Alternative Cancer Therapeutics: Unpatentable Compounds and Their Potential in Oncology
Abstract
1. Introduction
2. Patenting of Anticancer Compounds
| Novel Formulations | Developing new formulations of existing drugs can offer ways to improve their efficacy, reduce side effects, or enhance delivery to the target site. For instance, encapsulating a drug in a nanoparticle might improve its solubility or allow it to target cancer cells more effectively. Innovative formulations can be patentable, as they present new and non-obvious solutions [20,21]. |
| Drug Delivery Systems | Similar to novel formulations, advancements in drug delivery systems offer significant opportunities for patentability. Targeted delivery mechanisms, time-release capsules, and transdermal patches that improve the drug’s performance or patient experience can be patented. Such systems can transform how a drug is administered and delivered, making a substantial difference in treatment outcomes [22,23]. |
| Synthetic Derivatives | Even if a natural compound itself cannot be patented, chemically modified derivatives that show improved properties (higher potency or lower toxicity) may be. Researchers often focus on altering the molecular structure of known compounds to create new, patentable entities that retain or enhance the desired anticancer activity [24,25]. |
| Combination Therapies | Patenting the use of known drugs in combination can be another avenue for innovation. If two or more drugs are found to work synergistically, where their combined effect is greater than the sum of their individual effects, this combination can be patented. This approach opens new therapeutic avenues and extends the commercial life of existing drugs [26,27,28]. |
| Methods of Use | Even when the compounds themselves are not new, novel applications or methods of using them can be patentable. Discovering and proving a new use for an existing drug, such as using a known medication in treating a different type of cancer than it was originally approved for, can lead to patent protection for that specific application [29,30]. |
| Production Processes | Innovations in the methods for manufacturing or synthesizing anticancer compounds can also be protected by patents. Efficient, scalable, and environmentally friendly production methods that are novel and non-obvious offer significant competitive advantages and are valuable in the patent landscape [31,32]. |
3. Alternative Cancer Therapeutics: Combinatorial Approach and Patentability
4. Patient Perspectives and Ethical Considerations in Alternative Cancer Therapeutics
5. Cancer Chemopreventive Agents
| Compound | Stage Affected | Mechanism of Action | Source |
|---|---|---|---|
| Curcumin | Initiation and Promotion | Downregulates multiple survival signals; inhibits transcription factors, including NF-κB. | Curcuma longa, turmeric plant [48,49] |
| Epigallocatechin-3 Gallate | Initiation | Reduces tumor invasiveness; sensitizes cells to other treatments like tamoxifen. | Prevalent in green tea [50,51] |
| Resveratrol | Initiation and Promotion | Causes G1/S phase arrest, downregulates COX-1 and COX-2, and inhibits multiple signaling pathways. | Grapes, fruits, nuts [52,53] |
| Tryptanthrin | Promotion | Suppresses PMA-induced proliferation; downregulates pro-tumorigenic signaling pathways. | Strobilanthes cusia, other medicinal plants [54,55] |
| Kaempferol | Initiation and Promotion | Inhibits growth of cancer cells and induces autophagic cell death via signaling pathways. | Abundant in vegetables and medicinal herbs [56,57] |
| 6-Gingerol | Initiation and Promotion | Induces apoptosis, inhibits MAPK/AP-1 signaling, and scavenges chemical carcinogens. | Zingiber officinale, ginger plant [58,59,60] |
| Emodin | Initiation | Inhibits AP-1 and NF-κB signaling pathways; inhibits angiogenesis and metastasis. | Rheum palmatum, Polygonum cuspidatum, and other plants [61,62,63] |
6. Therapeutic Potential of Unpatentable Compounds in Cancer Treatment
6.1. Metals and Minerals
6.2. Organic Compounds
6.3. Natural Products
6.4. Off-Patent and Repurposed Drugs
6.5. Small Molecules
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286, Erratum in Biostatistics 2019, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.C.; Baines, A.C.; Gong, Y.; Moore, R.; Pamuk, G.E.; Saber, H.; Subedee, A.; Thompson, M.D.; Xiao, W.; Pazdur, R.; et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat. Rev. Drug Discov. 2023, 22, 625–640. [Google Scholar] [CrossRef]
- Saha, C.N.; Bhattacharya, S. Intellectual property rights: An overview and implications in pharmaceutical industry. J. Adv. Pharm. Technol. Res. 2011, 2, 88–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Root-Bernstein, R.S. The development and dissemination of non-patentable therapies (NPTs). Perspect. Biol. Med. 1995, 39, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem. Toxicol. 2017, 107 Pt A, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.R.; Kaplan, W.; Orbinski, J.; Harland-Logan, S.; N-Marandi, S. Are patents impeding medical care and innovation? PLoS Med. 2010, 7, e1000208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Shelton, L.M. Cancer as a metabolic disease. Nutr. Metab. 2010, 7, 7. [Google Scholar] [CrossRef]
- Dyvik, E.H. International Intellectual Property Index 2023. 2024. Available online: https://www.statista.com/statistics/257583/gipc-international-intellectual-property-index (accessed on 3 August 2024).
- US Chamber of Commerce. International IP Index. 2023. Available online: https://www.uschamber.com/assets/documents/GIPC_IPIndex2023_FullReport_final.pdf (accessed on 3 August 2024).
