | 16.0 |
| 18871 | 2682.8 | 24,031.9 | 11,976.9 | 1776.3 | 30.9 | 24.3 | 23.8 |
| 8989 | 817.1 | 20,195.2 | 14,486.0 | 1218.8 | 26.3 | 9.7 | 12.1 |
| 20939 | 920.0 | 17,861.9 | 15,500.0 | 476.5 | 38.0 | 11.8 | 19.3 |
| 39114 | 3458.6 | 25,000.0 | 12,282.1 | 1824.2 | 32.8 | 26.8 | 26.2 |
| 40906 | 1083.2 | 38,147.6 | 14,961.7 | 1596.1 | 58.9 | 20.8 | 19.4 |
| 48993 | – | 1126.8 | 15,407.5 | 120.4 | 38.7 | 3.1 | 14.1 |
| 48307 | 1260.3 | 20,852.7 | 1510.7 | 1280.8 | 9.5 | 5.7 | 6.2 |
| 9439 | 1513.2 | 26,116.5 | 22,206.3 | 1784.9 | 41.3 | 7.2 | 0.8 |
| 10448 | 478.7 | 24,237.2 | 14,659.1 | 1299.0 | 3.4 | 1.9 | 4.3 |
| 27980 | 733.4 | 2223.4 | 5345.6 | 835.3 | 9.3 | 17.5 | 1.9 |
| 12645 | 483.6 | 2605.4 | 5258.3 | 729.8 | 7.0 | 7.2 | 3.9 |
| 7593 | 1123.9 | 2110.4 | 14,905.5 | 298.9 | 26.8 | 8.6 | 13.2 |
| ID Patient | IC50 with Combinations of LL-37 with Chemotherapy, in μM | ||||
|---|---|---|---|---|---|
| DOX | CARB | TMZ | CIS | ETO | |
| 11081 | 1832.7 | 1645.3 | 5150.0 | 485.3 | 13.8 |
| 11961 | 227.2 | 55,122.9 | 54,577.5 | 4825.5 | 12.7 |
| 6770 | 160.1 | 26,481.5 | 1507.7 | 790.5 | 14.2 |
| 7934 | 160.0 | 1360.0 | 1550.0 | 800.0 | 19.5 |
| 49142 | 989.4 | 27,187.6 | 10,247.5 | 1594.8 | 11.8 |
| 25873 | 15.1 | 3307.9 | 9580.8 | 238.3 | 40.0 |
| 57595 | 4852.6 | 1424.0 | 689.6 | 388.2 | 6.6 |
| 55068 | 262.5 | 1234.5 | 5993.7 | 1201.9 | 37.4 |
| 15159 | 563.5 | 24,100.4 | 5494.4 | 634.2 | 2.89 |
| 62642 | 91.9 | 48,281.2 | 24,523.8 | 984.5 | 37.7 |
| 60886 | 910.7 | 742.5 | 365.1 | 919.3 | 12.6 |
| 114495 | 178.9 | 35,771.3 | 37,621.4 | 351.9 | 0.96 |
| ID Patient | IC50 the Combinations of PG-1 with Chemotherapy, μM | ||||
|---|---|---|---|---|---|
| DOX | CARB | TMZ | CIS | ETO | |
| 11081 | 1380.4 | 23,637.9 | 15,166.3 | 1118.6 | 37.1 |
| 6770 | 219.4 | 23,963.5 | 4424.8 | 750.4 | 6.4 |
| 7934 | 50.0 | 2269.4 | 2518.5 | 700.0 | 0.9 |
| 49142 | 1230.3 | 32,964.1 | 6117.6 | 1575.4 | 12.5 |
| 25873 | 74.9 | 362.2 | 4980.5 | 59.4 | 0.8 |
| 57595 | 41.2 | 14,674.4 | 2.8 | 3851.7 | 16.6 |
| 55068 | 215.8 | 37,804.8 | 1698.3 | 840.6 | 14.2 |
| 15159 | 179.1 | 1648.5 | 5297.4 | 315.3 | 4.7 |
| 62642 | 2299.2 | 44,725.1 | 38,364.4 | 3649.8 | 73.5 |
| 60886 | 30.6 | 3312.5 | 375.5 | 2295.3 | 25.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernov, A.N.; Skliar, S.S.; Kim, A.V.; Tsapieva, A.; Pyurveev, S.S.; Filatenkova, T.A.; Matsko, M.V.; Ivanov, S.D.; Shamova, O.V.; Suvorov, A.N. Glioblastoma Multiforme: Sensitivity to Antimicrobial Peptides LL-37 and PG-1, and Their Combination with Chemotherapy for Predicting the Overall Survival of Patients. Pharmaceutics 2024, 16, 1234. https://doi.org/10.3390/pharmaceutics16091234
Chernov AN, Skliar SS, Kim AV, Tsapieva A, Pyurveev SS, Filatenkova TA, Matsko MV, Ivanov SD, Shamova OV, Suvorov AN. Glioblastoma Multiforme: Sensitivity to Antimicrobial Peptides LL-37 and PG-1, and Their Combination with Chemotherapy for Predicting the Overall Survival of Patients. Pharmaceutics. 2024; 16(9):1234. https://doi.org/10.3390/pharmaceutics16091234
Chicago/Turabian StyleChernov, Alexander N., Sofia S. Skliar, Alexander V. Kim, Anna Tsapieva, Sarng S. Pyurveev, Tatiana A. Filatenkova, Marina V. Matsko, Sergey D. Ivanov, Olga V. Shamova, and Alexander N. Suvorov. 2024. "Glioblastoma Multiforme: Sensitivity to Antimicrobial Peptides LL-37 and PG-1, and Their Combination with Chemotherapy for Predicting the Overall Survival of Patients" Pharmaceutics 16, no. 9: 1234. https://doi.org/10.3390/pharmaceutics16091234
APA StyleChernov, A. N., Skliar, S. S., Kim, A. V., Tsapieva, A., Pyurveev, S. S., Filatenkova, T. A., Matsko, M. V., Ivanov, S. D., Shamova, O. V., & Suvorov, A. N. (2024). Glioblastoma Multiforme: Sensitivity to Antimicrobial Peptides LL-37 and PG-1, and Their Combination with Chemotherapy for Predicting the Overall Survival of Patients. Pharmaceutics, 16(9), 1234. https://doi.org/10.3390/pharmaceutics16091234