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hinkson, I.V.; Madej, B.; Stahlberg, E.A. Accelerating Therapeutics for Opportunities in Medicine: A Paradigm Shift in Drug Discovery. Front. Pharmacol. 2020, 11, 770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almouaalamy, N.A.; Banjar, L.A.; Alshaikh, H.M.; Altowairqi, J.M.; Alharbi, N.M.; Alghamdi, W.A. The prevalence and pattern of complementary and alternative medicine use among cancer patients in a tertiary oncology center: A cross-sectional study. Ann. Med. Surg. 2023, 85, 5420–5427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buckner, C.A.; Lafrenie, R.M.; Dénommée, J.A.; Caswell, J.M.; Want, D.A. Complementary and alternative medicine use in patients before and after a cancer diagnosis. Curr. Oncol. 2018, 25, e275–e281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, A.A.; Johnson, J. Research use exemptions to patent infringement for drug discovery and development in the United States. Cold Spring Harb. Perspect. Med. 2014, 5, a020933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, D.G. A Drug Discovery Perspective on FDA Expedited Development and Incentive Programs. J. Med. Chem. 2024, 67, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Chirmule, N.; Feng, H.; Cyril, E.; Ghalsasi, V.V.; Choudhury, M.C. Orphan drug development: Challenges, regulation, and success stories. J. Biosci. 2024, 49, 30. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Mandal, A.K.; Kumar, M.; Dwivedi, K.; Singh, D. Prospective Challenges for Patenting and Clinical Trials of Anticancer Compounds from Natural Products: Coherent Review. Recent Pat. Anticancer Drug Discov. 2023, 18, 470–494. [Google Scholar] [CrossRef] [PubMed]
- Cvek, B. Multiple deadlocks in the development of nonprofit drugs. Drug Discov. Today 2022, 27, 2411–2414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Murteira, S.; Ghezaiel, Z.; Karray, S.; Lamure, M. Drug reformulations and repositioning in pharmaceutical industry and its impact on market access: Reassessment of nomenclature. J. Mark. Access Health Policy 2013, 1, 21131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.H.; et al. Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kang, H.; Lyu, L.; Xiong, W.; Hu, Y. A target map of clinical combination therapies in oncology: An analysis of clinicaltrials.gov. Discov. Oncol. 2023, 14, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhatia, K.; Bhumika, T.V.; Das, A. Combinatorial drug therapy in cancer—New insights. Life Sci. 2020, 258, 118134. [Google Scholar] [CrossRef] [PubMed]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012, 30, 679–692. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Rooman, I.; Huys, I. Exploring new uses for existing drugs: Innovative mechanisms to fund independent clinical research. Trials 2021, 22, 322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowak-Sliwinska, P.; Scapozza, L.; Ruiz i Altaba, A. Drug repurposing in oncology: Compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 434–454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, A.M.D.C.; Schneider-Stock, R.; Aiello, F. Editorial: Design, Synthesis, and Preclinical Testing of Innovative Anti-Cancer Compounds with a High Level of Selectivity of Action and Low Toxicity. Front. Mol. Biosci. 2022, 9, 859821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dogra, A.; Kumar, J. Biosynthesis of anticancer phytochemical compounds and their chemistry. Front. Pharmacol. 2023, 14, 1136779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wode, K.; Sharp, L.; Fransson, P.; Nordberg, J.H. Communication About Complementary and Alternative Medicine When Patients Decline Conventional Cancer Treatment: Patients’ and Physicians’ Experiences. Oncologist 2023, 28, e774–e783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, P.; Jhawat, V.; Mathur, P.; Dutt, R. Innovation in cancer therapeutics and regulatory perspectives. Med. Oncol. 2022, 39, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mülder, D.T.; O’Mahony, J.F.; Doubeni, C.A.; Lansdorp-Vogelaar, I.; Schermer, M.H.N. The Ethics of Cancer Screening Based on Race and Ethnicity. Ann. Intern. Med. 2024, 177, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Swetha, M.; Keerthana, C.K.; Rayginia, T.P.; Anto, R.J. Cancer Chemoprevention: A Strategic Approach Using Phytochemicals. Front. Pharmacol. 2022, 12, 809308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.; Kong, A.N. Dietary cancer-chemopreventive compounds: From signaling and gene expression to pharmacological effects. Trends Pharmacol. Sci. 2005, 26, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, S. Dietary phytochemicals and their role in cancer chemoprevention. J. Cancer Metastasis Treat. 2021, 7, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langner, E.; Rzeski, W. Dietary derived compounds in cancer chemoprevention. Contemp. Oncol. 2012, 16, 394–400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, Y.; Hou, R.; Yu, J.; Xing, C.; Zhuang, C.; Qu, Z. Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis. Nutrients 2023, 15, 491. [Google Scholar] [CrossRef]
- Albanes, D.; Heinonen, O.P.; Huttunen, J.K.; Taylor, P.R.; Virtamo, J.; Edwards, B.K.; Haapakoski, J.; Rautalahti, M.; Hartman, A.M.; Palmgren, J. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am. J. Clin. Nutr. 1995, 62 (Suppl. 6), 1427S–1430S. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Brasky, T.M.; Baik, C.S.; Slatore, C.G.; Potter, J.D.; White, E. Non-steroidal anti-inflammatory drugs and small cell lung cancer risk in the VITAL study. Lung Cancer 2012, 77, 260–264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sirotkin, A.V.; Ovcharenko, D.; Benco, A.; Mlyncek, M. Protein kinases controlling PCNA and p53 expression in human ovarian cells. Funct. Integr. Genom. 2009, 9, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Sachse, C.; Krausz, E.; Krönke, A.; Hannus, M.; Walsh, A.; Grabner, A.; Ovcharenko, D.; Dorris, D.; Trudel, C.; Sönnichsen, B.; et al. High-throughput RNA interference strategies for target discovery and validation by using synthetic short interfering RNAs: Functional genomics investigations of biological pathways. Methods Enzymol. 2005, 392, 242–277. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Ovcharenko, D.; Mlyncek, M. Identification of protein kinases that control ovarian hormone release by selective siRNAs. J. Mol. Endocrinol. 2010, 44, 45–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sirotkin, A.V.; Alexa, R.; Kišová, G.; Harrath, A.H.; Alwasel, S.; Ovcharenko, D.; Mlynček, M. MicroRNAs control transcription factor NF-kB (p65) expression in human ovarian cells. Funct. Integr. Genom. 2015, 15, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin. Cancer Biol. 2022, 80, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, Y.; Zhang, G.; Li, C.; Long, H.; Chen, D.; Li, Z.; Ouyang, G.; Zhang, W.; Zhang, Y.; Wang, Z. Discovery of Tryptanthrin and Its Derivatives and Its Activities against NSCLC In Vitro via Both Apoptosis and Autophagy Pathways. Int. J. Mol. Sci. 2023, 24, 1450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, X. Recent advances of tryptanthrin and its derivatives as potential anticancer agents. RSC Med. Chem. 2024, 15, 1127–1147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaur, S.; Mendonca, P.; Soliman, K.F.A. The Anticancer Effects and Therapeutic Potential of Kaempferol in Triple-Negative Breast Cancer. Nutrients 2024, 16, 2392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimarães, J.T.; Keating, E.; Martel, F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef] [PubMed]
- Furlan, V.; Bren, U. Protective Effects of [6]-Gingerol Against Chemical Carcinogens: Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joo, J.H.; Hong, S.S.; Cho, Y.R.; Seo, D.W. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of AKT and p38MAPK activity. Oncol. Rep. 2016, 35, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Vinod, B.S.; Antony, J.; Nair, H.H.; Puliyappadamba, V.T.; Saikia, M.; Narayanan, S.S.; Bevin, A.; Anto, R.J. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013, 4, e505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakalli-Tecim, E.; Uyar-Arpaci, P.; Guray, N.T. Identification of Potential Therapeutic Genes and Pathways in Phytoestrogen Emodin Treated Breast Cancer Cell Lines via Network Biology Approaches. Nutr. Cancer 2022, 74, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Stompor-Gorący, M. The Health Benefits of Emodin, a Natural Anthraquinone Derived from Rhubarb-A Summary Update. Int. J. Mol. Sci. 2021, 22, 9522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoppe, C.; Kutschan, S.; Dörfler, J.; Büntzel, J.; Büntzel, J.; Huebner, J. Zinc as a complementary treatment for cancer patients: A systematic review. Clin. Exp. Med. 2021, 21, 297–313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olsen, V.; Mørland, J. Forgiftning med arsen [Arsenic poisoning]. Tidsskr. Nor. Laegeforen. 2004, 124, 2750–2753. [Google Scholar] [PubMed]
- Hosseininasab, S.S.; Naderifar, M.; Akbarizadeh, M.R.; Hashemi, N.; Ghaderi, M.; Pajavand, H.; Satarzadeh, N.; Dousari, A.S. Synthesized arsenic nanoparticles and their high potential in biomedical applications: A review. Biotechnol. Bioeng. 2024, 121, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, X.; Chang, X.; Liang, Z.; Lv, L.; Shan, M.; Lu, Q.; Wen, Z.; Gust, R.; Liu, W. Recent development of gold(I) and gold(III) complexes as therapeutic agents for cancer diseases. Chem. Soc. Rev. 2022, 51, 5518–5556. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Luo, M.; Liu, H.; Wei, S. Recent Advances of Gold Compounds in Anticancer Immunity. Front. Chem. 2020, 8, 543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium Compounds as Novel Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, P.; Wang, P.; Chen, H.; Xu, Y.; Ge, J.; Tian, Z.; Yan, Z. Potential of Copper and Copper Compounds for Anticancer Applications. Pharmaceuticals 2023, 16, 234. [Google Scholar] [CrossRef]
- Molinaro, C.; Martoriati, A.; Pelinski, L.; Cailliau, K. Copper Complexes as Anticancer Agents Targeting Topoisomerases I and II. Cancers 2020, 12, 2863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Lin, W. Platinum-based combination nanomedicines for cancer therapy. Curr. Opin. Chem. Biol. 2023, 74, 102290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ausina, P.; Branco, J.R.; Demaria, T.M.; Esteves, A.M.; Leandro, J.G.B.; Ochioni, A.C.; Mendonça, A.P.M.; Palhano, F.L.; Oliveira, M.F.; Abou-Kheir, W.; et al. Acetylsalicylic acid and salicylic acid present anticancer properties against melanoma by promoting nitric oxide-dependent endoplasmic reticulum stress and apoptosis. Sci. Rep. 2020, 10, 19617. [Google Scholar] [CrossRef] [PubMed]
- Grancher, A.; Michel, P.; Di Fiore, F.; Sefrioui, D. Colorectal cancer chemoprevention: Is aspirin still in the game? Cancer Biol. Ther. 2022, 23, 446–461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Chen, H.; Hu, B.; Shi, J.; Chen, Y.; Huang, K. New insights into the therapeutic potentials of statins in cancer. Front. Pharmacol. 2023, 14, 1188926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaky, M.Y.; Fan, C.; Zhang, H.; Sun, X.-F. Unraveling the Anticancer Potential of Statins: Mechanisms and Clinical Significance. Cancers 2023, 15, 4787. [Google Scholar] [CrossRef]
- Cao, M.; Duan, Z.; Wang, X.; Gong, P.; Zhang, L.; Ruan, B. Curcumin Promotes Diabetic Foot Ulcer Wound Healing by Inhibiting miR-152-3p and activating the FBN1/TGF-β Pathway. Mol. Biotechnol. 2024, 66, 1266–1278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsimafeyeu, I.; Makhov, P.; Ovcharenko, D.; Smith, J.; Khochenkova, Y.; Olshanskaya, A.; Khochenkov, D. A novel anti-FGFR1 monoclonal antibody OM-RCA-01 exhibits potent antitumor activity and enhances the efficacy of immune checkpoint inhibitors in lung cancer models. Immuno-Oncol. Technol. 2024, 23, 100725. [Google Scholar] [CrossRef]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, J.; Cui, S.; Li, S.; Du, C.; Tian, J.; Wan, S.; Qian, Z.; Gu, Y.; Chen, W.R.; Wang, G. Targeted Cancer Therapy with a 2-Deoxyglucose–Based Adriamycin Complex. Cancer Res. 2013, 73, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Simanek, E.E. Cancer Therapies Utilizing the Camptothecins: A Review of the in Vivo Literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, C.; Li, X.; Chen, F.; Li, G. Camptothecin enhances the anti-tumor effect of low-dose apatinib combined with PD-1 inhibitor on hepatocellular carcinoma. Sci. Rep. 2024, 14, 7140, Erratum in Sci. Rep. 2024, 14, 8787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heider, C.G.; Itenberg, S.A.; Rao, J.; Ma, H.; Wu, X. Mechanisms of Cannabidiol (CBD) in Cancer Treatment: A Review. Biology 2022, 11, 817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ivanov, V.N.; Wu, J.; Wang, T.J.C.; Hei, T.K. Inhibition of ATM kinase upregulates levels of cell death induced by cannabidiol and γ-irradiation in human glioblastoma cells. Oncotarget 2019, 10, 825–846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pathak, K.; Pathak, M.P.; Saikia, R.; Gogoi, U.; Sahariah, J.J.; Zothantluanga, J.H.; Samanta, A.; Das, A. Cancer Chemotherapy via Natural Bioactive Compounds. Curr. Drug Discov. Technol. 2022, 19, e310322202888. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Nanni, M.; Rodin, G.; Li, M.; Caruso, R. The use of antidepressants in oncology: A review and practical tips for oncologists. Ann. Oncol. 2018, 29, 101–111. [Google Scholar] [CrossRef]
- Solek, P.; Koszla, O.; Mytych, J.; Badura, J.; Chelminiak, Z.; Cuprys, M.; Fraczek, J.; Tabecka-Lonczynska, A.; Koziorowski, M. Neuronal life or death linked to depression treatment: The interplay between drugs and their stress-related outcomes relate to single or combined drug therapies. Apoptosis 2019, 24, 773–784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, L.; Zhao, Y.; Schwarz, B.; Mysliwietz, J.; Hartig, R.; Camaj, P.; Bao, Q.; Jauch, K.W.; Guba, M.; Ellwart, J.W.; et al. Verapamil inhibits tumor progression of chemotherapy-resistant pancreatic cancer side population cells. Int. J. Oncol. 2016, 49, 99–110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pluchino, K.M.; Hall, M.D.; Goldsborough, A.S.; Callaghan, R.; Gottesman, M.M. Collateral sensitivity as a strategy against cancer multidrug resistance. Drug Resist. Updat. 2012, 15, 98–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, M.; Hu, X.; Wang, Y.; Yao, X.; Zhang, W.; Yu, C.; Cheng, F.; Li, J.; Fang, Q. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol. Res. 2021, 163, 105207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Intuyod, K.; Hahnvajanawong, C.; Pinlaor, P.; Pinlaor, S. Anti-parasitic Drug Ivermectin Exhibits Potent Anticancer Activity Against Gemcitabine-resistant Cholangiocarcinoma In Vitro. Anticancer. Res. 2019, 39, 4837–4843. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.K. Medicine. How thalidomide works against cancer. Science 2014, 343, 256–257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, S.; Wang, F.; Hsieh, T.C.; Wu, J.M.; Wu, E. Thalidomide-a notorious sedative to a wonder anticancer drug. Curr. Med. Chem. 2013, 20, 4102–4108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- AboYoussef, A.M.; Khalaf, M.M.; Malak, M.N.; Hamzawy, M.A. Repurposing of sildenafil as antitumour; induction of cyclic guanosine monophosphate/protein kinase G pathway, caspase-dependent apoptosis and pivotal reduction of Nuclear factor kappa light chain enhancer of activated B cells in lung cancer. J. Pharm. Pharmacol. 2021, 73, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Elsherbeny, A.; Pittalà, V.; Fallica, A.N.; Alghamdi, M.A.; Greish, K. The Potential Role of Sildenafil in Cancer Management through EPR Augmentation. J. Pers. Med. 2021, 11, 585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pantziarka, P.; Bouche, G.; Sukhatme, V.; Meheus, L.; Rooman, I.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)-Propranolol as an anti-cancer agent. Ecancermedicalscience 2016, 10, 680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fjæstad, K.Y.; Rømer, A.M.A.; Goitea, V.; Johansen, A.Z.; Thorseth, M.-L.; Carretta, M.; Engelholm, L.H.; Grøntved, L.; Junker, N.; Madsen, D.H. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene 2022, 41, 1364–1375. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Hou, L.; Wang, W.; Li, K.; Zhang, Z.; Du, B.; Kong, D. Digoxin exerts anticancer activity on human nonsmall cell lung cancer cells by blocking PI3K/AKT pathway. Biosci. Rep. 2021, 41, BSR20211056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tai, C.J.; Yang, Y.H.; Tseng, T.G.; Chang, F.R.; Wang, H.C. Association between Digoxin Use and Cancer Incidence: A Propensity Score-Matched Cohort Study with Competing Risk Analysis. Front. Pharmacol. 2021, 12, 564097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chong, K.; Subramanian, A.; Sharma, A.; Mokbel, K. Measuring IGF-1, ER-α and EGFR expression can predict tamoxifen-resistance in ER-positive breast cancer. Anticancer Res. 2011, 31, 23–32. [Google Scholar] [PubMed]
- Buijs, S.M.; Koolen, S.L.W.; Mathijssen, R.H.J.; Jager, A. Tamoxifen Dose De-Escalation: An Effective Strategy for Reducing Adverse Effects? Drugs 2024, 84, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Zhu, Y.; Wu, Z.; Cui, C.; Cai, F. Anticancer Mechanisms of Salinomycin in Breast Cancer and Its Clinical Applications. Front. Oncol. 2021, 11, 654428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qi, D.; Liu, Y.; Li, J.; Huang, J.H.; Hu, X.; Wu, E. Salinomycin as a potent anticancer stem cell agent: State of the art and future directions. Med. Res. Rev. 2022, 42, 1037–1063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Podhorecka, M.; Ibanez, B.; Dmoszyńska, A. Metformin—Its potential anti-cancer and anti-aging effects. Postep. Hig. Med. Dosw. 2017, 71, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Aljofan, M.; Riethmacher, D. Anticancer activity of metformin: A systematic review of the literature. Future Sci. OA 2019, 5, FSO410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiao, Y.; Hannafon, B.N.; Ding, W.Q. Disulfiram’s Anticancer Activity: Evidence and Mechanisms. Anticancer Agents Med. Chem. 2016, 16, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.; Rohondia, S.; Khan, R.; Dou, Q.P. Repurposing Disulfiram as an Anti-Cancer Agent: Updated Review on Literature and Patents. Recent Pat. Anticancer Drug Discov. 2019, 14, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.X.; Han, B.O.; Fang, W.H.; Tran, H.D.; Hoang, C.; Shaw, D.G.; Nguyen, T.Q. The Rationality of Implementation of Dimethyl Sulfoxide as Differentiation-inducing Agent in Cancer Therapy. Cancer Diagn. Progn. 2023, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osman, A.M.M.; Alqahtani, A.A.; Damanhouri, Z.A.; Al-Harthy, S.E.; ElShal, M.F.; Ramadan, W.S.; Kamel, F.; Osman, M.A.M.; Khan, L.M. Dimethylsulfoxide excerbates cisplatin-induced cytotoxicity in Ehrlich ascites carcinoma cells. Cancer Cell Int. 2015, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and Antioxidant Effects of Vitamin C in Cancer in correspondence to Its Dietary and Pharmacological Concentrations. Oxid. Med. Cell. Longev. 2019, 2019, 7286737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uetaki, M.; Tabata, S.; Nakasuka, F.; Soga, T.; Tomita, M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci. Rep. 2015, 5, 13896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mussa, A.; Mohd Idris, R.A.; Ahmed, N.; Ahmad, S.; Murtadha, A.H.; Tengku Din, T.A.D.A.A.; Yean, C.Y.; Wan Abdul Rahman, W.F.; Mat Lazim, N.; Uskoković, V.; et al. High-Dose Vitamin C for Cancer Therapy. Pharmaceuticals 2022, 15, 711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zasowska-Nowak, A.; Nowak, P.J.; Ciałkowska-Rysz, A. High-Dose Vitamin C in Advanced-Stage Cancer Patients. Nutrients 2021, 13, 735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michelakis, E.D.; Webster, L.; Mackey, J.R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer 2008, 99, 989–994. [Google Scholar] [CrossRef]
- Tataranni, T.; Piccoli, C. Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications. Oxid. Med. Cell. Longev. 2019, 2019, 8201079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brandsma, D.; Dorlo, T.P.; Haanen, J.H.; Beijnen, J.H.; Boogerd, W. Severe encephalopathy and polyneuropathy induced by dichloroacetate. J. Neurol. 2010, 257, 2099–2100. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, D.; Chitjian, C.; Kashkin, A.; Fanelli, A.; Ovcharenko, V. Two dichloric compounds inhibit in vivo U87 xenograft tumor growth. Cancer Biol. Ther. 2019, 20, 1281–1289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feldman, R.C.; Hyman, D.A.; Price, W.N.; Ratain, M.J. Negative innovation: When patents are bad for patients. Nat. Biotechnol. 2021, 39, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.E.; Hassanein, M.; Razvi, Y.; Shaul, R.Z.; Denburg, A. Institutional Priority-Setting for Novel Drugs and Therapeutics: A Qualitative Systematic Review. Int. J. Health Policy Manag. 2024, 13, 7494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schucht, P.; Roccaro-Waldmeyer, D.M.; Murek, M.; Zubak, I.; Goldberg, J.; Falk, S.; Dahlweid, F.M.; Raabe, A. Exploring Novel Funding Strategies for Innovative Medical Research: The HORAO Crowdfunding Campaign. J. Med. Internet Res. 2020, 22, e19715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Tao, X.; Ji, B.; Wang, R.; Sörensen, S. The Success of Cancer Crowdfunding Campaigns: Project and Text Analysis. J. Med. Internet Res. 2023, 25, e44197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zenone, M.; Snyder, J.; Caulfield, T. Crowdfunding Cannabidiol (CBD) for Cancer: Hype and Misinformation on GoFundMe. Am. J. Public Health 2020, 110, S294–S299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riaz, I.B.; Khan, M.A.; Haddad, T.C. Potential application of artificial intelligence in cancer therapy. Curr. Opin. Oncol. 2024, 36, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Tarique, M. Artificial Intelligence (AI) and Nuclear Features from the Fine Needle Aspirated (FNA) Tissue Samples to Recognize Breast Cancer. J. Imaging 2024, 10, 201. [Google Scholar] [CrossRef]
- Ziegelmayer, S.; Marka, A.W.; Strenzke, M.; Lemke, T.; Rosenkranz, H.; Scherer, B.; Huber, T.; Weiss, K.; Makowski, M.R.; Karampinos, D.C.; et al. Speed and efficiency: Evaluating pulmonary nodule detection with AI-enhanced 3D gradient echo imaging. Eur. Radiol. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Z.; Gong, C.; Zhang, J.; Zhao, J.; Zhang, X.; Liu, X.; Li, B.; Li, R.; Shi, Z.; et al. Targeting treatment resistance: Unveiling the potential of RNA methylation regulators and TG-101,209 in pan-cancer neoadjuvant therapy. J. Exp. Clin. Cancer Res. 2024, 43, 232. [Google Scholar] [CrossRef]
- Pasha, S.A.; Khalid, A.; Levy, T.; Demyan, L.; Hartman, S.; Newman, E.; Weiss, M.J.; King, D.A.; Zanos, T.; Melis, M. Machine learning to predict completion of treatment for pancreatic cancer. J. Surg. Oncol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Goumas, G.; Dardavesis, T.I.; Syrigos, K.; Syrigos, N.; Simou, E. Chatbots in Cancer Applications, Advantages and Disadvantages: All that Glitters Is Not Gold. J. Pers. Med. 2024, 14, 877. [Google Scholar] [CrossRef]
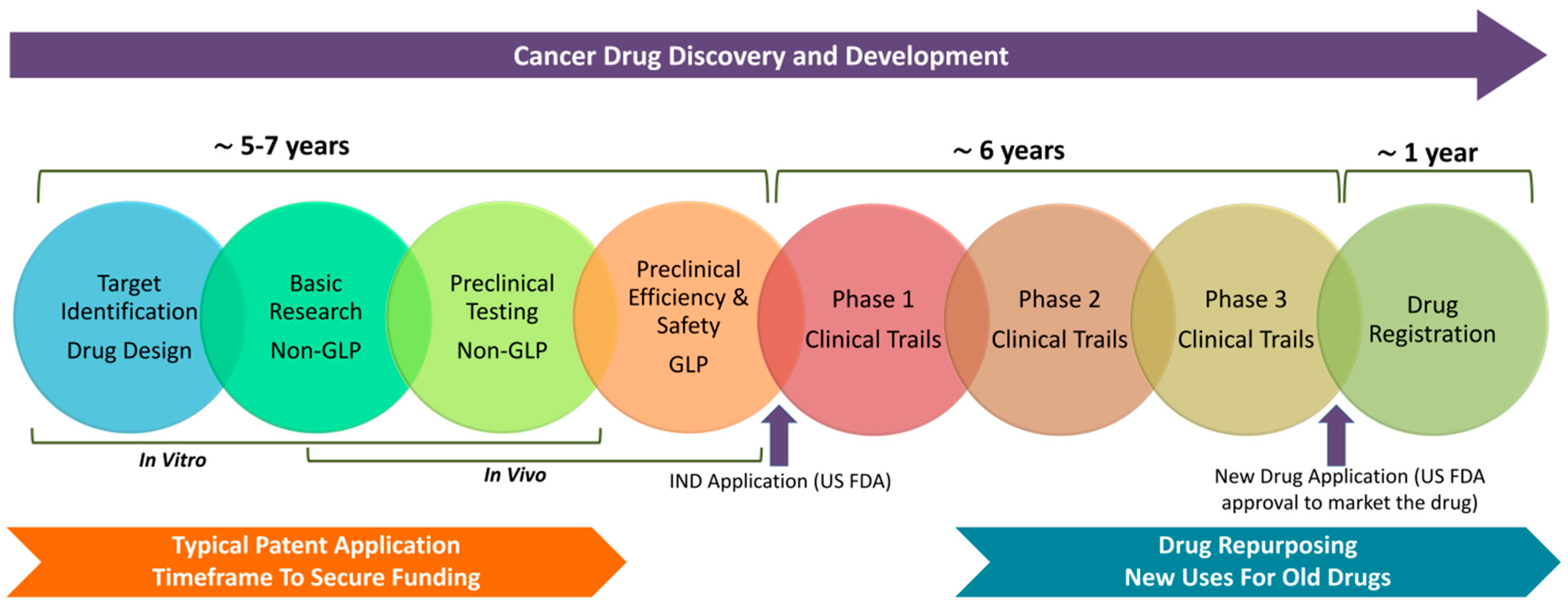
| Expanded Definitions of Novelty and Non-Obviousness | Allow incremental innovations and broaden non-obviousness for significant clinical benefits. |
| Patent Eligibility for Naturally Derived Substances | Allow patents for novel formulations and combinations with conventional drugs. |
| Reduced Stringency for Early-Stage Innovations | Allow provisional patents for early-stage therapies, with a fast-track for rare cancers. |
| Incentivizing Research on Synergistic Combinations | Allow less stringent patenting criteria for multi-modal approaches and synergistic combinations |
| Data-Driven Patents for Personalized Medicine | Grant patents for precision medicine and AI-driven combination therapies. |
| Collaboration and Shared Patents | Encourage co-patent models and patent pools for collaborative development. |
| Incentives for Open Innovation and Public Health Benefits | Extend patent periods for unmet needs and offer tax credits for socially responsible licensing. |
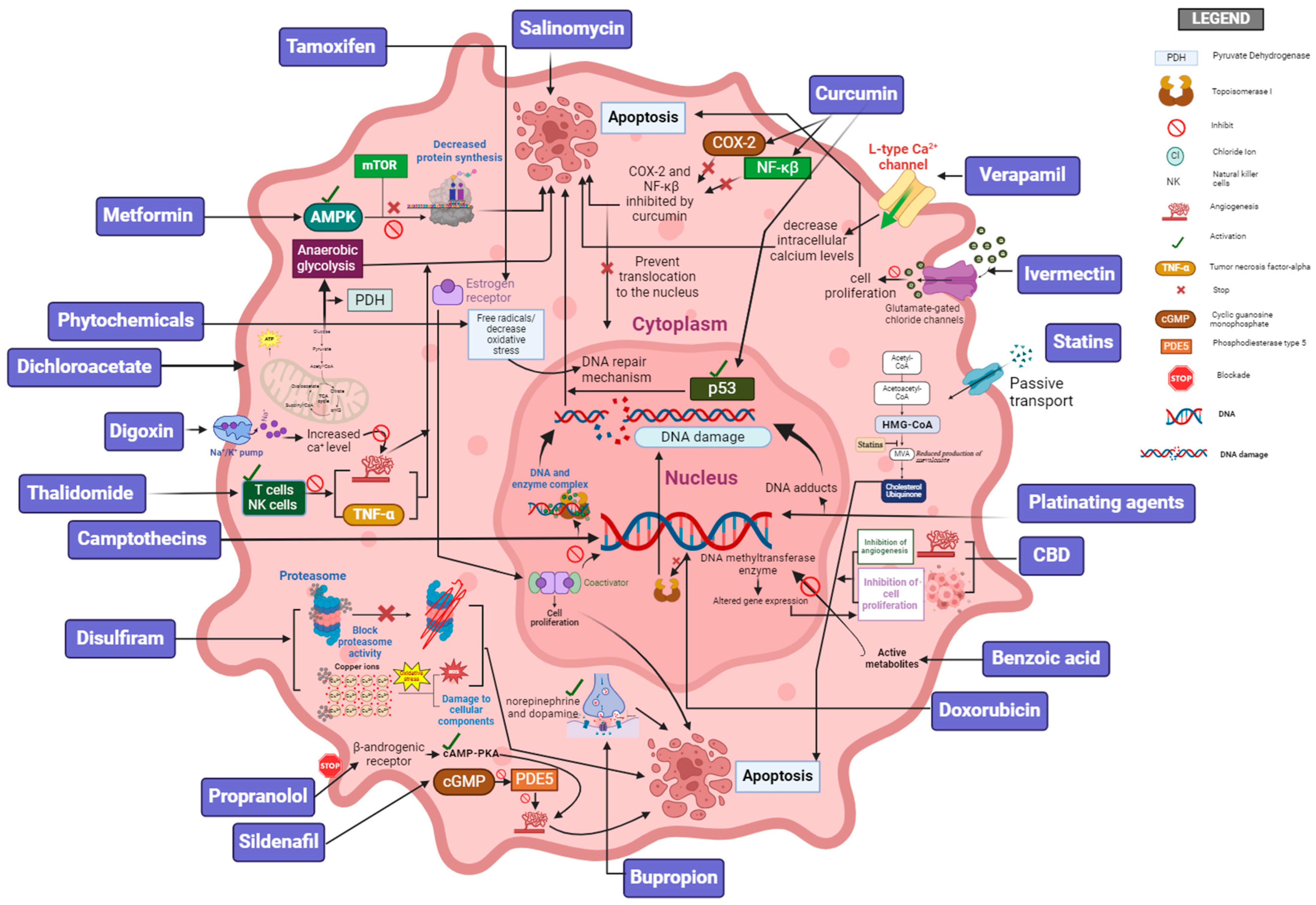
| Compound | Mechanism of Action |
|---|---|
| Adriamycin (e.g., Doxorubicin) | This drug intercalates into the DNA, disrupting the function of topoisomerase II, which leads to breaks in double-stranded DNA and prevents DNA and RNA synthesis. These drugs also generate free radicals, causing additional damage to cellular components. Adriamycin is used in the treatment of a wide variety of cancers, including breast cancer, lymphomas, and leukemia. |
| Platinating agents (e.g., Cisplatin, Carboplatin) | These are chemotherapy drugs that work by forming platinum–DNA adducts, which in turn interfere with DNA replication and transcription. This leads to cell cycle arrest and apoptosis. Platinates are used to treat various cancers, including lung, ovarian, and testicular cancer. |
| Camptothecins (e.g., Irinotecan, Topotecan) | These drugs inhibit the enzyme topoisomerase I, which is essential for DNA replication. By stabilizing the temporary breaks that topoisomerase I creates in the DNA double helix, camptothecins cause DNA damage that leads to cell death. Camptothecins are used in the treatment of colorectal, ovarian, and small cell lung cancer, among other conditions. |
| Salicylic Acid (e.g., Aspirin) | Salicylic acid inhibits the activity of cyclooxygenase enzymes (COX-1 and COX-2), leading to a reduction in the synthesis of prostaglandins, which are involved in inflammation and may play a role in cancer progression. It works through activation of the AKT/mTOR and AMPK-dependent pathways and is associated with a reduced risk of colorectal cancer. |
| Statins (e.g., Simvastatin, Pitavastatin) | Statins affect RAS and Rho isoprenylation, signal transduction, and DNA synthesis. Statins regulate autophagy, which plays a crucial role in the tumor suppressive process. Statins can induce ferroptosis and pyroptosis. |
| Antidepressants (e.g., Bupropion, Duloxetine) | These drugs impact cancer cell growth, death, and spread by affecting serotonin pathways, especially through the 5-HT1A and 5-HT2A/2C receptors. SSRIs and TCAs encourage cancer cell death by influencing apoptotic pathways and boosting the tumor suppressor gene p53. They reduce VEGF expression, potentially slowing tumor growth by affecting natural killer cells and enhancing phagocytosis. |
| Verapamil | A calcium channel blocker, verapamil has demonstrated potential anticancer effects through its interference with multidrug resistance (MDR) pathways and plays a functional role in cell cycle alteration. |
| Curcumin | The active component of turmeric, curcumin exhibits anti-inflammatory and anticancer properties, likely through the modulation of various molecular targets, including NF-κB, COX-2, and p53. Clinical utility has been limited by poor bioavailability, prompting research into formulation strategies. |
| Phytochemicals (e.g., Cannabidiol, CBD) | Phytocannabinoids from the cannabis plant exhibit a variety of proposed mechanisms, including anti-inflammatory, antioxidant, and anticonvulsant effects. CBD has been shown to induce apoptosis and inhibit cancer cell proliferation and angiogenesis via multiple cellular pathways. |
| Ivermectin | Ivermectin is a macrolide antiparasitic drug that is widely used for the treatment of many parasitic diseases. It suppresses cancer cell proliferation, cell cycle arrest, metastasis, and proliferation via inhibition of the PAK1 signaling and WNT/TCF pathway. |
| Thalidomide | Originally developed as a sedative, thalidomide has been repurposed to exhibit significant anticancer properties through a multifaceted mechanism of action, including cytokine action and modulating the release of inflammatory mediators like TNF-α. |
| Sildenafil | Sildenafil is a drug primarily prescribed for the treatment of erectile dysfunction. It exerts its biological effects through the inhibition of phosphodiesterase PDE-5. |
| Propranolol | Propranolol induces apoptosis and inhibits cancer cell proliferation by blocking the β-adrenergic receptors, thereby disrupting growth factor signaling. It exhibits anti-angiogenic properties by reducing factors essential for tumor blood vessel formation. |
| Digoxin | Digoxin, known for treating heart conditions, exhibits potential anticancer effects by inhibiting the Na+/K+-ATPase pump, affecting cellular ion balance, and increasing intracellular calcium. Digoxin targets the HIF-1α and AKT/mTOR pathways, essential in hypoxic tumor environments and cell proliferation. |
| Tamoxifen | A selective estrogen receptor modulator (SERM), tamoxifen is crucial in treating estrogen receptor-positive breast cancer due to its ability to block estrogen’s effects on breast tissue. Tamoxifen binds to estrogen receptors, preventing estrogen from promoting cancer cell growth. It modulates gene expression to inhibit cell proliferation and induce apoptosis, contributing to its anticancer effects. |
| Salinomycin | Identified as a potential cancer stem cell (CSC) targeting agent, salinomycin selectively kills CSCs over non-stem cancer cells, potentially offering a way to overcome resistance and prevent tumor recurrence. |
| Metformin | Originally developed as an antidiabetic drug, metformin has been observed to exhibit anticancer effects, likely through the activation of AMP-activated protein kinase (AMPK), which in turn inhibits the mTOR pathway, leading to decreased protein synthesis and cell proliferation. |
| Disulfiram | Used in the treatment of alcohol dependence, disulfiram has shown potential as an anticancer agent by inhibiting proteasome, leading to the accumulation of misfolded proteins and inducing cancer cell death. Disulfiram also likely chelates copper, forming a complex that induces oxidative stress and kills cancer cells. |
| Dichloroacetate | This drug modulates the activity of pyruvate dehydrogenase, an enzyme that influences cellular metabolism. By shifting cancer cell metabolism from anaerobic glycolysis to glucose oxidation, DCA promotes apoptosis in cancer cells, likely through the Warburg effect, a characteristic metabolic alteration observed in many cancer cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovcharenko, D.; Mukhin, D.; Ovcharenko, G. Alternative Cancer Therapeutics: Unpatentable Compounds and Their Potential in Oncology. Pharmaceutics 2024, 16, 1237. https://doi.org/10.3390/pharmaceutics16091237
Ovcharenko D, Mukhin D, Ovcharenko G. Alternative Cancer Therapeutics: Unpatentable Compounds and Their Potential in Oncology. Pharmaceutics. 2024; 16(9):1237. https://doi.org/10.3390/pharmaceutics16091237
Chicago/Turabian StyleOvcharenko, Dmitriy, Dmitry Mukhin, and Galina Ovcharenko. 2024. "Alternative Cancer Therapeutics: Unpatentable Compounds and Their Potential in Oncology" Pharmaceutics 16, no. 9: 1237. https://doi.org/10.3390/pharmaceutics16091237
APA StyleOvcharenko, D., Mukhin, D., & Ovcharenko, G. (2024). Alternative Cancer Therapeutics: Unpatentable Compounds and Their Potential in Oncology. Pharmaceutics, 16(9), 1237. https://doi.org/10.3390/pharmaceutics16091237







